Abstract
Background and purpose:
Superoxide (O2•−), derived from nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, is associated with acute respiratory distress syndrome (ARDS). NADPH oxidase activity and expression are blocked by nitric oxide (NO) and sildenafil. As another gas, hydrogen sulphide (H2S) is formed by blood vessels, the effect of sodium hydrosulphide (NaHS) and the H2S-donating derivative of sildenafil, ACS6, on O2•− formation and the expression of gp91phox (a catalytic subunit of NADPH oxidase) in porcine pulmonary arterial endothelial cells (PAECs) was investigated.
Experimental approach:
PAECs were incubated with 10 ng mL−1 tumour necrosis factor-α (TNFα) (±NaHS or ACS6), both of which released H2S, for 2 h or 16 h. O2•− was measured. Expression of gp91phox was measured by western blotting and the role of cyclic AMP (cAMP) and/or cyclic GMP was assessed using protein kinase inhibitors.
Key results:
After either 2- or 16-h incubations, O2•− formation by PAECs was inhibited by NaHS or ACS6, with IC50 values of about 10 nM and less than 1 nM, respectively. Both 100 nM NaHS and 1 nM ACS6 completely inhibited gp91phox expression induced by TNFα. The effects of NaHS were blocked by the inhibition of protein kinase A (PKA), but not PKG, and not by the inhibition of guanylyl cyclase. Effects of ACS6 were blocked by inhibition of both PKA and PKG. Both NaHS and ACS6 augmented cAMP formation.
Conclusion and implications:
H2S inhibited O2•− formation and upregulation of NADPH oxidase in PAECs through the adenylyl cyclase-PKA pathway. ACS6 may be effective in treating ARDS through both elevation of cAMP and inhibition of phosphodiesterase type 5 activity.
Keywords: superoxide, NADPH oxidase, hydrogen sulphide, endothelial cells, cAMP
Introduction
Acute respiratory distress syndrome (ARDS), a severe form of acute lung injury, is a common complication in critically ill patients and is associated with significant morbidity and mortality and is characterized by a rapid and time-dependent worsening of pulmonary hypertension (Metnitz et al., 1999; Ware and Matthay, 2000; Weinacker and Vaszar, 2001). Although ARDS can be initiated by a number of causal factors that include sepsis, shock, trauma and multiple transfusions, the pathological and clinical manifestations of the syndrome are very similar (Ware and Matthay, 2000; Weinacker and Vaszar, 2001). Of the pathological mechanisms underlying ARDS, oxidative stress, including superoxide (O2•−) formation, may have a critical function (Chabot et al., 1998; Folkaerts et al., 2001; Stuart-Smith and Jeremy, 2001; Dweik, 2005). Apart from directly eliciting vasoconstriction, O2•− reacts with nitric oxide (NO) to form peroxynitrite and other reactive nitrogen species, effectively reducing NO bioavailability (Folkaerts et al., 2001; Stuart-Smith and Jeremy, 2001; Muzaffar et al., 2003, 2004a, 2004b; Dweik, 2005).
It has become increasingly apparent that a major inducible source of vascular O2•− in ARDS may be nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), the expression of which is rapidly upregulated in intact pulmonary artery and in vascular smooth muscle cells (VSMCs) and endothelial cells derived from pulmonary artery by diverse factors associated with ARDS (Muzaffar et al., 2005a). These include cytokines, thomboxane A2, isoprostanes F2α, hypoxia, re-oxygenation following hypoxia and O2•− itself (Li and Shah, 2001; Cai et al., 2003; Muzaffar et al., 2003, 2005a, 2005b, 2005c, 2006). Conversely, NO donors and iloprost (a stable analogue of prostacyclin (PGI2)) inhibit O2•− formation through the inhibition of NADPH oxidase expression (Muzaffar et al., 2004a, 2004b). These effects are mediated through two major pathways: (i) the NO-cyclic GMP (cGMP)-protein kinase G (PKG) and (ii) the PGI2-cyclic AMP (cAMP)-protein kinase A (PKA) pathway. In turn, inhaled NO and PGI2 have been advocated as a potentially effective means of treating ARDS (Cepkova and Matthay, 2006).
It is now established that hydrogen sulphide (H2S) elicits effects that include vasodilation (Zhao et al., 2001; Zhao and Wang, 2002; Moore et al., 2003; Cheng et al., 2004; Beltowski, 2005) and the inhibition of VSMC proliferation (Du et al., 2004; Yang et al., 2004a, 2004b). This has positioned H2S in the family of protective transmitters that are also gases, together with nitric oxide (NO) and carbon monoxide (CO) (Pryor et al., 2006). Relaxation of arteries elicited by H2S is partially dependent on a functional endothelium and is calcium dependent (Zhao and Wang, 2002; Cheng et al., 2004; Beltowski, 2005). The vasodilator effect of H2S appears to be mediated, in part, by KATP channels (Zhao and Wang, 2002; Beltowski, 2005). NO also interacts synergistically with H2S to promote vasodilation (Hosoki et al., 1997). However, Whiteman et al. (2006) reported that H2S reacts with NO that may result in the constriction of blood vessels. In addition, decreased H2S generation has been demonstrated in the vasculature of spontaneously hypertensive rats (Beltowski, 2005). In experimental hypertension induced by NO synthase blockade and in hypoxia-induced pulmonary hypertension, the administration of exogenous H2S donors has significant therapeutic effects (Chen et al., 2004; Qingyou et al., 2004). As mentioned, H2S possesses other ‘vasculoprotective' properties, including the inhibition of VSMC proliferation (Du et al., 2004; Yang et al., 2004a, 2004b).
As H2S appears to have a function, similar to that of NO, in modulating vascular function, it is possible that H2S may also inhibit the expression and activity of NADPH oxidase. To test this proposal, the effect of sodium hydrosulphide (NaHS) on O2•− formation and expression of the catalytic subunit of NADPH oxidase (gp91phox) by porcine cultured pulmonary endothelial cells was studied. The mediatory roles of cAMP and cGMP were studied using inhibitors of PKA, PKG and guanylyl cyclase as well as the measurement of cyclic nucleotides. The effect of a novel H2S-donating derivative of sildenafil (ACS6) on these systems was also investigated, as we have demonstrated that sildenafil is a potent inhibitor of NADPH oxidase expression and O2•− formation in vascular tissue (Koupparis et al., 2005; Muzaffar et al., 2005b). ACS6 may have dual actions and therefore more potent therapeutic effects. The release of H2S from ACS6 was also studied under a variety of conditions.
Methods
All animal procedures were in compliance with the rules and regulations of Bristol University and the UK Home Office. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Preparation of PAECs
Endothelial cells were prepared as previously described (Muzaffar et al., 2003, 2004a, 2004b). Lungs were obtained from White Landrace male pigs of body weight ranging from 20 to 35 kg. Pigs were anaesthetized with an intravenous injection of ketamine hydrochloride (10 mg kg−1; Ketaset injection, Fort Dodge Animal Health, Southampton, UK) and inhaled halothane (1–2% v/v in oxygen), exsanguinated and had their lungs removed. Pulmonary arteries (3–4 mm diameter) were dissected out immediately and rinsed in Dulbecco's modified Eagles' medium (GibcoBRL, Paisley, UK). Pulmonary arterial endothelial cells (PAECs) were cultured by the explant method as previously described (Muzaffar et al., 2004a, 2004b). PAECs were grown and maintained in endothelial cell growth medium (PromoCell, Heidelberg, Germany) at 37 °C in a 95% air–5% CO2 incubator. When confluent, cells were harvested by trypsinization. All subsequent experiments were carried out using cells at passage 4.
