Abstract
Background and purpose:
To characterize the in vitro motor patterns and the neurotransmitters released by enteric motor neurons (EMNs) in the human sigmoid colon.
Experimental approach:
Sigmoid circular strips were studied in organ baths. EMNs were stimulated by electrical field stimulation (EFS) and through nicotinic ACh receptors.
Key results:
Strips developed weak spontaneous rhythmic contractions (3.67±0.49 g, 2.54±0.15 min) unaffected by the neurotoxin tetrodotoxin (TTX; 1 μM). EFS induced strong contractions during (on, 56%) or after electrical stimulus (off, 44%), both abolished by TTX. Nicotine (1–100 μM) inhibited spontaneous contractions. Latency of off-contractions and nicotine responses were reduced by NG-nitro-L-arginine (1 mM) and blocked after further addition of apamin (1 μM) or the P2Y1 receptor antagonist MRS 2179 (10 μM) and were unaffected by the P2X antagonist NF279 (10 μM) or α-chymotrypsin (10 U mL−1). Amplitude of on- and off-contractions was reduced by atropine (1 μM) and the selective NK2 receptor antagonist Bz-Ala-Ala-D-Trp-Phe-D-Pro-Pro-Nle-NH2 (1 μM). MRS 2179 reduced the amplitude of EFS on- and off-contractions without altering direct muscular contractions induced by ACh (1 nM–1 mM) or substance P (1 nM–10 μM).
Conclusions and implications:
Latency of EFS-induced off-contractions and inhibition of spontaneous motility by nicotine are caused by stimulation of inhibitory EMNs coreleasing NO and a purine acting at muscular P2Y1 receptors through apamin-sensitive K+ channels. EFS-induced on- and off-contractions are caused by stimulation of excitatory EMNs coreleasing ACh and tachykinins acting on muscular muscarinic and NK2 receptors. Prejunctional P2Y1 receptors might modulate the activity of excitatory EMNs. P2Y1 and NK2 receptors might be therapeutic targets for colonic motor disorders.
Keywords: Cotransmission, enteric motor neurons, human colon, motility, neurotransmitters, in vitro colonic motor patterns, organ baths
Introduction
Human colonic motor activity mediates transport and evacuation of luminal contents and is characterized by two distinct types of phasic motor events that are regulated by intrinsic mechanisms: (a) rhythmic contractions allowing mixing of luminal contents and (b) giant migrating contractions (GMCs) that can propagate over long segments of the colon-producing mass movements and propulsion of stools (Rao et al., 2001; Sarna, 2006). The origin, regulatory mechanisms and spatial and temporal characteristics of these contractions are not fully understood, limiting the development of specific drugs to treat colonic motor disorders (Sarna, 2006).
There is substantial evidence to suggest that the cells responsible for generating rhythmic phasic contractions (RPCs) in human and animal models are the interstitial cells of Cajal (ICC; Thuneberg, 1982). ICCs have been identified at the submucosal edge of the human colonic circular muscle layer (Rumessen, 1996) where they might have a pacemaker function, originating slow waves and a continuous rhythm of 2–4 contractions per minute of smooth muscle cells (Rae et al., 1988). The origin of propulsive sigmoid motility is much more complex and involves the activation of extrinsic parasympathetic and sympathetic nerves and/or interneurons in the myenteric plexus, which supply excitatory and inhibitory enteric motor neurons (EMNs) (Hinds et al., 2006). EMNs project to the circular and longitudinal smooth muscle and mediate simple stereotyped reflexes such as peristaltic reflex and GMCs at a local level (Sarna, 2006).
Neuromuscular neurotransmission in the human colon has not been fully characterized. Studies in the 1990s reported that inhibitory neurotransmission in human colonic EMNs involves a nitrergic as well as a non-nitrergic and apamin-sensitive neurotransmitter, probably ATP or a related purine (Boeckxstaens et al., 1993; Keef et al., 1993). P2-purinoceptors in the gastrointestinal tract are divided into two categories: P2X receptors are ligand-gated ion channels and P2Y receptors are G-protein-coupled receptors, with varying effects on organs (Ralevic and Burnstock, 1990). We have recently found that P2Y1 receptors mediate the fast inhibitory junction potential in the human colon (Gallego et al., 2006). P2X purinoceptor agonists cause muscle contraction in the guinea pig ileum (Matsuo et al., 1997) and induce muscle relaxation in the taenia coli and proximal colon (Bultmann et al., 1996). Excitatory neuromuscular transmission seems to be mostly cholinergic in the gastrointestinal tract (Sarna, 2006); however, some recent studies have suggested that tachykinins may be the main excitatory neurotransmitters in human sigmoid colon (Cao et al., 2000, 2006). Both NK1 and NK2 receptors are expressed in human colon smooth muscle and the most prominent subtype involved in colonic contraction is not fully understood (Lecci et al., 2006). In addition, little is known about the regulatory mechanisms of excitatory and inhibitory neuromuscular transmission that control motility in human sigmoid colon (Grider, 1989; Hinds et al., 2006; Cellek et al., 2007). We found recently that P2Y1 receptors are localized in EMNs in the human colon (Gallego et al., 2006) and that P2Y1 agonists can stimulate EMNs in the guinea pig ileum (Gallego et al., 2008). Sacral parasympathetic neurons and interneurons have been shown to provide excitatory input to EMNs in the myenteric plexus, synapsing with nicotinic ACh receptors (nAChRs) on excitatory and inhibitory intrinsic EMNs (Venkova et al., 2002; Sarna, 2006). However, recent studies show that most EMNs receive a mixed fast excitatory synaptic input mediated by both nACh and P2X receptors in other areas of the gastrointestinal tract (Galligan and North, 2004).
