Abstract
Background and purpose:
The xanthine oxidase inhibitors allopurinol and oxypurinol are used to treat hyperuricaemia, whereas loop and thiazide diuretics can cause iatrogenic hyperuricaemia. Some uricosuric drugs and salicylate have a bimodal action on urate renal excretion. The mechanisms of action of these hypo- and hyperuricaemic drugs on the handling of urate in renal tubules have not been fully elucidated. Recently, we identified the multidrug resistance protein (MRP) 4 as a luminal efflux transporter for urate in the proximal tubule.
Experimental approach:
Here, we studied the effect of these drugs on [14C]urate transport using human embryonic kidney 293 cells overexpressing human MRP4 and in membrane vesicles isolated from these cells.
Key results:
Allopurinol stimulated MRP4-mediated cellular urate efflux and allopurinol and oxypurinol both markedly stimulated urate transport by MRP4 in membrane vesicles. Bumetanide and torasemide had no effect, whereas furosemide, chlorothiazide, hydrochlorothiazide, salicylate, benzbromarone and sulfinpyrazone inhibited urate transport, at concentrations ranging from nanomolar up to millimolar. Probenecid stimulated urate transport at 0.1 μM and inhibited transport at higher concentrations.
Conclusions and implications:
These data suggest that inhibition of MRP4-mediated urate efflux by furosemide and thiazide diuretics could have an important function in their hyperuricaemic mechanisms. Furthermore, stimulation of MRP4-mediated renal urate efflux could be a new mechanism in the hypouricaemic action of allopurinol and oxypurinol. In conclusion, MRP4 may provide a potential target for drugs affecting urate homoeostasis, which needs to be further evaluated in vivo.
Keywords: multidrug resistance protein, urate transport, hyperuricaemia, diuretic, uricosuric
Introduction
The kidney has an important function in the elimination of uric acid (urate). After filtration of nearly all plasma urate through the glomerulus, it undergoes complex bidirectional steps of reabsorption and secretion in the proximal tubule, resulting in a net urinary excretion of less than 10% of the filtered amount. Uric acid, the final breakdown product of purines in humans, has important biological functions as an endogenous danger signal in the immune system and a free radical scavenger, involved in antioxidant protection and determining longevity (Hediger et al., 2005). It is a weak organic acid, which, at physiological plasma pH, exists in a monovalent anionic form. Normally, the human plasma uric acid concentration is about 300 μM (5 mg dl−1). Hyperuricaemia is characterized by a persistent increase of plasma concentrations above 400 μM, in which monosodium urate monohydrate crystals may be deposited in tissues causing several pathological conditions, including acute gouty arthritis, urolithiasis and obstructive uropathy (Terkeltaub et al., 2006).
Multiple transporters in the proximal tubule are involved in regulating renal urate excretion. The apical organic anion transporter (OAT) 4 (Hagos et al., 2007) and urate transporter (URAT) 1 (Enomoto et al., 2002) reabsorb filtered urate from the primary urine into the tubular cells. At the basolateral membrane, OAT1 (Ichida et al., 2003) and OAT3 (Bakhiya et al., 2003) are involved in the active uptake of plasma urate as a first step in its tubular secretion. Recently, we identified urate as a substrate of the ATP-dependent unidirectional efflux transporter, multidrug resistance protein 4 (MRP4/ABCC4), which is localized to the apical membrane of proximal tubule cells (Van Aubel et al., 2002, 2005). To date, MRP4 is the only known human transporter that may contribute to the secretion of urate across the apical membrane. As urate is almost completely reabsorbed after its glomerular filtration (Anzai et al., 2007), net urinary excretion is mainly dependent on tubular secretion. Recently, it was reported that the angiotensin II receptor blocker losartan may influence plasma urate levels through an inhibitory effect on renal MRP4-mediated transport (Sato et al., 2008). Other compounds that can alter serum urate concentrations include drugs used in the treatment of gout and hyperuricaemia, such as xanthine oxidase inhibitors and uricosurics (Hoskison and Wortmann, 2006), as well as drugs that can cause iatrogenic hyperuricaemia, such as loop (Brater, 1996) and thiazide (Huen and Goldfarb, 2007) diuretics. The non-steroidal anti-inflammatory drug, salicylate, has long been known to have a biphasic action on urate plasma concentration (Yu and Gutman, 1959). The goal of this work was to investigate the effect of these drugs on MRP4-mediated urate transport. Using human embryonic kidney (HEK) 293 cells overexpressing human MRP4 and membrane vesicles isolated from these cells, we show that many of these drugs can alter urate transport by MRP4, which could contribute to their overall effects on handling of urate by the kidney.
Materials and methods
Generation of human MRP4 baculovirus and transduction of HEK293 cells
Generation of human MRP4 baculovirus was performed as described previously (El-Sheikh et al., 2007). Briefly, the Bac-to-Bac system, normally used for protein production in insect cells, was made suitable for protein expression in mammalian cells. The human MRP4 and the enhanced yellow fluorescent protein (EYFP; control) were cloned into the Gateway entry vector (Invitrogen, Breda, the Netherlands). Baculoviruses were produced as described in the Bac-to-Bac manual. To transduce HEK293 cells with human MRP4 baculovirus, cells were cultured in 182 cm2 flasks containing Dulbecco's modified Eagle's medium supplemented with 10% foetal calf serum at 37 °C under 5% CO2-humidified air until 70% confluence. The medium was replaced by 1.5 ml of virus and 3.5 ml of medium and the cells were incubated for 30 min at 37 °C, after which 20 ml of medium was added. Sodium butyrate (5 mM) was added after 24 h of transduction.
Time-dependent diffusion and efflux assays in MRP4 cells
For cellular transport assays, HEK-MRP4 cells as well as control cells were harvested after 2 days of transfection and seeded in collagen precoated 96-well plates, at a density of 2.5 × 104 cells per well. After 24 h, the medium was removed and plates were incubated on ice with 50 μM [14C]urate in Hank's buffered salt solution (HBSS) medium (containing 1 mM CaCl2, 0.5 mM MgCl2, 0.4 mM MgSO4, 5 mM KCl, 0.4 mM KH2PO4, 4 mM NaHCO3, 138 mM NaCl and 0.3 mM Na2HPO4) for testing the diffusion step. After 15 s, 10 min, 20 min and 30 min, the diffusion was stopped by removing the diffusion medium and washing the cells with ice-cold HBSS buffer. Cells were then lysed in 0.1% Triton and 4 ml of scintillation fluid was added to measure the amount of [14C]urate in the cells, using liquid scintillation radioactivity counting. For testing time-dependent efflux of [14C]urate, cells were incubated in 50 μM of the radiolabelled compound for 30 min on ice. After removing the diffusion medium and washing the cells with ice-cold HBSS buffer, the efflux step was started by adding 100 μl of HBSS per well and incubating the cells at 37 °C for 15 s, 2 min, 3 min, 5 min and 10 min. The efflux medium was then removed and placed in vials to count the radioactivity. Each experiment was performed in triplicate.