Effect of drugs on O2•− formation
PAECs were incubated with tumour necrosis factor-α (TNFα) (10 ng mL−1) (±NaHS or ACS6; 10 pM–1 μM) for 16 h at 37 °C in a 95% air–5% CO2 incubator. Following incubation, cells were washed three times with Dulbecco's modified Eagles' medium to remove drugs. The washed cells were equilibrated in Dulbecco's modified Eagles' medium without phenol red for 10 min at 37 °C in a 95% air–5% CO2 incubator (Heraeus, HERAcell; Kandro Laboratory Products, Langesnbold, Germany). Horse heart cytochrome c (20 μM) (or partially acetylated cytochrome c) with or without 500 U mL−1 copper zinc superoxide dismutase was added and the cells were incubated at 37 °C in a 95% air–5% CO2 incubator for 1 h. The reaction medium was removed and reduction of cytochrome c was determined at 550 nm in an Anthos Lucy 1 spectrometer (Lab-tech International, Ringmer, East Sussex, UK) and converted to micromoles of O2•− using ΔE550 nm=21.1 mM−1 cm−1 as the extinction coefficient. The reduction of cytochrome c that was inhibited with superoxide dismutase, reflected actual O2•− release. Cells were then washed with phosphate-buffered saline, lysed with 0.1% v/v Triton X-100 and total protein content was measured using BCA protein assay kit (Pierce, Rockford, IL, USA). Data are expressed as micromoles of O2•− per milligram protein per hour.
To study the acute effects of NaHS or ACS6 on O2•− formation, PAECs were incubated with 10 ng mL−1 TNFα alone for 16 h, washed 3 × with phosphate-buffered saline and then further incubated with NaHS or ACS6 (both 10 pM–1 μM) for 2 h at 37 °C in a 95% air–5% CO2 incubator and O2•− measured as above. In some experiments, cells were incubated with the suboptimal concentrations of NaHS and sildenafil citrate to assess their combined effect on O2•− release.
O2•− measurement by different methods
To validate the data obtained by cytochrome c assay, O2•− production was also measured either using nitroblue tetrazolium (NBT) reduction assay as described by Lee et al. (2003) or by chemiluminescence, using 2-methyl-6-phenyl-3,7-dihydroimidazo[1,2-α]pyrazin-3-one (CLA).
CLA-dependent chemiluminescence
Following drug treatment, PAECs cultured in 96-well solid white plates were rinsed in phosphate-buffered saline and equilibrated in pre-warmed Hanks' balanced salt solution for 15 min with or without superoxide dismutase (50 U mL−1) to assess the specificity of the assay. CLA-enhanced chemiluminescence was measured, as an index of O2•− production, using a thermostatically (37 °C) controlled plate luminometer. CLA (5 μM final concentration) was programmed to be dispensed automatically into each well immediately before the chemiluminescence detection. Signals from each well were recorded for 10 s continuously. At the end of the experiment, cells were lysed and total protein content was measured using BCA protein assay kit. Data were expressed as arbitrary units per milligram protein per second.
NBT reduction assay
Following drug treatment, PAECs cultured in six-well plates were rinsed in phosphate-buffered saline and incubated with 1 mg mL−1 NBT for 1 h to allow superoxide generated by the cell to reduce NBT to blue formazan. Microscopic examination and photography verified the generation of insoluble formazan as dark purple granules. After removal of the medium, the cells were lysed in 0.1% Triton X-100, and formazan was dissolved with two volumes of 2 M KOH and dimethyl sulphoxide. The optical density at 630 nm was measured spectrophotometrically. NBT reduction was expressed as a percentage of basal.
Phosphodiesterase type 5 activity assay
To determine whether ACS6 retained its activity as an inhibitor of phosphodiesterase type 5 (PDE5), the hydrolysis of cGMP was determined using a modification of the assay method of Thompson and Appleman (1971). The assay consists of a two-step isotopic procedure. In the first step, cyclic [3H]GMP is hydrolysed to 5′-[3H]GMP by PDE. In the second step, 5′-[3H]GMP is further hydrolysed to [3H]guanosine by the snake venom nucleotidase. Prior to commencing the assay, 100 μL PAEC lysate was incubated at 37 °C for 10 min in the absence and presence of sildenafil or ACS6, both at 100 nM, to define PDE5 activity. The assay was initiated by adding 50 μL test mix (0.2 μCi [3H]cGMP, 1 μmol L−1 cGMP, 10 mmol L−1 Tris pH 7.4, 5 mmol L−1 MgCl2, 100 μmol L−1 EGTA). The reaction was carried out at 37 °C for 30 min and terminated by immediate boiling for 2 min followed by cooling on ice. Next, 25 μL (1 mg mL−1) of snake venom (Crotalus atrox) was added to all samples and incubated at 37 °C for 30 min. A 400 μL of anion exchange resin slurry (1 g AG 1-8X resin/1.1 mL ethanol/1.1 mL double distilled H2O) was added to each reaction mixture, vortexed and kept at room temperature for 20 min. The resin binds to all charged nucleotides and leaves [3H]guanosine as the only labelled compound to be counted. The samples were centrifuged at 16 000 g for 2 min to precipitate all the resin. A 150-μL aliquot of the supernatant was then added to 5 mL of a scintillation cocktail and the radioactivity was measured by liquid scintillation counting.
Measurement of hydrogen sulphide release
Release of H2S from ACS6 and NaHS was measured by the methylene blue method (Siegel, 1965; Yang et al., 2007). ACS6 (50 μL) or NaHS (1–40 μM) was added to zinc acetate (1% w/v, 250 μL) and NaOH (10 mM, 200 μL) to trap H2S. After 30 min, the reaction was terminated by adding 250 μL of N-N-dimethyl-p-phenylenediamine sulphate (20 mM in 7.2 M HCl) and 250 μL of FeCl3 (30 mM in 1.2 M HCl). The reaction mixture was kept in the dark for 1 h. H2S in the sample interacts with N-N-dimethyl-p-phenylenediamine sulphate and FeCl3 to form methylene blue and the absorbance of the resulting solution was determined at 670 nm. H2S concentration in the sample solution was calculated against the calibration curve of sodium sulphide (Na2S).
In time course experiments, zinc acetate and NaOH were added to NaHS or ACS6 solutions (both at 10 μM) at various time intervals. The following experimental steps were carried out as stated above.
