Abstract
Background and purpose:
The epithelial sodium channel (ENaC) is a key regulator of airway mucosal hydration and mucus clearance. Negative regulation of airway ENaC function is predicted to be of clinical benefit in the cystic fibrosis lung. The aim of this study was to develop a small animal model to enable the direct assessment of airway ENaC function in vivo.
Experimental approach:
Tracheal potential difference (TPD) was utilized as a measure of airway epithelial ion transport in the guinea-pig. ENaC activity in the trachea was established with a dose–response assessment to a panel of well-characterized direct and indirect pharmacological modulators of ENaC function, delivered by intra-tracheal (i.t.) instillation.
Key results:
The TPD in anaesthetized guinea-pigs was attenuated by the direct ENaC blockers: amiloride, benzamil and CF552 with ED50 values of 16, 14 and 0.2 μg kg−1 (i.t.), respectively. 5-(N-Ethyl-N-isopropyl) amiloride, a structurally related compound but devoid of activity on ENaC, was without effect on the TPD. Intra-tracheal dosing of the Kunitz-type serine protease inhibitors aprotinin and placental bikunin, which have previously been demonstrated to inhibit proteolytic activation of ENaC, likewise potently attenuated TPD in guinea-pigs, whereas α1-antitrypsin and soya bean trypsin inhibitor were without effect.
Conclusions and implications:
The pharmacological sensitivity of the TPD to amiloride analogues and also to serine protease inhibitors are both consistent with that of ENaC activity in the guinea-pig trachea. The guinea-pig TPD therefore represents a suitable in vivo model of human airway epithelial ion transport.
Keywords: ion transport, cystic fibrosis, mucokinetic, ENaC, animal model, amiloride, benzamil, CF552, aprotinin, channel-activating protease
Introduction
The epithelial sodium channel (ENaC) is composed of three subunits (α, β and γ) (Canessa et al., 1994) that are expressed in the apical membrane of several fluid absorptive epithelia including cortical collecting duct, colon and airway (Kellenberger and Schild, 2002). In the human airways, ENaC-mediated Na+ absorption (Knowles et al., 1981; Davies et al., 2005) has a key function in the regulation of mucosal hydration and as such contributes directly to effective mucus clearance (Boucher, 2007). The central regulatory function that ENaC has in the maintenance of airway mucus clearance can be observed in two genetic diseases, cystic fibrosis (CF) and pseudohypoaldosteronism type 1. In CF, ENaC-mediated Na+ hyperabsorption in the absence of normal anion secretion is widely believed to lead to the dessication of the airway lumen that results in mucostasis establishing the environment for chronic respiratory infections and overt inflammation (Boucher, 2007). In contrast, systemic loss of function mutations of ENaC in pseudohypoaldosteronism type 1 patients, result in a ‘hyper-hydration' of the airway lumen and an enhancement of mucociliary clearance of three- to fourfold above that of a normal healthy individual (Kerem et al., 1999).
As such, negative regulation of ENaC function in the airway represents a therapeutic opportunity with potential utility in the treatment of CF lung disease and additional diseases associated with impaired mucus clearance. Acute inhalation of the K+ sparing diuretic amiloride, an ENaC blocker, has been demonstrated to enhance mucociliary clearance and cough clearance in CF patients (Köhler et al., 1986; App et al., 1990). In a 25 week study, inhaled amiloride was also reported to show clinical benefit in CF patients (Knowles et al., 1990), although subsequent studies failed to validate these observations (Graham et al., 1993; Bowler et al., 1995). This lack of robust clinical benefit with inhaled amiloride has subsequently been ascribed to the poor potency of the compound and a suboptimal pharmacokinetic profile (Hofmann et al., 1997). As such, the identification of novel negative regulators of ENaC function, more suited to inhaled delivery, will enable the concept to be further tested in the clinic.
Epithelial ion transport processes can be directly measured in vitro and in vivo using the short-circuit current (ISC) and epithelial potential difference (PD) techniques, respectively. Primary cultures of nasal, tracheal and bronchial epithelial cells have been utilized in vitro to study ENaC function using the ISC method (Bridges et al., 2001; Donaldson et al., 2002; Hirsh et al., 2004). Nasal potential difference has been used clinically in both the diagnosis of CF and in the testing of candidate therapeutics (Knowles et al., 1981; Rubenstein and Zeitlin, 1998). Nasal potential difference and tracheal potential difference (TPD) have been measured in a number of laboratory species, including mice, rats, guinea-pigs and pigs (Takahashi et al., 1995; Grubb and Boucher, 1999; Tomlinson et al., 1999; Salinas et al., 2004), where the pharmacological modulation of predominantly Cl− transport has been examined. Although an amiloride-sensitive component of the nasal potential difference has been described in many of these species (Grubb and Boucher, 1999; Tomlinson et al., 1999; Salinas et al., 2004), the authors are not aware of any in vivo studies that have directly studied ENaC regulation in airways distal to the nose. An indirect assessment of the function of ENaC in the airway epithelium has been made in conscious sheep, where inhaled dosing with the direct ENaC blocker amiloride and its analogues benzamil and CF552 (Hirsh et al., 2004, 2008) was demonstrated to enhance the lung clearance of 99mTc-labelled particles using γ-scintigraphy. Furthermore, P2Y-receptor agonists, agents that both stimulate anion secretion and negatively regulate ENaC in the airway (Mason et al., 1991; Devor and Pilewski, 1999), also promoted mucus clearance in the sheep model (Sabater et al., 1999).