Effect of drugs on [14C]urate efflux from MRP4 cells
To evaluate the effect of different drugs on [14C]urate efflux, HEK-MRP4 cells as well as control cells were incubated in HBSS containing 50 μM of [14C]urate for 30 min in the presence or absence of 100 μM of the tested drug. Drugs were dissolved in dimethyl sulphoxide, with the exception of salicylate, which was dissolved in water. The concentration of dimethyl sulphoxide was fixed at 0.1% in all samples. This concentration was tested and was shown to exert no significant effect on the assay. After removing the diffusion medium and washing the cells with ice-cold HBSS buffer, 100 μl of HBSS efflux buffer was added per well and plates were incubated at 37 °C in a water bath for 3 min. The efflux medium was then removed and placed in vials to count the radioactivity. Net MRP4-dependent transport was calculated by subtracting background values measured in control cells.
Isolation of membrane vesicles and protein analysis
Three days after cell transduction with MRP4 or EYFP-control virus, the cells were harvested by centrifugation at 3000 g for 30 min. The cell pellets were resuspended in ice-cold homogenization buffer (0.5 mM sodium phosphate, 0.1 mM EDTA, pH 7.4) supplemented with protease inhibitors (100 μM phenylmethylsulphonyl fluoride, 5 μg ml−1 aprotinin, 5 μg ml−1 leupeptin, 1 μM pepstatin, 1 μM N-(trans-epoxy-succinyl)-L-leucine 4-guanidinobutylamide) and shaken at 4 °C for 60 min, followed by centrifugation at 4 °C and 100 000 g for 30 min. The pellets were homogenized in ice-cold Tris-HEPES buffer (10 mM Tris-HEPES, 250 mM sucrose, pH 7.4) with a tight-fitting Dounce homogenizer (30 strokes). After centrifugation at 500 g and 4 °C for 20 min, the supernatant was centrifuged at 100 000 g and 4 °C for 60 min. The resulting pellet was resuspended in TS buffer and passed through a 27-gauge needle 30 times. The protein concentration was determined using Bio-Rad protein assay kit. Crude membrane vesicles were dispensed in aliquots, frozen in liquid nitrogen and stored at −80 °C until use.
Vesicular transport assays
Uptake of [14C]urate into membrane vesicles was performed as described previously (Van Aubel et al., 2005). In brief, membrane vesicles were prewarmed for 1 min at 37 °C and added to TS buffer, supplemented with an ATP-regeneration mixture (4 mM ATP, 10 mM MgCl2, 10 mM creatine phosphate, 100 mg ml−1 creatine kinase) in a final volume of 60 μl. The reaction mixture was incubated at 37 °C for the indicated times, and samples were diluted in 900 μl of ice-cold TS buffer. Diluted samples were filtered through 0.45-μm-pore NC filters that had been pre-incubated with TS buffer, by a filtration device (Millipore, Bedford, MA, USA). Liquid scintillation counting was performed after adding 4 ml of scintillation fluid, and the uptake of [14C]urate into membrane vesicles was studied by measuring radioactivity associated with the filters. In control experiments, ATP was substituted with AMP. Net ATP-dependent transport was calculated by subtracting values measured in the presence of AMP from those measured in the presence of ATP. Measurements were corrected for the amount of [14C]urate bound to the filters (usually <2% of total radioactivity). Each experiment was performed in triplicate.
Effect of drugs on [14C]urate transport in MRP4 vesicles
To evaluate the effect of different drug concentrations on [14C]urate transport mediated by MRP4, the transport assay mentioned previously was performed using HEK-MRP4 vesicles incubated in 100 μM [14C]urate, in the presence or absence of different concentrations of drugs tested. All drugs were dissolved in dimethyl sulphoxide, with the exception of salicylate, which was dissolved in water. The final concentration of dimethyl sulphoxide in the incubation medium was adjusted to 1%, which did not have any significant effect on the vesicular transport assay.
Data analysis
All data were expressed as means±s.e.mean. IC50 values of drugs tested were obtained from curve fitting of the resulting concentration-inhibition curves by non-linear regression analysis using GraphPad Prism software version 4.03 (GraphPad Software Inc., San Diego, CA, USA). One-way ANOVA, Dunnett's multiple comparison and extra sum-of-squares F-test for model comparison were performed using the same software.
Materials
[8-14C]urate (53 mCi mmol−1) was purchased from Moravek Inc. (Brea, CA, USA). The Bac-to-Bac and Gateway system were purchased from Invitrogen (Breda, The Netherlands). NC45 filters were obtained from Schleicher and Schuell (Den Bosch, The Netherlands). Creatine phosphate and creatine kinase were purchased from Roche Diagnostics (Almere, The Netherlands). Protein concentrations were determined with an assay kit from Bio-Rad Laboratories (Veenendaal, The Netherlands). All drugs tested were purchased from Sigma-Aldrich (St Louis, MO, USA), with the exception of torasemide, which was extracted using methanol from Torasemid 10 mg tablets (Actavis, Germany), and its purity was confirmed using mass spectrometry and proton nuclear magnetic resonance.
