Abstract
Background and purpose:
trans-Resveratrol, a non-flavonoid polyphenol found abundantly in red wine possesses antiproliferative and anti-inflammatory activity in various inflammatory disease conditions. However, the effect of trans-resveratrol on eosinophil activation in relation to allergy has not been investigated.
Experimental approach:
Human eosinophils were isolated and purified from whole blood and incubated for 16 h with trans-resveratrol. Eosinophil chemotaxis, activation and degranulation, and apoptosis were investigated. The effect of trans-resveratrol on the inhibition of p38 and ERK1/2 activation was examined.
Key results:
Treatment of human eosinophils with trans-resveratrol at concentrations <100 μM for 16 h did not induce eosinophil apoptosis. Similar results were seen after 24 h and 48 h incubations. trans-Resveratrol (<100 μM) significantly inhibited eosinophil peroxidase release after activation with IL-5 (IC50=2.9±0.9 μM) or C5a (IC50=3.9±0.5 μM) after 5 min priming with cytochalasin B (CB). Similarly, the production of leukotriene C4 after stimulation with calcium ionophore, and eosinophil chemotaxis in response to eotaxin, as well as CD11b upregulation and CD62 L shedding was also significantly reduced by trans-resveratrol, at concentrations above 5 μM. All the activators induced p38 and ERK1/2 phosphorylation maximal at 2 min of activation. trans-Resveratrol potently inhibited p38 and ERK1/2 activation after calcium ionophore and CB and C5a activation.
Conclusions and implications:
trans-Resveratrol is effective at inhibiting human eosinophil activation and degranulation at concentrations <100 μM, while not inducing apoptosis. This potent anti-inflammatory activity of trans-resveratrol and possibly its metabolites on eosinophils may be worth investigating for the treatment of eosinophil-related allergic diseases.
Keywords: eosinophils, phytochemical, eosinophil activation, chemotaxis, apoptosis, asthma
Introduction
Eosinophils have an important function in the development of many allergic diseases, such as asthma, dermatitis and rhinitis. The association of eosinophils with the pathophysiology of these diseases has been intensively investigated. The activation and recruitment of eosinophils into sites of allergic inflammation are characteristic hallmarks of allergic diseases (Bousquet et al., 1990). Large numbers of activated tissue eosinophils are typically observed in airway biopsies, sputum and bronchoalveolar lavage fluid obtained from asthmatic patients (Wardlaw et al., 2000). Eosinophils are recruited by specific eosinophil chemoattractants such as eotaxin, nonspecific chemoattractants such as PAF or even the eosinophil chemoattractant factor for anaphylaxis (selectively chemotactic for eosinophils) released from mast cells. When eosinophils are activated, eosinophil degranulation occurs and destructive enzymes, including major basic protein, eosinophil peroxidase (EPO) and eosinophil cationic protein slowly erode cilia and the underlying epithelial layer. This can induce airway hyper-responsiveness from exposed nerve endings and an increased sensitivity to bronchoconstricting agents, a characteristic feature of allergic asthma (Sugimoto et al., 2003). Despite some studies which have indicated that anti-IL-5 therapies do not inhibit airway hyperreactivity (Leckie et al., 2000), tissue eosinophils are still thought to have an important function in allergic responses (O'Byrne et al., 2001).
Extensive efforts have been made to discover new anti-inflammatory therapies, more or equally effective to corticosteroids, which can be used in the treatment of allergy (Walsh et al., 2003). trans-Resveratrol is a naturally occurring phytoalexin found in several plants, and at especially high concentration in grapevines and red wine. trans-Resveratrol is strongly cardioprotective and also possesses chemopreventive, chemotherapeutic, neuroprotective and anti-inflammatory (Martin et al., 2006) properties. This compound exerts many of its diverse biological effects by acting as an antioxidant and free radical scavenger, and by controlling crucial gene transcription factors such as nuclear factor-κB and AP-1, upstream transcriptional regulators such as PKC and mitogen-activated protein (MAP) kinase, as well as downstream products such as p53, Bax and COX-2 (see Pervaiz, 2003). trans-Resveratrol has been found to be capable of inhibiting the release of inflammatory cytokines, reactive oxygen species (ROS) and inflammatory gene expression (Cavallaro et al., 2003; Birrell et al., 2005), as well as chemotaxis from neutrophils and monocytes. trans-Resveratrol may be useful and effective in the treatment of asthma and chronic obstructive pulmonary disease, as it inhibits interleukin (IL)-8 and granulocyte-macrophage colony-stimulating factor release, as well as nuclear factor-κB activity from A549 lung cancer cells (Donnelly et al., 2004). However, the functional effect of trans-resveratrol on primary human eosinophil activation and function in relation to allergy has not been studied previously.
In this study, the effect of trans-resveratrol on eosinophil function and activation was investigated. Our results demonstrate that this compound potently inhibits eosinophil activation and may be a useful compound in the prophylactic or symptomatic treatment of allergy or asthma.
Materials and methods
Eosinophil isolation
Venous blood was drawn from healthy non-atopic human volunteers under informed consent approved by the Institutional review board of the National University of Singapore. Blood was centrifuged to obtain the leukocyte-rich buffy coat, diluted in phosphate-buffered saline containing 0.2% BSA (Sigma, St Louis, MO, USA) and 5 mM EDTA before overlaying on isotonic Percoll (1.082 g mL−1) (Pharmacia, Uppsala, Sweden). After centrifugation, pellets were pooled and erythocytes were removed by hypotonic lysis before eosinophil purification performed using negative selection (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the isolated eosinophils was more than 98% as determined by Kimura staining, and the viability was more than 97% as shown by Trypan blue exclusion test.
Eosinophil culture
Freshly isolated eosinophils (1 × 106 cells mL−1) were cultured in RPMI medium (Sigma, St Louis, MO, USA) supplemented with 10% foetal bovine serum, 1% L-glutamine and 1% penicillin–streptomycin. Cells were incubated with or without trans-resveratrol (Sigma) (0–500 μM in 0.05% dimethylsulphoxide) and with or without human recombinant IL-5 (0–10 ng mL−1) (Peprotech, Rocky Hill, NJ, USA) treatment, in a humidified cell culture incubator at 37 °C and 5% CO2 overnight (14–16 h). In some experiments, eosinophils were treated instead with 1 μM dexamethasone in the same conditions as above for 14–16 h. All vehicle controls were performed using media with 0.05% dimethylsulphoxide. Eosinophils were activated with different activating agents, and concentration–response curves were obtained to determine concentrations, which produced submaximal activation. Stimuli used were calcium ionophore A23187 (10−5 M), eotaxin and 5 μg mL−1 cytochalasin B (CB) for 5 min, before the stimulation with C5a (10−8 M) or IL-5 (20 ng mL−1).