ACS6, unlike NaHS, does not release H2S in neutral solution but it can do so chemically in alkaline solution or enzymically as demonstrated for the same sulphurated moiety by Li et al. (2007). To determine H2S release in neutral solution, ACS6 (10 μM) was incubated with PAEC lysates prepared in RIPA buffer (50 mM Tris-HCl, pH 8; 150 mM NaCl; 1% NP-40; 0.5% sodium deoxycholate; 0.1% SDS; 2 mM EDTA) with protease and phosphatase inhibitor cocktails for 10 min at 37 °C. N-N-Dimethyl-p-phenylenediamine sulphate (400 μL) (20 mM in 7.2 M HCl) and FeCl3 (400 μL) (30 mM in 1.2 M HCl) were directly added to the test mix and further incubated at 37 °C in the dark. At the end of 2-h incubation, 250 μL of 10% trichloroacetic acid was to precipitate proteins, followed by centrifugation at 10 000 g for 10 min. H2S in the resulting supernatant was measured as stated above. To clarify further the mode of action of ACS6, the effect of the ‘spent' molecule (that is, after incubation in the absence of cells) was also studied. Thus, ACS6 dissolved in incubation buffer was incubated for 16 h and the effect on O2•− release by cells studied as described above.
Western blot analysis
For western blot analysis, following 16-h incubation with TNFα (±NaHS or ACS6; 100 pM–10 μM), as described above, PAECs were washed and lysed with Tris buffer (100 mM, pH 6.8) containing 1% glycerol and 1% SDS. Extracts were boiled at a 1:1 ratio with Tris (125 mM, pH 6.8 containing 4% w/v SDS; 10% v/v glycerol; 4% v/v 2-mercaptoethanol; 2 mg mL−1 bromophenol blue). Total cell lysates of equal protein (40 μg) were loaded onto 10% Tris-glycine SDS gels and separated by electrophoresis. After transfer to nitrocellulose, the blots were primed with a specific gp91phox monoclonal antibody (1:500 dilution; BD Biosciences, Oxford, UK). The blots were then incubated with goat anti-mouse antibody conjugated to horseradish peroxidase (1:2000 dilution) and developed by enhanced chemiluminescence (Amersham International). Rainbow markers (10–250 kDa; Amersham International) were used for molecular weight determination.
Effect of PKG and PKA inhibitors on O2•− formation
The role of cGMP and/or cAMP in mediating the inhibitory effects of NaHS and ACS6 on O2•− formation by PAECs was further investigated using the PKG peptide inhibitor, DT-3 (100 nM) or the PKA peptide inhibitor, 14–22 amide peptide (100 nM). The effect of the guanylyl cyclase inhibitor, 1H-{1,2,4}oxadiazolo{4,3-a}quinoxalin-1-one (ODQ; 100 nM) was also investigated.
Cells were pre-incubated for 1 h with PKG or PKA inhibitors or ODQ prior to the addition of TNFα+NaHS or TNFα+ACS6 for 16 h at 37 °C in a 95% air–5% CO2 incubator. Following washing of the cells, the production of O2•− was then measured by ferricytochrome c assay as described above. In studies on the acute effects of drugs, PAECs were at first incubated with 10 ng mL−1 TNFα alone for 16 h, washed and then incubated with NaHS or ACS6 for 2 h at 37 °C. The PKA or PKG inhibitors were added 1 h prior to the addition of NaHS or ACS6. O2•− was then measured by ferricytochrome c assay, as above.
cAMP measurements
Intracellular cAMP was measured using enzyme-linked immunoassay kit (R&D Systems, Abingdon, UK) following incubation of PAECs with drugs. PAECs were cultured in six-well plates and, on reaching confluence, were treated with various concentrations of NaHS or ACS6 (1 nM–1 μM) for 16 h. All incubations were carried out in the presence of the broad-spectrum PDE inhibitor, isobutylmethylxanthine (250 μM), to inhibit hydrolysis of cAMP. After incubation, media were removed and 100 μL of lysis buffer provided in the kit containing isobutylmethylxanthine was added to the cells and incubated for 15 min to extract cAMP. cAMP concentrations were then measured according to the manufacturer's instructions. The protein content was determined by the BCA protein assay kit and cAMP levels were expressed as picomoles per milligram protein per millilitre.
Data analysis
Data are expressed as mean±s.e.mean and n indicates the number of animals used. Dose–response data for NaHS and ACS6 in Figure 2 were best fit by nonlinear regression (sigmoidal dose–response, variable slope) to the following equation: Y=bottom + (top − bottom)/(1 + 10(logIC50−X)H), where IC50 is the concentration of NaHS or ACS6 resulting in 50% inhibition, X is the log of NaHS or ACS6 concentration, Y is the response and H is the Hill coefficient. The endothelial cells stimulated with TNFα alone were assigned for determinations of the zero value for IC50 calculations. The difference between the NaHS and ACS6 IC50 values with 95% confidence interval (CI) was analysed using the F-test (P<0.05). Data analysis was done using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, USA).
The remaining data were analysed by Student's unpaired t-test or one-way factorial ANOVA. A P-value of less than 0.05 was considered statistically significant. Multiple group comparisons were made using one-way ANOVA.
Drugs
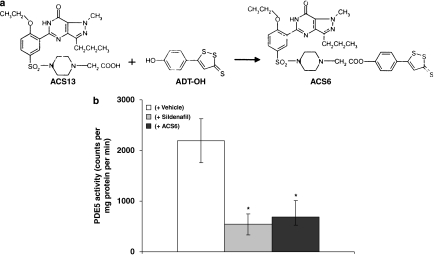
TNFα was purchased from R&D Systems. PKA inhibitor, 14–22 amide peptide, and PKG inhibitor, DT-3 peptide, were purchased from Calbiochem (Nottingham, UK). 1-Piperazineacetic acid 4-[[3-(4,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulphonyl]-,4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl ester, ACS6, was supplied by CTG Pharma, Milan, Italy (Figure 1a; see below for synthesis). Sildenafil citrate was supplied by University of Milan. Cyclic [3H]GMP was purchased from Amersham Biosciences (Little Chalfont, UK). All other drugs were purchased from Sigma Chemical Co. (Poole, Dorset, UK) unless otherwise stated.
Figure 1.
(a) Synthesis and structure of ACS6. (b) Effect of ACS6 or sildenafil on the activity of phosphodiesterase type 5 (PDE5). Each point is the mean±s.e.mean, n=6. *P<0.01; significantly inhibited compared with vehicle alone.