Results
Time-dependent diffusion and efflux of [14C]urate by HEK-MRP4 cells
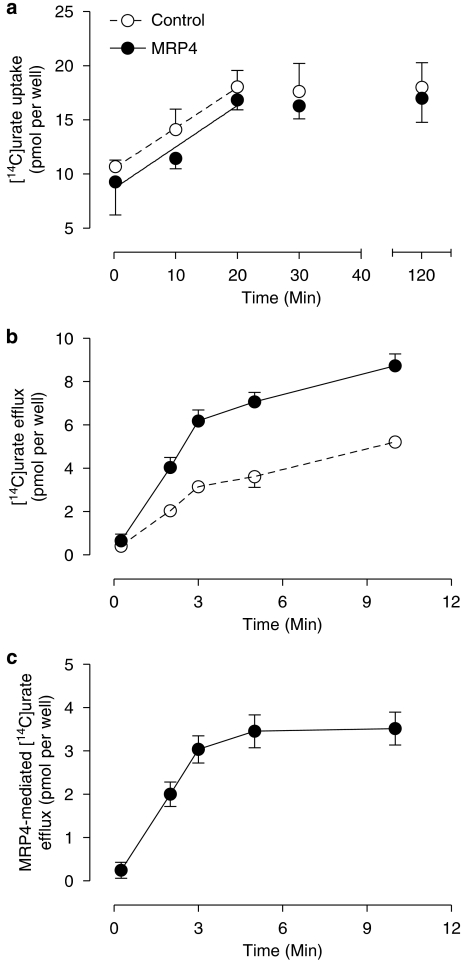
Human embryonic kidney 293 cells overexpressing MRP4 were incubated on ice in HBSS medium containing 50 μM [14C]urate. The relative contribution of MRP4 to overall transport was assessed in parallel experiments performed on HEK293 cells transfected with the control vector expressing EYFP. Diffusion of [14C]urate into both type of cells was similar and increased linearly up to 20 min, after which it remained stable for 2 h (Figure 1a). Thus, loading the cells for 30 min on ice was chosen before the efflux assay was started. Time-dependent efflux of [14C]urate from HEK-MRP4 and control cells was measured at 15 s, 2 min, 3 min, 5 min and 10 min (Figure 1b). Net MRP4-dependent urate transport was calculated from the difference between HEK-MRP4 and HEK-EYFP efflux and appeared to increase linearly up to 3 min (Figure 1c).
Figure 1.
Time-dependent uptake and efflux of [14C]urate in multidrug resistance protein (MRP) 4 cells. To compare the urate uptake in human embryonic kidney (HEK)-MRP4 cells with that in control cells, cells were incubated for 15 s, 10 min, 20 min, 30 min and 2 h at 4 °C with HBSS diffusion medium containing 50 μM [14C]urate (a). After incubation of MRP4 and control cells with 50 μM [14C]urate for 30 min at 4 °C, efflux was tested by incubating the cells in HBSS efflux medium at 37 °C for 15 s, 2 min, 3 min, 5 min and 10 min (b). MRP4-mediated [14C]urate efflux was calculated by subtracting control cell levels (c).
Effect of hypouricaemic and hyperuricaemic drugs on MRP4-mediated [14C]urate efflux
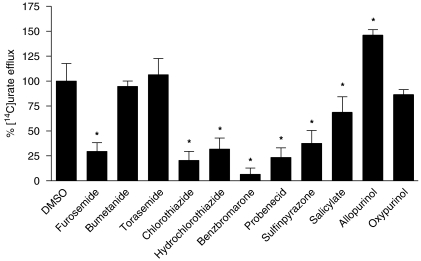
The effect of different drugs that can alter serum uric acid was tested on urate efflux from HEK-MRP4 and HEK-EYFP control cells. Cells were pre-incubated on ice for 30 min in HBSS medium containing 50 μM [14C]urate in the presence or absence of 100 μM of the drugs tested (Figure 2). MRP4-dependent urate transport was calculated from the difference between HEK-MRP4 and HEK-EYFP efflux in the presence and absence of the drugs. Urate efflux without the tested drugs was set at 100%. It was assumed that, in the same way as urate, the tested drugs entered the cells by diffusion, but as uptake was not measured, intracellular concentrations were not known. Furosemide, chlorothiazide, hydrochlorothiazide, benzbromarone, probenecid, sulfinpyrazone and salicylate reduced MRP4-mediated [14C]urate transport to 29±9, 21±9, 32±11, 7±6, 23±10, 38±13 and 69±16% of control MRP4 urate transport, respectively, whereas bumetanide, torasemide and oxypurinol had no significant effect. Interestingly, allopurinol stimulated [14C]urate MRP4 efflux by 146±5%.
Figure 2.
Effect of hypouricaemic and hyperuricaemic drugs on [14C]urate efflux from multidrug resistance protein (MRP) 4 cells. MRP4 and control cells were incubated with 50 μM [14C]urate at 4 °C for 30 min, in the presence or absence of 100 μM concentration of the drugs tested. [14C]Urate efflux was measured after 3 min at 37 °C. Net MRP4-mediated efflux was calculated by subtracting the background control levels. Urate efflux was expressed as a percentage of uptake measured in the presence of the solvent (0.1% in all samples). Values are shown as mean and s.e.mean of three different experiments. *Statistically significant changes (P<0.01) analysed by one-way ANOVA and Dunnett's multiple comparison test.
Time-dependent uptake of [14C]urate in MRP4 vesicles
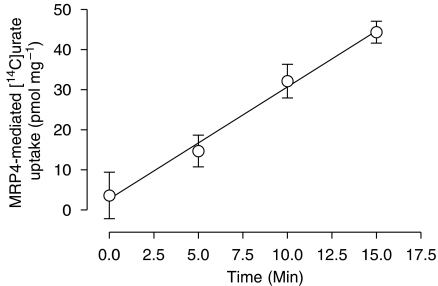
Membrane vesicles isolated from HEK293 cells overexpressing human MRP4 were incubated for 0, 5, 10 and 15 min at 37 °C in uptake medium containing 100 μM [14C]urate. Parallel experiments were performed on control membrane vesicles isolated from HEK-EYFP cells. ATP-dependent transport was determined by subtracting the values obtained in the presence of AMP from those in the presence of ATP. MRP4-dependent urate transport was calculated by subtracting the non-specific uptake in control vesicles, and is shown in Figure 3. Transport of [14C]urate increased with time, and the uptake was linear over the first 15 min of the assay. Accordingly, a 10-min incubation period was chosen for the kinetic and interaction experiments.
Figure 3.
Time-dependent uptake of [14C]urate in multidrug resistance protein (MRP) 4 membrane vesicles. Time course of the uptake of [14C]urate in control human embryonic kidney (HEK)-EYFP and MRP4 overexpressing membrane vesicles was measured at a concentration of 100 μM. After incubation for 0 min, 5 min, 10 min and 15 min at 37 °C, the ATP-dependent [14C]urate uptake was calculated by subtracting the uptake in the presence of AMP from uptake in the presence of ATP for MRP4 and control vesicles. MRP4-mediated [14C]urate transport was calculated by subtracting the non-specific uptake in control vesicles and was analysed by linear regression analysis.