The aim of this study, therefore, is (a) to assess the physiological mechanisms responsible for the various types of phasic motor events (RPCs and neurally mediated contractions) in human sigmoid colon in vitro and (b) to characterize, in vitro, the neurotransmitters involved in the mechanical responses induced by the stimulation of excitatory and inhibitory EMNs in the myenteric plexus by (i) electrical field stimulation (EFS) and (ii) stimulation of nACh and P2X receptors. We found that the effects of stimulation of inhibitory EMNs are mediated by the corelease of NO and a purine acting at muscular P2Y1 receptors, the effects of stimulation of excitatory EMNs are mediated by the corelease of ACh and tachykinins acting on muscular muscarinic and NK2 receptors and also found that prejunctional P2Y1 receptors might modulate the activity of excitatory EMNs. Our results suggest that P2Y1 and NK2 receptors might be therapeutic targets for colonic motor disorders.
Materials and methods
Tissue specimens
The experimental protocols were approved by the Ethical Review Board of the Hospital de Mataró, Barcelona, Spain. Informed consent was obtained from all patients before the use of their tissues. Samples of human sigmoid colon were obtained from 71 patients without symptoms of major clinical motility disorders who underwent surgery for rectal cancer (T2/T3 stages, age range: 32–85 years, 45% women). All strips were obtained from macroscopically non-invaded regions.
Preparation of circular muscle strips and isometric tension recording
The colonic segment was cut open longitudinally along the mesenteric border, the mucosal layer was removed and transmural muscle strips (3 mm wide by 10 mm long) were cut in the direction of circular muscular fibres. A silk thread was attached to either end of the strips and they were placed in 15 mL organ baths filled with Krebs solution (37 °C, bubbled with a mixture of 5% CO2/95% O2, pH 7.4). The Krebs solution contained (in mM): glucose 10.1; NaCl 115.48; NaHCO3 21.9; KCl 4.61; NaH2PO4 1.14; CaCl2 2.50 and MgSO4 1.16. Strips were positioned between two parallel platinum wire electrodes 10 mm apart and changes in tension were measured using isometric force transducers, recorded on a chart recorder (model 03 Force Transducer and model 7 Series Polygraph, Grass Instruments Co., Quincy, MA, USA) and digitized (AcqNowledge, MP100, Biopac Systems, Inc, Goleta, CA, USA). After an equilibration period of 1 h (Gallego et al., 2006), strips developed spontaneous phasic contractions and the experiment started. In each experiment, up to six strips from the same specimen were simultaneously studied.
Experimental design
Our experiments included (a) the study of origin and modulation of spontaneous RPCs, (b) the assessment of mechanical responses induced by the stimulation of EMNs by EFS and (c) the study of the responses induced by agonists of nACh or P2X receptors. The EFS was applied by an electrical stimulator (Model S88, Grass Instruments Co.) and a power booster (Stimu-Splitter II, Med-Lab Instruments, Loveland, CO, USA) to obtain six identical and undistorted signals. The 10-s trains of pulses of 0.4 ms duration at 1–40 Hz and 26 V (∼100 mA) were delivered to the electrodes (Farre et al., 2006) and simultaneously recorded with tension tracings through a synchronized transistor–transistor logic signal between the electrical stimulator Grass S88 and the computerized Biopac system. nAChRs were stimulated by nicotine (1–100 μM) and P2X receptors by the specific agonist α-β-methylene-ATP (α-β-met-ATP; 1–100 μM). Neural origin of responses was assessed by the neural blocker tetrodotoxin (TTX; 1 μM), which interrupts Na+ action potentials along the axon of EMNs. The mechanical responses following the stimulation of EMNs were characterized by antagonists such as NG-nitro-L-arginine (L-NNA; 1 mM), apamin (1 μM), the selective P2Y1 receptor antagonist (RA), MRS 2179 (10 μM), the selective P2X1,2,3-RA, NF279 (10 μM), the peptidase, α-chymotrypsin (α-CMT, 10 U mL−1) that cleaves vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP) at the level of tyrosine residues and the selective NK2-RA, Bz-Ala-Ala-D-Trp-Phe-D-Pro-Pro-Nle-NH2 (NK2-RA) and the NK1-RA ((S)1-{2-[3-(3,4-dichlorophenyl)-1-(3-isopropoxyphenyl-acetyl)-piperidin-3-yl]ethyl}-4-phenyl-1 azoniabicyclo(2.2.2.) octane chloride; (SR 140333). The effect of agonists for the proposed putative excitatory and inhibitory neurotransmitters such as ACh (0.01–100 μM), substance P (SP; 0.001–10 μM), sodium nitroprusside (0.001–10 μM) and the specific P2Y1 receptor agonist 2MeSATP (1–100 μM) were also studied.
Data analysis and statistical procedures
Spontaneous RPCs were measured by determining the area under the curve (AUC) of force development vs time (in g min−1) and paired Student's t-tests were used to assess the effect of antagonists and agonists of the proposed putative neurotransmitters (Gallego et al., 2006). The effect of antagonists on RPCs was assessed following a 20-min incubation, the last 10-min period being analysed. Data were normalized with respect to a control period (10 min before the addition of the antagonist) and concentration–response curves were analysed by two-way ANOVA (Farre et al., 2006). The effect of agonists was measured as the AUC of the 2-min period following their addition to the bath and normalized to the control period. The concentration–response curve for each selected agonist was computer fitted using non-linear regression and the EC50 was calculated (GraphPad Prism, Version 4.01, GraphPad Software Inc, La Jolla, CA, USA). Latency of EFS contractions was defined as the period of time from the beginning of EFS to the onset of contraction and maximal amplitude of EFS contractions (in g) was also measured. The effect of specific antagonists for excitatory and inhibitory neurotransmitters on latency and amplitude of on- and off-contractions was analysed by two-way ANOVA for repeated measures. When the two-way ANOVA was significant, the Bonferroni test was carried out to determine the frequencies or concentrations of statistically different responses (Farre et al., 2006). Data are expressed as mean±s.e.mean. P<0.05 was considered statistically significant.