Synthesis of 4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl 2-(4-(4-ethoxy-3-(1-methyl-7-oxo-3-propyl-6, 7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)phenylsuphonyl)piperazin-1-yl)acetate (S-sildenafil; ACS6)
A 1 M solution of dicyclohexylcarbodiimide (0.7 mL) in dichloromethane was added to a solution of 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione (ADTOH, 127 mg, 0.54 mM) prepared by demethylation of anetholetrithione (ADT) as previously described (Bottcher et al., 1951), 2-(4-(4-ethoxy-3-(1-methyl-7-oxo-3-propyl-6,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)phenylsulphonyl)piperazin-1-yl)acetic acid (ACS13, 240 mg, 0.54 mM) prepared according to Bell (US patent 5 346 901) and Kim et al. (2001), and 4-dimethylaminopyridine (2.5 mg) in anhydrous CH2Cl2 (25 mL). The mixture was stirred for 3 h at room temperature, and was then filtered and the organic solution was extracted first with 0.1 N NaOH and finally with water. After removal of the solvent, the product was chromatographed on silica gel with dichloromethane/methanol (99:1) and the obtained solid compound was washed first with EtOH and then with Et2O. (yield: 48% m.p.: 188–189 °C (Buchi apparatus)). The identity of the product was confirmed as 4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl-2-(4-(4-ethoxy-3-(1-methyl-7-oxo-3-propyl-6,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl) phenylsulphonyl) piperazin-1-yl)acetate by 1H NMR (Varian Mercuri 300, CDCl3): δ=10.80 (s, 1H, collapses with D2O); 8.81 (d, 1H, J=2.64 Hz); 7.81 (dd, 1H, J=8.79, 2.35 Hz); 7.67 (d, 2H, J=8.5 Hz); 7.38 (s, 1H); 7.25 (d, 2H, J=8.5 Hz); 7.17 (d, 1H, J=9.09 Hz); 4.39 (q, 2H, J=7.04 Hz); 4.28 (s, 3H); 3.72 (s, 2H); 3.36 (bs, 4H); 3.09 (br s, 4H); 2.93 (t, 2H, J=7.62 Hz); 1.95–1.80 (m, 2H); 1.65 (t, 3H, J=7.04 Hz); 1.02 (t, 3H, J=7.32 Hz) and by elemental analysis (Carlo Erba EA-1100CHNS-O instrument) calculated for C32H34N6O6S4: C% 52.87; H% 4.71; N% 11.56 and S% 17.64; found: C% 52.54; H% 4.74; N% 11.40 and S% 17.81.
Results
Effect on phosphodiesterase activity and release of hydrogen sulphide
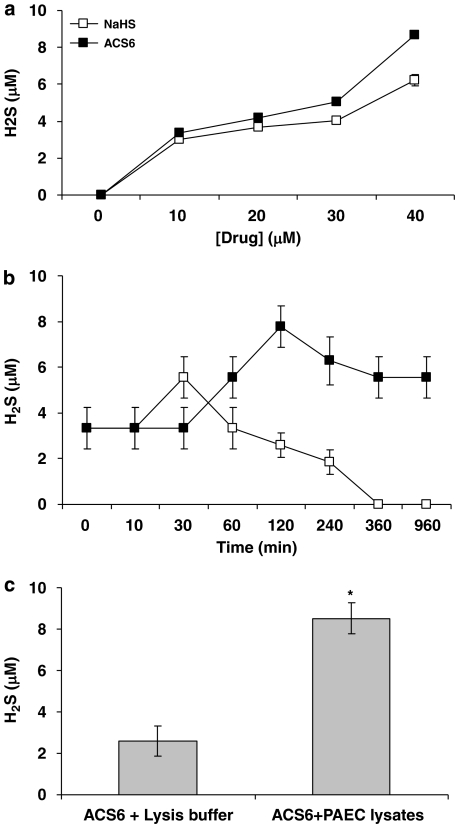
ACS6 inhibited PDE5 activity as effectively as sildenafil (Figure 1b), indicating that ACS6 retains its capacity to inhibit PDE5, despite being chemically modified. As mentioned above in the Methods section, H2S is not released from ACS6 spontaneously in neutral solution, unlike NaHS, but it can be released chemically in alkaline solution or enzymatically as demonstrated for the same sulphurated moiety by Li et al. (2007). Indeed ACS6 and NaHS released detectable amounts of H2S in alkaline solution, in a concentration-dependent manner (Figure 2a). Furthermore, H2S released from ACS6 was greater than that released from NaHS, a widely used water-soluble H2S donor (Figure 2a), due to the presence of three sulphur atoms in the molecule.
Figure 2.
H2S release from NaHS and ACS6 in vitro measured by the methylene blue assay, (a) concentration–response, (b) as a function of time. (c) H2S release from ACS6 in the absence or presence of pulmonary arterial endothelial cell (PAEC) lysates. Each point is the mean±s.e.mean, n=6. *P<0.01; significantly increased compared with ACS6 alone.
In addition, H2S concentration detected in any given solution of NaHS or ACS6 was fourfold less than the starting concentration of that solution (Figure 2a). This is in line with the previous finding, at least for NaHS, which dissociates to Na+ and HS− in solution, HS− then associates with H+ to form H2S (Wang, 2002). These authors stated that in physiological saline, ∼33% of the H2S exists as the undissociated form (H2S), and the remaining ∼66% exists as HS− in equilibrium with H2S, of a molar concentration of NaHS.
The timescale of maximal H2S release from 10 μM ACS6 in alkaline solution differed from that of 10 μM NaHS (Figure 2b). The maximal H2S release from ACS6 was after 2 h in alkaline solution and detectable levels were measured after 16 h (Figure 2b). The maximal H2S release from NaHS was after 30 min in alkaline solution and even though some levels were detected after 4 h, no H2S was detected after 16 h (Figure 2b).
As stated above, ACS6 only releases H2S chemically in alkaline solution (Figures 2a and b) or enzymatically. Thus, 10 μM ACS6 was incubated with PAEC lysates at 37 °C and after 2 h significantly greater levels of H2S were measured from this test incubation than that of 10 μM ACS6 in lysis buffer (Figure 2c).