An in vivo model in a small laboratory species that enabled a direct assessment of ENaC activity in the airway epithelium distal to the nose would be of benefit in the development of novel negative regulators of ENaC function in the airway. To this end, the aims of this study were (1) to probe the TPD in both rat and guinea-pig for any evidence of ENaC function, (2) to establish the pharmacological profile of established, direct ENaC blockers on the TPD when delivered directly into the airways and (3) to assess any putative sensitivity of airway ENaC in vivo to serine protease inhibition as had been proposed by several in vitro studies using primary cultures of human airway epithelial cells (Bridges et al., 2001; Donaldson et al., 2002). These studies have established the guinea-pig TPD as a suitable in vivo model of the human airway epithelium in terms of ENaC-function and its pharmacological regulation.
Materials and methods
Male Dunkin Hartley guinea-pigs (300–650 g) and male Wistar or Sprague–Dawley rats (250–450 g) (Harlan, UK) were used during the course of these studies. All studies were performed in accordance with the guidelines of the United Kingdom Home Office on the operation of the Animals (Scientific Procedures) Act 1986 and were approved by the local Ethical Review Process. Animals were acclimatized for a minimum of 7 days before the study, maintained on a 12 h light–dark cycle at a temperature of 21±2 °C and humidity of 55±5%. Animals were allowed to access food (guinea-pigs: FD1 pellets; rats: RM1P), hay (autoclavable hay bales) and water ad libitum.
Inhaled and i.t. dosing of animals
Test compounds (amiloride, benzamil, 5-(N-ethyl-N-isopropyl)amiloride (EIPA), N-(3,5-diamino-6-chloropyrazine-2-carbonyl)-N′-4-[4-(2,3-dihydroxypropoxy)phenyl] butyl-guanidine (CF552), aprotinin, placental bikunin (HAI-2A), soya bean trypsin inhibitor (SBTI), α1-antitrypsin) or matched vehicles were administered to animals by either inhalation of an aerosol or by i.t. instillation of solutions. For aerosol dosing, animals were placed into a perspex chamber containing Alpha-Dri in which they could be confined unrestrained, and exposed to an aerosol of either vehicle (sterile water) or compound solution generated by a CR60—System 22 pump, pumping through a Cirrus nebuliser into the chamber for 60 min. For i.t. dosing, animals were placed under short-term anaesthesia using an inhaled halothane/N2O mixture. When a suitable depth of anaesthesia was obtained (loss of pedal and blink reflex), 200 μL of either vehicle (5% glucose solution), or compound was instilled using a modified oral gavage needle directly into the trachea. Animals were then allowed to recover and had full movement before preparation for the measurement of TPD.
Measurement of tracheal potential difference
Preparation of agar bridges
Bridges were prepared according to a previously reported method (Takahashi et al., 1995). Agar (3.5%) was dissolved in Hank's buffered salt solution (pH 7.4, 280–305 mosm kg−1 H2O) by heating in a water bath at 100 °C. A 30–40 cm length of polyethylene tubing, of which one end was bevelled at an angle of 20–60°, was then filled with the agar ensuring no air bubbles were formed or trapped within the tubing. The bevelled tip of the tube was then dipped in the liquid agar, creating a small bulge. The exploring and reference electrodes were constructed of an Hank's buffered salt solution/agar bridge linked to a calomel half cell by immersion in 1 M KCl.
Tracheal potential difference measurements
At various times after the completion of dosing with test compounds, guinea-pigs were first administered Hypnovel (midazolam 5 mg mL−1) 1 mL kg−1 (i.p) as a preanaesthetic agent followed 10 min later by a 1 mL kg−1 (i.p.) injection of Narketan10 (100 mg mL−1 ketamine) and Rompun (25 mg mL−1 xylazine) in water for injections mixed at a ratio of 2:1:1, respectively. Rats were administered an i.p. injection (8 mL kg−1) of a 1:1:2 mix of Hypnorm (fentanyl 0.32 mg mL−1, fluanisone 10 mg mL−1) and Hypnovel in water for injections. Once under surgical anaesthesia, animals were placed supine and the throat area was shaved. The trachea was visualized near the sternum using minimal blunt dissection. A lateral incision was made in the trachea near the sternum. The bevelled end of the exploring electrode was inserted into the lumen of the cephalad region of the trachea, ensuring that respiration was still possible. The bevelled end of the reference agar bridge electrode was placed adjacent to the trachea in electrical contact with the flesh of the throat, and the area was moistened with Hank's buffered salt solution. PD measurements were taken by an IsoMil (WPI, Stevenage, UK) through a PowerLab 16/S, and recorded on data capture software Chart for Windows (PowerLab, ADInstruments, Chalgrove, UK). Stable PD values were recorded at a distance of either 5 mm (distal) or 25 mm (proximal) from the tracheal incision. Data are expressed as mean absolute PD values±s.e.mean (mV) and were compared using either Student's t-test (single group to group comparisons) or a one-way ANOVA followed by Dunnett's post hoc test (for comparisons of multiple doses to a single-vehicle control) with significance assumed when P<0.05. Dose–response data were fitted using GraphPad Prism (GraphPad Software Inc.), and the dose required to induce 50% of the compound effect (ED50) was calculated.
Cell culture and short-circuit current assay
Human bronchial epithelial cell culture
Human bronchial epithelial (HBE) cells were cultured at an air–liquid interface based upon a previously described method (Atherton et al., 2003) on Snapwell inserts. The method of Atherton et al. (2003) was followed with the exception that from the first day of establishment of an air–liquid interface, HBE cells were fed with a DMEM:HAMS F-12 (1:1) media containing 2% Ultroser G with gentamycin (50 μg mL−1) and amphotericin B (50 ng mL−1), rather than the BEGM-supplement-based media previously described. Cells were used for ISC assay between days 7 and 21 after the establishment of the air–liquid interface.