Mediator analysis
Eosinophil peroxidase was quantified using o-phenylenediamine as substrate. After activation, cell-free supernatants were obtained and mixed with an equal volume of substrate (0.4 mg mL−1 o-phenylenediamine dihydrochloride (Sigma) in 0.05 M Tris-HCl pH 8 supplemented with 0.012% H2O2). A volume of 4 M H2SO4 was added and absorbance was read at 450 nm. To determine total EPO, 0.1% Triton X-100 was added to the substrate. Cysteinyl leukotrienes (CysLT) (LTC4/D4/E4) were measured using an AChE competitive enzyme immunoassay kit, following the manufacturer's instructions (GE Amersham, Buckinghamshire, England).
Chemotaxis assay
Eosinophils were washed once with fresh cell culture medium before chemotaxis was performed using ChemoTx disposable plates (5 μm pore size, NeuroProbe, Gaithersburg, MD, USA). A volume of 25 μl of cell suspension (3 × 106 cells mL−1) was added on each filter site with the bottom well filled with 29 μl of cell culture medium with or without 10 ng mL−1 of eotaxin (Peprotech, Rocky Hill, NJ, USA). The cells were then incubated for 1.5 h in a humidified cell culture incubator at 37 °C and 5% CO2. After incubation, the cells were gently removed and the top filters were gently cleaned. Migrated cells were viewed and counted under × 10 magnification with a microscope with imaging function (Nikon ACT-2U, Melville, NY, USA), and the migration index was calculated by dividing the number of cells migrated towards eotaxin by the number of cells that migrated to media alone.
Flow cytometric analysis
Eosinophils were stained with monoclonal mouse anti-human CD11b antibody/anti-human CD62L antibody (BD Biosciences, San Jose, CA, USA) followed by polyclonal PE-conjugated rabbit anti-mouse IgG (Serotec, Oxford, UK) for 45 min. After the incubation, the cells were analysed by flow cytometry (Dako, Glostrup, Denmark), where a total of 10 000 gated cells were counted.
Western blotting
Treated cells were harvested at indicated times and lysed in a lysis buffer containing 75 mM NaCl, 10 mM Tris-HCl, 1 mM EDTA, 0.5% NP40, 0.5% Triton-X100, 1 μg mL−1 leupeptin, 1 μg mL−1 aprotinin, 1 μM phenylmethylsulphonyl fluoride, 1 mM Na3VO4 and protease inhibitor cocktail (Pierce, Rockford, IL, USA). Protein concentration was determined by the Bradford assay. A total of 30 μg of proteins per sample were subjected to western blot analysis. Blots were incubated with the antibodies specific for either phospho-ERK1/2 (Thr202/Tyr204), ERK1/2, phospho-p38 (Thr180/Tyr182), p38 (all from Cell Signaling Technology, Beverly, MA, USA) and 1:7500 horseradish peroxidase-conjugated rabbit anti-mouse or mouse anti-rabbit secondary antibodies (Pierce). All the primary antibodies were used at the final concentration of 1 μg mL−1. To detect phosphorylated proteins, the same blots were probed with phospho-specific antibodies, stripped and reprobed with antibodies against total proteins. All blots were incubated with mouse antibody against β-actin (Sigma) to confirm equal protein loading. Bands were visualized with a Chemiluminescent Reagent Plus detection system (Perkin-Elmer, Boston, MA, USA), as described by the manufacturer. For quantitative analysis of western blots, the density of bands corresponding to protein blotting with the antibody under study was normalized to the amount of respective total protein using ImageJ software (National Institute of Health, USA). After normalization, the expression level of proteins was expressed as fold changes relative to the vehicle control.
Annexin-V-FITC and propidium iodide staining
Externalization of inner membrane phosphatidylserine was assessed by the Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences, San Jose, CA, USA) according to the recommended protocol with slight modifications. Briefly, after drug treatment, cells were washed twice with cold phosphate-buffered saline and resuspended in 100 μl binding buffer. Annexin-V-FITC and propidium iodide (PI) were added, gently vortexed and the cells were incubated for 15 min at 25 °C in the dark before adding 200 μl binding buffer. Cells were analysed by flow cytometry within 1 h. Apoptotic cells were annexin V positive, PI negative and were expressed as a percentage of all cells.
Caspase-3 activity assay
Eosinophils were resuspended and incubated in 50 μl of Cell Lysis Buffer (BD Biosciences Pharmingen) for 20 min before the addition of 50 μl of cold 2 × reaction buffer (20 mM HEPES, 4 mM EDTA, 20 mM KCl and 3 mM MgCl2 in distilled water) with 10 mM dithiothreitol and 1 μl of 10 mM DEVD-AFC (substrate for caspase-3; Alexis, Lausen, Switzerland). The sample was incubated with the substrate solution for 1 h in a dark humidified incubator at 37 °C and 5% CO2. After the incubation, 1 μl of the sample was used for protein assay, whereas the rest was used to determine the caspase-3 activity by measuring the fluorescence intensity (excitation at 400 nm and emission at 505 nm) using a spectrofluorometer (GENios, Tecan, Switzerland). The relative fluorescence unit (RFU) for each sample was normalized against its respective protein content to obtain RFU per μg of protein (RFU μg−1).
Statistical analyses
Data are expressed as means±s.e. mean of n human donors per group. Data were tested for normality, and paired sets of data (treated versus control) were compared using paired Student's t-test (two-tailed). Comparisons between multiple treatment groups from the same experiment were made using one way ANOVA. When significant differences were found between groups, Tukey's post hoc test was used to test significance. In all cases, P<0.05 was accepted as statistically significant.