Effect on superoxide formation
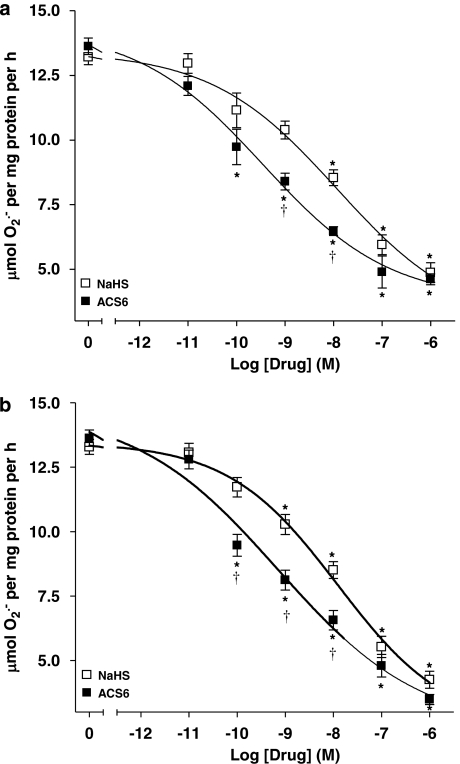
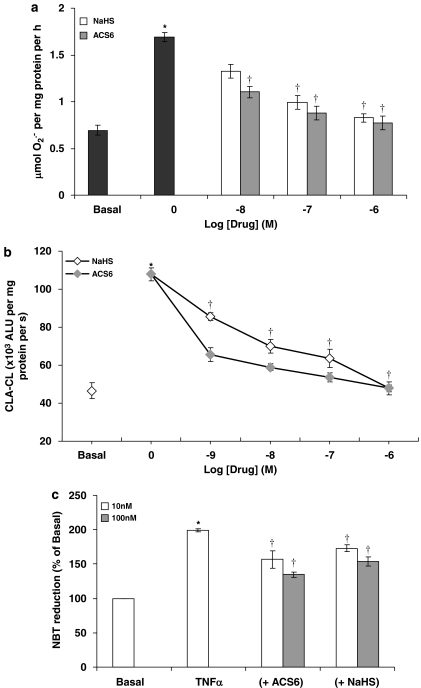
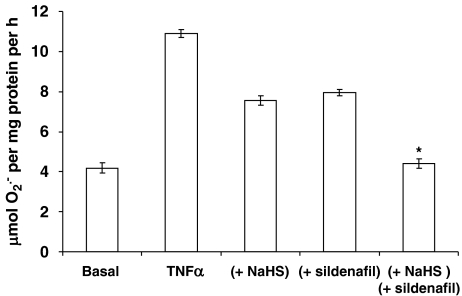
Using the ferricytochrome c assay, TNFα was a potent stimulator of O2•− formation by PAECs following 16-h incubation (Figure 3). Both NaHS and ACS6 inhibited the formation of O2•− in PAECs in a concentration-dependent manner when co-incubated with TNFα over 16-h incubation (Figure 3a). ACS6 (IC50: 0.35 nM with 95% CI of 0.10–1.25 nM) was significantly (P<0.0131) more potent than NaHS (IC50: 11.75 nM with 95% CI of 1.7–120 nM) (Figure 3a). In the acute studies (2 h exposure) following 16-h incubation with TNFα, ACS6 and NaHS inhibited O2•− formation in a concentration-dependent manner (Figure 3b). Again, ACS6 (IC50: 0.69 nM with 95% CI of 0.18–2.73 nM) was more potent than NaHS (IC50: 12.39 nM with 95% CI of 2.66–57.73 nM). Both ACS6 and H2S had no significant effect on basal superoxide release (data not shown). The data obtained by the cytochrome c assay was validated by various other well-established O2•− detection methods. Acetylated cytochrome c reduction, CLA-enhanced chemiluminescence or NBT reduction, all detected significantly increased O2•− generation in response to TNFα, which was inhibited by NaHS and more potently by ACS6 in a concentration-dependent manner (Figure 4). Furthermore, incubation of cells for 16 h with a combination of NaHS and the PDE5 inhibitor, sildenafil, at suboptimal concentrations, completely inhibited O2•− formation (not significantly different from basal values) from PAECs (Figure 5). This demonstrates that the inhibitory effect of ACS6 is mediated by a dual action, that is, inhibition of PDE5 and its H2S moiety. Furthermore, incubation of cells with ACS6 (at up to 10 μM) that had been pre-incubated in the absence of cells for 16 h had no effect on O2•− release by PAECs (data not shown).
Figure 3.
Comparison of the inhibitory effects of NaHS and ACS6 on tumour necrosis factor-α (TNFα) (0; 10 ng mL−1)-induced O2•− formation from pulmonary artery endothelial cells: (a) co-incubated over 16-h incubation period and (b) incubated for 2 h only following 16-h incubation with 10 ng mL−1 TNFα alone. Experimental data were averaged from six independent experiments carried out in different batches of cells. Data were normalized to TNFα responses and the curves were obtained fitting data to the sigmoidal dose–response equation described in Methods section. *P<0.01; significantly inhibited compared with TNFα-treated cells (0). †P<0.05; significantly greater inhibition than NaHS.
Figure 4.
Comparison of the inhibitory effects of NaHS and ACS6 on tumour necrosis factor-α (TNFα) (0; 10 ng mL−1)-induced O2•− formation from pulmonary artery endothelial cells as detected by (a) acetylated cytochrome c reduction, (b) CLA-enhanced chemiluminescence or (c) nitroblue tetrazolium reduction. Each point is the mean±s.e.mean, n=6. *P<0.01; significantly increased compared with basal. †P<0.05; significantly inhibited compared with TNFα-treated cells (0).
Figure 5.
Combined effect of NaHS (10 nM) and sildenafil citrate (10 nM) on tumour necrosis factor-α (TNFα) (10 ng mL−1)-induced O2•− formation from pulmonary arterial endothelial cell (PAEC) after 16-h incubation. Each point is the mean±s.e.mean, n=6. *P<0.01; significantly inhibited compared with NaHS or sildenafil alone.
Role of PKA and PKG
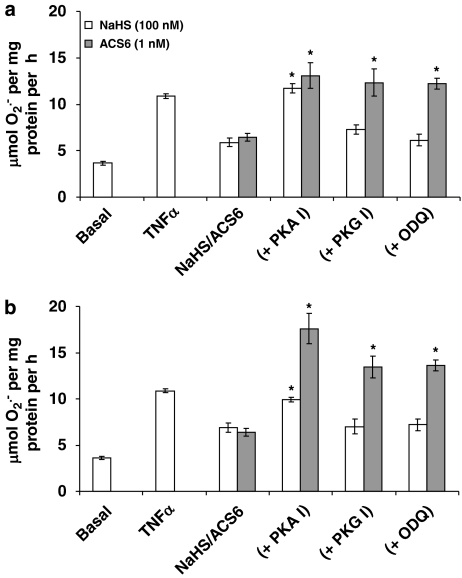
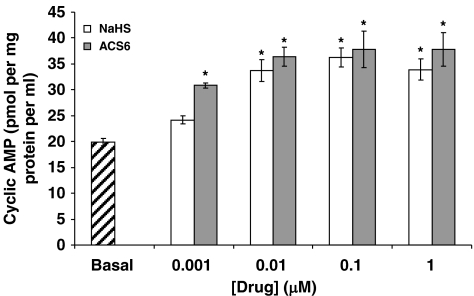
The inhibitory effect of NaHS on O2•− formation when co-incubated with TNFα over 16-h incubation was blocked by the PKA peptide inhibitor but not by the PKG peptide inhibitor or ODQ (Figure 6). Furthermore, NaHS significantly increased cAMP release from PAECs following overnight incubation (Figure 7). These data indicate that H2S activates adenylyl cyclase to generate cAMP and therefore activate PKA. By contrast, the inhibitory effect of ACS6 was blocked by both the PKA peptide inhibitor and by the PKG peptide inhibitor and ODQ (Figure 6). ACS6 also elevated cAMP levels in PAECs (Figure 7).
Figure 6.
Effect of protein kinase A (PKA) inhibition (PKA I; 14–22 amide peptide sequence; 100 nM), protein kinase G (PKG) inhibition (PKG I; DT-3 peptide; 100 nM) or guanylyl cyclase inhibition (ODQ; 100 nM) on the (a) longer term (16 h) and (b) acute (2 h) inhibition of tumour necrosis factor-α (TNFα) (10 ng mL−1)-induced O2•− formation from pulmonary arterial endothelial cell (PAEC) by H2S (100 nM) or ACS6 (1 nM). Each point is the mean±s.e.mean, n=6. *P<0.01; significantly increased compared with H2S/ACS6 alone.
Figure 7.
Effect of H2S and ACS6 on cyclic AMP (cAMP) formation in pulmonary arterial endothelial cells (PAECs) after 16-h incubation. Each point is the mean±s.e.mean, n=6. *P<0.01; significantly increased compared with controls.