Transiently transfected rat and guinea-pig ENaC in Fisher rat thyroid cells
The rat ENaC α1, β1 and γ1 subunit cDNAs were reverse transcription PCR (RT-PCR)-amplified from a commercially available rat kidney cDNA library. Primers were designed based on database sequences NM031548 (α1), NM012648 (β1) and NM017046 (γ1). The guinea-pig ENaC α1 cDNA was RT-PCR-amplified from a commercially available guinea-pig kidney cDNA library using primers based on the GenBank database sequences AF071230 (cavia cobya) and AJ249596 (cavia porcelus). The guinea-pig ENaC β1 and γ1 subunit cDNAs were RT-PCR amplified from a commercially available guinea-pig kidney cDNA library. The primers were designed, based on the alignments of human, mouse and rat sequences as, at the time, no guinea-pig sequences were deposited in the databases for β1 or γ1. All amplified PCR fragments were cloned in the pCR4-Topo vector and sequenced. Sequence verified inserts for all six subunits were subsequently subcloned by RT-PCR in the pXOON expression vector (from the start to stop codon only and introducing the general Kozak consensus sequence GCCACC immediately upstream of the start codon). The pXOON vector is a modified (extended multiple cloning site) pXOOM vector, which is a dual-function vector for protein expression in both mammalian cells and Xenopus laevis oocytes (Jespersen et al., 2002). The integrity of all six clones was confirmed by restriction enzyme digests and by double-stranded sequencing. The sequences for all six subunits were deposited in GenBank; rat ENaC α1=EU627515, rat ENaC β1=EU627516, rat ENaC γ1=EU627517, guinea-pig α1=EU627512, guinea-pig β1=EU627513, guinea-pig γ1=EU627514.
Fisher rat thyroid (FRT) cells were cultured in T162 cm2 flasks with F-12 Ham nutrient-mix supplemented with 10% foetal bovine serum, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin (growth media) at 37 °C in a humidified atmosphere of 5% CO2 (Sheppard et al., 1994). FRT cells were transiently transfected with the plasmids encoding either rat or guinea-pig ENaC subunits using Fugene 6 according to the manufacturer's conditions. Transfections were performed in growth media further supplemented with amiloride (10 μM). After overnight transfection, the FRT cells were trypsinized and seeded onto snapwell inserts at 1.65 × 105 cells per insert using growth media supplemented with dexamethasone (30 nM) and amiloride (10 μM). The inserts were fed on both the apical, and the basolateral side on day 1 post-seeding. Cells were used for ISC assay on day 2.
Short-circuit current assay
At the stated time in culture, the ion transport properties of HBE or transfected FRT cells were examined using the short-circuit current technique. Cells on Snapwell inserts were mounted in vertical diffusion chambers and were bathed with continuously gassed Ringer solution (5% CO2 in O2; pH 7.4) maintained at 37 °C containing (in mM): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 CaCl2, 1.2 MgCl2 and 10 glucose. The solution osmolarity was always between 280 and 300 mosmol kg−1 H2O for all physiological salt solutions used. Cells were voltage clamped to 0 mV (model EVC4000; WPI) and the ISC was measured. Transepithelial resistance (RT) was measured by applying a 1 or 2 mV pulse at 30 s intervals and calculating RT by Ohm's law. Data were recorded using a PowerLab workstation (ADInstruments). Direct ENaC blocker compounds were added to the apical chamber from a 1000-fold stock solution (prepared in dimethylsulphoxide) to achieve a cumulative concentration–response in terms of the inhibition of the basal ISC. At the completion of the concentration–response, a supramaximal concentration of amiloride (10 μM human; 30 μM guinea-pig/rat) was added. IC50 values were calculated (GraphPad Prism) assuming that the cumulative current inhibition achieved with the test compound and supramaximal amiloride concentration reflected the total ENaC-mediated ISC.
Materials
FD1 pellets, Alpha-Dri, RM1P and autoclavable hay bales, were obtained from Lillico (Crawley, UK). Sterile water, water for injections and 5% glucose solution, were from Baxter Healthcare (Northampton, UK); halothane, Merial (Harlow, UK). The CR60—System 22 pump was from Medic-Aid Ltd (Bognor Regis, UK); Cirrus nebuliser, Sunrise Medical (Stourbridge, UK); modified oral gavage needle, Vet-Tech Solutions (Macclesfield, UK); polyethylene tubing, Portex (London, UK); calomel half cell, Fisher (Waltham, MA, USA). Hypnovel (midazolam 5 mg mL−1) was from Roche (Welwyn Garden City, UK); Narketan10 (100 mg mL−1 ketamine), Vetoquinol (Buckingham, UK); Rompun (25 mg mL−1 xylazine), Bayer (Uxbridge, UK); Hypnorm (fentanyl 0.32 mg mL−1, fluanisone 10 mg mL−1), Janssen (High Wycombe, UK). HBE cells were from Lonza (Slough, UK); Ultroser G, Pall BioSepra (Cergy St Christophe, France). The rat kidney cDNA library was obtained from Biochain Institute Inc. (Hayward, CA, USA) and the guinea-pig kidney cDNA library from AMS Biotechnology (Abingdon, UK). The pCR4-Topo was from Invitrogen (Paisley, UK); Fugene 6, Roche Biosciences (Palo Alto, CA, USA). Unless stated otherwise, cell culture media (including Hank's buffered salt solution) and plastics were purchased from Invitrogen (Paisley, UK) and Fisher Scientific (Loughborough, UK), respectively. Chemicals were purchased from Sigma (Poole, UK) with the exceptions of CF552 and placental bikunin that were produced in-house. A truncated form of placental bikunin was used as described previously (Bridges et al., 2001).
All drug and molecular target nomenclature conforms with the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008).