Results
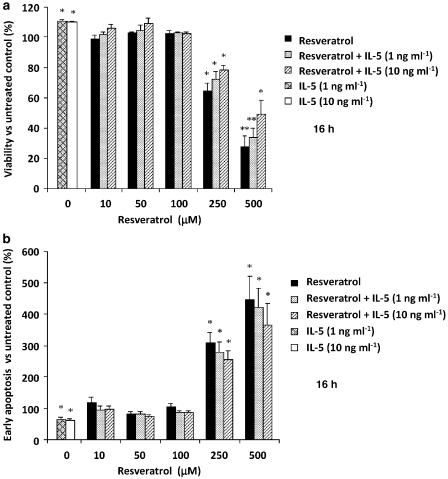
The effect of trans-resveratrol on eosinophil apoptosis was first investigated. Freshly isolated human eosinophils were cultured for 16 h with or without trans-resveratrol (1–500 μM), with and without human recombinant IL-5 (0–10 ng mL−1) to maintain viability. Cells were immediately analysed by flow cytometry. Viable cells were negative for both annexin-V-FITC and PI, whereas cells in early apoptotic stage were annexin-V-FITC positive but negative for PI, and cells in late apoptotic and necrotic stages were positive for both. At concentrations of trans-resveratrol of 1–100 μM, no reduction of eosinophil viability was observed after 16 h of incubation (Figure 1a). A significant reduction in cell viability was observed only at higher concentrations of trans-resveratrol (P<0.05), accompanied by an increase in early apoptotic cells (Figure 1b). The addition of IL-5 only partially rescued eosinophils from trans-resveratrol-induced cell death and had no effect on cell viability when incubated with lower concentrations of trans-resveratrol. Treatment with IL-5 alone reduced spontaneous cell apoptosis as compared with untreated cells.
Figure 1.
Effect of 16 h trans-resveratrol treatment on cell viability and apoptosis. Eosinophils were treated with or without trans-resveratrol (0–500 μM) and with or without human recombinant interleukin (IL)-5 (0–10 ng mL−1), after which the cells were immediately stained with annexin-V-FITC and propidium iodide (PI) and analysed by flow cytometry. (a) Cell viability was expressed as the percentage of viable (annexin-V-FITC negative and PI negative) in the test sample. (b) Early apoptosis was expressed as the percentage of early apoptotic cells (annexin-V-FITC positive and PI negative) in the test sample. Data are means±s.e.mean percent of n=5–7 per treatment group relative to the vehicle control. *P<0.05 and **P<0.01 versus untreated dimethylsulphoxide control.
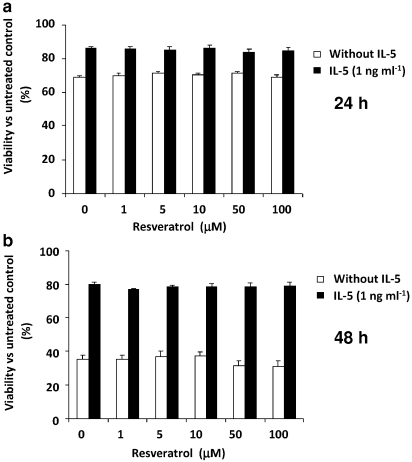
To determine whether trans-resveratrol induced apoptosis after a longer incubation, eosinophils were treated with low-moderate concentrations of trans-resveratrol (1–100 μM) for 24 h and 48 h. At both time points, no significant changes in eosinophil survival were observed, when cells were treated with trans-resveratrol at all concentrations (Figure 2). IL-5 was effective at rescuing eosinophil death, as eosinophils progressively became more susceptible to spontaneous apoptosis with extending time in culture. Without IL-5, the percentage viability of eosinophils decreased drastically from 83.8±0.9% at 16 h to 68.7±1.2% at 24 h and to 35.4±2.4% at 48 h. But in the presence of 1 ng mL−1 IL-5, cell viability was more stable 87.6±2.1% at 16 h, 85.9±1.3% at 24 h and 79.7±1.5% at 48 h.
Figure 2.
Effect of longer (24 and 48 h) trans-resveratrol treatment on eosinophil apoptosis. Eosinophils were treated with or without trans-resveratrol (0–100 μM) and treated as indicated in Figure 1 for (a) 24 or 48 h (b). Data are means±s.e.mean percent of n=4 per treatment group relative to the vehicle control. Values were not statistically significant versus dimethylsulphoxide control.
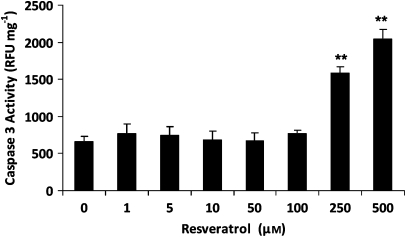
To further confirm that moderate (1–100 μM) concentrations of trans-resveratrol did not induce apoptosis, a caspase-3 activity assay was performed after incubation with trans-resveratrol. Once again, only high concentrations of trans-resveratrol (250–500 μM) induced significant caspase-3 activity, causing a 2.4- and 3-fold increase in caspase-3 activity, respectively (Figure 3). These results demonstrate that trans-resveratrol induces eosinophil apoptosis only at concentrations higher than 100 μM. Thus, 1–100 μM of trans-resveratrol were used in subsequent functional assays.
Figure 3.
Effect of trans-resveratrol treatment on caspase-3 activity Eosinophils were treated with trans-resveratrol (0–500 μM) for 16 h and cell lysates were obtained for caspase-3 activity assay. The results were expressed in relative fluorescence unit (RFU) and normalized against the corresponding total protein concentration (mg) in each sample. All measurements were performed in duplicate. Data are means±s.e.mean RFU mg−1 of n=5 per treatment group. **P<0.001 versus untreated dimethylsulphoxide control.
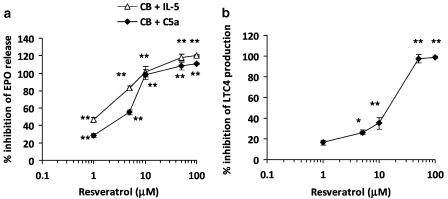
To investigate the effect of trans-resveratrol on eosinophil activation and degranulation, EPO release was analysed. Freshly isolated human eosinophils were cultured in cell medium with or without trans-resveratrol (1–100 μM) for 16 h. After removal of trans-resveratrol, eosinophils were pretreated with CB (5 μg mL−1) for 5 min followed by either C5a (10−8 M) or IL-5 (20 ng mL−1) for 1 h. Submaximal concentrations of activators were chosen from concentration–response curves (data not shown). Stimulation with CB and C5a induced a fivefold increase in EPO release (56.2±0.5 versus 11.4±0.5% EPO release, P<0.01) when compared with the unactivated control. When eosinophils were treated with trans-resveratrol, degranulation induced by CB and C5a was markedly reduced in a concentration-dependent manner (P<0.001; Figure 4a). The activation of eosinophils by CB and IL-5 was also inhibited by trans-resveratrol (P<0.001). A total of 1 μM of trans-resveratrol effectively blocked EPO release by both stimuli (C5a: 28.4±4.9% inhibition, IL-5: 20.8±3.3% inhibition, P<0.01 versus activated control). The trans-resveratrol IC50 (concentration of trans-resveratrol that produced a 50% inhibition) for EPO release was 3.9±0.5 and 2.9±0.9 μM for C5a and IL-5, respectively. The activity of trans-resveratrol on eosinophil activation was compared with the glucocorticoid, dexamethasone. Treatment of eosinophils with dexamethasone (1 μM) induced a 75.2% inhibition of EPO release induced by CB and C5a.