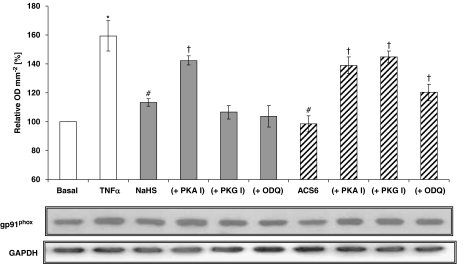
NaHS and ACS6 also blocked gp91phox expression induced by TNFα in PAECs in a concentration-dependent manner when co-incubated with TNFα over 16-h incubation (Figure 8). The PKA peptide inhibitor reversed the inhibitory effects of both NaHS and ACS6 on gp91phox expression, whereas the PKG peptide inhibitor only reversed the inhibitory effect of ACS6 (Figure 8).
Figure 8.
Western blot analysis of NADPH oxidase in pulmonary arterial endothelial cell (PAEC) lysates using a monoclonal antibody directed against the extracellular epitope of gp91phox-subunit of mouse NADPH oxidase. Cells were either not treated or treated with tumour necrosis factor-α (TNFα) (10 ng mL−1) for 16 h with one of the following: H2S (100 nM); ACS6 (1 nM); or combination of H2S/ACS6 with protein kinase A (PKA) inhibitor (PKA I; 100 nM), protein kinase G (PKG) inhibitor (PKG I; 100 nM) or guanylyl cyclase inhibitor (ODQ; 100 nM). The middle panel shows the representative blot and the upper panel the results of the densitometric analyses of six blots (expressed as % of basal values of OD mm−2). GAPDH expression was used as a loading control (lower panel). *P<0.05; significantly increased compared with basal value. #P<0.05; significantly inhibited compared with TNFα-treated value. †P<0.01; significantly increased compared with corresponding H2S/ACS6 only values.
Discussion
The results of this study demonstrate that NaHS is a potent inhibitor of O2•− formation induced by TNFα in porcine PAECs (PAECs), both acutely (2 h) and over the longer term (16 h). NaHS also inhibited gp91phox expression induced by TNFα over the longer term. These effects were blocked by inhibitors of PKA, but not by inhibitors of PKG. As NaHS also promoted the formation of cAMP, it is concluded that these effects of NaHS are mediated by the adenylyl cyclase-cAMP-PKA pathways. It should be stressed that NaHS was found to be a relatively weak stimulator of cAMP formation when compared with the levels promoted by iloprost, for example. Direct activation of PKA by NaHS cannot, therefore, be ruled out. As the principal source of O2•− in PAECs, in response to TNFα, is NADPH oxidase (Muzaffar et al., 2003) and NaHS releases H2S, these data indicate that H2S inhibits O2•− formation by blocking the upregulation and activation of NADPH oxidase. It should be stressed that the NADPH oxidase of endothelial cells comprises several subunits that include gp91phox, p22phox, p47phox, p67phox and p40phox, which must be assembled into a complex at the plasma membrane to be active (Li and Shah, 2001). NADPH oxidase also requires phosphorylated Rac1 for full activity (Hordijk, 2006). Thus, NaHS may well act on the expression of other components of the NADPH oxidase complex. Indeed, in recent studies we have demonstrated that NaHS inhibits Rac1 activity in human VSMCs (Muzaffar et al., 2008).
These effects of NaHS are similar to those of iloprost (a stable analogue of PGI2), which inhibits NADPH oxidase expression and concomitant O2•− formation, an effect also blocked by the inhibition of PKA (Muzaffar et al., 2004b). PGI2 is an important endogenous vasculoprotective factor that promotes vasodilation, inhibits platelet and leukocyte activity and adhesion, conserves NO bioavailability by preventing NADPH oxidase expression and inhibits VSMC replication (Jeremy et al., 1996). As such, a reduction of PGI2 formation has been widely implicated in cardiovascular diseases (Jeremy et al., 1996) and in ARDS (Muzaffar et al., 2005b). Thus, H2S appears to be akin to PGI2 rather than NO (at least in terms of intracellular protective mechanisms). An important facet of this study is that the inhibitory effects of NaHS on NO and O2•− formation were attained at concentrations that are similar to the amount of H2S generated by vascular tissues, which is in the region of 4 nM g−1 min−1 (Zhao et al., 2001).
This study also demonstrated that ACS6 (a putative H2S-donating derivative of sildenafil) is a potent inhibitor of O2•− formation both acutely and, over the longer term, of gp91phox expression induced by TNFα over the longer term in PAECs. We demonstrated that ACS6 possesses the capacity to generate comparable amounts of H2S to NaHS, that release from ACS6 is protracted compared with NaHS and perhaps most importantly that H2S is released from ACS6 when incubated with lysates prepared from cultured PAECs. This indicates that, once taken up by cells, ACS6 would release H2S intracellularly. In addition, incubation of ACS6 in buffer for 16 h (in the absence of cells), during which time all H2S would have been released from the drug (‘depleted' ACS6), had no effect on O2•− formation. This confirms that H2S release from ACS6 is crucial for the biological actions of the drug reported here.
This effect of ACS6 was far more potent than with NaHS alone, indicating that both the sildenafil and the sulphurated moieties of the drug are eliciting these effects. This is supported by the observation that the effects of ACS6 were blocked by both PKA peptide and PKG peptide inhibitors. The reversal of effects of ACS6 with PKA inhibition is indicative of the H2S moiety of the drug eliciting its effects through the activation of adenylyl cyclase and/or activation of PKA. With regard to the sildenafil moiety of ACS6, we previously demonstrated that sildenafil inhibits the formation of O2•− and gp91phox expression through an augmentation of cGMP levels and of PKG activation but not of PKA activation (Muzaffar et al., 2005b). We also demonstrated that NO, through a cGMP-PKG-dependent pathway, inhibits the formation of O2•− and gp91phox expression in PAECs (Muzaffar et al., 2004a). As sildenafil is a PDE5 inhibitor and PDE5 hydrolyses cGMP to inactive GMP (Jeremy et al., 1997), it was concluded that the inhibitory effect of sildenafil on NOX is mediated by augmentation of the of NO-PKG axis. Thus, the blockade of effects of ACS6 by the PKG inhibitor is indicative of the sildenafil moiety of ACS6 also coming into play in the present system and also that ACS6 retains its PDE5 inhibitory capacity despite having been modified chemically.
It is also notable that the potency of ACS6 was greater than that of sildenafil alone or NaHS alone. We previously demonstrated that sildenafil inhibits NADPH oxidase expression and O2•− at an IC50 of around 5 nM (Muzaffar et al., 2005b), whereas in this study the drug was effective at 0.1 nM. This is indicative of an additive, if not synergistic, interaction between the innate PDE5 inhibitory capacity and the H2S-donating capacity of ACS6. In this study, when added together at submaximal concentrations, the inhibitory effect of NaHS and sildenafil was greater than the effect elicited by the two moieties alone. Derivatization of sildenafil with a sulphur moiety may also confer additional pharmacological properties, such as more efficient cellular uptake and increased affinity for target protein (that is, PDE5) that increase its potency. Such studies are beyond the scope of this study but warrant further consideration.
