Abstract
Various caseinoglycopeptide derivatives prepared from mammalian milk were evaluated as inhibitors of hemagglutinations mediated by Actinomyces viscosus Ny1, Streptococcus sanguis OMZ9, and, for comparative purposes, plant lectins from Arachis hypogaea and Bauhinia purpurea. It was found that recognition of the beta-D-galactose-(1----3)-2-acetamido-2-deoxy-D-galactose carbohydrate chain by Actinomyces viscosus Ny1 organisms and Arachis hypogaea and B. purpurea agglutinins had similar structural requirements; in all cases, the desialylated bovine caseinoglycomacropeptide, on which several units of the above mentioned disaccharide are clustered, behaved as the most potent hemagglutination inhibitor. By contrast, none of the preparations tested inhibited erythrocyte agglutination by S. sanguis OMZ9. Thus, the desialylated bovine caseinoglycomacropeptide acts as a potent and specific inhibitor of oral Actinomyces adhesion to cell membranes (a soft surface) and could be used as a probe for the study of recognition mechanisms mediated by Actinomyces galactose-binding lectins. During the present study, both native and desialylated variants of the same bovine glycomacropeptide also totally prevented the adhesion of Actinomyces viscosus Ny1, S. sanguis OMZ9, and S. mutans OMZ176 to polystyrene surfaces. Comparative evaluations of various structurally different compounds gave the following results. Neither mono- nor disaccharides related to caseinoglycopeptide carbohydrates prevented adhesion; highly positively or negatively charged polypeptides and polysaccharides were either not or only moderately active. Besides these glycomacropeptides, an inhibitory activity was also exhibited by other mucin-type glycoproteins carrying short O-linked carbohydrate chains (including bovine submaxillary mucin), polyethylene glycol, and bovine serum albumin. Consequently, caseinoglycopeptide prevention of oral bacterial adhesion to polystyrene tubes (a hard surface) takes place with no species specificity and can be compared to nonspecific inhibition exhibited by various polymers with very different structural characteristics.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALAIS C., JOLLES P. [Comparative study of the caseinoglycopeptides formed by the action of rennin on caseins from the cow, sheep and goat. II. Study of the non-peptide portion]. Biochim Biophys Acta. 1961 Aug 5;51:315–322. doi: 10.1016/0006-3002(61)90172-x. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., Cisar J. O., Sandberg A. L. A 160-kilodalton epithelial cell surface glycoprotein recognized by plant lectins that inhibit the adherence of Actinomyces naeslundii. Infect Immun. 1986 Jun;52(3):840–845. doi: 10.1128/iai.52.3.840-845.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. J., Cisar J. O., Vatter A. E., Sandberg A. L. Lectin-dependent attachment of Actinomyces naeslundii to receptors on epithelial cells. Infect Immun. 1984 Nov;46(2):459–464. doi: 10.1128/iai.46.2.459-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., David V. A., Curl S. H., Vatter A. E. Exclusive presence of lactose-sensitive fimbriae on a typical strain (WVU45) of Actinomyces naeslundii. Infect Immun. 1984 Nov;46(2):453–458. doi: 10.1128/iai.46.2.453-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Wheeler T. T., Cisar J. O. Specific inhibition of adsorption of Actinomyces viscosus T14V to saliva-treated hydroxyapatite by antibody against type 1 fimbriae. Infect Immun. 1984 Feb;43(2):497–501. doi: 10.1128/iai.43.2.497-501.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Fillery E. D., Chan K. H., Grove D. A. Sialidase-enhanced lectin-like mechanism for Actinomyces viscosus and Actinomyces naeslundii hemagglutination. Infect Immun. 1980 Feb;27(2):335–343. doi: 10.1128/iai.27.2.335-343.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