Figure 4.
trans-Resveratrol inhibits degranulation and mediator production from activated eosinophils. Eosinophils were treated with trans-resveratrol (0–100 μM) for 16 h and activated with (a) cytochalasin B (CB, 5 μg mL−1) for 5 min followed by C5a (10−8 M) or interleukin (IL)-5 (20 ng mL−1) and eosinophil peroxidase (EPO) release was measured and normalized against total cellular EPO. (b) 10−5 M calcium ionophore A23187 for 3 h and the supernatants were collected for LTC4 ELISA. Data are expressed in the amount of LTC4 released (pg mL−1). Data are means±s.e.mean percent of n=5 per treatment group in triplicate. *P<0.05, **P<0.01 versus the untreated activated control after ANOVA analysis (P<0.01).
The effect of trans-resveratrol on the release of CysLTs (LTC4/D4/E4; assayed as LTC4) release after calcium ionophore (A23187) activation was also investigated (Figure 4b). Basal release of LTC4 in unactivated eosinophils was 8 pg mL−1, whereas activation with A23187 for 3 h induced the release of 1235±94 pg mL−1 LTC4. Treatment with trans-resveratrol significantly inhibited LTC4 production in a concentration-dependent manner, with an IC50 of 18.8±3.4 μM. Similarly, incubation of eosinophils with dexamethasone (1 μM) inhibited LTC4 production significantly (46.4% inhibition of LTC4 production). These data indicate that trans-resveratrol is effective at inhibiting eosinophil degranulation and mediator production in vitro, at a level comparable with glucocorticoids.
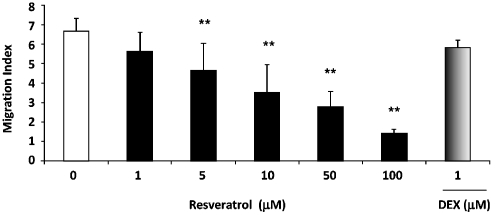
trans-Resveratrol was next tested for its effects on eosinophil migration in response to eotaxin, a potent eosinophil chemokine (Figure 5). A migration index was obtained by normalizing the number of cells migrating towards eotaxin with the number of cells migrating towards vehicle control. The migration index of the eotaxin positive control was 6.7±0.3. A concentration-dependent response (P<0.001) was observed after cells were incubated with trans-resveratrol overnight, with a 4.8-fold inhibition in migration (1.43±0.09 migration index, P<0.01) at the highest concentration used (100 μM). At 5 μM, trans-resveratrol significantly inhibited eosinophil migration to eotaxin (31.2±6.3% inhibition, P<0.01 versus basal control), whereas 1 μM dexamethasone did not significantly inhibit eosinophil migration.
Figure 5.
trans-Resveratrol reduced eosinophil chemotaxis towards eotaxin. Eosinophils were treated with trans-resveratrol (0–100 μM) or dexamethasone (1 μM) for 16 h before the chemotaxis assay was performed with the ChemoTx chemotaxis system. Eotaxin (10 ng mL−1) was used as the chemoattractant. All measurements were performed in triplicate. The data were expressed as the migration index, which was normalized against the number of randomly migrated cells in the vehicle control sample. Data are means±s.e.mean of the migration index of n=5 per treatment group. *P<0.05 and **P<0.01 versus untreated vehicle control after ANOVA analysis (P<0.01).
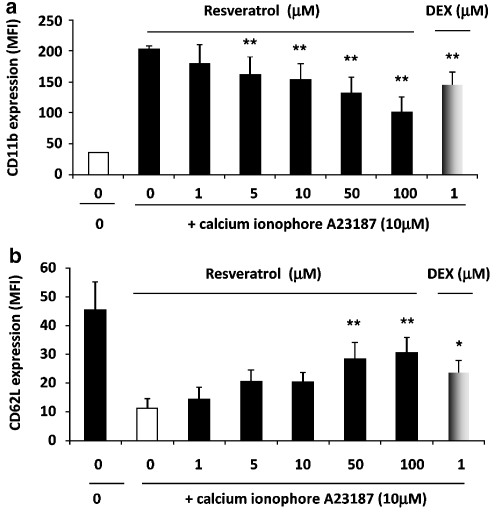
The effect of trans-resveratrol on the expression of CD11b and CD62L on human eosinophils was next investigated. Eosinophils were treated with trans-resveratrol (0–100 μM) for 16 h, followed by stimulation with 10−5 M calcium ionophore A23187 for 1 h. Activation of vehicle-treated eosinophils induced a 3.5-fold upregulation of surface CD11b expression compared with the unactivated cells (P<0.01; Figure 6a). Pretreatment of eosinophils with increasing concentrations of trans-resveratrol resulted in a concentration-dependent suppression of CD11b upregulation (P<0.001) upon subsequent cell activation. The inhibition was marked at 100 μM (65.3±8.1% inhibition, P<0.01) and statistically significant at 5 μM (26.8±3.6% inhibition, P<0.01). At all the concentrations used, trans-resveratrol did not affect the basal CD11b expression or total CD11b expression (data not shown). Treatment of eosinophils with dexamethasone (1 μM) significantly inhibited CD11b expression after activation (28.3% inhibition, P<0.05). This was comparable with the inhibition by trans-resveratrol at 10 μM.