Drugs
NG-nitro-L-arginine, phentolamine, ACh, α-β-met-ATP, sodium nitroprusside, α-CMT and nicotine were obtained from Sigma Chemical Co. (St Louis, MO, USA). SR 140333 was a kind gift of Sanofi Recherche, Montpellier Cédex, France. TTX, atropine sulphate, propranolol and apamin were obtained from the Research Biochemicals International (Natick, MA, USA); MRS 2179, NF 279 and 2MeSATP from Tocris Cookson Inc. (Ellisville, MO, USA) and Bz-Ala-Ala-D-Trp-Phe-D-Pro-Pro-Nle-NH2 (NK2-RA) from Bachem (Torrance, CA, USA). All drugs were dissolved in distilled water except for SR 14033, which were dissolved with ethanol <5% vol/vol and L-NNA, which was dissolved in Krebs solution by sonication. Drug and receptor nomenclature conform to the guidelines of the British Journal of Pharmacology (Alexander et al., 2008).
Results
Spontaneous RPCs
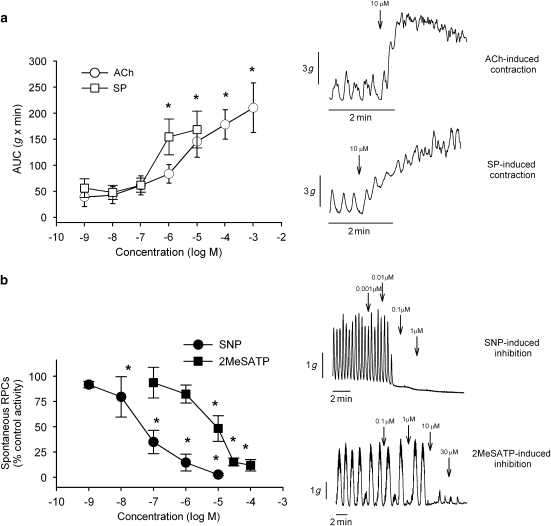
Circular strips of human sigmoid colon developed spontaneous RPCs after 1-h equilibration. The AUC, amplitude and frequency of RPCs were 64±7.9 g min−1, 3.7±0.5 g and 2.5±0.2 contractions per minute (n=31). Up to 30-min incubation of strips with the neural blocker TTX (1 μM; n=17) did not cause a significant change in this pattern of spontaneous motility (68±9 g min−1; NS), showing that RPCs have a non-neural origin (Figure 1a). L-NNA (1 mM, n=9) and apamin (1 μM, n=6) caused an increase in the activity (AUC) of spontaneous RPCs (Figure 1b, P<0.05). In contrast, α-CMT (10 U mL−1, n=4) did not affect RPCs (Figure 1b). Atropine (1 μM, n=12) and the NK2-RA (1 μM, n=7) caused a significant decrease in the activity (AUC) of spontaneous RPCs (P<0.05, Figure 1b). The specific P2Y1-RA, MRS 2179 (10 μM, n=7) also slightly decreased spontaneous RPCs (P<0.05), whereas the specific P2X-RA NF279 (10 μM, n=5) did not affect them (Figure 1b). In experiments during incubation of strips with TTX (1 μM), both ACh (log EC50 −5.33±0.18) and SP (log EC50 −6.36±0.20) induced a concentration-dependent contraction of colon strips (n=7, Figure 2a). The contraction induced by 10 μM SP was significantly antagonized by 74±6% by the NK2 antagonist and by 20±2% by the NK1 antagonist (P<0.005, n=7). The NO donor sodium nitroprusside (log IC50 −7.39±0.11) and the specific P2Y1 receptor agonist 2MeSATP (log IC50 4.77±0.15) inhibited spontaneous RPCs in a concentration-dependent manner (Figure 2b).
Figure 1.
Experimental recordings and summary diagrams showing (a) the effect of the neural blocker tetrodotoxin 1 μM and (b) the effect of antagonists for proposed excitatory and inhibitory neurotransmitters on spontaneous rhythmic phasic contractions (RPCs). Y-axis depicts the magnitude of contractions and results are expressed in percentage of basal control values. *P<0.05, **P<0.01.
Figure 2.
The effect of (a) the proposed excitatory neurotransmitters—ACh and substance P (SP) and (b) inhibitory neurotransmitters—the nitric oxide donor sodium nitroprusside (SNP) and the specific P2Y1 agonist 2MeSATP. Y-axis depicts the magnitude of contractions. *P<0.05 vs basal values.
Electrically induced responses
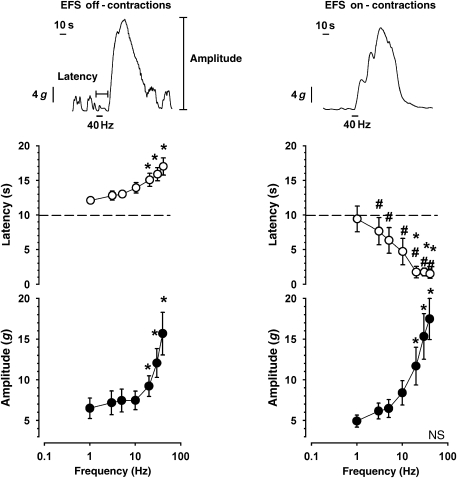
Up to 44% of the strips of the sigmoid colon responded to low frequencies of electrical stimulation with an off-contraction, which appeared after the electrical stimulus had terminated (Figure 3). Latency and amplitude of off-contractions were significantly enhanced with increasing frequencies of electrical stimulation above 20 Hz (Figure 3, left panel). In contrast, EFS induced an on-contraction during the stimulus in 56% of the colon strips, and increasing frequencies of electrical stimulation enhanced the amplitude of electrical on-contractions, whereas latency was significantly reduced above 20 Hz (Figure 3, right panel). EFS on- and off-responses were unaffected by 20-min exposure to hexamethonium (100 μM) and were fully blocked by TTX (1 μM; data not shown).
Figure 3.
Patterns of electric field stimulation (EFS)-induced responses in strips of human sigmoid colon. Left panel shows an off-contraction appearing briefly following EFS. Right panel shows an EFS-induced on-contraction during EFS. *P<0.05 vs 1 Hz; #P<0.05 vs off-contractions.