The mechanisms underlying the inhibition of NADPH oxidase protein expression at the transcriptional and/or translational level by ACS6 were not determined in this study. However, PKA and PKG (both activated by ACS6) phosphorylate other mediators and protein kinases that influence transcription and translation (Cho-Chung et al., 2002; Pilz and Broderick, 2005). Potential candidates for this role include cAMP response element (CRE)-binding protein that binds to CRE resulting in the transcription of a wide range of CRE-containing genes (Montminy, 1997; Daniel et al., 1998), activating transcription factor-1 (ATF-1) (Sauzeau et al., 2003) and the multifunctional transcription factor TFII-I (Casteel et al., 2002).
A paradox arising from this study is that H2S biosynthesis is markedly increased by inflammation, including LPS-induced inflammation of the lung, which is a model for ARDS (Li et al., 2005). However, acute inflammation (including sepsis) also results in the increased formation of NO (Vallance and Moncada, 1993) and dilatory eicosanoids, including PGI2 and PGE1 (Jeremy et al., 1994), which in turn have been widely implicated in mediating the systemic hypotension associated with sepsis. Such a role has also been ascribed to H2S, as H2S is a vasodilator (Li et al., 2005). A secondary paradox, however, is that inhaled NO and PGI2 have proven effective in treating ARDS (Cepkova and Matthay, 2006). But why should this be so when ARDS is associated with increased NO and eicosanoid formation? The answer may be that ARDS is characterized by pulmonary hypertension and is a dynamic, rapidly changing syndrome. Thus, it is possible that the initial increase in the formation of NO, PGI2 and H2S subsides to be overtaken by events that favour vasoconstriction, in particular, the overproduction of O2•−. Put another way, the upregulation of NADPH oxidase follows the initial surge of NO and PG formation. Certainly, in the later stages of ARDS, the bioactivity of these endogenous dilators may be negated by O2•− and other ROS. Perhaps this is why NO and PGI2 and (perhaps exogenous H2S) at this later stage, are therapeutically beneficial. It is also notable that another H2S-donating derivative, S-diclofenac, is a more potent inhibitor than diclofenac of pulmonary inflammation induced by endotoxin in rats (Li et al., 2007).
From a therapeutic perspective, the overproduction of O2•− may be associated with the pathophysiology of ARDS and other cardiovascular diseases that include ischaemia reperfusion injury, diabetic angiopathy, hypertension and atherogenesis (Li and Shah, 2001; Muzaffar et al., 2005a). Although ARDS is a notoriously difficult condition to treat, both inhaled NO and PGI2 have proven promisingly effective in treating ARDS. It was suggested that as sildenafil augments the inhibitory effect of NO on O2•− formation through augmentation of the cGMP-PKG axis, that sildenafil may be useful in treating ARDS, possibly in conjunction with inhaled NO. Similarly, PGI2 inhibits NADPH oxidase expression and activity and concomitant O2•− formation. Inhaled ACS6 may be particularly effective in treating ARDS, as it activates the cAMP-PKA axis and augments the cGMP-PKG system, simultaneously. Further studies are required to test these possibilities.
Acknowledgments
This research was funded by the British Heart Foundation (RFLS.RJ4313).
Abbreviations
- ARDS
acute respiratory distress syndrome
- NADPH
nicotinamide adenine dinucleotide phosphate
- NaHS
sodium hydrosulphide
- PAECs
pulmonary artery endothelial cells
- PDE5
phosphodiesterase type 5
- PGI2
prostacyclin
- PKA
protein kinase A
- PKG
protein kinase G
- O2•−
superoxide
Conflict of interest
Dr P Del Soldato is a shareholder of CTG Pharma, Milan, Italy. This company has patents on reagents used in this study. Professor A Sparatore received a grant from CTG Pharma.
References
- Beltowski J. Hydrogen sulfide as a biologically active mediator in the cardiovascular system. Postepy Hig Med Dosw (Online) 2005;58:285–291. [PubMed] [Google Scholar]
- Bottcher B, Bauer F, Trithiones V. Some new trithiones. Chem Ber. 1951;84:458–463. [Google Scholar]
- Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- Casteel DE, Zhuang S, Gudi T, Tang J, Vuica M, Desiderio S, et al. cGMP-dependent protein kinase Iβ physically and functionally interacts with the transcriptional regulator TFII-I. J Biol Chem. 2002;277:32003–32014. doi: 10.1074/jbc.M112332200. [DOI] [PubMed] [Google Scholar]
- Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intens Care Med. 2006;21:119–145. doi: 10.1177/0885066606287045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot F, Mitchell JA, Gutteridge JM, Evans AM. Reactive oxygen species in acute lung injury. Eur Respir J. 1998;11:745–757. [PubMed] [Google Scholar]
- Chen X, Jhee K-W, Kruger WD. Production of the neuromodulator H2S by cystathione beta synthase via the condensation of cysteine and homocysteine. J Biol Chem. 2004;279:52082–52086. doi: 10.1074/jbc.C400481200. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- Cho-Chung YS, Nesterova M, Becker KG, Srivastava R, Park YG, Lee YN, et al. Dissecting the circuitry of protein kinase A and cAMP signaling in cancer genesis: antisense, microarray, gene overexpression, and transcription factor decoy. Ann NY Acad Sci. 2002;968:22–36. doi: 10.1111/j.1749-6632.2002.tb04324.x. [DOI] [PubMed] [Google Scholar]
- Daniel PB, Walker WH, Habener JF. Cyclic AMP signaling and gene regulation. Annu Rev Nutr. 1998;18:353–383. doi: 10.1146/annurev.nutr.18.1.353. [DOI] [PubMed] [Google Scholar]
- Du J, Hui Y, Cheung Y, Bin G, Jiang H, Chen X, et al. The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cell. Heart Vessels. 2004;19:75–80. doi: 10.1007/s00380-003-0743-7. [DOI] [PubMed] [Google Scholar]
- Dweik RA. Nitric oxide, hypoxia, and superoxide: the good, the bad, and the ugly. Thorax. 2005;60:265–267. doi: 10.1136/thx.2004.038471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkaerts G, Kloek J, Muijsers RB, Nijkamp FP. Reactive nitrogen and oxygen species in airway inflammation. Eur J Pharmacol. 2001;429:251–262. doi: 10.1016/s0014-2999(01)01324-3. [DOI] [PubMed] [Google Scholar]
- Hordijk PL. Regulation of NADPH oxidases: the role of rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- Jeremy JY, Ballard S, Naylor A, Angelini GD. Effect of sildenafil (VIAGRA™), a specific inhibitor of cGMP phosphodiesterase on cAMP and cGMP formation by the rabbit corpus cavernosa, in vitro. Br J Urol. 1997;79:958–963. doi: 10.1046/j.1464-410x.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- Jeremy JY, Jackson CL, Bryan AJ, Angelin GD. Eicosanoids, fatty acids and restenosis following balloon angioplasty and vein graft surgery. Prostagl Leukotr Essential Fatty Acids. 1996;54:385–402. doi: 10.1016/s0952-3278(96)90022-8. [DOI] [PubMed] [Google Scholar]
