Abstract
Background and purpose:
Mechanisms associated with the enhanced contractile response to endothelin-1 in hyperinsulinaemic diabetes have been examined using the rat aorta. Functions for angiotensin II, endothelin-1 receptor expression and extracellular signal-regulated kinase (ERK) have been investigated.
Experimental approach:
Streptozotocin-induced diabetic rats were infused with angiotensin II or, following insulin treatment, were treated with losartan, an angiotensin II receptor antagonist. Contractions of aortic strips with or without endothelium, in response to endothelin-1 and angiotensin II, were examined in vitro. Aortic ETA receptors and ERK/MEK expression were measured by western blotting.
Key results:
Insulin-treated diabetic rats exhibited increases in plasma insulin, angiotensin II and endothelin-1. The systolic blood pressure and endothelin-1-induced contractile responses in aortae in vitro were enhanced in insulin-treated diabetic rats and blunted by chronic losartan administration. LY294002 (phosphatidylinositol 3-kinase inhibitor) and/or PD98059 (MEK inhibitor) diminished the enhanced contractile response to endothelin-1 in aortae from insulin-treated diabetic rats. ETA and ETB receptors, ERK-1/2 and MEK-1/2 protein expression and endothelin-1-stimulated ERK phosphorylation were all increased in aortae from insulin-treated diabetic rats. Such increases were blunted by chronic losartan administration. Endothelin-1-induced contraction was significantly higher in aortae from angiotensin II-infused diabetic rats. angiotensin II-infusion increased ERK phosphorylation, but the expression of endothelin receptors and ERK/MEK proteins remained unchanged.
Conclusions and implications:
These results suggest that the combination of high plasma angiotensin II and insulin with a diabetic state induced enhancement of endothelin-1-induced vasoconstriction, ETA receptor expression and ERK expression/activity in the aorta. Losartan improved both the diabetes-related abnormalites and the diabetic hypertension.
Keywords: angiotensin, endothelin, hyperinsulinaemia, diabetes, contraction
Introduction
Diabetes mellitus is an important risk factor for increased blood pressure and for the development of atherosclerosis (Cohen, 1995; De Vriese et al., 2000; Eckel et al., 2002). Many of the vascular complications seen in type 1 and type 2 diabetes arise from hyperglycaemia that cannot be completely prevented using the methods of blood glucose control that are at present available (Poston and Taylor, 1995; Koivisto et al., 1996; Feener and King, 1997; Orchard et al., 2003). Among these complications, diabetes-accelerated atherosclerosis and hypertension are most likely multifactorial in origin, with hyperinsulinaemia being one of several possible causative factors (Reaven, 1995; Snell-Bergeon et al., 2003). Indeed, systemic hyperinsulinaemia is inevitable during the insulin treatment of type 1 diabetes mellitus and it may have an important function in the progression of coronary artery disease (Reaven, 1995; Snell-Bergeon et al., 2003). One possibility raised by previous in vitro studies is that the hyperinsulinaemia may result in an increased sensitivity of blood vessels to vasoconstrictors such as angiotensin II (Ang II) or catecholamines (Gans et al., 1991; Hall et al., 1995; Kobayashi and Kamata, 1999). We have previously demonstrated that in insulin-treated (IT) type 1 diabetic rats with systemic hyperinsulinaemia, treatment with an Ang II type 1 (AT1; Alexander et al., 2008) receptor blocker can almost totally prevent the development of the hypertension and enhanced catecholamine-induced vasoconstriction, which are associated with an increase in phosphatidylinositol 3-kinase (PI3-K) activity (Kobayashi et al., 2006). The biological effects of PI3-K are exerted through the activation of several downstream effectors, including the mitogen-activated protein kinase (MAPK) family (Yamboliev et al., 2000; Pearson et al., 2001; Takeda et al., 2001). An interesting question for this study was whether stimulation of PI3-K is followed by the activation of the downstream kinase MAPK family, and whether this is, in turn, linked to vascular hypereactivity.
The MAPK family, which consists of three isoforms, have a central function in the intracellular signal transduction initiated by extracellular stimuli, including growth factors and hormones (Pearson et al., 2001). The isoforms, extracellular signal-regulated kinase (ERK) and mitogen-activated/ERK-activating kinase (MEK), are activated by those receptor agonists (including Ang II and endothelin-1 (ET-1)) that induce smooth muscle contraction (Meloche et al., 2000; Roberts, 2001; Kim et al., 2004; Matsumoto et al., 2006). Both Ang II and ET-1 are vasoactive peptides that may be involved in the pathogenesis of cardiovascular diseases. Indeed, enhanced levels of Ang II and ET-1 have been reported in diabetic subjects with vascular complications, and also in diabetic rats (Kirilov et al., 1994; Goto et al., 1996; Kanie et al., 2003). Further, there is evidence to suggest that ET-1 and Ang II, as activators of G-protein-coupled receptors, exhibit cross-talk with ERK activity in vascular smooth muscle cells (Touyz et al., 1999; Hong et al., 2004; Yogi et al., 2007). Interestingly, Touyz et al. (1999) have suggested that in aortae from spontaneously hypertensive rats, increases in ERK 1/2 activity are associated with an increase in Ang II-induced contractility. Thus, we speculated that an increased plasma Ang II and/or ET-1 level and/or an abnormality of the MAP pathway system might be related to the enhancement of vascular contractility previously observed in IT type1 diabetic rats.
Although the hyperinsulinaemia associated with diabetes may make important contributions to the development of cardiovascular disorders, as described in several models of the disease, there is evidence that the elevation of plasma insulin found in patients with insulinomas or in control rats subjected to high-dose insulin treatment is not in itself sufficient to cause hypertension or vascular dysfunction (Hall et al., 1995; Kobayashi et al., 2006, 2007). Moreover, we suspect that for vascular dysfunction to develop, a high insulin level and an established diabetic state may need to coexist (Kobayashi et al., 2006, 2007). If so, high insulin alone would not be sufficient to cause increased blood pressure and enhanced aortic contraction in the rat; instead, we suspect that a high insulin level, a diabetic state and other factors (including Ang II and ET-1) need to exist together to cause an enhancement of contractility (by an as yet unknown mechanism).
The aim of this study was to investigate the causal relationship between Ang II, ET-1 and the MEK/ERK system in the enhancement of aortic contraction that occurs in IT type 1 diabetes. We examined in vivo whether Ang II itself, insulin itself, or their combination, might alter MEK/ERK activity, ET-1 receptor expression and/or vascular contractility in diabetic rats. We also asked whether chronic AT1 receptor blockade or ET-1 (ETA and ETB) receptor blockade might blunt certain diabetes-related abnormalities, seen in association with systemic hyperinsulinaemia.
Materials and methods
Animals and experimental design
This study was approved by the Hoshi University Animal Care and Use Committee, and all procedures were in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals adopted by the Committee on the Care and Use of Laboratory Animals of Hoshi University (which is accredited by the Ministry of Education, Culture, Sports, Science and Technology, Japan).
