Abstract
Background and purpose:
Allergic inflammation and autoimmune diseases, such as atopic dermatitis, psoriasis and multiple sclerosis (MS), involve both mast cell and T-cell activation. However, possible interactions between the two and the mechanism of such activations are largely unknown.
Experimental approach:
Human umbilical cord blood-derived cultured mast cells (hCBMCs) and Jurkat T cells were incubated separately or together, following activation with myelin basic protein (MBP), as well as with or without pretreatment with the flavonoid luteolin for 15 min. The supernatant fluid was assayed for inflammatory mediators released from mast cells and interleukin (IL)-2 release from Jurkat cells.
Key results:
MBP (10 μM) stimulates hCBMCs to release IL-6, IL-8, transforming growth factor (TGF)-β1, tumour necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF), histamine and tryptase (n=6, P<0.05). Addition of mast cells to Jurkat cells activated by anti-CD3/anti-CD28 increases IL-2 release by 30-fold (n=3, P<0.05). MBP-stimulated mast cells and their supernatant fluid further increase Jurkat cell IL-2 release (n=3, P<0.05). Separation of mast cells and activated Jurkat cells by a Transwell permeable membrane inhibits Jurkat cell stimulation by 60%. Pretreatment of Jurkat cells with a TNF-neutralizing antibody reduces IL-2 release by another 40%. Luteolin pretreatment inhibits mast cell activation (n=3–6, P<0.05), Jurkat cell activation and mast cell-dependent Jurkat cell stimulation (n=3, P<0.05).
Conclusions and implications:
Mast cells can stimulate activated Jurkat cells. This interaction is inhibited by luteolin, suggesting that this flavonoid may be useful in the treatment of autoimmune diseases.
Keywords: cell activation, cell–cell contact, cytokines, inflammation, Jurkat cells, mast cells, myelin basic protein, multiple sclerosis, TNF-α, T cells
Introduction
CD4+ T cells can develop into T helper cells (Th1 and Th2 cells) characterized by the production of different cytokines, such as interleukin-2 (IL-2) and interferon-γ from Th1 cells, and IL-4 and IL-13 from Th2 cells. Th2 cytokines are associated with allergic reactions and maturation of mast cells, but have recently also been implicated in multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE) (Pedotti et al., 2003). Mast cells are involved in allergy as well as in innate and acquired immunity (Galli et al., 2005), including T-cell-mediated disorders (Gregory et al., 2006) and autoimmune disorders (Benoist and Mathis, 2002). Mast cells and T cells could interact in a variety of immune responses (Pedotti et al., 2003; Nakae et al., 2005), including many inflammatory diseases (Theoharides and Kalogeromitros, 2006). Mast cells are also located at the blood–brain barrier (BBB), especially in the choroid plexus, diencephalon and the third ventricle (Silver et al., 1996), as well as at MS plaques (Ibrahim et al., 1996). There, mast cells could regulate BBB permeability (Esposito et al., 2001), disruption of which precedes clinical or pathological signs of MS (Stone et al., 1995); this could attract T cells and superactivate them.
Multiple sclerosis is the second most common neurological disorder leading to severe disability in almost half a million people in the United States (Noseworthy et al., 2000). MS is characterized by inflammation and demyelination of the CNS mediated by infiltration of CD4+ Th1 cells, macrophages, B cells and mast cells (Frohman et al., 2006). However, it is still not known how T cells enter the brain and are sensitized to induce brain inflammation. This process is dependent on several factors, including IL-8, which regulates recruitment and activation of leukocytes, as well as the expression of vascular adhesion molecules that permit leukocyte transmigration (Mirowska-Guzel et al., 2006). Involvement of mast cells in the pathophysiology of MS is based on both anatomical and biochemical evidence (Krüger, 2001; Brown et al., 2002). Mast cell tryptase is elevated in the CSF of MS patients (Rozniecki et al., 1995), and can cause widespread inflammation by stimulating protease-activated receptors (Molino et al., 1997). Release of myelin basic protein (MBP) or other myelin breakdown products could also induce rat mast cell degranulation (Johnson et al., 1988; Theoharides et al., 1993). Moreover, genes highly upregulated in MS plaques include mast cell-associated molecules, such as tryptase, the IgE receptor (FcɛRI) and the histamine H1 receptor (Lock et al., 2002).