Results
Species crossreactivity of direct ENaC blockers
The potencies of amiloride and benzamil on human and rat ENaC have been described previously (Bridges et al., 1989; Hirsh et al., 2004), although only limited data are available for guinea-pig (Liebold et al., 1996). CF552 represents a recently described ENaC blocker with potency data available for human, canine and ovine ENaC (Hirsh et al., 2008) but not guinea-pig or rat. To enable the interpretation of in vivo data, the potencies of these compounds were assessed in cell-based ISC assays. In addition, EIPA, a related pyrazine-acylguanidine compound that is inactive on ENaC, was studied. The cultured human bronchial epithelial cells and transiently transfected FRT cells (expressing rat or guinea-pig ENaC) displayed a spontaneous basal ISC that was sensitive to inhibition by direct ENaC blockers. Non-transfected FRT cells displayed no basal or amiloride-sensitive ISC (data not shown). The potencies of the ENaC blockers were maintained across species with a potency order of CF552>benzamil>amiloride≫EIPA (Table 1), consistent with the established pharmacology of ENaC (Kleyman and Cragoe, 1988).
Table 1.
ENaC blocker potency on human, guinea-pig and rat ENaC in vitro
| Compound | Human | Guinea-pig | Rat |
|---|---|---|---|
| Amiloride | 6.66±0.13 (93) | 6.27±0.30 (37) | 6.33±0.20 (24) |
| Benzamil | 7.70±0.22 (9) | 7.42±0.03 (30) | 7.55±0.28 (15) |
| CF552 | 8.62±0.15 (12) | 8.42±0.31 (32) | 8.46±0.18 (14) |
| EIPA | <4.52 (4) | <4.52 (4) | <4.52 (6) |
pIC50 data for selected ENaC blockers on human (bronchial epithelial cells), and guinea-pig or rat ENaC in transiently transfected Fisher Rat Thyroid cells. Mean pIC50 values±s.d. (n-value) calculated from short-circuit current assays are shown.
Comparison of rat and guinea-pig TPD: sensitivity to inhaled benzamil
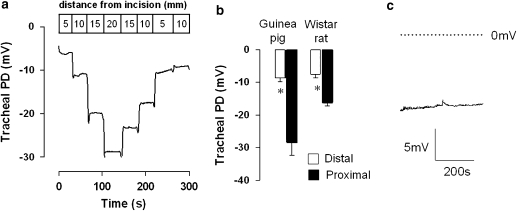
The guinea-pig and rat (Wistar and Sprague Dawley) displayed a spontaneous, lumen-negative TPD in vivo. The absolute TPD value was, however, dependent upon the electrode placement in the trachea. The TPD measured in the distal trachea (5 mm from the tracheal incision) was consistently smaller than that measured in the proximal region in both species (Figure 1). Figure 1a illustrates the effect of ‘walking' the exploring electrode up the trachea of a naive, untreated guinea-pig, from the distal to proximal regions. In this example, the TPD increased as the electrode was moved proximally, and as it was then withdrawn from the trachea, the TPD reduced. The raw data traces shown in Figures 1a and c illustrate the stability of the PD in the guinea-pig trachea over a period of several minutes. A similar stable PD was consistently observed in the rat (data not shown).
Figure 1.
The tracheal potential difference (TPD) measured in both guinea-pig and rat in vivo is dependent upon the site of placement of the exploring electrode. A sample raw data trace illustrates the effect of moving the exploring electrode in 5 mm increments from the distal to proximal trachea in the guinea-pig (a). The TPD measured in the proximal tracheal was consistently larger than that in the distal region, in both guinea-pig and rat (b). Mean data±s.e.mean are shown. * Indicates P<0.01 with n=6–9 animals per group. (c) A typical raw data trace from a guinea-pig study, further illustrating the stability of the TPD over several minutes.
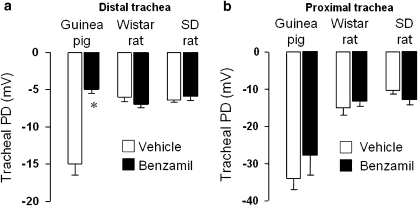
Having established a methodology for the assessment of the spontaneous TPD, our next aim was to test for any evidence of an ENaC-mediated component to this PD in both rat and guinea-pig. Animals were exposed to an aerosol of benzamil (100 μM) or vehicle (sterile water for injections) for 60 min after which the TPD was measured in both the distal and proximal portions of the trachea in the same animals (Figure 2). Control, vehicle-exposed guinea-pigs showed a spontaneous TPD in the distal trachea, whereas benzamil-treated animals displayed a significantly reduced TPD (Figure 2a). There was no significant effect of benzamil on the proximal tracheal PD in the same animals (Figure 2b). Wistar rats did not show any sensitivity of either the distal or proximal TPD to inhaled benzamil (Figures 2a and b, respectively). Likewise, Sprague–Dawley rats failed to display any evidence of an ENaC-mediated component to the TPD (Figures 2a and b). The guinea-pig was therefore used for subsequent studies to establish the pharmacological profile of the benzamil-sensitive portion of the distal TPD.
Figure 2.
Mean absolute potential difference values±s.e.mean recorded in both the distal (a) and proximal (b) regions of the guinea-pig, Wistar or Sprague–Dawley (SD) rat trachea, immediately following a 60 min exposure to an aerosol of vehicle (water for injections) or benzamil (100 μM). Benzamil significantly attenuated the potential difference in the distal trachea of guinea-pigs only; n=6–13 animals per group. * Indicates P<0.001, using Student's two-tailed t-test.
Pharmacological profile of direct ENaC blockers on guinea-pig tracheal potential difference
All subsequent studies were performed using i.t. dosing of test compounds. This route of administration, in contrast to inhalation of aerosol, enabled an accurate regulation of delivered dose. Amiloride, benzamil and CF552 all dose-dependently attenuated the guinea-pig TPD (Table 2); ED50 values were assessed at 60 min following compound administration. In contrast, the ENaC-inactive amiloride-analogue, EIPA, did not significantly attenuate the TPD at the highest dose that could be administered in solution, 300 μg kg−1.
Table 2.