Concentration-dependent transport of [14C]urate by MRP4 vesicles
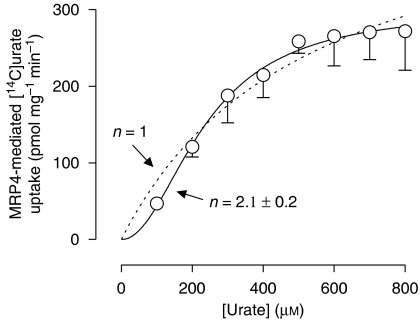
Membrane vesicles isolated from control and MRP4 overexpressing HEK293 cells were incubated for 10 min at 37 °C with [14C]-labelled and [14C]-unlabelled urate to the final concentrations indicated (Figure 4). ATP-dependent [14C]urate transport was calculated by subtracting the values obtained in the presence of AMP from those in the presence of ATP, and after correction for ATP-dependent transport in control vesicles, MRP4 showed a positive cooperative allosteric interaction with urate, which is in accordance with previous results (Van Aubel et al., 2005). The data were described significantly better according to a Hill equation with a coefficient of 2.1±0.2 as compared with a simple single-binding site model (P<0.005, F-test). The urate concentration at half-maximum uptake rate (Km) was 237±14 μM, and the maximum transport rate (Vmax) was 300±11 pmol mg−1 min−1.
Figure 4.
Concentration-dependent uptake of [14C]urate in multidrug resistance protein (MRP) 4 membrane vesicles. The uptake rates of urate by MRP4 and human embryonic kidney (HEK)-EYFP control vesicles were measured at different concentrations of [14C]urate. Net ATP-dependent transport was calculated by subtracting background levels in the absence of ATP (substituted by AMP). MRP4-dependent transport was determined by subtracting control transport, and non-linear regression analysis was performed. Data were described significantly better according to a Hill equation with a slope factor (n) of 2.1 (solid line) as compared with a model with n=1 (dotted line), indicating positive cooperativity (P<0.005, F-test). Values are shown as mean and s.e.mean of three different experiments performed each in triplicate.
Effect of hypouricaemic and hyperuricaemic drugs on MRP4-mediated [14C]urate transport
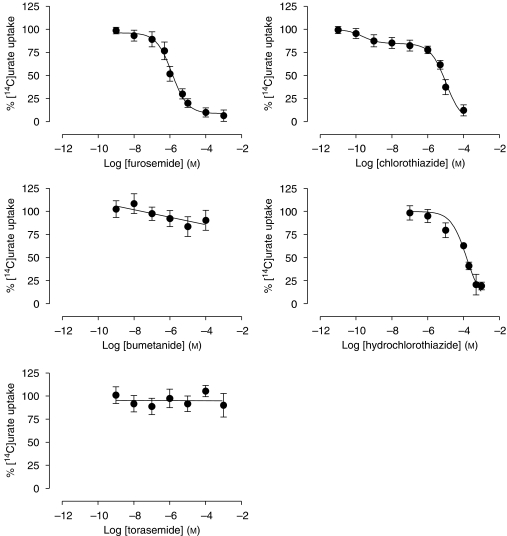
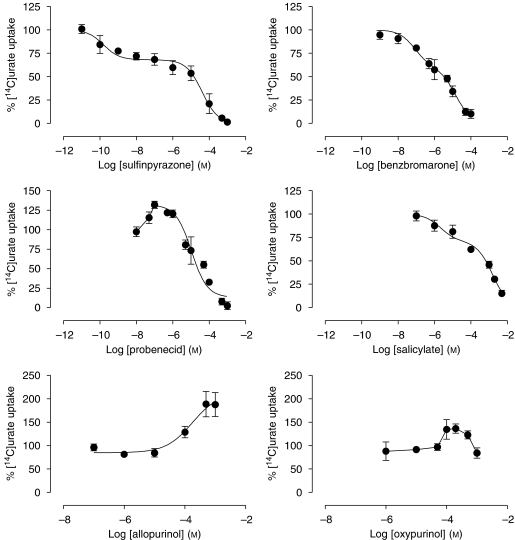
Human embryonic kidney-MRP4 membrane vesicles were incubated with 100 μM [14C]urate at 37 °C for 10 min, in the presence or absence of increasing concentrations of the different drugs tested (Figures 5 and 6). No significant effect of these drugs was observed on urate uptake in control vesicles (results not shown). Furosemide, chlorothiazide, hydrochlorothiazide, benzbromarone, sulfinpyrazone and salicylate inhibited [14C]urate transport with different inhibitory potencies (Table 1). With the exception of furosemide, the inhibition curves of these drugs could not be described simply according to a one-site competition model but were fitted significantly better using the two-site model. Probenecid stimulated MRP4-mediated urate transport up to 132±8% at a concentration of 0.1 μM and acted as an inhibitor at higher concentrations, thereby producing a bell-shaped curve. Both allopurinol and oxypurinol significantly stimulated transport up to 190±50 and 136±17%, respectively, in the high micromolar–low millimolar range, whereas bumetanide and torasemide had no effect at all concentrations tested.
Figure 5.
Effect of diuretics on multidrug resistance protein (MRP) 4-mediated [14C]urate uptake in membrane vesicles. The uptake of 100 μM [14C]urate by MRP4 and human embryonic kidney (HEK)-EYFP control membrane vesicles was measured at 37 °C for 10 min, in the presence or absence of different drugs. ATP-dependent uptake was calculated by subtracting the background (AMP) from the ATP-dependent uptake. Respective transport rates were expressed as a percentage of uptake measured in the absence of the drug against log drug concentration. Values are shown as mean and s.e.mean of three different experiments performed each in triplicate.
Figure 6.
Effect of sulfinpyrazone, benzbromarone, probenecid, salicylate, allopurinol and oxypurinol on multidrug resistance protein (MRP) 4-mediated [14C]urate uptake in membrane vesicles. Membrane vesicles were incubated with 100 μM [14C]urate at 37 °C for 10 min, in the presence or absence of different concentrations of the drugs tested. Uptake was measured by subtracting the background (AMP) from the ATP-dependent uptake. Respective transport rates were expressed as a percentage of uptake measured in the absence of the drug against log drug concentration. Values are shown as mean and s.e.mean of three different experiments performed each in triplicate.
Table 1.
IC50 values of various drugs tested on MRP4-mediated [14C]urate transport
| Drugs | MRP4-mediated urate transport (μM) |
|---|---|
| Furosemide | 1.29±0.01 |
| Bumetanide | NI |
| Torasemide | NI |
| Chlorothiazide | 0.24 × 10−3±0.005 × 10−3 (H) |
| 10.4±0.1 (L) | |
| Hydrochlorothiazide | 1.9±0.1 (H) |
| 220±3 (L) | |
| Salicylate | 2.1±0.1 (H) |
| 1547±25 (L) | |
| Sulfinpyrazone | 0.16±0.02 (H) |
| 40±1 (L) | |
| Benzbromarone | 0.104±0.01 (H) |
| 15.6±0.2 (L) | |
| Probenecid | 132±0.2a |
| Allopurinolb | NI |
| Oxypurinolb | NI |
Abbreviation: NI, no inhibition.