Male Wistar rats (8 weeks old) received a single injection through the tail vein of streptozotocin (STZ) 65 mg kg−1 dissolved in a citrate buffer to induce diabetes (Kamata et al., 1989; Kobayashi and Kamata, 1999). Rats were considered diabetic if their blood glucose exceeded 4 g L−1 at 7 days after the injection of STZ. The STZ-induced diabetic rats (8 weeks after STZ injection) and the age-matched control (citrate buffer-injected) rats were treated with a gradually increasing daily subcutaneous dose of insulin (human crystalline insulin zinc, 5–30 U kg−1 day−1; Novo Nordisk, Agsværd, Denmark) for 2 weeks (Kobayashi et al., 2006). In all subsequent experiments, the rats referred to as ‘controls' are age-matched controls. The IT-diabetic rats received a daily evening injection (at 1700 hours) of insulin in doses of 5–30 U kg−1 day−1 individually adjusted to maintain urine sugar levels at below 2.5 g L−1 (measured using a urine sugar tester; Bayer HealthCare, NY, USA). For chronic losartan (Nulotan; 25 mg kg−1 day−1 at 1700 hours, p.o.; Banyu Co. Ltd, Tsukuba, Japan) +insulin or chronic J104132 (10 mg kg−1 day−1 at 1700 hours, p.o.) +insulin cotreatment, the appropriate drug was administered concomitantly with the above-described insulin treatment for 2 weeks. J104132 is a potent orally active mixed antagonist of ETA and ETB receptors (Nishikibe et al., 1999). When J104132 was administered to rats at 10 mg kg−1 p.o., its mean residence time in the systemic circulation was 3 h. We previously reported that chronic J104132 administration at a dose of 10 mg kg−1 day−1 improves diabetic endothelial dysfunction (Kanie and Kamata, 2002). For continuous stimulation with Ang II, some STZ-induced diabetic and control rats (17 weeks old) were treated with Ang II (288 μg kg−1 day−1) by way of an osmotic mini-pump (2ML2; Alzet, Palo Alto, CA, USA) for 2 weeks, as described previously (Taguchi et al., 2007). Thus, we studied eight groups: controls, diabetic, IT controls, IT diabetic, losartan-treated IT diabetic, J104132-treated IT diabetic, Ang II-infused control and Ang II-infused diabetic rats. Food and water were provided ad libitum.
Measurement of plasma glucose, insulin, angiotensin II and endothelin-1 and systolic blood pressure
Rats were anaesthetized with diethyl ether and killed decapitation each morning (at 0900 hours). Then, blood samples were collected. Plasma glucose, insulin, Ang II and ET-1 levels and systemic blood pressure were measured as described previously (Kobayashi and Kamata, 2001; Kanie et al., 2003; Kobayashi et al., 2006). Plasma glucose was determined by the use of a commercially available enzyme kit (Wako Chemical Company, Osaka, Japan). Plasma insulin was measured by enzyme immunoassay (Shibayagi, Gunma, Japan). Plasma Ang II was eluted with methanol using C-18 columns (Cayman Chemical, MI, USA) and measured using a commercially available Ang II enzyme immunoassay (SPI-BIO, Massy Cedex, France) according to the manufacturer's instructions. Plasma ET-1 was eluted with methanol using Amprep C2 columns (Amersham International Plc, Buckinghamshire, UK) and measured using a commercially available ET-1 enzyme immunoassay (Amersham Biosciences UK Ltd, Buckinghamshire, England) according to the manufacturer's instructions.
For blood pressure measurements, rats were kept in a constant temperature hot box at 37 °C for 15 min. Its blood pressure was then measured by the tail-cuff method using a blood pressure analyzer (BP-98A; Softron, Tokyo, Japan) at least 5 min after the rat had been put in a restrainer for the purpose of measuring.
Measurement of isometric force in aortic tissue
After decapitation (at 0900 hours; see above), aortas were excised. Then, each aorta (cut into helical strips) was then placed in a bath containing 10 mL modified Krebs–Henseleit solution, with one end of each strip being connected to a tissue holder and the other to a force displacement transducer, as described previously (Kobayashi and Kamata, 1999; Kobayashi et al., 2006).
Vascular responses to angiotensin II and endothelin-1
For the contraction studies, ET-1 (10−10–10−7 M) or Ang II (10−10–10−6 M) was added cumulatively to the bath until a maximal response was achieved. To investigate the effect of drug treatments on the ET-1-induced contractile response—namely, 10−6 M BQ123 (ETA receptor antagonist), 10−6 M BQ788 (ETB receptor antagonist), 2 × 10−5 M LY294002 (PI3-K inhibitor), or 5 × 10−5 M PD98059 (ERK/MEK inhibitor)—the strip was incubated for 30 min in the appropriate medium containing the drug before the cumulative addition of agonist. In some strips, the endothelium was mechanically removed, successful removal being functionally confirmed by the absence of relaxation to acetylcholine (10−5 M).
Immunohistochemistry for ETA/B receptors
Immunohistochemistry was carried out using a previously described method (Kobayashi et al., 2007). Aortic strips were embedded in O C T Compound (Sakura, Torrance, CA, USA). After a wash with this compound, slides were treated with 10 mM citric acid and then microwave-heated (for 1 min) to recover antigenicity. Non-specific binding was blocked by treatment with a drop of normal horse serum in Block ace (Dainippon Pharmaceutical, Osaka, Japan) for 20 min before incubation with monoclonal anti-ETA or ETB receptor antibodies (1:100, BD Biosciences, San Jose, CA, USA) in Block ace (Dainippon Pharmaceutical) overnight at 4 °C. Tissue sections were then incubated for 30 min at room temperature with an antimouse immunoglobulin G CY3 conjugate (1:100, Sigma, St Louis, MO, USA) secondary antibody. Sections of rat aorta embedded in VECTASHIELD (Vector Laboratories, Burlingame, CA, USA) were examined using a fluorescence microscope.
Measurements of the expressions of ETA and ETB receptors, ERK-1/2, MEK-1/2 and phosphorylated-ERK by western blotting
For measurement of ERK activity, aortic tissues were suspended in an organ bath and incubated with 10−8 M ET-1 for 10 min. For inhibitor experiments, tissues were pretreated with 5 × 10−5 PD98059 or 2 × 10−5 M LY294002 for 30 min before the addition of ET-1. Western blotting was performed using a previously described method (Kobayashi et al., 2006). Briefly, aortae (5 mm long) were homogenized in ice-cold lysis buffer, and samples (24 μg protein per lane) were resolved by electrophoresis on SDS-polyacrylamide gel electrophoresis gels, then transferred onto polyvinylidene difluoride (PVDF) membranes. Next, the membrane was incubated with an antibody to ETA receptors (1:1000; Abcam, Cambridge, UK), ETB receptors (1:1500; Abcam), ERK-1/2 (1:1000; BD Biosciences), MEK-1/2 (1:2500; BD Biosciences), phosphorylated-ERK (1:1000; BD Biosciences), or β-actin (1:5000; Sigma) in blocking solution. Finally, horseradish peroxidase-conjugated, antirabbit antibody or antimouse antibody (Vector Laboratories) was used at a 1:10 000 dilution in Tween phosphate-buffered saline, followed by detection using SuperSignal (PIERCE).