The effects of ENaC blocker compounds on the guinea-pig tracheal potential difference
| Compound | ED50 (μg kg−1; i.t.) | Dose (μg kg−1; i.t.) | TPD (mV) | n |
|---|---|---|---|---|
| Amiloride | 16 | 0 | −10.5±1.1 | 10 |
| 3.0 | −9.8±1.2 | 6 | ||
| 30 | −6.0±0.4a | 8 | ||
| 300 | −4.4±0.2a | 4 | ||
| 3000 | −4.3±0.4a | 4 | ||
| Benzamil | 14 | 0 | −8.8±0.5 | 20 |
| 1.0 | −10.1±1.4 | 7 | ||
| 10 | −6.0±0.4 | 5 | ||
| 100 | −4.3±0.6a | 6 | ||
| 1000 | −3.0±0.5a | 6 | ||
| 10 000 | −2.9±0.9a | 6 | ||
| CF552 | 0.2 | 0 | −10.5±1.0 | 9 |
| 0.01 | −9.8±0.9 | 4 | ||
| 0.1 | −7.8±0.9 | 4 | ||
| 1.0 | −7.1±0.4b | 4 | ||
| 10 | −5.1±0.3a | 4 | ||
| 100 | −5.2±0.4a | 6 | ||
| EIPA | >300 | 0 | −9.7±1.3 | 5 |
| 300 | −10.0±0.6 | 5 | ||
Mean absolute tracheal potential difference values±s.e.mean obtained in groups of guinea-pigs dosed with either vehicle (0.2 mL 5% dextrose) or test compound by i.t. instillation. One-way ANOVA followed by Dunnett's multiple comparison test was used to test for differences between compound-treated and vehicle control groups.
P<0.01.
P<0.05.
EIPA was tested up to the limit of solubility in 5% dextrose.
The effects of serine protease inhibitors on the guinea-pig tracheal potential difference
The broad spectrum serine protease inhibitors aprotinin and placental bikunin have been demonstrated to attenuate ENaC activity in primary cultures of human airway epithelial cells (Bridges et al., 2001; Donaldson et al., 2002). In contrast, SBTI and α1-antitrypsin failed to significantly influence ENaC activity in these cell types (Bridges et al., 2001). The in vivo experimental design assumed that the rate of onset of action of the protease inhibitors would be similar in the guinea-pig to that observed in primary cultures of human airway epithelial cells. In primary cultures of human airway epithelial cells, a 90 min incubation with protease inhibitor was sufficient to see an apparent maximal inhibition of amiloride-sensitive ISC. As such, the TPD was assessed at 2 h after i.t. dosing with each of the serine protease inhibitors. Aprotinin and placental bikunin both attenuated the TPD (Table 3). In both cases, the TPD was reduced by ∼5 mV, similar to the magnitude of PD inhibition observed with the direct ENaC blockers. In contrast to aprotinin and placental bikunin, SBTI and α1-antitrypsin did not significantly affect the TPD at doses of 16 000 and 14 500 pmol kg−1, respectively.
Table 3.
The effects of serine protease inhibitors on the guinea-pig tracheal potential difference in vivo
| Compound (Mw) | ED50 (μg kg−1; i.t.) | ED50 (pmol kg−1; i.t.) | Dose (pmol kg−1; i.t.) | TPD (mV) | n |
|---|---|---|---|---|---|
| Aprotinin (6.5 kDa) | 0.26 | 38 | 0 | −10.0±0.8 | 11 |
| 15 | −8.2±1.1 | 6 | |||
| 153 | −6.0±0.6a | 4 | |||
| 1530 | −4.8±1.1b | 4 | |||
| 15 300 | −4.7±0.6b | 7 | |||
| Placental bikunin (21.0 kDa) | 0.19 | 8.8 | 0 | −10.8±0.6 | 8 |
| 1.5 | −9.8±0.6 | 5 | |||
| 15 | −7.0±0.7b | 5 | |||
| 150 | −5.0±0.4b | 6 | |||
| 1500 | −4.7±0.4b | 7 | |||
| SBTI (20.1 kDa) | >320 | >16 000 | 0 | −10.8±0.9c | 8 |
| 16 000 | −10.8±1.0 | 9 | |||
| α1-antitrypsin (51.3 kDa) | >744 | >14 500 | 0 | −10.8±0.9c | 8 |
| 14 500 | −9.8±0.9 | 9 |
Mean absolute tracheal potential difference values±s.e.mean obtained in groups of guinea-pigs dosed with either vehicle (0.2 mL 5% dextrose) or serine protease inhibitor by i.t. instillation. One-way ANOVA followed by Dunnett's multiple comparison test was used to test for differences between compound-treated and vehicle control groups.
P<0.05.
P<0.01.
Common, shared control group.
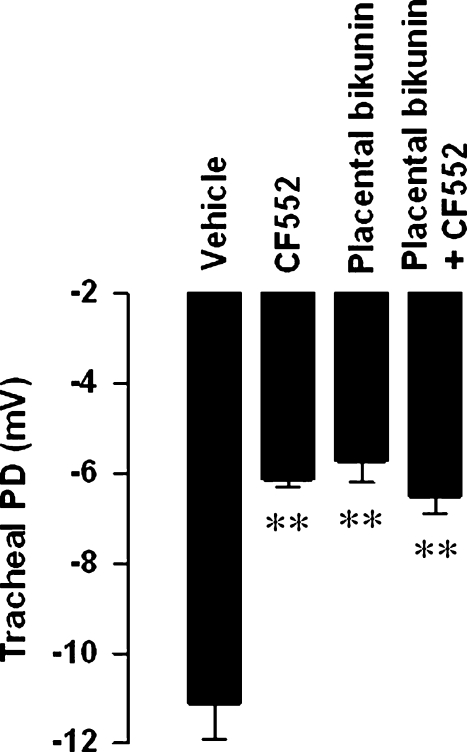
Studies were then undertaken to establish whether the serine protease inhibitor-sensitive component of the TPD was ENaC-mediated. The effects of placental bikunin and CF552 were examined when dosed independently and when combined in a single-dose formulation. At 2 h after dosing, both CF552 (2 mg kg−1; i.t.) and placental bikunin (15 nmol kg−1; i.t.) significantly attenuated the TPD (Figure 3). When co-administered, there was no greater attenuation of the TPD than that was obtained with either compound alone (Figure 3).