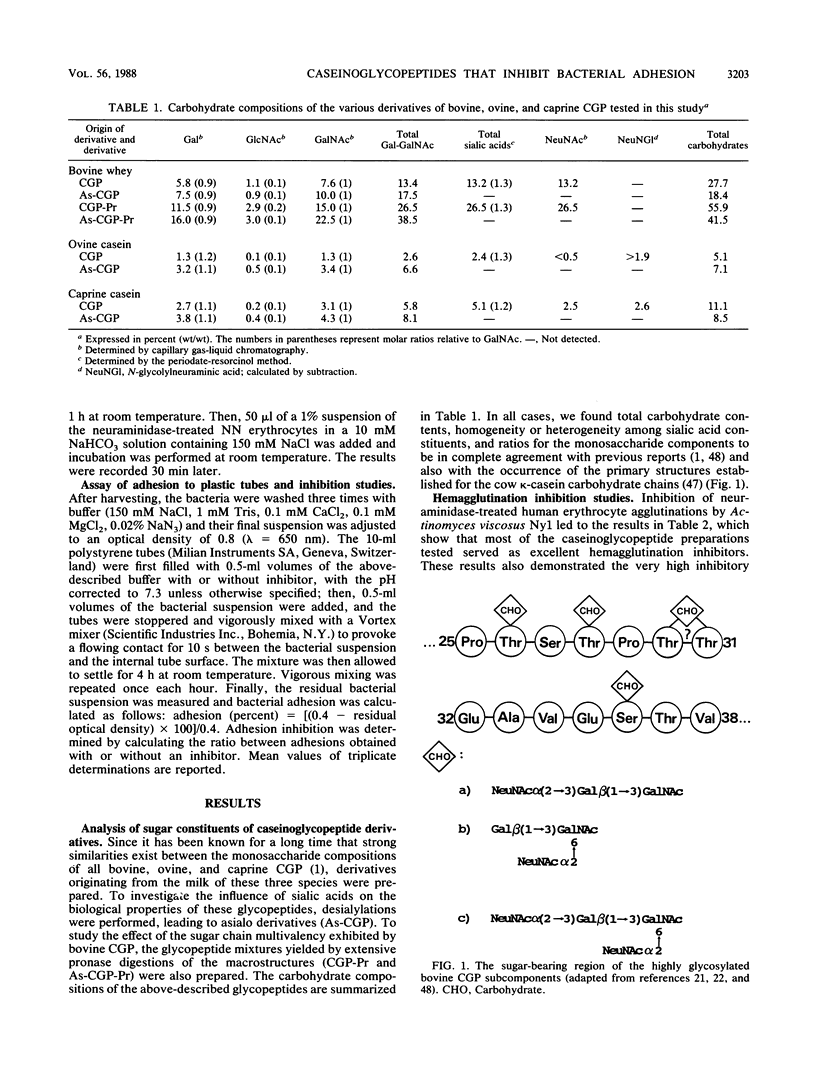
- Fournet B., Fiat A. M., Alais C., Jollès P. Cow kappa-casein: structure of the carbohydrate portion. Biochim Biophys Acta. 1979 Feb 26;576(2):339–346. doi: 10.1016/0005-2795(79)90409-4. [DOI] [PubMed] [Google Scholar]
- Heeb M. J., Costello A. H., Gabriel O. Characterization of a galactose-specific lectin from Actinomyces viscosus by a model aggregation system. Infect Immun. 1982 Dec;38(3):993–1002. doi: 10.1128/iai.38.3.993-1002.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeb M. J., Marini A. M., Gabriel O. Factors affecting binding of galacto ligands to Actinomyces viscosus lectin. Infect Immun. 1985 Jan;47(1):61–67. doi: 10.1128/iai.47.1.61-67.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOLLES P., LAIS C., JOLLES J. [Comparative study of the caseino-glycopeptides formed by the action of rennin on caseins from the cow, sheep and goat. I. Study of the peptide portion]. Biochim Biophys Acta. 1961 Aug 5;51:309–314. doi: 10.1016/0006-3002(61)90171-8. [DOI] [PubMed] [Google Scholar]
- Jost R., Monti J. C. Partial enzymatic hydrolysis of whey protein by trypsin. J Dairy Sci. 1977 Sep;60(9):1387–1393. doi: 10.3168/jds.S0022-0302(77)84041-1. [DOI] [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- Kaifu R., Osawa T. Syntheses of O-beta-D-galactopyranosyl-(1 leads to 3)-0-(2-acetamido-2-deoxy-alpha(and -beta)-D-galactopyranosyl)-N-tosyl-L-serine and their interaction with D-galactose-binding lectins. Carbohydr Res. 1979 Mar;69:79–88. doi: 10.1016/s0008-6215(00)85753-5. [DOI] [PubMed] [Google Scholar]
- Kanamori M., Doi H., Ideno S., Ibuki F. Presence of O-glycosidic linkage through serine residue in kappa-casein component from bovine mature milk. J Nutr Sci Vitaminol (Tokyo) 1981;27(3):231–241. doi: 10.3177/jnsv.27.231. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Multigeneric aggregations among oral bacteria: a network of independent cell-to-cell interactions. J Bacteriol. 1986 Nov;168(2):851–859. doi: 10.1128/jb.168.2.851-859.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Barlow J. J., Matta K. L. Structural preferences of beta-galactoside-reactive lectins on Actinomyces viscosus T14V and Actinomyces naeslundii WVU45. Infect Immun. 1983 Aug;41(2):848–850. doi: 10.1128/iai.41.2.848-850.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Vatter A. E., Cisar J. O., McNeil M. R., Bush C. A., Tjoa S. S., Fennessey P. V. A polysaccharide from Streptococcus sanguis 34 that inhibits coaggregation of S. sanguis 34 with Actinomyces viscosus T14V. J Bacteriol. 1988 May;170(5):2229–2235. doi: 10.1128/jb.170.5.2229-2235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergenhagen S. E., Sandberg A. L., Chassy B. M., Brennan M. J., Yeung M. K., Donkersloot J. A., Cisar J. O. Molecular basis of bacterial adhesion in the oral cavity. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S467–S474. doi: 10.1093/clinids/9.supplement_5.s467. [DOI] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Tabak L. A., Reddy M. S. Specificity of salivary-bacterial interactions: II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAc alpha 2, 3Ga1 beta 1, 3Ga1NAc sequence. Biochem Biophys Res Commun. 1982 May 31;106(2):390–396. doi: 10.1016/0006-291x(82)91122-6. [DOI] [PubMed] [Google Scholar]