EFS-induced off-contractions
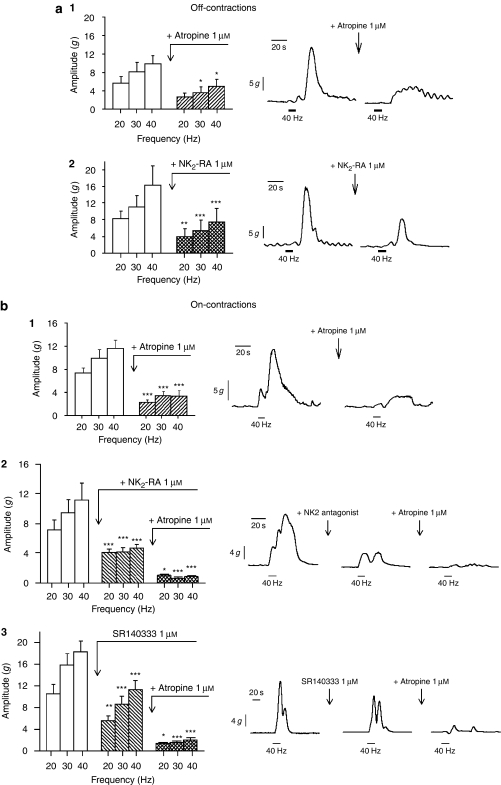
Inhibition of NOS by L-NNA caused a significant reduction in the duration of latency at 20–40 Hz (P<0.001; n=16) and an increase in the amplitude of EFS-induced off-contractions at high frequencies of electrical stimulation (P<0.05, Figure 4). Sequential addition of atropine did not modify latency and reduced the amplitude of off-contractions (P<0.01). Finally, addition of apamin caused a further reduction in latency (P<0.001), inducing the appearance of on-contractions during EFS at 10–40 Hz and increasing the amplitude of these contractions (P<0.05; Figure 4). In separate experiments (Figure 5), we found that in the presence of L-NNA and atropine, the PY1-RA MRS 2179 (10 μM) strongly reduced latency (P<0.001; n=7) and amplitude (P<0.001) of EFS responses; but the P2X antagonist NF279 (10 μM) did not cause any significant change in the latency or the amplitude of the EFS responses (n=9, NS). Also the protease α-CMT (10 U mL−1) had no effect on latency and slightly increased amplitude of contraction (n=7, P<0.001, Figure 5). In further experiments to explore the mediators of off-contractions, we found that atropine or the NK2 antagonist, given alone, significantly reduced the amplitude at 30–40 Hz (P<0.05; n=7) or 20–40 Hz (P<0.001; n=5, see Figure 6a), respectively. However, the NK1 antagonist SR 140333 did not significantly affect amplitude of off-contractions (−22±7% at 20 Hz to −9±2% at 40 Hz, NS). We also found that neither propranolol (1 μM, n=6) nor phentolamine (1 μM, n=6) affected the latency or amplitude of EFS-induced off-contractions (data not shown).
Figure 4.
Pharmacological characterization of off-contractions induced by electric field stimulation (EFS). Latency was significantly reduced by NG-nitro-L-arginine (L-NNA), unaffected by the sequential addition of atropine, and further reduced after the addition of apamin leading to an on-contraction during the electrical stimulus. Amplitude of EFS-induced off-contractions was significantly enhanced by L-NNA, reduced by atropine and the residual contraction was enhanced by sequential addition of apamin *P<0.05 statistical significance between the addition of the current and previous drug.
Figure 5.
Characterization of non-nitrergic responses in strips showing electric field stimulation (EFS)-induced off-contractions. Strips were incubated with NG-nitro-L-arginine (L-NNA; 1 mM) and atropine (1 μM) before drug addition. Left panels show quantitative effects and right panels show typical recordings. (a) MRS 2179 (10 μM) significantly reduced both latency and amplitude. (b) NF279 (10 μM) had no effect on EFS-induced responses. (c) α-chymotrypsin (α-CMT; 10 U mL−1) increased the amplitude of off-contractions. ***P<0.001.
Figure 6.
Characterization of electric field stimulation (EFS)-induced (a) off- and (b) on-contractions. Atropine (1 μM) and the NK2-RA (1 μM) significantly reduced the amplitude of EFS off- and on-contractions. *P<0.05, ***P<0.001.
EFS-induced on-contractions
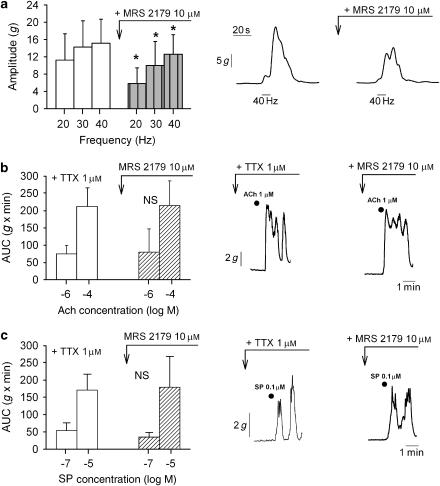
Atropine significantly reduced the amplitude of EFS-induced on-contractions at all frequencies of EFS (P<0.05; n=6). The specific NK2 antagonist NK2-RA also significantly decreased the amplitude of EFS-induced on-contractions (P<0.05; n=7, Figure 6b.2). The sequential addition of NK2-RA and atropine almost abolished the amplitude of EFS on-contractions at all frequencies of electrical stimulation (residual contraction at 40 Hz 0.8±0.2 g; P<0.001, Figure 6b.2). SR 140333 also antagonized the amplitude of EFS-induced on-contractions at frequencies of 20 Hz (−47±3%), 30 Hz (−45±4%) and 40 Hz (−38.2±4.3%), and the sequential addition of atropine abolished EFS responses (residual contraction <2±0.45 g, Figure 6b.3). As observed with with the off-contractions (Figure 5a), the P2Y1-RA MRS 2179 also significantly reduced the amplitude of the electrically induced on-contraction (P<0.05; n=7, Figure 7a). In contrast, MRS 2179 did not affect the contraction induced by direct effect of ACh or SP on smooth muscle cells in strips pretreated with the neural blocker TTX (n=8, NS, Figures 7b and 7c).
Figure 7.
Presynaptic modulation of electric field stimulation (EFS)-induced contractions. MRS 2179 (10 μM) significantly reduced the amplitude of EFS-induced on-contractions (a). In contrast, MRS 2179 did not alter the direct contraction induced by ACh (b) and substance P (c) on smooth muscle cells. *P<0.05.