Figure 3.
Mean absolute potential difference values±s.e.mean illustrating the attenuation of the basal potential difference in the guinea-pig trachea following i.t. instillation of vehicle (0.2 mL 5% dextrose), CF552 (2 mg kg−1), placental bikunin (15 nmol kg−1) or a combination of CF552 with placental bikunin. One-way ANOVA followed by a Dunnett's multiple comparison test was used to determine differences between compound-treated and vehicle control groups. ** Indicates P<0.01; n=5–6 animals per group.
Discussion
There now exists good evidence to link airway mucosal hydration with mucus clearance in human diseases, most notably in CF (Boucher, 2007). Modulators of airway epithelial ion transport processes therefore represent approaches to enhance mucosal hydration and thereby promote mucus clearance. The overall aim of this study was to establish a small animal model that was suitable for the study of negative regulators of ENaC function in the airways, in vivo.
In this study, rat and guinea-pig both displayed a stable, spontaneous, lumen-negative TPD consistent with published data (Takahashi et al., 1995). However, the data indicated that the PD could vary considerably depending upon the placement of the exploring electrode in the trachea. When placed in the proximal tracheal, PD measurements of ⩾20 or ⩾10 mV were observed in guinea-pig and rat, respectively (Figures 1 and 2), that are consistent with published values (Takahashi et al., 1995). TPD measurements made in the distal trachea revealed a consistently lower PD value than that observed in the proximal region.
Based upon its commercial availability and approximate 10-fold superior potency over amiloride in all species examined (Table 1; Kleyman and Cragoe, 1988; Bridges et al., 1989; Liebold et al., 1996), benzamil was selected for an initial evaluation of TPD sensitivity in both rat and guinea-pig with compound delivery by inhalation of a nebulized solution. In the guinea-pig, only the distal PD was sensitive to inhaled benzamil, consistent with an ENaC-mediated component of this TPD (Figure 2a). In the rat, the TPD was insensitive to benzamil, irrespective of the site of electrode placement. As only the guinea-pig distal trachea displayed a benzamil-sensitive component to the PD, this species was selected for all subsequent studies.
Direct i.t. instillation of test compounds was preferred over inhalation of a nebulized solution for all subsequent studies because it enabled an accurate and efficient delivery of dose. The i.t. dosing required the guinea-pigs to be placed under a short acting (<5 min) halothane/N2O anaesthesia. Halothane has been previously reported to attenuate airway epithelial ion transport processes including amiloride-sensitive Na+ transport both in vitro and in vivo (Molliex et al., 1998; Rezaiguia-Delclaux et al., 1998; Roch et al., 2006), although there is evidence that these observations may be species-dependent (Nielsen et al., 2000). Because of the short period of exposure to halothane, and with the TPD being measured 60 min after dosing, any putative impact of halothane on tracheal epithelial ion transport processes and therefore TPD was anticipated to be of limited significance and would have been controlled through the use of time-matched vehicle-treated animals. TPD was measured in guinea-pigs 60 min after i.t. dosing with test compound or vehicle, at which time a clear ENaC-blocker sensitive component of the spontaneous PD was evident. Dose–response studies revealed a blocker potency order in vivo of CF552≫benzamil=amiloride≫EIPA that was inconsistent with the in vitro potency order of CF552>benzamil>amiloride≫EIPA. In contrast to the in vitro paradigm, the pharmacokinetics of these blockers will probably have a significant impact on the in vivo potency, as efficacy was assessed 60 min after dosing. As such, it is possible that benzamil could be cleared from the airway lumen more rapidly than amiloride, resulting in the higher than predicted ED50 value. In-house in vitro permeability assays using cultured HBE cells indicate a significantly greater apical to basolateral permeability of benzamil when compared with amiloride (data not shown) that would be consistent with a faster lumenal clearance of benzamil in vivo. Likewise, inhaled benzamil and amiloride demonstrated a similar in vivo potency in the sheep mucociliary clearance model despite a 10-fold greater in vitro potency of benzamil over amiloride in this species (Hirsh et al., 2004). Together these data are consistent with an ENaC-mediated component of the TPD in the guinea-pig.