Current available MS therapies are not curative. Certain naturally occurring flavonoids (Kimata et al., 2000; Middleton et al., 2000; Kumazawa et al., 2006) inhibit the release of pro-inflammatory molecules from human mast cells (Kempuraj et al., 2005) and can suppress EAE (Hendriks et al., 2004). Here, we investigated whether human mast cells can affect Jurkat T-cell activation either through direct contact and/or mediators released in response to stimulation by MBP, and whether these processes could be blocked by pretreatment with the flavonoid, luteolin.
Materials and methods
Isolation of CD34+ cells and mast cell culture
Human umbilical cord blood was collected at Tufts Medical Center (Boston, MA, USA), as approved by the Institution's Human Investigation Review Board, in tubes containing 10 U mL−1 heparin. The isolation of haematopoietic stem cells (CD34+) was performed by positive selection of CD34+/AC133+ cells by magnetic cell sorting using an AC133+ cell isolation kit (Miltenyi Biotec, Auburn, CA, USA) as reported earlier (Kempuraj et al., 1999). CD34+ cells were suspended in Iscove's modified Dulbecco's medium (GIBCO BRL, Grand Island, NY, USA), supplemented with 200 ng mL−1 recombinant human stem cell factor, 50 ng mL−1 IL-6, 2% foetal bovine serum (BioWhittaker, Walkersville, MD, USA), 5 × 10−5 M 2-mercaptoethanol, and 1% penicillin–streptomycin (GIBCO BRL) for 12–16 weeks. IL-4 (20 ng mL−1) was used for 2 weeks before the experiment. The purity of human umbilical cord blood-derived cultured mast cells (hCBMCs) was evaluated by immunocytochemical staining for tryptase as described earlier (Kempuraj et al., 1999). Mast cells cultured for 10–16 weeks (100% purity) were used for the experiments.
Jurkat cell culture
Jurkat cells, Clone E6-1 (The American Type Culture Collection—ATCC number TIB-152) were cultured in RPMI-1640 medium with 2 mM L-glutamine adjusted to contain 1.5 g L−1 sodium bicarbonate, 4.4 g L−1 glucose, 10 mM HEPES, 1.0 mM sodium pyruvate and 10% foetal bovine serum at 37 °C in 95% air/5% CO2 atmosphere. Doubling time was 48 h. Culture medium was changed every 3 days. Cells were maintained at a concentration of about 1 × 106 cells per mL. Jurkat cell activation was assayed by the release of IL-2, the only cytokine released from these cells.
Sensitization of mast cells and cytokine assay
hCBMCs were washed with Dulbecco's phosphate buffered saline from GIBCO BRL and plain culture medium (without any growth factors), once in each, and were resuspended in serum-free complete culture medium. The cells (106 cells per mL) were then incubated with human myeloma IgE (2 μg mL−1, Chemicon International Inc., Temecula, CA, USA) at 37 °C for 48 h in 24-well Falcon cell culture plates (Becton Dickinson, Franklin Lakes, NJ, USA). These sensitized hCBMCs were stimulated for the experiments using anti-human IgE (10 μg mL−1) for 1 or 24 h as specified below. For the cytokine assay, sensitized hCBMCs were washed with Dulbecco's phosphate buffered saline, sterile human Tyrode's buffer and plain culture medium, once in each, and were resuspended in complete culture medium. The hCBMCs (2 × 105 cells per well) were plated in 96-well round-bottom Falcon cell culture plates (Becton Dickinson) and incubated for 15 min at 37 °C in 95% air/5% CO2 atmosphere. MBP was added and the cells were incubated for 24 h at 37 °C. At the end of this incubation, the supernatant fluid was gently collected from the wells and stored at −80 °C until IL-6, IL-8, transforming growth factor-β 1 (TGF-β1), tumour necrosis factor-α (TNF-α) and vascular endothelial growth factor (VEGF) were measured by ELISA using commercial kits (Quantikine, R&D Systems, Minneapolis, MN, USA). The minimum detectable level of IL-6, IL-8, TGF-β1, TNF-α and VEGF was typically 0.70, 3.5, 4.6, 5.1 and 5.0 pg mL−1, respectively. Control cells were treated with equal volume of culture medium; cells were also stimulated with anti-IgE alone for comparison.