Membrane vesicles were incubated with 100 μM [14C]urate at 37 °C for 10 min, in the presence or absence of different concentrations of the drugs tested. [14C]urate uptake was measured by subtracting the background (AMP) from the ATP-dependent uptake. Each value represents the mean±s.e.mean of data obtained from three separate experiments, each performed in triplicate, calculated by non-linear regression analysis of the data from Figures 5 and 6. High (H)- and low (L)-affinity IC50 were determined according to a two-site competition model.
At low probenecid concentration (0.1 μM), urate transport was stimulated by 132±8%.
Stimulation of urate transport by190±50% at 200 μM allopurinol, and 136±17% at 100 μM oxypurinol, respectively.
Discussion
Renal excretion of urate is one of the main factors controlling its plasma levels. Previously, we identified urate as a substrate of the renal proximal tubule efflux transporter MRP4 (Van Aubel et al., 2005), but hardly any information is available on the effect of hyper- and hypouricaemic drugs on this transport step. Recently, angiotensin II receptor blockers have been proposed to modulate plasma urate partially through their effect on MRP4-mediated urate efflux (Sato et al., 2008). In this work, we investigated the effects of diuretics, xanthine oxidase inhibitors and uricosuric drugs on urate efflux by MRP4. Using membrane vesicles isolated from HEK293 cells overexpressing human MRP4, we confirmed our previous findings that MRP4-mediated urate transport is characterized by a positive cooperative allosteric interaction (Van Aubel et al., 2005). In addition, we showed that different types of drug known to alter serum urate levels could affect MRP4-mediated urate transport, which could contribute to their overall effects on renal urate excretion.
Several loop and thiazide diuretics are known to decrease the fractional excretion of urate and cause hyperuricaemia as a side effect (Feig, 1986; Huen and Goldfarb, 2007). One of the suggested underlying mechanisms is their effect on renal urate secretion. Recently, hydrochlorothiazide and furosemide were identified as substrates of human MRP4 (Hasegawa et al., 2007). In the same study, bumetanide inhibited MRP4-mediated dehydroepiandrosterone sulphate transport. Here, we found that furosemide and hydrochlorothiazide inhibited MRP4-mediated urate transport, whereas bumetanide had no effect. Apparently, bumetanide is an inhibitor of dehydroepiandrosterone sulphate, but not urate, transport. This is in line with our previous findings, in which we have shown that a compound can inhibit one MRP4 substrate although not affecting the other because of multiple binding sites (Van Aubel et al., 2005). Also torasemide had no effect on MRP4-mediated urate transport. It has been reported that both bumetanide (Hasannejad et al., 2004) and torasemide (Vormfelde et al., 2006) are substrates of OAT4, and their exchange for urate through this transporter may contribute to their hyperuricaemic effects.
The xanthine oxidase inhibitor allopurinol and its active metabolite oxypurinol are used in the treatment of hyperuricaemia-related conditions. Here, we showed that both oxypurinol and allopurinol significantly stimulated MRP4-mediated urate transport, suggesting a new mechanism that may contribute to their plasma urate lowering effect. This is in agreement with the complex transport kinetics encountered previously (Van Aubel et al., 2005; El-Sheikh et al., 2007), which could be explained by the presence of two independent transport sites and an allosteric site, interacting in a positively cooperative manner. Whether the stimulatory effect of oxypurinol and allopurinol is on account of co-transport or an allosteric effect needs further investigation. Oxypurinol may also affect renal urate handling as a substrate of URAT1 exchanger (Iwanaga et al., 2005), whereas allopurinol was reported to have no significant effect on URAT1-mediated urate exchange (Enomoto et al., 2002).
The anti-inflammatory drug salicylate can cause urate retention in plasma at doses less than 300 mg per day (Louthrenoo et al., 2002), which is reversed to uricosuria at a dose higher than 3 g per day. The mechanism of the uricosuric effect at high doses has been partially explained by inhibition from the luminal side of URAT1-mediated urate reabsorption (Enomoto et al., 2002). In addition, salicylate itself is a substrate of URAT1 (Anzai et al., 2007), where it can possibly compete with urate for reabsorption. Here, we showed that salicylate was able to inhibit MRP4-mediated urate transport, with a high-affinity site with an IC50 of 2 μM, which is an attainable concentration even at very low doses of salicylate. Nevertheless, the contribution of the high-affinity site to overall urate excretion may be of minor relevance, and it is more likely that salicylate exerts its low-dose hyperuricaemic effect through interacting with OAT1 and OAT3 (Apiwattanakul et al., 1999; Khamdang et al., 2002).
Similar biphasic effects on renal urate handling have been described for other uricosuric drugs like probenecid and sulfinpyrazone (Yu and Gutman, 1955). The main uricosuric mechanism proposed for these drugs is inhibition of urate reabsorption through URAT1 (Enomoto et al., 2002), with benzbromarone showing the highest inhibitory potency (Yu et al., 2007). In addition, benzbromarone and sulfinpyrazone are reported to inhibit the low-affinity urate reuptake transporter OAT4 (Hagos et al., 2007), whereas probenecid inhibits both OAT1 and OAT3 transport activity (Takeda et al., 2001). In this study, benzbromarone and sulfinpyrazone inhibited MRP4-mediated urate transport according to a two-site binding model. Probenecid acted as an inhibitor of urate transport at concentrations above 5 μM, but stimulated transport up to 132% at low concentrations (0.1 μM). The inhibition of MRP4-mediated urate efflux by these drugs may be another factor in their paradoxical effects on renal urate handling. At higher concentrations, their inhibitory effect on MRP4 is probably of minor importance compared with the strong uricosuric action through inhibition of URAT1- and OAT4-mediated urate reabsorption.
Although transport studies performed in isolated membrane vesicles overexpressing a specific transporter lack regulatory and feedback phenomena that are present in a cellular environment, they provide a useful tool to investigate transport mechanisms and inhibition kinetics. In this work, we studied the effect of different drugs on human MRP4-mediated urate transport in both intact cells and isolated membrane vesicles. Our observations were similar, with the exception of oxypurinol, which did not show a significant effect on urate efflux from intact cells, whereas it stimulated MRP4-mediated urate transport in vesicular transport studies. It is possible that in the intact cellular system, oxypurinol may not have reached a sufficient concentration to stimulate MRP4 urate efflux.