- Neeser J. R. G.l.c. of O-methyloxime and alditol acetate derivatives of neutral sugars, hexosamines, and sialic acids: "one-pot" quantitative determination of the carbohydrate constituents of glycoproteins and a study of the selectivity of alkaline borohydride reductions. Carbohydr Res. 1985 May 15;138(2):189–198. doi: 10.1016/0008-6215(85)85102-8. [DOI] [PubMed] [Google Scholar]
- Neeser J. R., Schweizer T. F. A quantitative determination by capillary gas-liquid chromatography of neutral and amino sugars (as O-methyloxime acetates), and a study on hydrolytic conditions for glycoproteins and polysaccharides in order to increase sugar recoveries. Anal Biochem. 1984 Oct;142(1):58–67. doi: 10.1016/0003-2697(84)90516-5. [DOI] [PubMed] [Google Scholar]
- Pahud J. J., Monti J. C., Jost R. Allergenicity of whey protein: its modification by tryptic in vitro hydrolysis of the protein. J Pediatr Gastroenterol Nutr. 1985 Jun;4(3):408–413. [PubMed] [Google Scholar]
- Pereira M. E., Kabat E. A., Lotan R., Sharon N. Immunochemical studies on the specificity of the peanut (Arachis hypogaea) agglutinin. Carbohydr Res. 1976 Oct;51(1):107–118. doi: 10.1016/s0008-6215(00)84040-9. [DOI] [PubMed] [Google Scholar]
- Pratt-Terpstra I. H., Weerkamp A. H., Busscher H. J. Adhesion of oral streptococci from a flowing suspension to uncoated and albumin-coated surfaces. J Gen Microbiol. 1987 Nov;133(11):3199–3206. doi: 10.1099/00221287-133-11-3199. [DOI] [PubMed] [Google Scholar]
- Reddy M. S., Levine M. J., Prakobphol A. Oligosaccharide structures of the low-molecular-weight salivary mucin from a normal individual and one with cystic fibrosis. J Dent Res. 1985 Jan;64(1):33–36. doi: 10.1177/00220345850640010601. [DOI] [PubMed] [Google Scholar]
- Revis G. J., Vatter A. E., Crowle A. J., Cisar J. O. Antibodies against the Ag2 fimbriae of Actinomyces viscosus T14V inhibit lactose-sensitive bacterial adherence. Infect Immun. 1982 Jun;36(3):1217–1222. doi: 10.1128/iai.36.3.1217-1222.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg A. L., Mudrick L. L., Cisar J. O., Brennan M. J., Mergenhagen S. E., Vatter A. E. Type 2 fimbrial lectin-mediated phagocytosis of oral Actinomyces spp. by polymorphonuclear leukocytes. Infect Immun. 1986 Nov;54(2):472–476. doi: 10.1128/iai.54.2.472-476.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg A. L., Mudrick L. L., Cisar J. O., Metcalf J. A., Malech H. L. Stimulation of superoxide and lactoferrin release from polymorphonuclear leukocytes by the type 2 fimbrial lectin of Actinomyces viscosus T14V. Infect Immun. 1988 Jan;56(1):267–269. doi: 10.1128/iai.56.1.267-269.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Halbeek H., Dorland L., Vliegenthart J. F., Fiat A. M., Jolles P. A 360-MHz 1H-NMR study of three oligosaccharides isolated from cow kappa-casein. Biochim Biophys Acta. 1980 Jun 26;623(2):295–300. doi: 10.1016/0005-2795(80)90257-3. [DOI] [PubMed] [Google Scholar]