Statistical analysis
The contractile force developed by aortic strips is expressed in milligram tension per milligram tissue. Data are mean±s.e.mean Statistical differences were assessed using Dunnett's test for multiple comparisons after a one-way ANOVA, a probability level of P<0.05 being regarded as significant. Statistical comparisons between concentration–response curves were made using a two-way ANOVA with a Bonferroni correction performed post hoc to correct for multiple comparisons; P<0.05 was considered significant.
Drugs
Angiotensin II, STZ (–), BQ123, BQ788 and LY294002 were all purchased from Sigma Chemical Co. J104132 and losartan were from Banyu Co. Ltd (Tsukuba, Japan), insulin was from Novo Nordisk, PD98059 was from Calbiochem-Novabiochem (La Jolla, CA, USA) and ET-1 was from Peptide Institute (Osaka, Japan).
Results
Plasma glucose, insulin, angiotensin II, endothelin-1 and systolic blood pressure
As indicated in Table 1, body weight was significantly lower in non-IT-diabetic rats than in age-matched controls, an effect partially reversed in all IT groups, irrespective of drug therapy. Non-fasting plasma glucose was significantly elevated in non-IT-diabetic rats, where insulin (5–30 U kg−1 day−1 for 2 weeks) reduced plasma glucose to the levels seen in age-matched controls, irrespective of drug therapy. Plasma insulin was significantly lower in non-IT-diabetic rats and significantly higher in each of the IT groups. In all subsequent experiments, the rats referred to as ‘IT-diabetic' had received insulin (5–30 U kg−1 day−1 for 2 weeks). Plasma insulin was significantly reduced by losartan treatment in the IT-diabetic rat.
Table 1.
Plasma glucose and insulin levels in age-matched controls, untreated diabetic, insulin-treated controls and insulin-treated diabetic rats (some treated with insulin+losartan or insulin+J104132)
| Parameters | Age-matched control (8) | Untreated diabetic (8) | Insulin-treated control (8) | Insulin-treated diabetic (8) | Losartan+insulin-treated diabetic (8) | J104132+insulin-treated diabetic (6) |
|---|---|---|---|---|---|---|
| Body weight (g) | 524±16 | 268±11* | 553±19 | 401± 21*,# | 387±18*,# | 396±19*,# |
| Glucose (g L−1) | 1.33±0.12 | 5.42±0.25* | 0.84±0.10* | 1.05± 0.16# | 0.95±0.14# | 1.13±0.15# |
| Insulin (ng mL−1) | 4.6±0.7 | 0.3±0.2 | 15.9±1.8* | 18.7±1.4*,# | 14.3±1.6*,#,† | 18.1±1.8*,# |
Values in the table are means±s.e.mean. The number of determinations is shown in parenthesis, in each column heading. *P<0.05 vs control. #P<0.05 versus untreated diabetic. †P<0.05 versus insulin-treated diabetic.
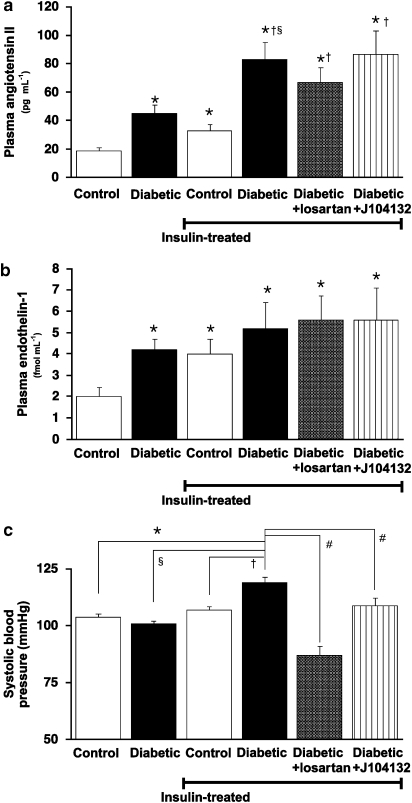
Plasma Ang II and plasma ET-1 were significantly higher in diabetic than control rats and were further raised by insulin therapy, particularly for plasma Ang II in IT-diabetic rats. Both plasma Ang II and plasma ET-1 remained unaffected by the chronic administration of either losartan or J104132 (Figures 1a and b). The increase in systolic blood pressure seen in IT-diabetic rats was significantly reduced by the chronic administration of either losartan or J104132 (Figure 1c). Treatment with losartan reduced the systolic blood pressure to the levels below that present in non-diabetic control animals.
Figure 1.
Plasma angiotensin II (a) and endothelin-1 (b) and systolic blood pressure (c). Diabetic rats received insulin treatment (5–30 U kg−1 day−1 for 2 weeks) together with either the angiotensin receptor antagonist, losartan or the endothelin antagonist, J104132. Each column represents mean±s.e.mean from 8 to 10 experiments. *P<0.05 versus controls. §P<0.05 versus diabetic. †P<0.05 versus chronic insulin-treated controls. #P<0.05 versus chronic insulin+losartan-treated diabetic.
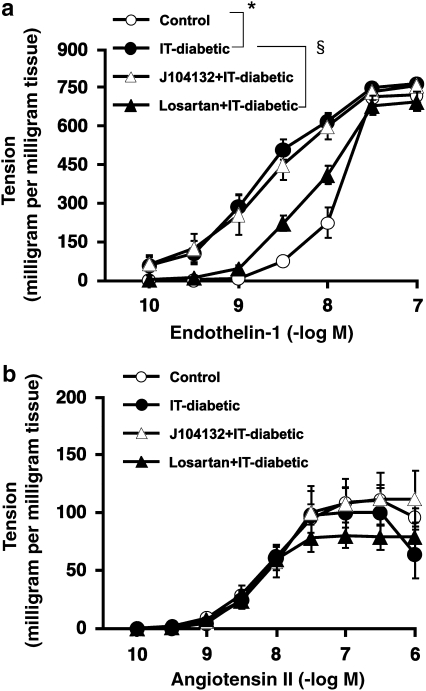
Effect of losartan or J104132 in vivo, on the contraction of endothelium-denuded aortic strips induced by endothelin-1 and angiotensin II in vitro
Aortic contraction induced by ET-1 was enhanced in endothelium-denuded strips obtained from IT-diabetic rats, an effect largely prevented by chronic treatment with losartan, an Ang receptor antagonist, but unaffected by the ET antagonist, J104132 (Figure 2a). In endothelium-denuded aortic strips, the contraction induced by Ang II was markedly weaker than that induced by ET-1. The sensitivity to Ang II remained unaffected by IT diabetes. In contrast to ET-1, aortic contraction induced by Ang II was not significantly modified in the IT-diabetic rat and remained unchanged by chronic treatment with either losartan or J104132 (Figure 2b).