Figure 6.
trans-Resveratrol inhibits CD11b upregulation and CD62L shedding in activated eosinophils. Eosinophils were treated with trans-resveratrol (0–100 μM) or dexamethasone (1 μM) for 16 h and eosinophils were either immunostained immediately or activated with 10−5 M calcium ionophore A23187 for 1 h before (a) CD11b-PE or (b) CD62L-PE immunostaining and analysed by flow cytometry. The data are represented in mean fluorescence intensity (MFI) after isotype control was subtracted. Data are means±s.e.mean of n=7 per treatment group. *P<0.05, **P<0.01 versus the untreated activated control after ANOVA analysis (P<0.01).
Similarly, after activation with A23187, cell surface expression of CD62L was reduced by 2.6-fold after the cells were activated (P<0.01; Figure 6b). After overnight incubation with trans-resveratrol, CD62L shedding was significantly reduced (P<0.001) with 50 and 100 μM of trans-resveratrol. These high concentrations of trans-resveratrol significantly inhibited CD62L shedding by 2.7- and 2.5-fold, respectively relative to the activated control, returning the level of surface CD62L to basal levels. As a reference, dexamethasone (1 μM) significantly inhibited CD62L shedding by 2.1-fold (P<0.05). As observed with CD11b, trans-resveratrol did not affect the basal expression of CD62L or total CD62L expression (data not shown).
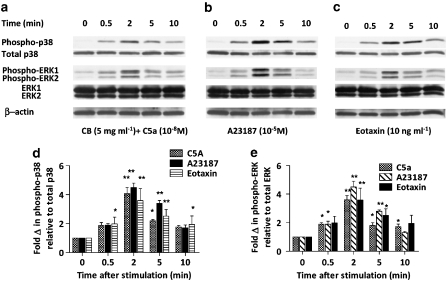
To elucidate the possible mechanism of action of trans-resveratrol, the MAP kinase signalling pathways in eosinophils were studied using western blotting. As the MAP kinase cascades are early upstream events and the phosphorylation of the kinases occur rapidly after cell activation, the kinetics of p38 and ERK1/2 MAP kinase phosphorylation was first studied. Eosinophils were cultured in vitro for 16 h and activated with CB+10−8 M C5a, 10−5 M calcium ionophore A23187 or 10 ng mL−1 eotaxin, which were all activating stimuli used in earlier functional assays for various time periods (0.5, 2, 5, 10 min). As illustrated in Figure 7, all three activating agents induced strong phosphorylation of p38 (Figure 7d) and ERK1/2 (Figure 7e) MAP kinases, as early as 0.5 min after the addition of the stimulus, maximal at 2 min and lasting over 5 min.
Figure 7.
p38 and ERK1/2 MAP kinase phosphorylation in activated eosinophils. Eosinophils were cultured in vitro for 16 h and stimulated with (a) CB+10−8 M C5a (as described in Figure 3), (b) 10−5 M calcium ionophore A23187or (c) 10 ng mL−1 eotaxin for 0, 0.5, 2, 5 or 10 min. After the indicated length of activating time, the cells were harvested and lysed for western blot analysis, as described in the Materials and methods. The same membrane was used to detect phospho- and total p38, phospho- and total ERK1/2 and β-actin. Representative images of five independent sets of experiments are shown. The bar graphs below each blot summarize (n=5) the fold change of phospho p38 (d) or ERK1/2 (e) versus no activation control after normalization to total p38 or ERK1/2, respectively. *P<0.05, **P<0.01 versus the untreated activated sample after ANOVA analysis (P<0.01).
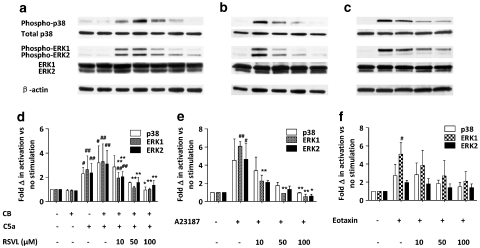
The effect of trans-resveratrol on the phosphorylation of p38 and ERK1/2 MAP kinases at 2 min of cell activation triggered by CB+C5a, A23187 or eotaxin was examined. Eosinophils were incubated with or without trans-resveratrol (10, 50, 100 μM) in vitro for 16 h before stimulation with one of the activating agents for exactly 2 min, and the whole cell lysate extracted for SDS-polyacrylamide gel electrophoresis followed by immunoblotting. Our findings suggest that trans-resveratrol effectively inhibited, in a dose-dependent manner, the phosphorylation of p38 as well as ERK1/2 MAP kinases, for all the stimuli used (Figure 8a–c). Figure 8a illustrates that CB alone does not activate p38 or ERK1/2, and addition of CB with C5a additionally activates both p38 and ERK1/2 activation. trans-Resveratrol almost completely abolished p38 and ERK1/2 phosphorylation stimulated by CB+C5a and A23187 back to baseline levels, while it was not as effective in eotaxin stimulated cells (Figure 8d–f, P<0.01). In fact, trans-resveratrol was more effective at inhibiting ERK1 activation than ERK2, as ERK2 activation was more sustained than ERK1 activation after trans-resveratrol treatment. These results demonstrate that trans-resveratrol effectively inhibits p38 and ERK1/2 activation after eosinophil activation, which may suggest a possible mechanism of action of its inhibitory functions in eosinophils.
Figure 8.
Effect of trans-resveratrol on p38 and ERK1/2 MAP kinase phosphorylation. Eosinophils were treated for 16 h with the indicated concentrations of trans-resveratrol before stimulation with CB+C5a (a,d), A23187 (b,e) or eotaxin (c,f) for 2 min (as described in Figure 7). The same membrane was used to detect phospho-p38/total p38, phospho-ERK1/2/total ERK1/2 and β-actin. Representative images of four independent sets of experiments are shown. The bar graphs below each blot summarize (n=4) the fold change of phospho p38 or ERK1/2 versus no activation control after normalization to total p38 or ERK1/2, respectively. #P<0.05, ##P<0.01 versus the negative control after ANOVA analysis (P<0.01); *P<0.05, **P<0.01 versus the untreated activated sample after ANOVA analysis (P<0.01).
Discussion
In this study, we show that trans-resveratrol inhibits a number of inflammatory functions, such as degranulation, migration and chemotaxis in human eosinophils, cells important in the pathogenesis of asthma (Gleich and Adolphson, 1986). Eosinophils contain granules of EPO and major basic protein, which are released upon degranulation. These mediators damage respiratory epithelium and provoke bronchospasm in asthmatic patients (Agosti et al., 1987). We found that trans-resveratrol was a potent inhibitor of eosinophil degranulation, indicated by the concentration-dependent inhibition of EPO release. Increased amounts of eosinophil granule proteins correlate with eosinophil number and clinical activity in atopic dermatitis and proteins oxidatively modified by EPO-generated catabolites have been detected in the lung lavage fluid of patients with severe asthma (MacPherson et al., 2001). EPO induces non-lytic detachment of pneumocytes, whereas major basic protein lyses alveolar epithelial cells causing damage to the epithelium (Ayars et al., 1985).