The next aim of this study was to evaluate any putative regulation of the TPD by serine protease inhibitors. The Kunitz-type macromolecular serine protease inhibitors aprotinin and placental bikunin have been previously demonstrated to attenuate ENaC activity in HBE cells derived from both normal and CF airways, whereas α1-antitrypsin and SBTI were without effect (Bridges et al., 2001; Donaldson et al., 2002). An assessment of this potential ‘channel-activating protease' (CAP) mechanism of ENaC regulation would further support the evidence for an ENaC-mediated component of the TPD in the guinea-pig and would also offer support for the in vitro to in vivo translation of this mechanism. The current model of CAP regulation of ENaC function in the airways proposes that ENaC is inserted into the apical membrane of the epithelial cells in an inactive state, and that an interaction (direct or indirect) with a CAP is required for activation. The airway CAP has been proposed to be the membrane-anchored trypsin-like serine protease, prostasin (PRSS8), based upon sequence homology to the xenopus CAP (Vallet et al., 1997), co-expression data (Donaldson et al., 2002) and gene silencing studies (Tong et al., 2004). The CAP-mediated activation of ENaC has been demonstrated to be as a consequence of an increase in channel open probability (Adebamiro et al., 2005). In the presence of a CAP inhibitor, such as aprotinin or placental bikunin, ENaC would not become activated upon insertion into the plasma membrane resulting in a steady decline in epithelial ENaC-mediated Na+ transport as active channel is internalized. In vitro, HBE cell studies have indicated a maximal effect of Kunitz-type inhibitors on ENaC by approximately 90 min after addition (Bridges et al., 2001). Two hours was therefore selected as the time point for assessing CAP inhibitor activity in vivo in the guinea-pig. At this time, both aprotinin and placental bikunin potently attenuated the TPD to a similar degree to that observed with the direct ENaC blockers, that is, ∼5 mV. In contrast, α1-antitrypsin and SBTI were without effect on the TPD. To confirm whether aprotinin and placental bikunin were attenuating the ENaC-mediated component of the TPD, the effects of CF552 and placental bikunin either alone or in combination were studied. That both the direct (CF552) and indirect (placental bikunin) blockers of ENaC attenuated the TPD to the same degree, and that in combination there was no greater effect than that with either agent alone, are consistent with activities on a common pathway, that is, ENaC. Together, these CAP inhibitor data are consistent with the published macromolecular inhibitor profile in the human airway epithelium and provide the first in vivo data to support a function for CAP regulation of ENaC in the airways.
The guinea-pig TPD model described here offers the potential to study both the potency and durability of negative regulators of ENaC function, whether delivered topically into the airways or following systemic exposure. Furthermore, the ability to directly monitor the activity of airway ENaC in vivo will enable a confirmation of compound mechanism. This system will therefore have the potential to complement models of mucociliary clearance, such as the conscious sheep, where the downstream consequence of attenuated ENaC function can be assessed.
Acknowledgments
We thank Zakiyya Ahmed for her technical assistance with the FRT assays, Drs Sabine Geisse and Frank Kolbinger (Novartis Protein Production Unit, Basel, Switzerland) for the preparation of placental bikunin and Dr Renato Tarchini (Solvias AG, Basel, Switzerland) for his support in the cloning of the rat and guinea-pig ENaC subunits.
Abbreviations
- CF552
N-(3,5-diamino-6-chloropyrazine-2-carbonyl)-N′-4-[4-(2,3-dihydroxypropoxy)phenyl] butyl-guanidine
- EIPA
5-(N-ethyl-N-isopropyl) amiloride
- ENaC
epithelial sodium channel
- FRT
Fisher rat thyroid
- HBE
human bronchial epithelial
- i.t.
intra-tracheal
- ISC
short-circuit current
- PD
potential difference
- SBTI
soya bean trypsin inhibitor
- TPD
tracheal potential difference
Conflict of interest
The authors state no conflict of interest.
References
- Adebamiro A, Cheng Y, Johnson JP, Bridges RJ. Endogenous protease activation of ENaC: effect of serine protease inhibition on ENaC single channel properties. J Gen Physiol. 2005;126:339–352. doi: 10.1085/jgp.200509285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC) Br J Pharmacol. 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- App EM, King M, Helfesrieder R, Köhler D, Matthys H. Acute and long-term amiloride inhalation in cystic fibrosis lung disease. A rational approach to cystic fibrosis therapy. Am Rev Respir Dis. 1990;141:605–612. doi: 10.1164/ajrccm/141.3.605. [DOI] [PubMed] [Google Scholar]
- Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol. 2003;285:L730–L739. doi: 10.1152/ajplung.00089.2003. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- Bowler IM, Kelman B, Worthington D, Littlewood JM, Watson A, Conway SP, et al. Nebulised amiloride in respiratory exacerbations of cystic fibrosis: a randomised controlled trial. Arch Dis Child. 1995;73:427–430. doi: 10.1136/adc.73.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RJ, Cragoe EJ, Jr, Frizzell RA, Benos DJ. Inhibition of colonic Na+ transport by amiloride analogues. Am J Physiol. 1989;256:C67–C74. doi: 10.1152/ajpcell.1989.256.1.C67. [DOI] [PubMed] [Google Scholar]
- Bridges RJ, Newton BB, Pilewski JM, Devor DC, Poll CT, Hall RL. Na+ transport in normal and CF human bronchial epithelial cells is inhibited by BAY 39-9437. Am J Physiol Lung Cell Mol Physiol. 2001;281:L16–L23. doi: 10.1152/ajplung.2001.281.1.L16. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Davies JC, Davies M, McShane D, Smith S, Chadwick S, Jaffe A, et al. Potential difference measurements in the lower airway of children with and without cystic fibrosis. Am J Respir Crit Care Med. 2005;171:1015–1019. doi: 10.1164/rccm.200408-1116OC. [DOI] [PubMed] [Google Scholar]