Figure 2.
Effect of endothelin-1 (a) and angiotensin II (b) on the contraction of endothelium-denuded rat aortic strips. Strips were obtained from control or insulin-treated diabetic (IT-diabetic) rats, receiving chronic treatment with either losartan or J104132. Ordinate shows increase in tension (expressed in milligram tension per milligram tissue) measured at the peak of the response. Values are mean±s.e.mean from 8 to 10 experiments. *P<0.05 versus control. §P<0.05 versus IT-diabetic.
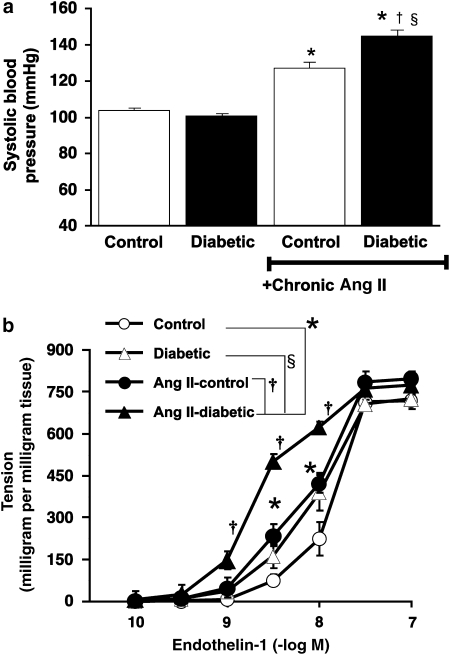
Effect of angiotensin II infusion on systolic blood pressure in vivo and the contraction of endothelium-denuded aortic strips induced by endothelin-1 in vitro
The significant increase in systolic blood pressure following Ang II infusion (288 μg kg−1 day−1) for 2 weeks in control rats was enhanced following Ang II infusion in diabetic animals (Figure 3a). The ET-1-induced contractile response of endothelium-denuded aortic strips was greater in strips obtained from control rats following Ang II infusion. The ET-1-induced contraction was further enhanced in endothelium-denuded aortic strips obtained from non-IT-diabetic rats following Ang II infusion (Figure 3b).
Figure 3.
Effect of an angiotensin II infusion in vivo on systolic blood pressure (a) and the contraction of endothelium-denuded rat aortic strips induced by endothelin-1 in vitro (b). Strips were obtained from control and diabetic animals in the absence of insulin treatment. Ordinate shows increase in tension (expressed in milligram tension per milligram tissue) measured at the peak of the response. Values are mean±s.e.mean from 8 to 10 experiments. *P<0.05 versus controls. †P<0.05 versus angiotensin II-untreated diabetic. §P<0.05 versus chronic angiotensin II-infused control.
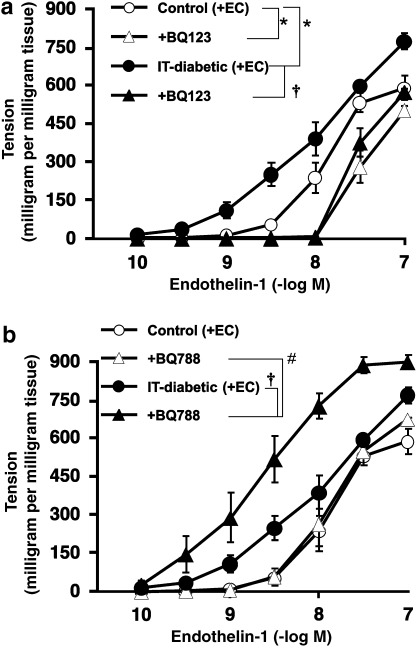
Effect of ET antagonists on the contraction of endothelium-intact aortic strips induced by endothelin-1 in vitro
Treatment with BQ123, an ETA receptor antagonist, led to a rightward shift in the dose–response curve for ET-1-induced contraction in endothelium-intact aortae from both control and IT-diabetic rats (Figure 4a). In the presence of BQ123, there was no significant difference between control and IT-diabetic rats. When BQ788, an ETB receptor antagonist, was added to endothelium-intact aortic strips from control rats, the ET-1-induced contraction was unchanged. In IT-diabetic aortae, the ET-1-induced contraction was enhanced by incubation with BQ788 (Figure 4b).
Figure 4.
Effect of endothelin antagonists, BQ123 (a) and BQ788 (b) on endothelin-1-induced contraction of endothelium-intact rat aortic strips in vitro. Endothelin antagonists were used at a concentration of 10−6 M. Ordinate shows increase in tension (expressed in milligram tension per milligram tissue) measured at the peak of the response. Values are mean±s.e.mean from 8 to 10 experiments. *P<0.05 versus controls. †P<0.05 vs IT-diabetic. #P<0.05 versus BQ788-treated control.
Effect of PI3-K and MEK/ERK inhibitors on the contraction of endothelium-denuded aortic strips induced by endothelin-1 in vitro
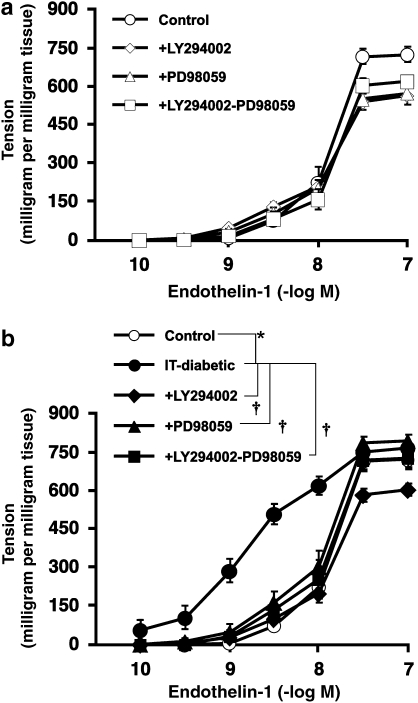
Incubation of endothelium-denuded control aortae with LY294002 alone, PD98059 alone or LY294002 plus PD98059 had no significant effect on ET-1-induced contraction (Figure 5a). In aortic strips from IT-diabetic rats, these inhibitors (either alone or together) inhibited the enhancement of contraction observed in IT-diabetic aortae. There was no significant difference in the ET-1-induced contraction among the LY294002-treated, PD98059-treated and LY294002+PD98059-cotreated groups (Figure 5b).
Figure 5.