Histamine and tryptase assays
hCBMCs were washed with Dulbecco's phosphate buffered saline and human Tyrode's buffer, once in each, and resuspended in sterile human Tyrode's buffer. The cell suspension (5 × 104 cells per tube, 500 μL per sample) was pre-incubated for 15 min at 37 °C in a shaking water bath and then MBP was added, and the cells were incubated for additional 60 min. Control cells were treated with equal volume of Tyrode's buffer without MBP. After the reaction, the cells were centrifuged and the supernatant solution was collected and acidified with 2% perchloric acid (final concentration, 2%), whereas the pellet was resuspended in 2% perchloric acid and the pellet samples were placed in a boiling water bath for 10 min to release all remaining cellular histamine, following which these tubes were again centrifuged to remove denatured proteins. Histamine levels in the supernatant and pellet were measured by a spectrometer (LS-5B Luminescence Spectrometer, Perkin-Elmer, Norwalk, CT, USA) at excitation 365 nm and emission 460 nm, as reported earlier (Kempuraj et al., 2005). Then the percentage of histamine release was calculated (amount in the supernatant fluid/total × 100). Part of the supernatant fluid was used for tryptase assay (Kempuraj et al., 2005) by a fluoroenzyme immunoassay using the Unicap tryptase assay kit and the Unicap 100 automated instrument (Pharmacia & Upjohn, Uppsala, Sweden).
Effect of mast cell–Jurkat cell co-culture, activation and IL-2 release
Jurkat cells (5 × 104 cells per 200 μL per well) were plated in flat-bottom 96-well culture plates. These cells were pre-incubated with anti-CD3/anti-CD28 (1 μg mL−1 each) for 3 h and an equal number of hCBMCs was added either together with Jurkat cells (50:50) or separate, as required. MBP was then added and the cells were further incubated for 48 h at 37 °C. After 48 h of incubation, the plates were centrifuged and the supernatant was collected and stored at −80 °C until IL-2 assay by ELISA (Quantikine, R&D Systems). The minimum detectable level of IL-2 was 7 pg mL−1. Pancreatic cells (INS-1 rat insulinoma) were also used as a control. Jurkat cells were also pretreated with luteolin for 15 min before activation as shown in the Results section.
Effect of mast cell–Jurkat cell contact and IL-2 release
To investigate the importance of cell-to-cell contact, mast cells were cultured with Jurkat cells separated by a Transwell permeable membrane with 6.5 mm diameter and 0.4 μm pore size (Costar, Corning Life Sciences, Wilkes-Barre, PA, USA). Jurkat cells were added to the lower well and stimulated with anti-CD3/anti-CD28, whereas mast cells stimulated by MBP (10 μM) were added to the upper well. After 48 h of incubation, the supernatant fluid was collected and assayed for IL-2 levels by ELISA.
Effect of mast cell mediators and IL-2 release
hCBMCs (5 × 104 cells) were incubated with MBP (10 μM) for 24 h and the supernatant fluid was collected. Supernatant aliquots (100 μL) were then added to Jurkat cells (5 × 104 cells) in Jurkat cell culture medium for 48 h, and the supernatant fluid was collected and assayed for IL-2 release by ELISA. In another set of experiments, to study the influence of TNF-α on Jurkat cell activation and IL-2 release, Jurkat cells (5 × 104 cells per 200 μL per well) were pre-incubated with anti-human TNF-α (25 ng mL−1, Sigma, St Louis, MO, USA) for 1 h before stimulation with anti-CD3/anti-CD28. After 48 h, the supernatant fluid was collected and assayed for IL-2 level.