Eosinophils are also a rich source of CysLTs released in large amounts when cells are activated through the 5-lipoxygenase (5-LO) pathway (Weller et al., 1983). CysLTs have been implicated in the pathophysiology of asthma, including mucus hypersecretion, increased microvascular permeability, inflammatory cell recruitment and oedema (Hay et al., 1995). In addition, CysLTs increase airway hyper-responsiveness and induce smooth muscle hypertrophy in both healthy and asthmatic patients (O'Hickey et al., 1991), and increased production of CysLTs have been detected in BAL samples from asthmatic patients (Lam et al., 1988). We found that trans-resveratrol inhibited CysLTs production significantly at low concentrations, very comparable to that of the glucocorticoid dexamethasone used as a reference compound. It is possible that both classes of drugs could be affecting arachidonic acid production following stimulation or the inhibition of PLA2, resulting in less prostaglandin and LT production.
The generation of inflammatory mediators, including chemotactic cytokines such as C5a and eotaxin, in response to tissue injury or inflammation will result in leukocyte chemotaxis and migration (Granger and Kubes, 1994). The release of these proinflammatory mediators induces rapid and severe changes in the adhesive properties of the endothelial cells lining the vessel walls in the surrounding vasculature, inducing increased adhesiveness between the endothelium and leukocytes. The recruitment of eosinophils from the blood into the tissues is an important coordinated step in the inflammatory response. Leukocyte recruitment from the blood into tissue sites of inflammation involves initial contact with the vascular endothelium under conditions of shear flow (Granger and Kubes, 1994). Next, leukocytes roll on the endothelium through selectins and eventually adhere and transmigrate through the endothelial layer, mediated mainly by integrins, such as CD11b and CD49d and their immunoglobulin-like family of ligands. trans-Resveratrol was able to functionally block eosinophil chemotaxis towards eotaxin in a dose-dependent manner. The inhibition of chemotaxis by trans-resveratrol was similarly observed in a study on human neutrophils (Rotondo et al., 1998). One possible explanation of this observation is that trans-resveratrol could block the shedding of CD62L and the upregulation of CD11b after activation, the first signs of leukocyte activation and adhesion molecules important in rolling and firm adhesion. This possibility is supported by our previous investigation (Lim et al., 2000), which demonstrated a correlation between reduced CD11b expression and chemotactic ability of eosinophils after glucocorticoid treatment. Furthermore, ligation of the β-subunit of the CD11b/CD18 complex triggers activation and degranulation of eosinophils; thus, the inhibition of CD11b/CD18 expression by trans-resveratrol could possibly reduce eosinophil activation as well. In this study, treatment of eosinophils with dexamethasone did not significantly inhibit eosinophil migration to eotaxin. This could have been on account of the short incubation time (16 h) used in this study, whereas longer incubation times (24–48 h) were used previously (Lim et al., 2000). As glucocorticoids are known to act through the transcriptional machinery, longer incubation times may be necessary for the inhibition of functional chemotaxis and migration.
The MAP kinases signalling pathway in eosinophils may be important in mediating the chemotaxis and activation of eosinophils (Kampen et al., 2000). Eotaxin and C5a have previously been shown to induce degranulation and chemotaxis of eosinophils through p44/42 (ERK1/2) and p38 MAP kinases (Boehme et al., 1999; Adachi et al., 2000). Similarly, calcium ionophore is also known to cause phosphorylation of p38 and ERK1/2 MAP kinases in eosinophils and several other cell types. Regulation of expression of the adhesion molecules is dependent on Ca2+ mobilization, as well as the activation of p38 and ERK1/2 MAP kinases. Either the depletion of Ca2+ or the inhibition of the MAP kinases was able to affect the rolling and adhesion of leukocytes. The p38 and ERK1/2 kinases are crucial in modulating the expression of adhesion molecules on the eosinophil surface. Thus, we examined if trans-resveratrol could inhibit the MAP kinases ERK1/2 and p38. To date, numerous reports have suggested the importance of p38 and ERK1/2 MAP kinase signalling pathways in leukocyte activation. In eosinophils, both p38 and ERK1/2 MAP kinases have been shown to be essential for a wide range of cellular activities such as eosinophil differentiation, cytokine production, degranulation, adhesion and chemotaxis (Boehme et al., 1999; Adachi et al., 2000). Indeed, our results demonstrated that trans-resveratrol effectively inhibited the dual phosphorylation of p38 and ERK1/2 MAP kinases induced by calcium ionophore and CB+C5a in a dose-dependent manner. It was not as effective on eotaxin activation, however, suggesting a correlation between the small inhibition on chemotaxis observed as well. Studies on the anti-inflammatory effects of trans-resveratrol on neutrophils, macrophages and lymphocytes have not focused on the MAP kinase signalling pathways, although anticancer studies have identified the regulation of MAP kinases as one of the mechanisms of action of trans-resveratrol (Shih et al., 2002). This is the first report of trans-resveratrol inhibiting the activation of p38 and ERK1/2 kinases in leukocytes. The anti-inflammatory effect of trans-resveratrol has been largely attributed to its ability to inhibit COX-2 and iNOS expression by suppressing the activities of transcription factors nuclear factor-κB and AP-1 (Zangrilli et al., 2000; Birrell et al., 2005). It may be possible that trans-resveratrol may be producing multiple effects on eosinophils by interfering with the activation of p38 and ERK1/2 MAP kinases, both of which have been associated with the execution of eosinophil effector functions.
