Abstract
Calcitonin gene-related peptide (CGRP) is expressed throughout the CNS and peripheral nervous system, consistent with control of vasodilatation, nociception, motor function, secretion and olfaction. αCGRP is prominently localized in primary afferent C and Aδ fibres of spinal and trigeminal ganglia. Activation of the trigeminal nerve results in antidromic release of CGRP, acting through a CGRP1 receptor. Antagonists of CGRP1 receptors reduce signalling in the trigeminovascular pathway at multiple sites, putatively inside the blood–brain barrier. Other ways of interacting with CGRP mechanisms have appeared limiting the availability of CGRP in the circulation with a specific CGRP antibody or with a CGRP-binding RNA-Spiegelmer. Either way reduces neurogenic inflammation and attenuates signalling within the trigeminovascular pathway. Specific CGRP receptor blockade has been shown to reduce the effect of released CGRP and to abort acute migraine attacks. The novel approach of reducing available CGRP is limited by the blood–brain barrier; its usefulness may be more as prophylaxis rather than as acute treatment of migraine.
Keywords: migraine, CGRP, trigeminovascular mechanisms, CGRP receptor antagonists, CGRP antibodies
Migraine is a common and debilitating episodic disorder with a prevalence of up to 15–20% of the adult population. It is more common in women than in men, and occurs in two major subtypes, migraine with aura and without aura. In migraine with aura (20% of the cases), the migraine headaches are preceded by a visual hallucination/illusion known as an aura. Typically, the aura begins in the visual field and expands during a 5–20-min period, and is usually followed by headache. The phenomenon is linked with ‘cortical spreading depression' and has been examined in depth in numerous neuroimaging studies. Migraine attacks are characterized by typically moderate or severe headaches which are unilateral and pulsating, lasting 4–72 h, often accompanied by nausea, phonophobia and photophobia (Goadsby et al., 2002).
Migraine has a strong genetic component, most likely with a multifactorial polygenic inheritance. It is only for familiar hemiplegic migraine, a rare autosomal dominant inherited subtype of migraine with aura, that a genetic link has been established. There are currently three loci associated with hemiplegic migraine; the CACNA1A gene at 19p13, the ATP1A2 gene at 1q23 and a sodium channel SCN1A gene. Common to these genes is a close relation to cerebral ion channel function. In theory, they could cause reduced or enhanced gain of function in the CNS (van den Maagdenberg et al., 2007). This has been taken as a strong evidence for depolarizations to spread across the cortex, and these are considered as key early events in migraine attacks. However, it is still unclear how this links to the trigeminovascular system, the part of the intracranial vasculature that is supplied with sensory nerve endings.
It is my belief that the key nociceptors are located on the cerebral vasculature, and these are activated by changes in vessel tone (vasoconstriction) or directly through intracerebral neuronal connections to the microcirculation. In support of this view is the recent demonstration that sensory axons in the cranial dura mater/meninges lack calcitonin gene-related peptide (CGRP) receptor elements (Levy et al., 2005; Lennerz et al., 2008), and hence CGRP is not involved in meningeal neurogenic inflammation. The release of CGRP in conjunction with migraine attacks has lead to the demonstration that triptans in part act to inhibit the release of CGRP (Goadsby and Edvinsson, 1993) and has stimulated the search for novel CGRP antagonists for migraine treatment (Edvinsson, 2004).
Calcitonin gene-related peptide is expressed throughout the CNS and peripheral nervous system. Two forms of CGRP are expressed; αCGRP is prominently localized in primary afferent C and Aδ fibres of spinal and trigeminal ganglia in various parts of the body, whereas ßCGRP is the main isoform located in the enteric nervous system. In CNS, there is a wide distribution of CGRP with the highest levels in striatum, amygdale, colliculi and cerebellum. Trigeminal nerve activation results in antidromic release of CGRP, acting through a CGRP1 receptor complex of calcitonin-like receptor—receptor activity-modifying proteins (RAMP1) and receptor component protein—adenylate cyclase to cause non-endothelium-mediated vasodilatation. At synapses in the trigeminal nucleus caudalis and at C1/C2 levels, CGRP acts on second-order neurons to transmit pain signals centrally through brainstem and midbrain to the thalamus and higher cortical pain regions.
The receptor components, calcitonin-like receptor and RAMPs, are widely expressed throughout the brain and in intracranial arteries. Despite this colocalization, RAMP1 seems to be functionally rate limiting for the CGRP receptor activity as seen in human cranial arteries. Calcitonin-like receptor and RAMP1 have recently been localized to trigeminal neurons, and suggested to have a prejunctional role controlling CGRP production in a feedback loop (Lennerz et al., 2008). CGRP does not induce neurogenic inflammation or sensitization at peripheral meningeal sites but relays nociceptive information from trigeminal primary afferent neurons to the second-order neurons in the spinal trigeminal nucleus neurons. Thus, a key question to be addressed is where do the CGRP antagonists act?
Recently developed CGRP receptor antagonists (olcegepant and telcagepant) act on the RAMP1 element and this could occur at central and peripheral sites to attenuate signaling within the trigeminovascular pathway. These antagonists are highly CGRP receptor specific and have been examined in clinical trials with positive outcomes (Olesen et al., 2004; Ho et al., 2008). However, despite their in vitro potency (0.1–10 nM), large amounts are necessary to obtain a significant antimigraine effect (5–600 mg) in vivo, possibly owing to high protein binding, low passage through the blood–brain barrier and being substrates for the efflux transport system P-glycoprotein pump. In comparing the effects of luminal and abluminal application on perfused middle cerebral arteries, we showed that the blood–brain barrier is, in part, responsible for the high doses needed in therapy (Edvinsson et al., 2007).
