Abstract
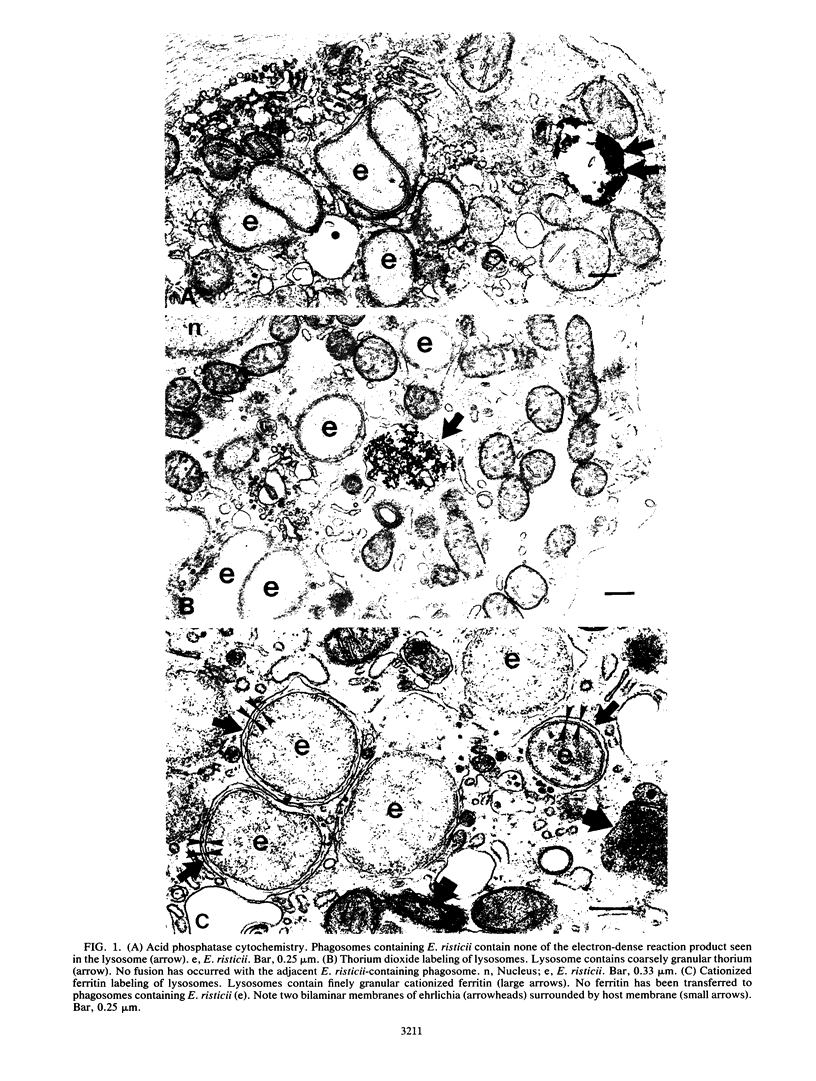
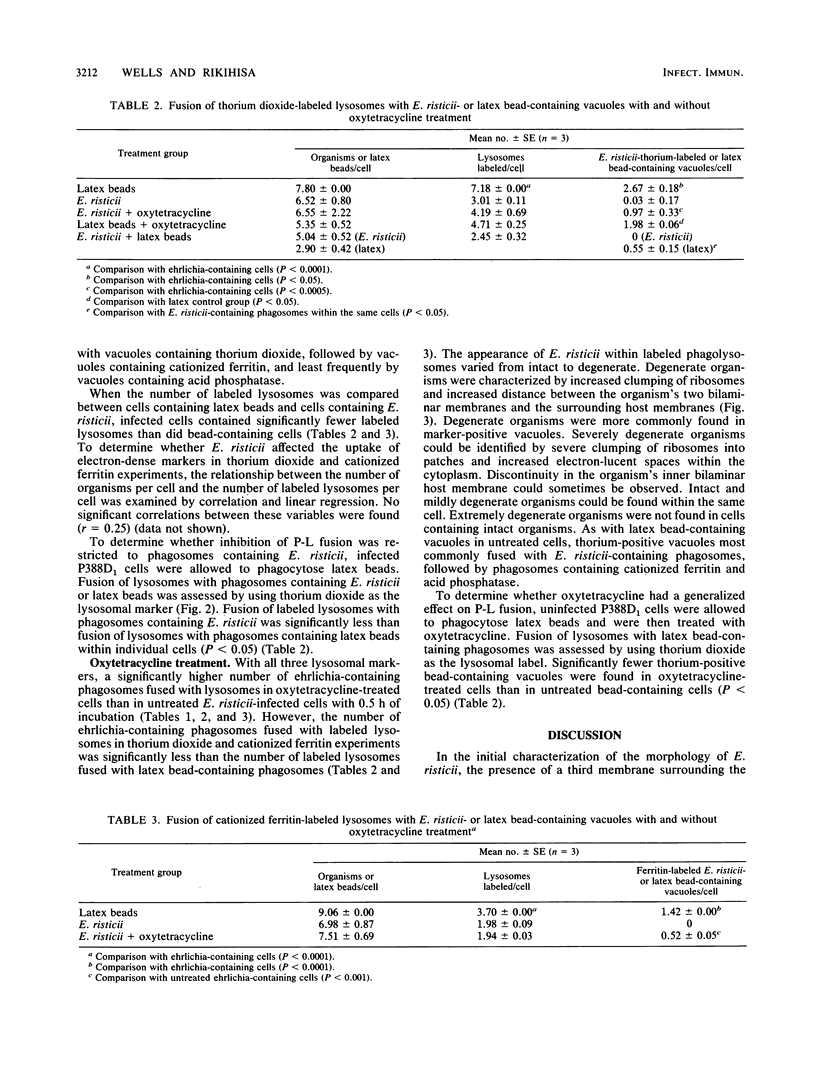
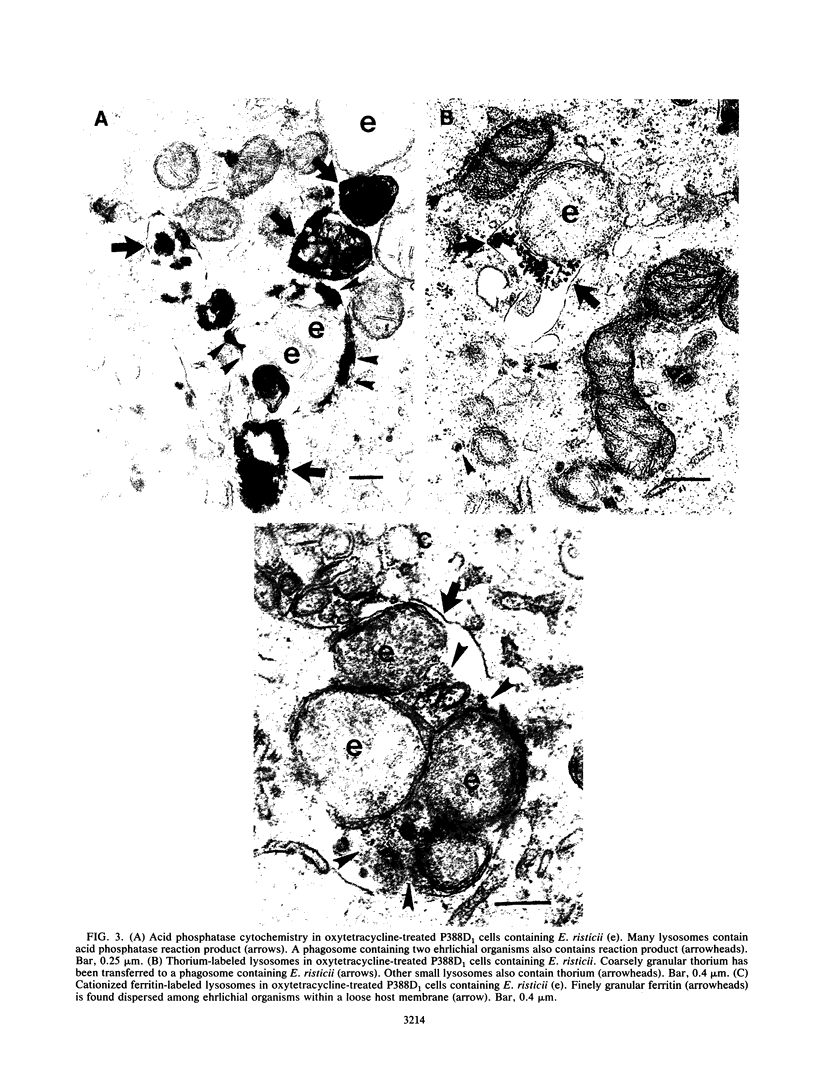
Fusion of lysosomes with phagosomes containing Ehrlichia risticii, an obligate intracellular parasite, was evaluated in P388D1 murine macrophagelike cells. Lysosomes in cells ranging in infectivity from 30 to 70% were labeled cytochemically with acid phosphatase or via endocytosis of thorium dioxide or cationized ferritin to document phagosome-lysosome (P-L) fusion in untreated cells and cells treated with oxytetracycline. Regardless of the marker used, P-L fusion was generally not observed in E. risticii-containing vacuoles in untreated cells, while significantly greater P-L fusion with ehrlichia-containing vacuoles was observed after oxytetracycline treatment. When latex beads were introduced into uninfected cell cultures, P-L fusion was observed with vacuoles containing latex. Fusion of lysosomes with latex-containing vacuoles in cells was significantly greater than