- Jeremy JY, Nystrom ML, Barradas MA, Mikhailidis DP. Eicosanoids and septicaemia. Prostagl Leukotr Essential Fatty Acids. 1994;50:287–297. doi: 10.1016/0952-3278(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Kim DK, Lee JY, Lee N, Ryu DH, Kim JS, Lee S, et al. Synthesis and phosphodiesterase inhibitory activity of new sildenafil analogues containing a carboxylic acid group in the 5′-sulfonamide moiety of a phenyl ring. Biorg Med Chem. 2001;9:3013–3021. doi: 10.1016/s0968-0896(01)00200-0. [DOI] [PubMed] [Google Scholar]
- Koupparis A, Jeremy JY, Muzaffar S, Shukla N. Sildenafil inhibits the formation of superoxide and the expression of gp91phox NADPH oxidase induced by the thomboxane A2 mimetic, U46619 in corpus cavernosal smooth muscle cells. Br J Urol Int. 2005;96:423–427. doi: 10.1111/j.1464-410X.2005.05643.x. [DOI] [PubMed] [Google Scholar]
- Lee HS, Son SM, Kim YK, Hong KW, Kim CD. NAD(P)H oxidase participates in the signalling events in high glucose-induced proliferation of vascular smooth muscle cells. Life Sci. 2003;72:2719–2730. doi: 10.1016/s0024-3205(03)00186-3. [DOI] [PubMed] [Google Scholar]
- Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, et al. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- Li L, Rossoni G, Sparatore A, Lee LC, Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Rad Biol Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Li JM, Shah AM. Differential NADPH- versus NADH-dependent superoxide production by phagocyte-type endothelial cell NADPH oxidase. Cardiovasc Res. 2001;52:477–486. doi: 10.1016/s0008-6363(01)00407-2. [DOI] [PubMed] [Google Scholar]
- Metnitz PG, Bartens C, Fischer M, Fridrich P, Steltzer H, Druml W. Antioxidant status in patients with acute respiratory distress syndrome. Intens Care Med. 1999;25:134–136. doi: 10.1007/s001340050813. [DOI] [PubMed] [Google Scholar]
- Moore PK, Bhatia M, Moochhala S. Hydrogen sulfide: from the smell of the past to the mediator of the future. Trends Pharmacol Sci. 2003;24:609–611. doi: 10.1016/j.tips.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- Muzaffar S, Jeremy JY, Angelini GD, Stuart-Smith K, Shukla N. The role of the endothelium and nitric oxide synthases in modulating superoxide formation induced by endotoxin and cytokines in porcine pulmonary arteries. Thorax. 2003;58:598–604. doi: 10.1136/thorax.58.7.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Nitroaspirins and SIN-1, but not aspirin, inhibit the expression of endotoxin- and cytokine-induced NADPH oxidase in vascular smooth muscle cells from pig pulmonary arteries. Circulation. 2004a;110:1140–1147. doi: 10.1161/01.CIR.0000139851.50067.E4. [DOI] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Nicotinamide adenine dinucleotide phosphate oxidase: a promiscuous therapeutic target for cardiovascular drugs. Trends Cardiovasc Med. 2005a;15:278–282. doi: 10.1016/j.tcm.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Sildenafil citrate and sildenafil nitrate inhibit superoxide formation and gp91phox expression in porcine pulmonary endothelial cells though a cyclic GMP dependent mechanism. Br J Pharmacol. 2005b;146:109–117. doi: 10.1038/sj.bjp.0706305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Hypoxia and the expression of gp91phox and endothelial nitric oxide synthase in the pulmonary artery. Thorax. 2005c;60:305–313. doi: 10.1136/thx.2003.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Superoxide auto-augments superoxide formation in isolated porcine pulmonary artery endothelial cells though upregulation of NADPH oxidase expression: inhibition with iloprost. Eur J Pharmacol. 2006;538:108–114. doi: 10.1016/j.ejphar.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Bond M, Newby AC, Sparatore A, Del Soldato P, et al. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac(1) activity in human vascular smooth muscle cells. J Vasc Res. 2008;45:521–528. doi: 10.1159/000129686. [DOI] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Lobo C, Angelini GD, Jeremy JY. Iloprost inhibits superoxide formation and NADPH oxidase expression induced by the thomboxane A2 analogue, U46619, and isoprostane F2α in cultured porcine pulmonary artery vascular smooth muscle cells. Br J Pharmacol. 2004b;141:488–496. doi: 10.1038/sj.bjp.0705626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz RB, Broderick KE. Role of cyclic GMP in gene regulation. Front Biosci. 2005;10:1239–1268. doi: 10.2741/1616. [DOI] [PubMed] [Google Scholar]
- Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, et al. Free radical biology and medicine: it's a gas, man. Am J Physiol Regul Integr Comp Physiol. 2006;291:R491–R511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- Qingyou Z, Jumbao D, Weijin Z, Hui Y, Chaoshu Y, Chunyu Z. Impact of hydrogen sulfide on carbon moNADPH oxidaseide/heme oxygenase pathway in the pathogenesis of hypoxic pulmonary hypertension. Biochem Biophys Res Commun. 2004;317:30–37. doi: 10.1016/j.bbrc.2004.02.176. [DOI] [PubMed] [Google Scholar]
- Sauzeau V, Rolli-Derkinderen M, Marionneau C, Loirand G, Pacaud P. RhoA expression is controlled by NO though cGMP-dependent protein kinase activation. J Biol Chem. 2003;278:9472–9480. doi: 10.1074/jbc.M212776200. [DOI] [PubMed] [Google Scholar]
- Siegel LM. A direct microdetermination for sulphide. Anal Biochem. 1965;11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- Stuart-Smith K, Jeremy JY. Microvessel damage in acute respiratory distress syndrome: the answer may not be NO. Br J Anaesth. 2001;87:272–279. doi: 10.1093/bja/87.2.272. [DOI] [PubMed] [Google Scholar]
- Thompson WJ, Appleman MM. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971;10:311–316. [PubMed] [Google Scholar]
- Vallance P, Moncada S. Role of endogenous nitric oxide in septic shock. New Horiz. 1993;1:77–86. [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter. FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Weinacker AB, Vaszar LT. Acute respiratory distress syndrome: physiology and new management strategies. Annu Rev Med. 2001;52:221–237. doi: 10.1146/annurev.med.52.1.221. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Li L, Kosteski I, Chu SH, Siau JL, Bhatia M, et al. Evidence for the formation of a novel nitrosothiol from the gaseous mediators, nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun. 2006;343:303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- Yang G, Cao K, Wu L, Wang R. Cystathionine gamma-lyase over-expression inhibits cell proliferation via a H2S-dependent modulation of ERK1/2 phosphorylation and p21Cip/WAK−1. J Biol Chem. 2004a;279:49199–49205. doi: 10.1074/jbc.M408997200. [DOI] [PubMed] [Google Scholar]
- Yang G, Yang W, Wu L, Wang R. H2S, endoplasmic reticulum stress and apoptosis of insulin-secreting beta cells. J Biol Chem. 2007;282:16567–16576. doi: 10.1074/jbc.M700605200. [DOI] [PubMed] [Google Scholar]
- Yang S, Sun X, Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004b;18:1782–1784. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]