To translate our findings to the clinical situation, it is important to realize the function of active proximal tubule transporters, mainly OATs, in concentrating each of the tested drugs intracellularly through basolateral uptake or apical reabsorption, thereby subjecting MRP4 to higher drug levels compared with that in plasma. This concentrating effect of OATs is associated with their function in renal toxicity (Sweet, 2005) and emphasizes the importance of apical MRPs as an efflux detoxifying route as well as a site for drug interactions. The maximum plasma steady-state concentrations and plasma protein binding of the drugs studied in this work as well as the transporters involved in their renal proximal tubular handling are summarized in Table 2. Most of these compounds have a high plasma protein binding. Therefore, unbound fractions are much lower than the total maximum steady state concentrations. However, as shown in Table 2, several organic anion uptake transporters may account for high intracellular concentrations in renal proximal tubule. We showed previously up to 20-fold accumulation of salicyluric acid and hydrochlorothiazide in rat kidney (Cox et al., 1989; Masereeuw et al., 1997), indicating that local proximal tubular concentrations may reach even higher levels. Furthermore, studies in Mrp4 knockout mice have shown that Mrp4 accounts for the luminal efflux of furosemide and hydrochlorothiazide at therapeutic plasma concentrations (Hasegawa et al., 2007). Accordingly, this may result in intracellular concentrations of the diuretics furosemide, chlorothiazide and hydrochlorothiazide that could be sufficient to inhibit MRP4-mediated urate transport at pharmacologically relevant concentrations.
Table 2.
Therapeutic plasma concentrations of hypo- and hyperuricaemic drugs and transporters regulating their renal proximal tubular handling
Clinically relevant maximum steady-state drug plasma concentrations (Cmax) and the abasolateral and bapical proximal tubular membrane transporters known to be involved in their renal handling.
In addition to the obvious effects on renal urate excretion, MRP4 presumably has a function in regulating urate levels in various tissues where it is expressed, including the liver (Gradhand et al., 2007), vascular smooth muscle (Mitani et al., 2003), brain (Hirrlinger et al., 2005), intestine (Zimmermann et al., 2005), blood cells (Kock et al., 2007) and placenta (Azzaroli et al., 2007). The function of MRP4 may have different implications depending on the tissue and side of cell membrane in which MRP4 is expressed. MRP4 expressed apically in the intestine might be a site where allopurinol stimulates the luminal efflux of urate (Shaw and Parsons, 1984; Li et al., 2007). In vascular smooth muscle, where urate is taken up by URAT1 (Price et al., 2006), efflux through MRP4 could have an important function in regulating intracellular urate levels. Interestingly, urate has been implicated in the pathogenesis of hypertension and microvascular diseases (Hediger et al., 2005), and stimulation of urate efflux may be a mechanism contributing to the beneficial effects of allopurinol in urate-induced vascular pathology (Mazzali et al., 2002; George et al., 2006). Furthermore, the inhibition of erythrocyte-mediated ATP-dependent urate transport by salicylate (Lucas-Heron and Fontenaille, 1979) may be explained by the interaction at the erythrocyte MRP4 level.
In summary, our study shows that MRP4-mediated efflux of urate can provide a potential target for drugs affecting plasma urate levels. In addition to their known effects on renal urate uptake transporters, altering MRP4-mediated urate secretion may be a new mechanism in the hyperuricaemic effects of diuretics and hypouricaemic effects of xanthine oxidase inhibitors. The human apical urate efflux transporter MRP4 could provide a new target for the development of drugs in the treatment of hyperuricaemia. Its relative importance as opposed to the other renal urate transporters needs to be verified in vivo. In addition, the results of this study also indicate that potential drug–drug interactions may occur between the studied hypo- and hyperuricaemic drugs and other MRP4 substrates.
Acknowledgments
This work was partially supported by a scholarship granted by the Egyptian Ministry of Higher Education to AAK El-Sheikh).
Abbreviations
- EYFP
enhanced yellow fluorescent protein
- HBSS
Hank's buffered salt solution
- HEK
human embryonic kidney
- MRP
multidrug resistance protein
- OAT
organic anion transporter
- URAT
urate transporter
Conflict of interest
The authors state no conflict of interest.
References
- Anzai N, Kanai Y, Endou H. New insights into renal transport of urate. Curr Opin Rheumatol. 2007;19:151–157. doi: 10.1097/BOR.0b013e328032781a. [DOI] [PubMed] [Google Scholar]
- Apiwattanakul N, Sekine T, Chairoungdua A, Kanai Y, Nakajima N, Sophasan S, et al. Transport properties of nonsteroidal anti-inflammatory drugs by organic anion transporter 1 expressed in Xenopus laevis oocytes. Mol Pharmacol. 1999;55:847–854. [PubMed] [Google Scholar]
- Azzaroli F, Mennone A, Feletti V, Simoni P, Baglivo E, Montagnani M, et al. Clinical trial: modulation of human placental multidrug resistance proteins in cholestasis of pregnancy by ursodeoxycholic acid. Aliment Pharmacol Ther. 2007;26:1139–1146. doi: 10.1111/j.1365-2036.2007.03462.x. [DOI] [PubMed] [Google Scholar]
- Bakhiya A, Bahn A, Burckhardt G, Wolff N. Human organic anion transporter 3 (hOAT3) can operate as an exchanger and mediate secretory urate flux. Cell Physiol Biochem. 2003;13:249–256. doi: 10.1159/000074539. [DOI] [PubMed] [Google Scholar]
- Bard RL, Bleske BE, Nicklas JM. Food: an unrecognized source of loop diuretic resistance. Pharmacotherapy. 2004;24:630–637. doi: 10.1592/phco.24.6.630.34736. [DOI] [PubMed] [Google Scholar]
- Brater DC. Benefits and risks of torasemide in congestive heart failure and essential hypertension. Drug Saf. 1996;14:104–120. doi: 10.2165/00002018-199614020-00005. [DOI] [PubMed] [Google Scholar]