Responses induced by stimulation of nAChRs
Stimulation of nAChRs by nicotine inhibited the spontaneous contractions in a concentration-dependent manner (log IC50 −5.22±0.32, n=22; see Figure 8a). At 100 μM, nicotine reduced the AUC of basal activity by 71±10% (P<0.001). In the presence of 1 μM TTX, nicotine inhibited spontaneous motility by only 43±14% (n=5, P<0.05). L-NNA antagonized the inhibition of spontaneous RPCs induced by 100 μM nicotine (n=6), and sequential addition of 1 μM apamin fully blocked the non-nitrergic, nicotine-induced effect and returned spontaneous motility to basal values (see Figure 9a). The non-nitrergic and apamin-sensitive component of nicotine responses was also fully antagonized by the specific P2Y1 antagonist MRS 2179 (n=8, p<0.005, Figure 9b) and unaffected by the specific P2X antagonist NF 279 (n=5; NS) or α-CMT (10 U mL−1) (n=7; NS, not shown).
Figure 8.
Nicotine (a) and α-β-methylene-ATP (α-β-met-ATP; b) abolished the spontaneous rhythmic phasic contractions (RPCs) in a concentration-dependent manner. *P<0.05 vs basal values.
Figure 9.
Inhibition of spontaneous motility induced by nicotine (100 μM) was significantly antagonized by NG-nitro-L-arginine (L-NNA; 1 mM) and fully blocked by the simultaneous addition of L-NNA and (a) apamin (1 μM) or (b) L-NNA and MRS 2179 (10 μM). *P<0.05; ***P<0.001.
Responses induced by α-β-met-ATP
Stimulation of purinergic receptors by α-β-met-ATP also inhibited spontaneous contractions in a concentration-dependent manner (log IC50 −5.29±0.15, n=7; Figure 8b). At 10 μM, α-β-met-ATP caused a 68±16% inhibition of spontaneous RPCs (P<0.05). In the presence of TTX 1 μM, α-β-met-ATP 10 μM caused a similar effect with 70±15% inhibition of spontaneous RPCs (n=5, NS). Inhibition of RPCs by α-β-met-ATP (10 μM) was unaffected by L-NNA suggesting that it is not mediated by nitrergic pathways (n=4, Figure 10a). Sequential addition of apamin fully antagonized the inhibitory effect of α-β-met-ATP (n=4, P<0.05, Figure 10a). Unexpectedly, the inhibitory effect of α-β-met-ATP (10 μM) was not affected by the P2X antagonist NF 279 (10 μM) and was fully antagonized by sequential addition of MRS 2179 (10 μM; P<0.001; n=5; Figure 10b).
Figure 10.
Mechanisms of inhibition of the spontaneous motility induced by α-β-methylene-ATP (α-β-met-ATP; 10 μM). *P<0.05; ***P<0.001.
Discussion
Circular strips of human sigmoid colon developed, in vitro, spontaneous RPCs of non-neural origin and responded to EFS with two distinct patterns of contractile responses of neural origin: (a) a phasic contraction, which began during EFS (on-contractions) or (b) a phasic contraction, which began after EFS (off-contractions). The amplitude of EFS-induced on- and off-contractions depends on the stimulation of excitatory EMNs releasing ACh and tachykinins, mainly acting on muscarinic and NK2 receptors located on smooth muscle cells. Our study also shows that inhibitory EMNs are also efficiently stimulated by nAChRs located in somatodendritic regions and nerve terminals inhibiting spontaneous RPCs by release of NO and a purine acting at muscular P2Y1 receptors. The participation of these mechanisms in the normal motor activity of the sigmoid colon should be considered to develop new pharmacological strategies to treat motility disorders of the human colon.
We found that strips of circular muscle from the sigmoid colon developed non-neural spontaneous RPCs (Huizinga et al., 1985). Our results on the non-neural origin of this pattern of motility agree with previous human studies (McKirdy et al., 2004) where RPCs were found to be caused by rhythmic oscillations of smooth muscle cell membrane potential (slow waves), originating in a pacemaker located at or near the submucosal border of the muscle layer and consisting of a network of ICCs (Pluja et al., 2001). Transmission of slow waves from ICCs to smooth muscle cells is mediated by direct connections through gap junctions (Ward and Sanders, 2006). In contrast, other recent studies found that the neurotransmitters released from excitatory EMNs might also mediate RPCs in the human colon (Cao et al., 2006). In this study, RPCs were not affected by TTX and were modulated by a slight tonic activity from both inhibitory and excitatory EMNs as L-NNA and apamin increased, and atropine and NK2-RA decreased RPCs, probably by the changes in basal tone of smooth muscle cells, excitability of ICCs or blockade of minimal amounts of neurotransmitters released by EMNs through potential action-independent mechanisms. The lack of effect of TTX demonstrated the non-neural origin of RPCs and a balanced effect of TTX on excitatory and inhibitory EMNs is possible.
In our study, EFS produced two distinct patterns of contractile responses: (a) contractions, which began during EFS (defined as on-contractions) and (b) contractions, which began after EFS (defined as off-contractions), both caused by the activation of EMNs as they were TTX sensitive (Cellek et al., 2006). The most common type of EFS-induced responses that we observed was on-contraction, a response very similar to that defined as ‘monophasic response' in previous studies, also in human colon (Cellek et al., 2006). The on-contractions are caused by the selective stimulation of excitatory EMNs as they were almost abolished by the sequential addition of atropine and tachykinin antagonists (Anand and Paterson, 1994). The cholinergic excitatory component of EFS-induced on-contractions that we observed in human sigmoid colon has been described before (Venkova et al., 2002; Hinds et al., 2006; Cellek et al., 2006, 2007). In contrast to the exclusive tachykinergic transmission observed by other authors (Cao et al., 2000, 2006), our data clearly suggest a cholinergic and tachykinergic excitatory coneurotransmission with effects of similar magnitude on human colonic motility. NK1 and NK2 tachykinin receptors have been found in the muscle layers of human colon (Gates et al., 1988) and NK2 receptors have been involved in electrically induced colonic contractions as well (Cao et al., 2000). In our study, both SR 14033 and the NK2-RA antagonized EFS on-contractions, and the SP-induced contraction was mainly antagonized by the NK2-RA, suggesting that NK2 receptors mainly mediate tachykinin responses in the human colon.