Eosinophils are terminally differentiated cells, which die rapidly in culture after 2 days, and IL-5 (Rothenberg et al., 1989) can enhance their survival in vitro for up to 2 weeks by blocking apoptosis. Investigations have shown that binding of these cytokines to their corresponding receptor leads to receptor dimerization and subsequent activation of the JAK2 kinase-Stat1α/Stat5 pathway, and the activation of Lyn and Syk tyrosine kinases and Src homology 2 tyrosine phosphatase with resulting activation of the Ras/ERK kinase pathways (Pazdrak et al., 1995). The final consequence would be upregulation of survival-promoting genes and the suppression of apoptosis-enhancing genes. In our studies, we have shown that the addition of IL-5 significantly reduced spontaneous apoptosis and promoted the survival of human eosinophils. Interestingly, at low concentrations (5–10 μM), trans-resveratrol did not affect eosinophil viability. This is in accordance with studies on macrophages and neutrophils, where trans-resveratrol did not affect their survival (Rotondo et al., 1998), and in human monocytes, where trans-resveratrol concentrations of up to 20 μM did not induce apoptosis, whereas 50 μM was slightly toxic (Losa, 2003). However, for cancer cells, 32 μM trans-resveratrol was shown to be lethal, causing extensive apoptosis after only 8 h incubation (Surh et al., 1999). Similarly, a previous study on an eosinophilic leukaemia cell line demonstrated that trans-resveratrol could induce a reduction in cell proliferation at 50 μM and significant apoptosis at 100 μM (Baatout et al., 2005). This discrepancy could be explained by the fact that the cell line used was a cancer cell line. This differential sensitivity of cancer versus non-cancer cells towards trans-resveratrol may be of clinical importance, as it suggests that chemotherapeutic doses of the compound can effectively target cancerous cells without compromising the survival of normal cells, therefore reducing unwanted side effects, which many anticancer drugs possess.
The average trans-resveratrol content in red wine is approximately 5 μg mL−1 (Gescher and Steward, 2003). Although it appears impossible to attain an effective concentration of trans-resveratrol through drinking wine, it is conceivable that regular consumption over a long period of time may increase trans-resveratrol concentrations within the body, possibly reaching pharmacological relevance. In addition, the use of red wine extract or trans-resveratrol as a dietary supplement has been suggested for cancer (Bemis et al., 2006) and cardiovascular diseases (Lekakis et al., 2005), and is undergoing clinical trials. As trans-resveratrol is metabolized after ingestion, it is not surprising that many do not see the benefit of using trans-resveratrol as a dietary supplement. Plasma peak levels of trans-resveratrol (30 min after ingestion) administered at a dose of 360 μg kg−1 in rats were 20 nM trans-resveratrol and 2 μM ‘total' trans-resveratrol (trans-resveratrol plus its metabolites) (Goldberg et al., 2003). Other studies showed that 2 and 50 mg kg−1 trans-resveratrol, given orally to rats, generated plasma peak values of 2 and 6.6 μM, respectively (Marier et al., 2002), whereas trans-resveratrol metabolite levels reached 105 μM. In humans, it has been reported that after oral administration of trans-resveratrol, only trace amounts were detected in the plasma (Walle et al., 2004). However, the bioavailability of its metabolites was not reported. Piceatannol, a hydroxylated trans-resveratrol metabolite, also possesses anticancer properties similar to the unmetabolized molecule (Wieder et al., 2001). Further studies are being performed to investigate the effects of trans-resveratrol metabolites on eosinophil function. If trans-resveratrol metabolites also possess similar anti-inflammatory activities to the parent compound as shown in our study, trans-resveratrol would be very effective when taken as a dietary supplement. Furthermore, it may be possible to use trans-resveratrol as an inhalant therapy similar to inhaled steroids, but its absorbance from the airways has not been studied.
In conclusion, we report that trans-resveratrol exhibits potent anti-inflammatory effects on eosinophils, key cells in the pathogenesis of allergic diseases such as asthma. This may have an impact on the current treatment of asthma and allergy and may encourage the use of trans-resveratrol in the prophylactic or symptomatic treatment of allergic diseases.
Acknowledgments
We thank Wong Kuan Yau and Komathi Paramasivam for expert technical assistance. These studies were funded by Grant numbers R-185-000-077-112 from the University Research Committee and R-185-000-105-213 from the National Medical Research Council of Singapore to LHK Lim.
Abbreviations
- CB
cytochalasin B
- CysLTs
cysteinyl leukotrienes
- EPO
eosinophil peroxidase
- MAP
mitogen-activated protein kinases
- PI
propidium iodide
- RFU
relative fluorescence unit
Conflict of interest
The authors state no conflict of interest.
References
- Adachi T, Choudhury BK, Stafford S, Sur S, Alam R. The differential role of extracellular signal-regulated kinases and p38 mitogen-activated protein kinase in eosinophil functions. J Immunol. 2000;165:2198–2204. doi: 10.4049/jimmunol.165.4.2198. [DOI] [PubMed] [Google Scholar]
- Agosti JM, Altman LC, Ayars GH, Loegering DA, Gleich GJ, Klebanoff SJ. The injurious effect of eosinophil peroxidase, hydrogen peroxide, and halides on pneumocytes in vitro. J Allergy Clin Immunol. 1987;79:496–504. doi: 10.1016/0091-6749(87)90368-x. [DOI] [PubMed] [Google Scholar]
- Ayars GH, Altman LC, Gleich GJ, Loegering DA, Baker CB. Eosinophil- and eosinophil granule-mediated pneumocyte injury. J Allergy Clin Immunol. 1985;76:595–604. doi: 10.1016/0091-6749(85)90781-x. [DOI] [PubMed] [Google Scholar]
- Baatout S, Derradji H, Jacquet P, Mergeay M. Increased radiation sensitivity of an eosinophilic cell line following treatment with epigallocatechin-gallate, resveratrol and curcuma. Int J Mol Med. 2005;15:337–352. [PubMed] [Google Scholar]
- Bemis DL, Katz AE, Buttyan R. Clinical trials of natural products as chemopreventive agents for prostate cancer. Expert Opin Investig Drugs. 2006;15:1191–1200. doi: 10.1517/13543784.15.10.1191. [DOI] [PubMed] [Google Scholar]
- Birrell MA, McCluskie K, Wong S, Donnelly LE, Barnes PJ, Belvisi MG. Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-kappaB-independent mechanism. FASEB J. 2005;19:840–841. doi: 10.1096/fj.04-2691fje. [DOI] [PubMed] [Google Scholar]