- Cox PG, Moons WM, Russel FG, van Ginneken CA. Renal handling of salicyluric acid in the isolated perfused rat kidney: evidence for accumulation in tubular cells. J Pharmacol Exp Ther. 1989;251:750–755. [PubMed] [Google Scholar]
- Dayton PG, Yu TF, Chen W, Berger L, West LA, Gutman AB. The physiological disposition of probenecid, including renal clearance, in man, studied by an improved method for its estimation in biological material. J Pharmacol Exp Ther. 1963;140:278–286. [PubMed] [Google Scholar]
- Dieterle W, Faigle JW, Mory H, Richter WJ, Theobald W. Biotransformation and pharmacokinetics of sulfinpyrazone (Anturan) in man. Eur J Clin Pharmacol. 1975;9:135–145. doi: 10.1007/BF00614010. [DOI] [PubMed] [Google Scholar]
- Dollery C. Therapeutic Drugs 1991aChurchill Livingstone: London, UK; A49–A52.(ed.)Allopurinol. Involume 1, pp. [Google Scholar]
- Dollery C. Therapeutic Drugs 1991bChurchill Livingstone: London, UK; S5–S6.(ed.)Salicylate. Involume 2, pp. [Google Scholar]
- El-Sheikh AAK, van den Heuvel JJMW, Koenderink JB, Russel FGM. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2-and MRP4/ABCC4-mediated methotrexate transport. J Pharmacol Exp Ther. 2007;320:229–235. doi: 10.1124/jpet.106.110379. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- Feig PU. Cellular mechanism of action of loop diuretics: implications for drug effectiveness and adverse effects. Am J Cardiol. 1986;57:14A–19A. doi: 10.1016/0002-9149(86)91001-5. [DOI] [PubMed] [Google Scholar]
- Ferber H, Vergin H, Hitzenberger G. Pharmacokinetics and biotransformation of benzbromarone in man. Eur J Clin Pharmacol. 1981;19:431–435. doi: 10.1007/BF00548587. [DOI] [PubMed] [Google Scholar]
- Friedman PA. Biochemistry and pharmacology of diuretics. Semin Nephrol. 1988;8:198–212. [PubMed] [Google Scholar]
- George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- Gradhand U, Lang T, Schaeffeler E, Glaeser H, Tegude H, Klein K, et al. Variability in human hepatic MRP4 expression: influence of cholestasis and genotype. Pharmacogenomics J. 2007;8:42–52. doi: 10.1038/sj.tpj.6500451. [DOI] [PubMed] [Google Scholar]
- Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol. 2007;18:430–439. doi: 10.1681/ASN.2006040415. [DOI] [PubMed] [Google Scholar]
- Hasannejad H, Takeda M, Taki K, Shin HJ, Babu E, Jutabha P, et al. Interactions of human organic anion transporters with diuretics. J Pharmacol Exp Ther. 2004;308:1021–1029. doi: 10.1124/jpet.103.059139. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Multidrug resistance-associated protein 4 is involved in the urinary excretion of hydrochlorothiazide and furosemide. J Am Soc Nephrol. 2007;18:37–45. doi: 10.1681/ASN.2005090966. [DOI] [PubMed] [Google Scholar]
- Hediger MA, Johnson RJ, Miyazaki H, Endou H. Molecular physiology of urate transport. Physiology (Bethesda) 2005;20:125–133. doi: 10.1152/physiol.00039.2004. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Moeller H, Kirchhoff F, Dringen R. Expression of multidrug resistance proteins (Mrps) in astrocytes of the mouse brain: a single cell RT-PCR study. Neurochem Res. 2005;30:1237–1244. doi: 10.1007/s11064-005-8795-y. [DOI] [PubMed] [Google Scholar]
- Hoskison TK, Wortmann RL. Advances in the management of gout and hyperuricaemia. Scand J Rheumatol. 2006;35:251–260. doi: 10.1080/03009740600855825. [DOI] [PubMed] [Google Scholar]
- Huen SC, Goldfarb DS. Adverse metabolic side effects of thiazides: implications for patients with calcium nephrolithiasis. J Urol. 2007;177:1238–1243. doi: 10.1016/j.juro.2006.11.040. [DOI] [PubMed] [Google Scholar]
- Ichida K, Hosoyamada M, Kimura H, Takeda M, Utsunomiya Y, Hosoya T, et al. Urate transport via human PAH transporter hOAT1 and its gene structure. Kidney Int. 2003;63:143–155. doi: 10.1046/j.1523-1755.2003.00710.x. [DOI] [PubMed] [Google Scholar]
- Iwanaga T, Kobayashi D, Hirayama M, Maeda T, Tamai I. Involvement of uric acid transporter in increased renal clearance of the xanthine oxidase inhibitor oxypurinol induced by a uricosuric agent, benzbromarone. Drug Metab Dispos. 2005;33:1791–1795. doi: 10.1124/dmd.105.006056. [DOI] [PubMed] [Google Scholar]
- Keystone EC, Paton TW, Littlejohn G, Verdejo A, Piper S, Wright LA, et al. Steady-state plasma levels of salicylate in patients with rheumatoid arthritis: effects of dosing interval and tablet strength. Can Med Assoc J. 1982;127:283–286. [PMC free article] [PubMed] [Google Scholar]
- Khamdang S, Takeda M, Noshiro R, Narikawa S, Enomoto A, Anzai N, et al. Interactions of human organic anion transporters and human organic cation transporters with nonsteroidal anti-inflammatory drugs. J Pharmacol Exp Ther. 2002;303:534–539. doi: 10.1124/jpet.102.037580. [DOI] [PubMed] [Google Scholar]
- Knauf H, Mutschler E. Clinical pharmacokinetics and pharmacodynamics of torasemide. Clin Pharmacokinet. 1998;34:1–24. doi: 10.2165/00003088-199834010-00001. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Ohshiro N, Sakai R, Ohbayashi M, Kohyama N, Yamamoto T. Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]) J Pharm Pharmacol. 2005;57:573–578. doi: 10.1211/0022357055966. [DOI] [PubMed] [Google Scholar]
- Kock K, Grube M, Jedlitschky G, Oevermann L, Siegmund W, Ritter CA, et al. Expression of adenosine triphosphate-binding cassette (ABC) drug transporters in peripheral blood cells: relevance for physiology and pharmacotherapy. Clin Pharmacokinet. 2007;46:449–470. doi: 10.2165/00003088-200746060-00001. [DOI] [PubMed] [Google Scholar]
- Li C, Krishnamurthy PC, Penmatsa H, Marrs KL, Wang XQ, Zaccolo M, et al. Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell. 2007;131:940–951. doi: 10.1016/j.cell.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louthrenoo W, Kasitanon N, Wichainun R, Sukitawut W. Effect of minidose aspirin on renal function and renal uric acid handling in healthy young adults. J Clin Rheumatol. 2002;8:299–304. doi: 10.1097/00124743-200212000-00003. [DOI] [PubMed] [Google Scholar]