Effect of LY294002, a PI3-kinase inhibitor and/or PD98059, an MEK/ERK inhibitor on endothelin-1-induced contraction of endothelium-denuded rat aortic strips from control (a) and insulin-treated diabetic (IT-diabetic) rats (b) in vitro. LY294002 was used at 2 × 10−5 M and PD98059 at 5 × 10−5 M. Ordinate shows increase in tension (expressed in milligram tension per milligram tissue) measured at the peak of the response. Values are mean±s.e.mean from 8 to 10 experiments. *P<0.05 versus controls. †P<0.05 versus IT-diabetic.
Expression of endothelin-1 receptor proteins
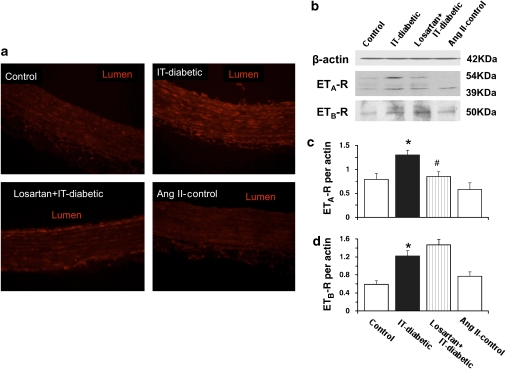
Using immunohistochemistry, we found that aortae obtained from IT-diabetic rats showed increased ETA/B receptor-positive staining within the medial smooth muscle, as well as within the endothelium (by comparison with the controls). In aortae from losartan-treated IT-diabetic rats, this increase in ETA/B receptor-positive staining was markedly attenuated in the medial smooth muscle, but not in the endothelium. Positive staining for ETA/B receptor was not significantly modified in aortae obtained from control animals following Ang II-infusion (Figure 6a).
Figure 6.
Immunohistochemical staining for endothelin-1 receptor (a) and western blots for ETA receptor (ETA-R; b and c) or ETB receptor (ETB-R; b and d) in endothelium-intact rat aorta. Representative sections of endothelin-1 receptor-positive staining is shown in red for aorta obtained from control rats, with and without angiotensin II infusion and for insulin-treated diabetic rats (IT-diabetic) with and without chronic losartan therapy. Calibration bar, 25 μm. Original magnification × 100. Representative western blots of ETA and ETB receptors from rat aorta (b) were quantified by scanning densitometry (c and d). For the ETA receptor, two bands (54 and 39 kDa) corresponding to native and glycosylated forms were detected. Values are mean±s.e.mean from 8 experiments. *P<0.05 versus controls. #P<0.05 versus IT-diabetic.
ETA (Figure 6b, upper) and ETBreceptors (Figure 6b, lower) were examined by western blotting. ETA receptor density was determined from two bands at 54 and 39 kDa (corresponding to the native and glycosylated forms of the receptor, respectively), as described previously (Ergul et al., 2003). The expressions of the ETA and ETBreceptors were significantly increased in aortae from IT-diabetic rats (compared with those from control rats). Losartan treatment greatly attenuated this increased ETA receptor expression, but did not alter the increased ETB receptor expression. In contrast, aortae from control rats exhibited no significant change in ETA or ETB receptor expressions following Ang II infusion (Figures 6b, c and d).
Expression of phosphorylated-ERK, ERK-1/2 and MEK-1/2
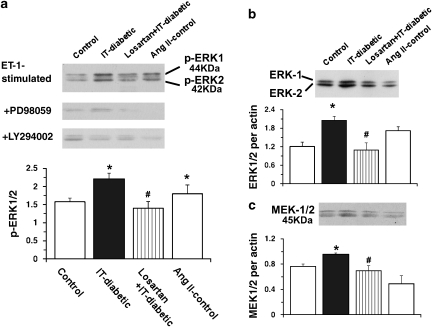
In the IT-diabetic group, the ET-1-stimulated ERK-phosphorylation level was significantly increased (versus the control group) and this increase was completely normalized by chronic losartan treatment (versus the IT-diabetic group; Figure 7a). In the Ang II-infused control group, the ET-1-stimulated ERK phosphorylation level was significantly increased (versus the control group). In each of the relevant groups, the increase in the ET-1-stimulated ERK phosphorylation level was abolished in the presence of either 5 × 10−5 M PD98059 or 2 × 10−5 M LY294002 (Figure 7a).
Figure 7.
Western blots for endothelin-1-induced ERK phosphorylation (a), ERK-1/2 (b) and MEK-1/2 (c) in endothelium-intact rat aortic strips. Strips were obtained from age-matched control, insulin-treated diabetic (IT-diabetic), losartan-treated IT-diabetic and angiotensin II (Ang II)-infused control rats. Top: representative blots of endothelin-1-induced phosphorylated ERK (p-ERK) are shown for strips in the absence and presence of PD98059 and LY294002 (a). Bands were quantified by scanning densitometry. Ratios were calculated for the optical density of ERK-1/2 or MEK-1/2 over that of β-actin. Values are mean±s.e.mean from 8 experiments. *P<0.05 versus controls. #P<0.05 versus IT-diabetic.
Next, we examined the expression of ERK-1/2 and MEK-1/2 proteins by western blot (Figures 7b and c). ERK-1/2 expression was greater in the IT-diabetic group than in the control group. Chronic treatment with losartan normalized this increased expression, the difference from the IT-diabetic group being significant (Figure 7b). Moreover, MEK-1/2 expression was greater in the IT-diabetic group than in the control group, and this increased expression was significantly lowered by chronic losartan treatment (Figure 7c). Neither the ERK-1/2 nor the MEK-1/2 expression level differed between the Ang II-infused control and control groups (Figures 7b and c).
Discussion
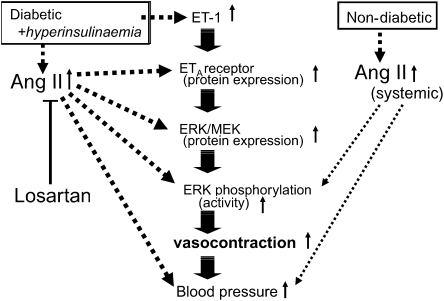
The main conclusion to be drawn from this study is that the mechanism underlying the enhancement of ET-1-induced aortic contraction observed in IT type 1 diabetic rats with hyperinsulinaemia involves both an increased ETA receptor expression and increased activity in the MEK/ERK pathway. Moreover, chronic AT1 receptor blockade blunted these increases and the hypertension. Interestingly, neither insulin alone nor Ang II alone is sufficient to cause increased ETA receptor or MEK/ERK protein expression in the rat aorta; instead, we suspect that a high Ang II level and a diabetic state need to exist together to cause enhancements of vasocontractility and blood pressure (Figure 8).
Figure 8.
Scheme showing the events described in the text (involving angiotensin II (Ang II), endothelin-1 (ET-1) and the MEK/ERK system) leading to the enhancement of aortic contraction and raised blood pressure in non-diabetics and diabetics.