- Boehme SA, Sullivan SK, Crowe PD, Santos M, Conlon PJ, Sriramarao P, et al. Activation of mitogen-activated protein kinase regulates eotaxin-induced eosinophil migration. J Immunol. 1999;163:1611–1618. [PubMed] [Google Scholar]
- Bousquet J, Chanez P, Lacoste JY, Barnéon G, Ghavanian N, Enander I, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- Cavallaro A, Ainis T, Bottari C, Fimiani V. Effect of resveratrol on some activities of isolated and in whole blood human neutrophils. Physiol Res. 2003;52:555–562. [PubMed] [Google Scholar]
- Donnelly LE, Newton R, Kennedy GE, Fenwick PS, Leung RH, Ito K, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol. 2004;287:L774–L783. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- Gescher AJ, Steward WP. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomarkers Prev. 2003;12:953–957. [PubMed] [Google Scholar]
- Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin.Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- Granger DN, Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol. 1994;55:662–675. [PubMed] [Google Scholar]
- Hay DW, Torphy TJ, Undem BJ. Cysteinyl leukotrienes in asthma: old mediators up to new tricks. Trends Pharmacol Sci. 1995;16:304–309. doi: 10.1016/s0165-6147(00)89059-8. [DOI] [PubMed] [Google Scholar]
- Kampen GT, Stafford S, Adachi T, Jinquan T, Quan S, Grant JA, et al. Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood. 2000;95:1911–1917. [PubMed] [Google Scholar]
- Lam S, Chan H, LeRiche JC, Chan-Yeung M, Salari H. Release of leukotrienes in patients with bronchial asthma. J Allergy Clin Immunol. 1988;81:711–717. doi: 10.1016/0091-6749(88)91043-3. [DOI] [PubMed] [Google Scholar]
- Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- Lekakis J, Rallidis LS, Andreadou I, Vamvakou G, Kazantzoglou G, Magiatis P, et al. Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:596–600. doi: 10.1097/00149831-200512000-00013. [DOI] [PubMed] [Google Scholar]
- Lim LH, Flower RJ, Perretti M, Das AM. Glucocorticoid receptor activation reduces CD11b and CD49d levels on murine eosinophils: characterization and functional relevance. Am J Respir Cell Mol Biol. 2000;22:693–701. doi: 10.1165/ajrcmb.22.6.3890. [DOI] [PubMed] [Google Scholar]
- Losa GA. Resveratrol modulates apoptosis and oxidation in human blood mononuclear cells. Eur J Clin Invest. 2003;33:818–823. doi: 10.1046/j.1365-2362.2003.01219.x. [DOI] [PubMed] [Google Scholar]
- MacPherson JC, Comhair SA, Erzurum SC, Klein DF, Lipscomb MF, Kavuru MS, et al. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol. 2001;166:5763–5772. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- Martin AR, Villegas I, Sanchez-Hidalgo M, de la Lastra CA. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharmacol. 2006;147:873–885. doi: 10.1038/sj.bjp.0706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Byrne PM, Inman MD, Parameswaran K. The trials and tribulations of IL-5, eosinophils, and allergic asthma. J Allergy Clin Immunol. 2001;108:503–508. doi: 10.1067/mai.2001.119149. [DOI] [PubMed] [Google Scholar]
- O'Hickey SP, Hawksworth RJ, Fong CY, Arm JP, Spur BW, Lee TH. Leukotrienes C4, D4, and E4 enhance histamine responsiveness in asthmatic airways. Am Rev Respir Dis. 1991;144:1053–1057. doi: 10.1164/ajrccm/144.5.1053. [DOI] [PubMed] [Google Scholar]
- Pazdrak K, Schreiber D, Forsythe P, Justement L, Alam R. The intracellular signal transduction mechanism of interleukin 5 in eosinophils: the involvement of lyn tyrosine kinase and the Ras-Raf-1-MEK-microtubule-associated protein kinase pathway. J Exp Med. 1995;181:1827–1834. doi: 10.1084/jem.181.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Petersen J, Stevens RL, Silberstein DS, McKenzie DT, Austen KF, et al. IL-5-dependent conversion of normodense human eosinophils to the hypodense phenotype uses 3T3 fibroblasts for enhanced viability, accelerated hypodensity, and sustained antibody-dependent cytotoxicity. J Immunol. 1989;143:2311–2316. [PubMed] [Google Scholar]
- Rotondo S, Rajtar G, Manarini S, Celardo A, Rotillo D, de Gaetano G, et al. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br J Pharmacol. 1998;123:1691–1699. doi: 10.1038/sj.bjp.0701784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih A, Davis FB, Lin HY, Davis PJ. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK- and p53-dependent mechanism. J Clin Endocrinol Metab. 2002;87:1223–1232. doi: 10.1210/jcem.87.3.8345. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Ogawa M, Tai N, Kamei C. Inhibitory effects of glucocorticoids on rat eosinophil superoxide generation and chemotaxis. Int Immunopharmacol. 2003;3:845–852. doi: 10.1016/S1567-5769(03)00055-9. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Hurh YJ, Kang JY, Lee E, Kong G, Lee SJ. Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL-60) cells. Cancer Lett. 1999;140:1–10. doi: 10.1016/s0304-3835(99)00039-7. [DOI] [PubMed] [Google Scholar]
- Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- Walsh GM, Sexton DW, Blaylock MG. Corticosteroids, eosinophils and bronchial epithelial cells: new insights into the resolution of inflammation in asthma. J Endocrinol. 2003;178:37–43. doi: 10.1677/joe.0.1780037. [DOI] [PubMed] [Google Scholar]
- Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic diseases. Br Med Bull. 2000;56:985–1003. doi: 10.1258/0007142001903490. [DOI] [PubMed] [Google Scholar]
- Weller PF, Lee CW, Foster DW, Corey EJ, Austen KF, Lewis RA. Generation and metabolism of 5-lipoxygenase pathway leukotrienes by human eosinophils: predominant production of leukotriene C4. Proc Natl Acad Sci USA. 1983;80:7626–7630. doi: 10.1073/pnas.80.24.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieder T, Prokop A, Bagci B. Piceatannol, a hydroxylated analog of the chemopreventive agent resveratrol, is a potent inducer of apoptosis in the lymphoma cell line BJAB and in primary, leukemic lymphoblasts. Leukemia. 2001;15:1735–1742. doi: 10.1038/sj.leu.2402284. [DOI] [PubMed] [Google Scholar]
- Zangrilli J, Robertson N, Shetty A, Wu J, Hastie A, Fish JE, et al. Effect of IL-5, glucocorticoid, and Fas ligation on Bcl-2 homologue expression and caspase activation in circulating human eosinophils. Clin Exp Immunol. 2000;120:12–21. doi: 10.1046/j.1365-2249.2000.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]