The off-contractions have been described in several gastrointestinal tissues (Kannan et al., 1985). Our off-contractions are very similar to the ‘biphasic response'—a relaxation, which began at the initiation of the EFS followed by a contraction—, which began at the termination of EFS, also described by Cellek et al. (2006) in previous studies in human colon. We did not observe relaxations in our study as in our experimental conditions; the strips of sigmoid colon did not develop the tone. The off-contractions are slightly more difficult to interpret. We found that latency of off-contractions was reduced by L-NNA and blocked by sequential addition of apamin, suggesting that the latency is caused by stimulation of inhibitory EMNs releasing NO and a second non-nitrergic neurotransmitter that acts through apamin-sensitive K+ channels. In addition, MRS 2179 reduced non-nitrergic latency suggesting that the second neurotransmitter involved in latency is ATP acting at P2Y1 receptors. In an earlier study, we found that P2Y1 receptors mediated the fast inhibitory junction potential in the human colon (Gallego et al., 2006). We also found that both L-NNA and apamin significantly enhanced the amplitude of electrically induced off-contractions, and both ACh and tachykinins released from excitatory EMNs are involved in this response as both atropine and NK2-RA decreased the amplitude of EFS-induced off-contractions to a similar extent. Supporting our results, both ACh and tachykinins are involved in the ascending contractile component of the peristaltic reflex in human ileum (Grider, 1989) and guinea pig colon (Tonini et al., 2001). Finally, an off-depolarization after the inhibitory period is plausible and might be added to the neuronal excitatory component. We found, in previous studies, that a positive P2Y1 receptor immunoreactivity was present in the smooth muscle layers as well as in some neurons of the myenteric plexus (Gallego et al., 2006). In addition, recent studies show that P2Y1 agonists can stimulate myenteric neurons of the guinea pig ileum (Gallego et al., 2008). In this study, we found a mechanism of prejunctional modulation of amplitude on- and off-contractions by P2Y1 receptors. The reduction in the amplitude of on-contractions caused by MRS 2179 can be explained by the blockade of presynaptic excitatory P2Y1 receptors on excitatory EMNs. Similar findings have also been reported in guinea pig ileum with prejunctional depression of EFS-induced contractions by purinergic antagonists (Bartho et al., 1997). The effect of MRS 2179 on off-contractions is more complex including a reduction in the latency period by the blockade of postjunctional inhibitory P2Y1 receptors on smooth muscle cells and a reduction in the amplitude of off-contractions by the blockade of presynaptic excitatory P2Y1 receptors on excitatory EMNs. This is supported by the fact that the blockade of muscular P2Y1 receptors by MRS 2179 did not affect the direct muscular contraction induced by ACh and SP.
Stimulation of nAChRs inhibited the spontaneous RPCs of sigmoid colon in a concentration-dependent manner. In vivo, intraluminal nicotine has been shown to significantly reduce both smooth muscle tone and contractile activity in human rectosigmoid colon (Green et al., 1999). This study shows that the induced inhibition is partially blocked by TTX, suggesting the presence of nicotinic receptors located on the soma and nerve terminals of EMNs (Galligan, 1999; Schneider et al., 2000; Galligan and North, 2004). However, no contraction was observed after the blockade of inhibitory responses, suggesting that nicotine selectively stimulated the inhibitory EMNs in our preparation. L-NNA partially antagonized the inhibition induced by nicotine, whereas apamin or MRS 2179 fully blocked the non-nitrergic relaxation, also indicating the inhibitory cotransmission. Taken together, our data support the idea that NO and a purine acting at P2Y1 receptors are the main neuromuscular inhibitory coneurotransmitters in human sigmoid colon. Finally, this study also shows that α-β-met-ATP inhibited spontaneous RPCs by a mechanism unaffected by TTX or L-NNA, suggesting a non-neural action (Storr et al., 2000). P2X receptors have been described in smooth muscle cells and ICCs (Ralevic and Burnstock, 1990). Apamin fully antagonized the inhibition induced by α-β-met-ATP, confirming the involvement of Ca2+-dependent K+ channels. A similar apamin-sensitive inhibitory effect of α-β-met-ATP has also been reported in rat ileum where it is mediated through P2X receptors located on smooth muscle cells (Storr et al., 2000). Unexpectedly, we found that the selective P2X antagonist NF279 did not antagonize the inhibitory effect of this agonist, whereas the further addition of the specific P2Y1-RA MRS 2179 fully blocked its action. The P2X purinoceptor is classified as a ligand-gated ion channel for which α-β-met-ATP is a potent selective agonist (Fredholm et al., 1994). However, the P2X receptor can present some atypical pharmacological properties and it has also been reported that α-β-met-ATP can act as an agonist at P2Y receptors as well (Storr et al., 2000). Recently, α-β-met-ATP has been confirmed as a potent agonist for P2Y11 receptors (King and Townsend-Nicholson, 2008). All these hypotheses are plausible and further studies are needed to explain the putative effects of P2X and P2Y receptors in the human colon.
Although it is always difficult to correlate and in vivo motor patterns, several parallelisms can be established here. Firstly, RPCs might be linked, primarily, to the mixture of colonic contents and the uniform exposure of the faeces to the absorbent colonic mucosal surface (Sarna, 2006). Secondly, on- and off-contractions are intense enough to be occlusive. In vitro on-contractions correlate well with in vivo simultaneous contractions—the most common pattern of pressure activity in the human colon—, which can slow the transit. In vitro off-contractions are the equivalent of GMCs, or high-amplitude propagating contractions observed in in vivo manometric studies (Rao et al., 2001). The off-contractions and GMCs involve the interaction of excitatory and inhibitory EMNs (Sarna, 2006). The activation of inhibitory EMNs might modulate the timing of the response of the excitatory EMNs, the descending inhibition of tone and RPCs and the progressive nature of the peristaltic contraction; in contrast, the activation of excitatory EMNs might determine the amplitude of the contraction. GMC fit the criteria of the standard peristaltic reflex produce mass movements of gas or faeces and are associated with the passage of stools (Rao et al., 2001; Sarna, 2006).