Circulating plasma insulin, Ang II and ET-1 levels have been shown to be higher in type 1 diabetic patients than in non-diabetic controls, and they may have important functions in the progression of coronary artery disease and of hypertension (Kirilov et al., 1994; Reaven, 1995; Goto et al., 1996; Kanie et al., 2003). In the diabetic model used in our experiments, the non-IT-diabetic rats had a raised plasma glucose level, whereas the IT-diabetic rats had (a) markedly raised plasma insulin, Ang II and ET-1 levels and (b) normalized plasma glucose levels. Moreover, chronic AT1 receptor blockade or ET-1 receptor blockade blunted the increase in blood pressure observed in the IT-diabetic group. These observations suggest that the increased plasma Ang II and ET-1 levels each caused an enhancement of blood pressure, but only when there was an elevated plasma insulin level (as seen in the present IT-diabetic state). Interestingly, such insulin or Ang II administration to the diabetic rats increased systolic blood pressure to values above those seen in the insulin- or Ang II-administered controls. We suggest that high plasma Ang II, high plasma ET-1 and a diabetic state need to exist together to cause an enhancement of blood pressure, but that chronic Ang II type1 receptor blockade or ET-1 receptor blockade can each limit or prevent the increase in blood pressure.
The powerful vasoconstrictor ET-1 is thought to have a pathological function in a number of vascular diseases (Goto et al., 1996). In this study, our IT-diabetic rats displayed enhanced ET-1-induced contractility (in both endothelium-intact and endothelium-denuded aortae), but not enhanced Ang II-induced contractility. ET-1 is widely known to stimulate ETA receptors on vascular smooth muscle cells to produce vasoconstriction and ETB receptors on endothelial cells to produce vasodilatation (Goto et al., 1996). Some studies have demonstrated the overexpression of the vascular ETA receptor in both insulin-resistant rats and high fat diet-induced obese mice, providing further support for the function of ET-1 and its receptors in vascular diseases related to insulin resistance and obesity (Jesmin et al., 2006; Mundy et al., 2007). We, therefore, wondered if the expressions of ET-1 receptors might be changed by IT-diabetes with hyperinsulinaemia. In fact, the expression of the ETA and ETB receptors in aortic tissue was increased in IT-diabetic, compared with control rats. Treatment with BQ123, an ETA receptor antagonist, shifted the dose–response curve for the ET-1-induced contraction to the right. Thus, the enhanced ET-1-induced contractile response seen in the IT-diabetic aorta may be the result of an increased expression of the ETA receptor in the smooth muscle cells.
Recently, we reported that the hyperinsulinaemia resulting from insulin treatment of diabetic rats enhanced catecholamine-induced contractility, possibly owing to the increased PI3-K activity (Kobayashi et al., 2006). Here, we found that a PI3-K inhibitor, LY294002, had a normalizing effect on the enhanced ET-1-induced contractions seen in aortae from IT-diabetic rats. Thus, our data suggest that the increase in ET-1 contractility observed in such rats may also be due to increased PI3-K activity. There is emerging evidence that in vascular smooth muscle cells, activation of MEK/ERK by some agonists is mediated by PI3-K (Yamboliev et al., 2000; Takeda et al., 2001). Here, we found that an MEK/ERK inhibitor, PD98059, had a normalizing effect on the enhanced ET-1-induced contractions in IT-diabetic rats. Interestingly, there was no difference in the ET-1 contraction among the LY294002-treated, PD98059-treated and LY294002+PD98059-treated groups. As cotreatment with a PI3-K inhibitor and MEK/ERK inhibitor had no greater effect on the ET-1-induced contractile response than the PI3-K inhibitor alone or the MEK/ERK inhibitor alone, the enhancement of aortic contractility seen in our IT-diabetic rats may be related to the increased activity in the PI3-K-associated MEK/ERK pathway. In addition, the ET-stimulated levels of ERK activity and ERK-1/2 and MEK-1/2 protein expressions were greatly increased in our IT-diabetic rats. Those results suggest that the increase in ET-1-induced contractility observed in the IT-diabetic rat aorta may be due to increases in ERK activity and MEK/ERK protein expressions. ET-1 actin at ETA receptors possesses the ability to activate the PI3-K/MEK/ERK pathway in human and rat vascular smooth muscle cells (Koide et al., 1992; Yang et al., 1999), but few reports specifically implicate the PI3-K/MEK/ERK pathway in the arterial contraction seen in response to ET-1. Reportedly, in aortae from deoxycorticosterone acetate -salt hypertensive rats, an MEK inhibitor slightly reduced ET-1-induced contraction, an effect that was partially dependent on activation of an MAPK pathway (Kim et al., 2004). As neither PI3-K nor MEK/ERK inhibitors had any effect on ET-1-induced contractile responses in our controls, the importance of the MEK/ERK pathway-dependent contraction may be specific to aortae from IT-diabetic rats. Thus, our data suggest that the increase in ET-1-induced contractility seen in the IT-diabetic rat may be attributable not only to the increased ETA receptor expression, but also to the increased activity of the PI3-K/MEK/ERK-pathway.
Mutual cross-talk between Ang II and ET-1 may exist in vascular smooth muscle cells and they may share many signalling components. Indeed, Ang II can induce ET-1 gene expression in rat aortic smooth muscle cells (Jesmin et al., 2006), and angiotensin-converting enzyme inhibition can decrease circulating ET-1 levels in diabetic models (Ortiz et al., 2001). In our study, chronic AT1 receptor blockade blunted the increases in ET-1-induced contractility and blood pressure observed in the IT-diabetic group, as well as those in ERK activity and MEK/ERK protein expression seen in that group. These observations suggest that in diabetic rats with systemic hyperinsulinaemia, an AT1 receptor blocker can almost totally prevent the development of the hypertension and ET-1 vascular hypereactivity that are associated with increases in PI3-K/MEK/ERK activity and ET-1 receptors. In contrast, chronic ET-1 receptor blockade normalized only the increase in blood pressure, with neither ET-1 receptor expression nor ERK activity being affected. Although both ET-1 and Ang II have been implicated in models of hypertension, several studies shown that in Ang II-infused rats, blood pressure may be increased through mechanisms other than direct Ang II-induced vasoconstriction (Ballew and Fink, 2001). Indeed, Ang II has many effects other than direct vasoconstriction. One action of Ang II, which receives increasing attention is its ability to increase the production of substances capable of inducing powerful contractions, such as ET-1 (Ballew and Fink, 2001; Ortiz et al., 2001). Furthermore, in rats made hypertensive by Ang II infusion, blocking either ET-1 or Ang II receptors ameliorated the hypertension, indicating that ET-1 has a causal function in Ang II-induced hypertension (Ballew and Fink, 2001; Ortiz et al., 2001). These results suggest that in diabetics with hyperinsulinaemia, systemic blood pressure may be enhanced by combined increases in the Ang II system and ET-1 system.