- Devor DC, Pilewski JM. UTP inhibits Na+ absorption in wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol. 1999;276:C827–C837. doi: 10.1152/ajpcell.1999.276.4.C827. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, et al. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem. 2002;277:8338–8345. doi: 10.1074/jbc.M105044200. [DOI] [PubMed] [Google Scholar]
- Graham A, Hasani A, Alton EW, Martin GP, Marriott C, Hodson ME, et al. No added benefit from nebulized amiloride in patients with cystic fibrosis. Eur Respir J. 1993;6:1243–1248. [PubMed] [Google Scholar]
- Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79 1 Suppl:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- Hirsh AJ, Sabater JR, Zamurs A, Smith RT, Paradiso AM, Hopkins S, et al. Evaluation of second generation amiloride analogs as therapy for cystic fibrosis lung disease. J Pharmacol Exp Ther. 2004;311:929–938. doi: 10.1124/jpet.104.071886. [DOI] [PubMed] [Google Scholar]
- Hirsh AJ, Zhang J, Zamurs A, Fleegle J, Thelin WR, Caldwell RA, et al. Pharmacological properties of N-(3,5-diamino-6-chloropyrazine-2-carbonyl)-N′-4-[4-(2,3-dihydroxypropoxy)phenyl]butyl-guanidine methanesulfonate (552-02), a novel epithelial sodium channel blocker with potential clinical efficacy for cystic fibrosis lung disease. J Pharmacol Exp Ther. 2008;325:77–88. doi: 10.1124/jpet.107.130443. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Senier I, Bittner P, Hüls G, Schwandt HJ, Lindemann H. Aerosolized amiloride: dose effect on nasal bioelectric properties, pharmacokinetics, and effect on sputum expectoration in patients with cystic fibrosis. J Aerosol Med. 1997;10:147–158. doi: 10.1089/jam.1997.10.147. [DOI] [PubMed] [Google Scholar]
- Jespersen T, Grunnet M, Angelo K, Klaerke DA, Olesen SP.Dual-function vector for protein expression in both mammalian cells and Xenopus laevis oocytes Biotechniques 200232536–538.540 [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kerem E, Bistritzer T, Hanukoglu A, Hofmann T, Zhou Z, Bennett W, et al. Pulmonary epithelial sodium-channel dysfunction and excess airway liquid in pseudohypoaldosteronism. N Engl J Med. 1999;341:156–162. doi: 10.1056/NEJM199907153410304. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981;305:1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- Knowles MR, Church NL, Waltner WE, Yankaskas JR, Gilligan P, King M, et al. A pilot study of aerosolized amiloride for the treatment of lung disease in cystic fibrosis. N Engl J Med. 1990;322:1189–1194. doi: 10.1056/NEJM199004263221704. [DOI] [PubMed] [Google Scholar]
- Köhler D, App E, Schmitz-Schumann M, Würtemberger G, Matthys H. Inhalation of amiloride improves the mucociliary and the cough clearance in patients with cystic fibroses. Eur J Respir Dis Suppl. 1986;146:319–326. [PubMed] [Google Scholar]
- Liebold KM, Reifarth FW, Clauss W, Weber W. cAMP-activation of amiloride-sensitive Na+ channels from guinea pig colon expressed in Xenopus oocytes Pflugers. Arch. 1996;431:913–922. doi: 10.1007/s004240050085. [DOI] [PubMed] [Google Scholar]
- Mason SJ, Paradiso AM, Boucher RC. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br J Pharmacol. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliex S, Dureuil B, Aubier M, Friedlander G, Desmonts JM, Clerici C. Halothane decreases Na,K-ATPase, and Na channel activity in alveolar type II cells. Anesthesiology. 1998;88:1606–1613. doi: 10.1097/00000542-199806000-00025. [DOI] [PubMed] [Google Scholar]
- Nielsen VG, Baird MS, Geary BT, Matalon S. Halothane does not decrease amiloride-sensitive alveolar fluid clearance in rabbits. Anesth Analg. 2000;90:1445–1449. doi: 10.1097/00000539-200006000-00036. [DOI] [PubMed] [Google Scholar]
- Rezaiguia-Delclaux S, Jayr C, Luo DF, Saïdi NE, Meignan M, Duvaldestin P. Halothane and isoflurane decrease alveolar epithelial fluid clearance in rats. Anesthesiology. 1998;88:751–760. doi: 10.1097/00000542-199803000-00027. [DOI] [PubMed] [Google Scholar]
- Roch A, Shlyonsky V, Goolaerts A, Mies F, Sariban-Sohraby S. Halothane directly modifies Na+ and K+ channel activities in cultured human alveolar epithelial cells. Mol Pharmacol. 2006;69:1755–1762. doi: 10.1124/mol.105.021485. [DOI] [PubMed] [Google Scholar]
- Rubenstein RC, Zeitlin PL. A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in deltaF508-homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. Am J Respir Crit Care Med. 1998;157:484–490. doi: 10.1164/ajrccm.157.2.9706088. [DOI] [PubMed] [Google Scholar]
- Sabater JR, Mao YM, Shaffer C, James MK, O'Riordan TG, Abraham WM. Aerosolization of P2Y(2)-receptor agonists enhances mucociliary clearance in sheep. J Appl Physiol. 1999;87:2191–2196. doi: 10.1152/jappl.1999.87.6.2191. [DOI] [PubMed] [Google Scholar]
- Salinas DB, Pedemonte N, Muanprasat C, Finkbeiner WF, Nielson DW, Verkman AS. CFTR involvement in nasal potential differences in mice and pigs studied using a thiazolidinone CFTR inhibitor. Am J Physiol Lung Cell Mol Physiol. 2004;287:L936–L943. doi: 10.1152/ajplung.00354.2003. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Carson MR, Ostedgaard LS, Denning GM, Welsh MJ. Expression of cystic fibrosis transmembrane conductance regulator in a model epithelium. Am J Physiol. 1994;266:L405–L413. doi: 10.1152/ajplung.1994.266.4.L405. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Freed AN, Croxton TL. Scaling of transepithelial potential difference in the mammalian trachea. Respir Physiol. 1995;99:19–27. doi: 10.1016/0034-5687(94)00076-c. [DOI] [PubMed] [Google Scholar]
- Tomlinson LA, Carpenter TC, Baker EH, Bridges JB, Weil JV. Hypoxia reduces airway epithelial sodium transport in rats. Am J Physiol. 1999;277 5 Part 1:L881–L886. doi: 10.1152/ajplung.1999.277.5.L881. [DOI] [PubMed] [Google Scholar]
- Tong Z, Illek B, Bhagwandin VJ, Verghese GM, Caughey GH. Prostasin, a membrane-anchored serine peptidase, regulates sodium currents in JME/CF15 cells, a cystic fibrosis airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2004;287:L928–L935. doi: 10.1152/ajplung.00160.2004. [DOI] [PubMed] [Google Scholar]
- Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]