In summary, the in vitro motor patterns in human sigmoid colon described in this study have an important myogenic component, excitatory neural responses involving muscarinic and NK2 receptors and inhibitory neural responses mediated by NO and ATP or a related purine acting on postjunctional P2Y1 receptors. A significant correlation between these in vitro patterns and those observed in colonic manometric studies in humans can be established (Rao et al., 2001) Our protocols may contribute to the understanding of colonic motility in health and disease and may even serve as an experimental in vitro model for the development of more specific prokinetic or spasmolytic treatments in human diseases caused and/or associated with alterations in colonic motility. Treatment with P2Y1 receptor agonists (Wood, 2006) or NK2-RAs (Lecci et al., 2006) might be considered in patients with irritable bowel syndrome and functional disorders affecting colonic motility.
Acknowledgments
We thank Dr Alex Sáenz and J Cases (Clínica Sagrada Familia, Barcelona), Dr Joan Martí Ragué (Clínica Sagrada Familia) and Dr Xavier Suñol (Hospital de Mataró) for providing human tissue; Mr Ricard Farré and Dr Pilar Hernández for preliminary studies; and Mrs Jane Lewis and Mrs Carolina Parada for revising the manuscript. This study was supported by a grant from the Fundació de Gastroenterologia Dr Francisco Vilardell, the Fundació Salut del Consorci Santari del Maresme, the Departament d'Universitats, Recerca i Societat de la Información (SGR2001–0214), the Fondo de Investigaciones Sanitarias del Ministerio de Sanidad y Consumo (IF063678–1), and by the Laboratorios Menarini SA. Ciberehd is funded by the Instituto de Salud Carlos III.
Abbreviations
- AUC
area under curve
- EFS
electrical field stimulation
- EMNs
enteric motor neurons
- GMC
giant migrating contractions
- ICC
interstitial cells of Cajal
- L-NNA
NG-nitro-L-arginine
- nAChR
nicotinic ACh receptors
- RPCs
rhythmic phasic contractions
- TTX
tetrodotoxin
Conflict of interest
P Clavé has served as a speaker for Menarini International and has received research funding from Laboratorios Menarini SA-Menarini Group, Badalona, Spain.
References
- Alexander SPH, Mathie A, Peters JA.Guide to receptors and channels (GRAC) Br J Pharmacol 2008153Suppl 2S1–S209.3rd edn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand N, Paterson WG. Role of nitric oxide in esophageal peristalsis. Am J Physiol. 1994;266:G123–G131. doi: 10.1152/ajpgi.1994.266.1.G123. [DOI] [PubMed] [Google Scholar]
- Bartho L, Lenard L, Jr, Maggi CA. Evidence for the involvement of P2-purinoceptors in the cholinergic contraction of the guinea-pig ileum. Br J Pharmacol. 1997;121:1507–1508. doi: 10.1038/sj.bjp.0701350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckxstaens GE, Pelckmans PA, Herman AG, Van Maercke YM. Involvement of nitric oxide in the inhibitory innervation of the human isolated colon. Gastroenterology. 1993;104:690–697. doi: 10.1016/0016-5085(93)91003-z. [DOI] [PubMed] [Google Scholar]
- Bultmann R, Dudeck O, Starke K. Evaluation of P2-purinoceptor antagonists at two relaxation-mediating P2-purinoceptors in guinea-pig taenia coli. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:445–451. doi: 10.1007/BF00261442. [DOI] [PubMed] [Google Scholar]
- Cao W, Harnett KM, Pricolo VE. NK2 receptor-mediated spontaneous phasic contractions in normal and ulcerative colitis human sigmoid colon. J Pharmacol Exp Ther. 2006;317:1349–1355. doi: 10.1124/jpet.105.097030. [DOI] [PubMed] [Google Scholar]
- Cao W, Pricolo VE, Zhang L, Behar J, Biancani P, Kirber MT. Gq-linked NK2 receptors mediate neurally induced contraction of human sigmoid circular smooth muscle. Gastroenterology. 2000;119:51–61. doi: 10.1053/gast.2000.8552. [DOI] [PubMed] [Google Scholar]
- Cellek S, John AK, Thangiah R, Dass NB, Bassil AK, Jarvie EM, et al. 5-HT4 receptor agonists enhance both cholinergic and nitrergic activities in human isolated colon circular muscle. Neurogastroenterol Motil. 2006;18:853–861. doi: 10.1111/j.1365-2982.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- Cellek S, Thangiah R, Bassil AK, Campbell CA, Gray KM, Stretton JL, et al. Demonstration of functional neuronal beta3-adrenoceptors within the enteric nervous system. Gastroenterology. 2007;133:175–183. doi: 10.1053/j.gastro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Farre R, Auli M, Lecea B, Martinez E, Clave P. Pharmacologic characterization of intrinsic mechanisms controlling tone and relaxation of porcine lower esophageal sphincter. J Pharmacol Exp Ther. 2006;316:1238–1248. doi: 10.1124/jpet.105.094482. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- Gallego D, Hernandez P, Clave P, Jimenez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G584–G594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- Gallego D, Vanden Berghe P, Farré R, Tack J, Jiménez M. P2Y1 receptors mediate inhibitory neuromuscular transmission and enteric neuronal activation in small intestine. Neurogastroenterol Motil. 2008;20:159–168. doi: 10.1111/j.1365-2982.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- Galligan JJ. Nerve terminal nicotinic cholinergic receptors on excitatory motoneurons in the myenteric plexus of guinea pig intestine. J Pharmacol Exp Ther. 1999;291:92–98. [PubMed] [Google Scholar]