Next, we considered whether an enhancement of plasma Ang II alone might increase ET-1-induced contraction through increases in MEK/ERK activity and ET-1 receptors. In our study, (a) the ET-1-induced contraction was greater in Ang II-infused rats than in Ang II-untreated rats and (b) Ang II-infused controls showed an increased ET-1-stimulated ERK phosphorylation level (versus Ang II-untreated controls). These results strongly suggest that the increased plasma Ang II level caused the observed enhancement of aortic contractility through the ERK pathway. Interestingly, Ang II-infused controls showed no change in the protein expressions of ETA/B receptors or MEK/ERK. This is important because several previous studies have shown that the increased MEK/ERK activity detected in aortae from the Ang II-infused rat and deoxycorticosterone acetate-salt hypertensive rat did not alter those protein expressions (Kim et al., 2004; Ding et al., 2007). Thus, Ang II alone is not sufficient to increase the protein expression of the ETA and ETB receptors, or MEK/ERK, in the rat aorta. Instead, we suggest that a high plasma Ang II level and a diabetic states have an additive effect on the ET-1-induced contractile response through different pathways. Generally, different growth factors and hormones, aside from insulin and Ang II, could also have important functions in the development of diabetic complications. In our study, both the ET-1-induced contractile response and blood pressure were higher in Ang II-infused diabetic rats than in Ang II-infused controls, strongly suggesting that a high Ang II level acts more potently in the diabetic state than in the non-diabetic state in increasing ET-1-induced contraction and blood pressure. This may be owing to the increases in the protein expressions of the ETA receptor, MEK-1/2 and ERK-1/2. It is unclear at present, however, which aspect of the diabetic state might be responsible for increasing ET-induced contraction in the aorta; the effect might conceivably result from changes in the physiological conditions and/or in the level of any of several other hormones.
Taken together, our findings indicate that in IT type 1 diabetic rats with systemic hyperinsulinaemia, treatment either with an AT1 receptor blocker or with an ET receptor blocker can prevent the development of the hypertension typically seen in that model. Furthermore, chronic AT1 receptor blockade blunted the increase in ET-induced contractility observed in such diabetics, through decreases in ETA receptor and MEK/ERK protein expressions/activities. We suggest that a high plasma Ang II level, a high plasma insulin level and a diabetic state need to exist together to cause an augmentation of ET-1-induced contraction through the ETA receptor /PI3-K/MEK/ERK pathway. The coexistence of these factors may accelerate the progression of systemic hypertension in diabetes.
Acknowledgments
This study was supported in part by the Ministry of Education, Science, Sports and Culture, Japan, and by the Open Research Center System, Japan.
Abbreviations
- ERK
extracellular signal-regulated kinase
- IT
insulin-treated
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated /ERK-activating kinase
- PI3-K
phosphatidylinositol 3-kinase
- STZ
streptozotocin
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153 Suppl. 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballew JR, Fink GD. Role of ET(A) receptors in experimental ANG II-induced hypertension in rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R150–R154. doi: 10.1152/ajpregu.2001.281.1.R150. [DOI] [PubMed] [Google Scholar]
- Cohen RA. The role of nitric oxide and other endothelium-derived vasoactive substances in vascular disease. Prog Cardiovasc Dis. 1995;38:105–128. doi: 10.1016/s0033-0620(05)80002-7. [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van De Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Chapman A, Boyd R, Wang HD. ERK activation contributes to regulation of spontaneous contractile tone via superoxide anion in isolated rat aorta of angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H2997–H3005. doi: 10.1152/ajpheart.00388.2006. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Wassef M, Chait A, Sobel B, Barrett E, King G, et al. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group II: pathogenesis of atherosclerosis in diabetes. Circulation. 2002;105:e138–e143. doi: 10.1161/01.cir.0000013954.65303.c5. [DOI] [PubMed] [Google Scholar]
- Ergul A, Portik-Dobos V, Giulumian AD, Molero MM, Fuchs LC. Stress upregulates arterial matrix metalloproteinase expression and activity via endothelin A receptor activation. Am J Physiol Heart Circ Physiol. 2003;285:H2225–H2232. doi: 10.1152/ajpheart.00133.2003. [DOI] [PubMed] [Google Scholar]
- Feener EP, King GL. Vascular dysfunction in diabetes mellitus. Lancet. 1997;350:9–13. doi: 10.1016/s0140-6736(97)90022-2. [DOI] [PubMed] [Google Scholar]
- Gans RO, Bilo HJ, von Maarschalkerweerd WW, Heine RJ, Nauta JJ, Donker AJ. Exogenous insulin augments in healthy volunteers the cardiovascular reactivity to noradrenaline but not to angiotensin II. J Clin Invest. 1991;88:512–518. doi: 10.1172/JCI115333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Hama H, Kasuya Y. Molecular pharmacology and pathophysiological significance of endothelin. Jpn J Pharmacol. 1996;72:261–290. doi: 10.1254/jjp.72.261. [DOI] [PubMed] [Google Scholar]
- Hall JE, Brands MW, Zappe DH, Galicia MA. Insulin resistance, hyperinsulinaemia, and hypertension: causes, consequences, or merely correlations. Proc Soc Exp Biol Med. 1995;208:317–329. doi: 10.3181/00379727-208-43862b. [DOI] [PubMed] [Google Scholar]
- Hong HJ, Chan P, Liu JC, Juan SH, Huang MT, Lin JG, et al. Angiotensin II induces endothelin-1 gene expression via extracellular signal-regulated kinase pathway in rat aortic smooth muscle cells. Cardiovasc Res. 2004;61:159–168. doi: 10.1016/j.cardiores.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Jesmin S, Hattori Y, Maeda S, Zaedi S, Sakuma I, Miyauchi T. Subdepressor dose of benidipine ameliorates diabetic cardiac remodeling accompanied by normalization of upregulated endothelin system in rats. Am J Physiol Heart Circ Physiol. 2006;290:H2146–H2154. doi: 10.1152/ajpheart.01142.2005. [DOI] [PubMed] [Google Scholar]