fusion of lysosomes with ehrlichia-containing vacuoles in the same infected cells. These findings indicate that E. risticii is able to inhibit P-L fusion, whereas oxytetracycline deprives organisms of this ability.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goren M. B. Phagocyte lysosomes: interactions with infectious agents, phagosomes, and experimental perturbations in function. Annu Rev Microbiol. 1977;31:507–533. doi: 10.1146/annurev.mi.31.100177.002451. [DOI] [PubMed] [Google Scholar]
- Kielian M. C., Cohn Z. A. Phagosome-lysosome fusion. Characterization of intracellular membrane fusion in mouse macrophages. J Cell Biol. 1980 Jun;85(3):754–765. doi: 10.1083/jcb.85.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Ito S. Entry of Rickettsia tsutsugamushi into polymorphonuclear leukocytes. Infect Immun. 1982 Oct;38(1):343–350. doi: 10.1128/iai.38.1.343-350.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Jiang B. M. In vitro susceptibilities of Ehrlichia risticii to eight antibiotics. Antimicrob Agents Chemother. 1988 Jul;32(7):986–991. doi: 10.1128/aac.32.7.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D. Causative ehrlichial organisms in Potomac horse fever. Infect Immun. 1985 Sep;49(3):513–517. doi: 10.1128/iai.49.3.513-517.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D., Cordes D. O. Ultrastructural study of ehrlichial organisms in the large colons of ponies infected with Potomac horse fever. Infect Immun. 1985 Sep;49(3):505–512. doi: 10.1128/iai.49.3.505-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L., Fuchs R., Male P., Mellman I. Two distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomes. Cell. 1988 Jan 15;52(1):73–83. doi: 10.1016/0092-8674(88)90532-6. [DOI] [PubMed] [Google Scholar]
- Weiss E. The biology of rickettsiae. Annu Rev Microbiol. 1982;36:345–370. doi: 10.1146/annurev.mi.36.100182.002021. [DOI] [PubMed] [Google Scholar]
- van der Valk P., Herman C. J. Leukocyte functions. Lab Invest. 1987 Feb;56(2):127–137. [PubMed] [Google Scholar]