- Galligan JJ, North RA. Pharmacology and function of nicotinic acetylcholine and P2X receptors in the enteric nervous system. Neurogastroenterol Motil. 2004;16:64–70. doi: 10.1111/j.1743-3150.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- Gates TS, Zimmerman RP, Mantyh CR, Vigna SR, Maggio JE, Welton ML. Substance P and substance K receptor binding sites in the human gastrointestinal tract: localization by autoradiography. Peptides. 1988;9:1207–1219. doi: 10.1016/0196-9781(88)90184-2. [DOI] [PubMed] [Google Scholar]
- Green JT, McKirdy HC, Rhodes J, Thomas GA, Evans BK. Intra-luminal nicotine reduces smooth muscle tone and contractile activity in the distal large bowel. Eur J Gastroenterol Hepatol. 1999;11:1299–1304. doi: 10.1097/00042737-199911000-00020. [DOI] [PubMed] [Google Scholar]
- Grider JR. Identification of neurotransmitters regulating intestinal peristaltic reflex in humans. Gastroenterology. 1989;97:1414–1419. doi: 10.1016/0016-5085(89)90384-3. [DOI] [PubMed] [Google Scholar]
- Hinds NM, Ullrich K, Smid SD. Cannabinoid 1 (CB1) receptors coupled to cholinergic motorneurones inhibit neurogenic circular muscle contractility in the human colon. Br J Pharmacol. 2006;148:191–199. doi: 10.1038/sj.bjp.0706710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Stern HS, Chow E, Diamant NE, el-Sharkawy TY. Electrophysiologic control of motility in the human colon. Gastroenterology. 1985;88:500–511. doi: 10.1016/0016-5085(85)90513-x. [DOI] [PubMed] [Google Scholar]
- Kannan MS, Jager LP, Daniel EE. Electrical properties of smooth muscle cell membrane of opossum esophagus. Am J Physiol. 1985;248:G342–G346. doi: 10.1152/ajpgi.1985.248.3.G342. [DOI] [PubMed] [Google Scholar]
- Keef KD, Du C, Ward SM, McGregor B, Sanders KM. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology. 1993;105:1009–1016. doi: 10.1016/0016-5085(93)90943-7. [DOI] [PubMed] [Google Scholar]
- King BF, Townsend-Nicholson A.Involvement of P2Y1 and P2Y11 purinoceptors in parasympathetic inhibition of colonic smooth muscle J Pharmacol Exp Ther 20083241055–1063.e-pub ahead of print 29 November 2007 [DOI] [PubMed] [Google Scholar]
- Lecci A, Capriati A, Altamura M, Maggi CA. Tachykinins and tachykinin receptors in the gut, with special reference to NK2 receptors in human. Auton Neurosci. 2006;126–127:232–249. doi: 10.1016/j.autneu.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Katsuragi T, Fujiki S, Sato C, Furukawa T. ATP release and contraction mediated by different P2-receptor subtypes in guinea-pig ileal smooth muscle. Br J Pharmacol. 1997;121:1744–1748. doi: 10.1038/sj.bjp.0701329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKirdy HC, Richardson CE, Green JT, Rhodes J, Williams GT, Marshall RW. Differential effect of nitric oxide synthase inhibition on sigmoid colon longitudinal and circular muscle responses to nicotine and nerve stimulation in vitro. Br J Surg. 2004;91:229–234. doi: 10.1002/bjs.4395. [DOI] [PubMed] [Google Scholar]
- Pluja L, Alberti E, Fernandez E, Mikkelsen HB, Thuneberg L, Jimenez M. Evidence supporting presence of two pacemakers in rat colon. Am J Physiol Gastrointest Liver Physiol. 2001;281:G255–G266. doi: 10.1152/ajpgi.2001.281.1.G255. [DOI] [PubMed] [Google Scholar]
- Rae MG, Fleming N, McGregor DB, Sanders KM, Keef KD. Control of motility patterns in the human colonic circular muscle layer by pacemaker activity. J Physiol. 1988;510:309–320. doi: 10.1111/j.1469-7793.1998.309bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1990;50:413–492. [PubMed] [Google Scholar]
- Rao SS, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G629–G639. doi: 10.1152/ajpgi.2001.280.4.G629. [DOI] [PubMed] [Google Scholar]
- Rumessen JJ. Ultrastructure of interstitial cells of Cajal at the colonic submuscular border in patients with ulcerative colitis. Gastroenterology. 1996;111:1447–1455. doi: 10.1016/s0016-5085(96)70005-7. [DOI] [PubMed] [Google Scholar]
- Sarna SK. Molecular, functional, and pharmacological targets for the development of gut promotility drugs. Am J Physiol Gastrointest Liver Physiol. 2006;291:G545–G555. doi: 10.1152/ajpgi.00122.2006. [DOI] [PubMed] [Google Scholar]
- Schneider DA, Perrone M, Galligan JJ. Nicotinic acetylcholine receptors at sites of neurotransmitter release to the guinea pig intestinal circular muscle. J Pharmacol Exp Ther. 2000;294:363–369. [PubMed] [Google Scholar]
- Storr M, Franck H, Saur D, Schusdziarra V, Allescher HD. Mechanisms of alpha, beta-methylene atp-induced inhibition in rat ileal smooth muscle: involvement of intracellular Ca2+ stores in purinergic inhibition. Clin Exp Pharmacol Physiol. 2000;27:771–779. doi: 10.1046/j.1440-1681.2000.03334.x. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells. Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- Tonini M, Spelta V, D'Agostino G, De Ponti F, De Giorgio R, anghellini V, et al. Tachykinin-dependent and -independent components of peristalsis in the guinea pig isolated distal colon. Gastroenterology. 2001;20:938–945. doi: 10.1053/gast.2001.22526. [DOI] [PubMed] [Google Scholar]
- Venkova K, Greenwood-Van Meeveld B, Krier J.Neural Control of the Large Intestine Innervation of the Gastrointestinal Tract 2002Taylor & Francis: London, pp; 171–187.In: Brookes SJ and Costa M (eds). [Google Scholar]
- Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006;576:675–682. doi: 10.1113/jphysiol.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD. The enteric purinergic P2Y1 receptor. Curr Opin Pharmacol. 2006;6:564–570. doi: 10.1016/j.coph.2006.06.006. [DOI] [PubMed] [Google Scholar]