- Kamata K, Miyata N, Kasuya Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br J Pharmacol. 1989;97:614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanie N, Kamata K. Effects of chronic administration of the novel endothelin antagonist J-104132 on endothelial dysfunction in streptozotocin-induced diabetic rat. Br J Pharmacol. 2002;135:1935–1942. doi: 10.1038/sj.bjp.0704659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanie N, Matsumoto T, Kobayashi T, Kamata K. Relationship between peroxisome proliferators-activated receptors (PPAR alpha and PPAR gamma) and endothelial dependent relaxation in streptozotocin-induced diabetic rats. Br J Pharmacol. 2003;140:23–32. doi: 10.1038/sj.bjp.0705414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Kim J, Bae YM, Cho SI, Kwon SC, Jung JY, et al. p38 mitogen-activated protein kinase contributes to the diminished aortic contraction by endothelin-1 in DOCA-salt hypertensive rats. Hypertension. 2004;43:1086–1091. doi: 10.1161/01.HYP.0000125995.85427.fd. [DOI] [PubMed] [Google Scholar]
- Kirilov G, Dakovska L, Borisova AM, Krivoshiev S, Nentchev N. Increased plasma endothelin levels in patients with insulin-dependent diabetes mellitus and end-stage vascular complications. Horm Metab Res. 1994;26:119–120. doi: 10.1055/s-2007-1000787. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hayashi Y, Taguchi K, Matsumoto T, Kamata K. Angiotensin II enhances contractile responses via PI3-kinase p110{delta} pathway in aortas from diabetic rats with systemic hyperinsulinemia. Am J Physiol Heart Circ Physiol. 2006;291:H846–H853. doi: 10.1152/ajpheart.01349.2005. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kamata K. Effect of insulin treatment on smooth muscle contractility and endothelium-dependent relaxation in rat aortae from established STZ-induced diabetes. Br J Pharmacol. 1999;127:835–842. doi: 10.1038/sj.bjp.0702554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Kamata K. Effect of chronic insulin treatment on NO metabolism and endothelium-dependent relaxation in aortae from established STZ-induced diabetic rats. Atherosclerosis. 2001;155:313–320. doi: 10.1016/s0021-9150(00)00583-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Taguchi K, Takenouchi Y, Matsumoto T, Kamata K. Insulin-induced impairment via peroxynitrite production of endothelium-dependent relaxation and sarco/endoplasmic reticulum Ca2+-ATPase function in aortas from diabetic rats. Free Radic Biol Med. 2007;43:431–443. doi: 10.1016/j.freeradbiomed.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Koide M, Kawahara Y, Tsuda T, Ishida Y, Shii K, Yokoyama M. Endothelin-1 stimulates tyrosine phosphorylation and the activities of two mitogen-activated protein kinases in cultured vascular smooth muscle cells. J Hypertens. 1992;10:1173–1182. doi: 10.1097/00004872-199210000-00010. [DOI] [PubMed] [Google Scholar]
- Koivisto VA, Stevens LK, Mattock M, Ebeling P, Muggeo M, Stephenson J, et al. Cardiovascular disease and its risk factors in IDDM in Europe. EURODIAB IDDM Complications Study Group. Diabetes Care. 1996;19:689–697. doi: 10.2337/diacare.19.7.689. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kobayashi T, Kamata K. Mechanisms underlying lysophosphatidylcholine-induced potentiation of vascular contractions in the Otsuka Long-Evans Tokushima Fatty (OLETF) rat aorta. Br J Pharmacol. 2006;149:931–941. doi: 10.1038/sj.bjp.0706937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche S, Landry J, Huot J, Houle F, Marceau F, Giasson E. p38 MAP kinase pathway regulates angiotensin II-induced contraction of rat vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2000;279:H741–H751. doi: 10.1152/ajpheart.2000.279.2.H741. [DOI] [PubMed] [Google Scholar]
- Mundy AL, Haas E, Bhattacharya I, Widmer CC, Kretz M, Hofmann-Lehmann R, et al. Fat intake modifies vascular responsiveness and receptor expression of vasoconstrictors: implications for diet-induced obesity. Cardiovasc Res. 2007;73:368–375. doi: 10.1016/j.cardiores.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Nishikibe M, Ohta H, Okada M, Ishikawa K, Hayama T, Fukuroda T, et al. Pharmacological properties of J-104132 (L-753, 037), a potent, orally active, mixed ETA/ETB endothelin receptor antagonist. J Pharmacol Exp Ther. 1999;289:1262–1270. [PubMed] [Google Scholar]
- Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26:1374–1379. doi: 10.2337/diacare.26.5.1374. [DOI] [PubMed] [Google Scholar]
- Ortiz MC, Sanabria E, Manriquez MC, Romero JC, Juncos LA. Role of endothelin and isoprostanes in slow pressor responses to angiotensin II. Hypertension. 2001;37:505–510. doi: 10.1161/01.hyp.37.2.505. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Poston L, Taylor PD. Endothelium-mediated vascular function in insulin-dependent diabetes mellitus. Clin Sci. 1995;88:245–255. doi: 10.1042/cs0880245. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75:473–486. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
- Roberts RE. Role of the extracellular signal-regulated kinase (Erk) signal transduction cascade in alpha(2) adrenoceptor-mediated vasoconstriction in porcine palmar lateral vein. Br J Pharmacol. 2001;133:859–866. doi: 10.1038/sj.bjp.0704149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell-Bergeon JK, Hokanson JE, Jensen L, MacKenzie T, Kinney G, Dabelea D, et al. Progression of coronary artery calcification in type 1 diabetes: the importance of glycemic control. Diabetes Care. 2003;26:2923–2928. doi: 10.2337/diacare.26.10.2923. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Kobayashi T, Hayashi Y, Matsumoto T, Kamata K. Enalapril improves impairment of SERCA-derived relaxation and enhancement of tyrosine nitration in diabetic rat aorta. Eur J Pharmacol. 2007;556:121–128. doi: 10.1016/j.ejphar.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Takeda K, Ichiki T, Tokunou T, Iino N, Takeshita A. 15-Deoxy-delta 12, 14-prostaglandin J2 and thiazolidinediones activate the MEK/ERK pathway through phosphatidylinositol 3-kinase in vascular smooth muscle cells. J Biol Chem. 2001;276:48950–48955. doi: 10.1074/jbc.M108722200. [DOI] [PubMed] [Google Scholar]
- Touyz RM, El Mabrouk M, He G, Wu XH, Schiffrin EL. Mitogen-activated protein/extracellular signal-regulated kinase inhibition attenuates angiotensin II-mediated signaling and contraction in spontaneously hypertensive rat vascular smooth muscle cells. Circ Res. 1999;84:505–515. doi: 10.1161/01.res.84.5.505. [DOI] [PubMed] [Google Scholar]
- Yamboliev IA, Wiesmann KM, Singer CA, Hedges JC, Gerthoffer WT. Phosphatidylinositol 3-kinases regulate ERK and p38 MAP kinases in canine colonic smooth muscle. Am J Physiol Cell Physiol. 2000;279:C352–C360. doi: 10.1152/ajpcell.2000.279.2.C352. [DOI] [PubMed] [Google Scholar]
- Yang Z, Krasnici N, Luscher TF. Endothelin-1 potentiates human smooth muscle cell growth to PDGF: effects of ETA and ETB receptor blockade. Circulation. 1999;100:5–8. doi: 10.1161/01.cir.100.1.5. [DOI] [PubMed] [Google Scholar]
- Yogi A, Callera GE, Montezano AC, Aranha AB, Tostes RC, Schiffrin EL, et al. Endothelin-1, but not Ang II, activates MAP kinases through c-Src independent Ras-Raf dependent pathways in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1960–1967. doi: 10.1161/ATVBAHA.107.146746. [DOI] [PubMed] [Google Scholar]