- Lucas-Heron B, Fontenaille C. Urate transport in human red blood cells. Activation by ATP. Biochim Biophys Acta. 1979;553:284–294. doi: 10.1016/0005-2736(79)90232-3. [DOI] [PubMed] [Google Scholar]
- Masereeuw R, Moons WM, Russel FG. Saturable accumulation and diuretic activity of hydrochlorothiazide in the isolated perfused rat kidney. Pharmacology. 1997;54:33–42. doi: 10.1159/000139467. [DOI] [PubMed] [Google Scholar]
- Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282:F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- Mitani A, Nakahara T, Sakamoto K, Ishii K. Expression of multidrug resistance protein 4 and 5 in the porcine coronary and pulmonary arteries. Eur J Pharmacol. 2003;466:223–224. doi: 10.1016/s0014-2999(03)01552-8. [DOI] [PubMed] [Google Scholar]
- Pentikainen PJ, Pasternack A, Lampainen E, Neuvonen PJ, Penttila A. Bumetanide kinetics in renal failure. Clin Pharmacol Ther. 1985;37:582–588. doi: 10.1038/clpt.1985.91. [DOI] [PubMed] [Google Scholar]
- Perel JM, Chen W, Dayton PG, Snell MM. Study of structure–activity relationships in regard to species difference in phenylbutazone series. Biochem Pharmacol. 1964;13:1305–1317. doi: 10.1016/0006-2952(64)90231-x. [DOI] [PubMed] [Google Scholar]
- Prandota J, Pruitt AW. Furosemide binding to human albumin and plasma of nephrotic children. Clin Pharmacol Ther. 1975;17:159–165. doi: 10.1002/cpt1975172159. [DOI] [PubMed] [Google Scholar]
- Price KL, Sautin YY, Long DA, Zhang L, Miyazaki H, Mu W, et al. Human vascular smooth muscle cells express a urate transporter. J Am Soc Nephrol. 2006;17:1791–1795. doi: 10.1681/ASN.2006030264. [DOI] [PubMed] [Google Scholar]
- Sato M, Iwanaga T, Mamada H, Ogihara T, Yabuuchi H, Maeda T, et al. Involvement of uric acid transporters in alteration of serum uric acid level by angiotensin II receptor blockers. Pharm Res. 2008;25:639–646. doi: 10.1007/s11095-007-9401-6. [DOI] [PubMed] [Google Scholar]
- Selen A, Amidon GL, Welling PG. Pharmacokinetics of probenecid following oral doses to human volunteers. J Pharm Sci. 1982;71:1238–1242. doi: 10.1002/jps.2600711114. [DOI] [PubMed] [Google Scholar]
- Shah VP, Walker MA, Hunt JP, Schuirmann D, Prasad VK, Cabana BE. Thiazides XI: partitioning of chlorothiazide in red blood cells after oral administration. Biopharm Drug Dispos. 1984;5:55–62. doi: 10.1002/bdd.2510050108. [DOI] [PubMed] [Google Scholar]
- Shaw MI, Parsons DS. Absorption and metabolism of allopurinol and oxypurinol by rat jejunum in vitro: effects on uric acid transport. Clin Sci (Lond) 1984;66:257–267. doi: 10.1042/cs0660257. [DOI] [PubMed] [Google Scholar]
- Sweet DH. Organic anion transporter (Slc22a) family members as mediators of toxicity. Toxicol Appl Pharmacol. 2005;204:198–215. doi: 10.1016/j.taap.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Takeda M, Narikawa S, Hosoyamada M, Cha SH, Sekine T, Endou H. Characterization of organic anion transport inhibitors using cells stably expressing human organic anion transporters. Eur J Pharmacol. 2001;419:113–120. doi: 10.1016/s0014-2999(01)00962-1. [DOI] [PubMed] [Google Scholar]
- Terkeltaub R, Bushinsky DA, Becker MA. Recent developments in our understanding of the renal basis of hyperuricemia and the development of novel antihyperuricemic therapeutics. Arthritis Res Ther. 2006;8 Suppl 1:S4. doi: 10.1186/ar1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnheim K, Krivanek P, Oberbauer R. Pharmacokinetics and pharmacodynamics of allopurinol in elderly and young subjects. Br J Clin Pharmacol. 1999;48:501–509. doi: 10.1046/j.1365-2125.1999.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol. 2002;13:595–603. doi: 10.1681/ASN.V133595. [DOI] [PubMed] [Google Scholar]
- Van Aubel RA, Smeets PH, van den Heuvel JJ, Russel FG. Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol Renal Physiol. 2005;288:F327–F333. doi: 10.1152/ajprenal.00133.2004. [DOI] [PubMed] [Google Scholar]
- Vormfelde SV, Schirmer M, Hagos Y, Toliat MR, Engelhardt S, Meineke I, et al. Torsemide renal clearance and genetic variation in luminal and basolateral organic anion transporters. Br J Clin Pharmacol. 2006;62:323–335. doi: 10.1111/j.1365-2125.2006.02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter-Sack I, de Vries JX, Ittensohn A, Kohlmeier M, Weber E. Benzbromarone disposition and uricosuric action; evidence for hydroxilation instead of debromination to benzarone. Klin Wochenschr. 1988;66:160–166. doi: 10.1007/BF01727785. [DOI] [PubMed] [Google Scholar]
- Weir SJ, Dimmitt DC, Lanman RC, Morrill MB, Geising DH. Steady-state pharmacokinetics of diltiazem and hydrochlorothiazide administered alone and in combination. Biopharm Drug Dispos. 1998;19:365–371. doi: 10.1002/(sici)1099-081x(199809)19:6<365::aid-bdd112>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Welling PG, Barbhaiya RH. Influence of food and fluid volume on chlorothiazide bioavailability: comparison of plasma and urinary excretion methods. J Pharm Sci. 1982;71:32–35. doi: 10.1002/jps.2600710108. [DOI] [PubMed] [Google Scholar]
- Yu TF, Gutman AB. Paradoxical retention of uric acid by uricosuric drugs in low dosage. Proc Soc Exp Biol Med. 1955;90:542–547. doi: 10.3181/00379727-90-22094. [DOI] [PubMed] [Google Scholar]
- Yu TF, Gutman AB. Study of the paradoxical effects of salicylate in low, intermediate and high dosage on the renal mechanisms for excretion of urate in man. J Clin Invest. 1959;38:1298–1315. doi: 10.1172/JCI103905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Fong WP, Cheng CH. Morin (3, 5, 7, 2′, 4′-pentahydroxyflavone) exhibits potent inhibitory actions on urate transport by the human urate anion transporter (hURAT1) expressed in human embryonic kidney cells. Drug Metab Dispos. 2007;35:981–986. doi: 10.1124/dmd.106.012187. [DOI] [PubMed] [Google Scholar]
- Zimmermann C, Gutmann H, Hruz P, Gutzwiller JP, Beglinger C, Drewe J. Mapping of multidrug resistance gene 1 and multidrug resistance-associated protein isoform 1 to 5 mRNA expression along the human intestinal tract. Drug Metab Dispos. 2005;33:219–224. doi: 10.1124/dmd.104.001354. [DOI] [PubMed] [Google Scholar]