Another approach to block the effects of CGRP is either the use of specific CGRP antibodies that bind excess of CGRP and thus hinders its effects (Zeller et al., 2008), or the use of a specific CGRP-binding RNA-Spiegelmer (a single-stranded mirror-image oligonucleotide) that is capable of tightly and specifically binding to CGRP and thus inhibiting its function (Denekas et al., 2006). In this issue of the British Journal of Pharmacology, Zeller et al. (2008) have revealed that the application of antibodies for CGRP is a possible way of reducing the effects of CGRP. Thus, chronic treatment with anti-CGRP antibodies exerted a long-lasting inhibition of neurogenic vasodilatation. The advantage with this approach is the long half-life of the effect, which opens up the possibility for its use in the prophylaxis of migraine.
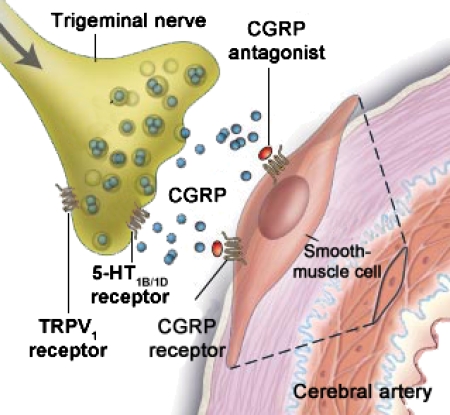
However, one crucial question remains—where are the antibodies acting? The CGRP1 receptors are present at the peripheral end of the trigeminovascular system, on smooth muscle cells of arteries (Figure 1) and on second-order neurons in the brain stem. CGRP1 receptor elements have been found in the trigeminal ganglion, but the question of autoreceptors or intraganglionic interactions has not been clarified as yet (Lennerz et al., 2008). Antagonists are considered to reduce signalling in the trigeminovascular pathway at multiple sites inside the blood–brain barrier. In addition, there might be other places such as in the CNS where the anti-CGRP molecules could act. Studies with the perfused middle cerebral artery using CGRP antibodies or the CGRP-Spiegelmer revealed that they were unable to penetrate the blood–brain barrier but were able to inhibit the responses to CGRP if given abluminally to the barrier (Edvinsson et al., 2007) or if studied in the dura mater or middle meningeal artery, which lack the blood–brain barrier (Juhl et al., 2007). Consequently, if the CGRP antibodies or the CGRP-Spiegelmer is evaluated in clinical trials, the results may provide further understanding of migraine pathophysiology and, in particular, the function of CGRP and the blood–brain barrier in this disorder.
Figure 1.
Localization of CGRP receptors at the peripheral end of the trigeminovascular system; CGRP1 receptors are located only at the smooth muscle cells, and at present, there is no evidence of CGRP1 receptors on the sensory axons (autoreceptors). The CGRP antibodies would reduce the availability of CGRP at the receptor sites (through the reduction of available CGRP at the receptor), whereas a CGRP antagonist competes with CGRP at the receptor site. Either way will limit the effect of activation of the trigeminal nerve in migraine attacks (modified from Durham 2004, with permission). CGRP, calcitonin gene-related peptide.
References
- Denekas T, Troltzsch M, Vater A, Klussmann S, Messlinger K. Inhibition of stimulated meningeal blood flow by a calcitonin gene-related peptide binding mirror-image RNA oligonucleotide. Br J Pharmacol. 2006;148:536–543. doi: 10.1038/sj.bjp.0706742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L. Blockade of CGRP receptors in the intracranial vasculature: a new target in the treatment of headache. Cephalalgia. 2004;24:611–622. doi: 10.1111/j.1468-2982.2003.00719.x. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Nilsson E, Jansen-Olesen I. Inhibitory effect of BIBN4096BS, a CGRP8-37, a CGRP antibody and an RNA-Spiegelmer on CGRP induced vasodilatation in the perfused and non-perfused rat middle cerebral artery. Br J Pharmacol. 2007;150:633–640. doi: 10.1038/sj.bjp.0707134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Lipton RB, Ferrari MD. Migraine—current understanding and treatment. N Engl J Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, et al. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70:1304–1312. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- Juhl L, Edvinsson L, Olesen J, Jansen-Olesen I. Effect of two novel CGRP-binding compounds in a closed cranial window rat model. Eur J Pharmacol. 2007;567:117–124. doi: 10.1016/j.ejphar.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, et al. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP). immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol. 2008;507:1277–1299. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Strassman AM. Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Ann Neurol. 2005;58:698–705. doi: 10.1002/ana.20619. [DOI] [PubMed] [Google Scholar]
- Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Haan J, Terwindt GM, Ferrari MD. Migraine: gene mutations and functional consequences. Curr Opin Neurol. 2007;20:299–305. doi: 10.1097/WCO.0b013e3281338d1f. [DOI] [PubMed] [Google Scholar]
- Zeller J, Poulsen KT, Sutton JE, Abdiche YN, Collier S, Chopra R, et al. CGRP function-blocking antibodies inhibit neurogenic vasodilation without affecting heart rate or arterial blood pressure in the rat Br J Pharmacol 20081551093–1103.(this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]