Statistics
Results are expressed as mean±s.d. and were individually compared either with control (for stimulation) or with the maximal stimulation (for inhibition) using the non-parametric Mann–Whitney U-test because it was not known if data would follow a normal distribution. However, they have also now been compared using paired Student's t-test. Significance is denoted by P<0.05. The percent inhibition by luteolin pretreatment was calculated after subtracting the spontaneous release value from the MBP-stimulated value and from the luteolin plus MBP treatment value. The percent inhibition was calculated with reference to MBP stimulation only.
Materials
MBP and luteolin were purchased from Sigma and were dissolved in sterile water and dimethyl sulphoxide, respectively, as 10−1 M stock concentrations. The maximum dimethyl sulphoxide concentration in the final working dilutions was <0.1%, and this concentration had no effect on the release of any of the mediators studied (results not presented). Luteolin did not affect the viability of either hCBMCs or Jurkat cells as determined by Trypan blue exclusion, for up to 24 h.
Cytokines and antibodies
Recombinant human stem cell factor was kindly provided by Amgen (Thousand Oaks, CA, USA). Anti-human IgE was purchased from DakoCytomation (Carpinteria, CA, USA). Human myeloma IgE, mouse anti-human mast cell tryptase monoclonal antibody as well as human IL-6 and IL-4 were purchased from Chemicon International Inc. Anti-human TNF-α was purchased from Sigma. Anti-human CD3 and anti-human CD28 were purchased from BD Pharmingen (Franklin Lakes, NJ, USA).
Results
Effect of MBP and luteolin on human mast cells
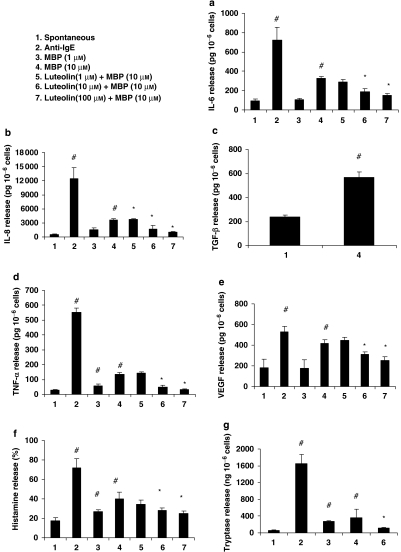
MBP at 10 μM increased (P<0.05) the release of IL-6 (Figure 1a), IL-8 (Figure 1b), TGF-β1 (Figure 1c), TNF-α (Figure 1d), VEGF (Figure 1e), histamine (Figure 1f) and tryptase (Figure 1g). Anti-IgE, used as a positive control, was more potent in increasing (P<0.05) the release of all mediators, compared with controls (Figure 1). Pre-incubation of hCBMCs for 15 min with luteolin (10 and 100 μM) significantly inhibited (P<0.05) MBP-stimulated release of IL-6 (62 and 81% inhibition), IL-8 (62% and 87% inhibition), TNF-α (80 and 98% inhibition), VEGF (46 and 70% inhibition) and histamine (52 and 66% inhibition); luteolin at 10 μM also inhibited tryptase release (81% inhibition). Neither anti-IgE stimulation nor luteolin inhibition was performed for TGF-β1 release.
Figure 1.
MBP stimulates hCBMCs to release increased amounts of inflammatory molecules: (a) IL-6 (n=6), (b) IL-8 (n=6), (c) TGF-β1 (n=3), (d) TNF-α (n=6), (e) VEGF (n=6), (f) histamine (n=6) and (g) tryptase (n=6) compared with control cells. hCBMCs were treated with MBP (1–10 μM) for 24 h at 37 °C in a CO2 incubator for IL-6, IL-8, TGF-β1, TNF-α and VEGF release, and 1 h at 37 °C in a shaking water bath for histamine and tryptase release. IL-6, IL-8, TGF-β1, TNF-α and VEGF were measured in the supernatant fluid using commercial ELISA kits. Histamine was measured fluorometrically and tryptase was measured by fluoroenzyme immunoassay. In some samples, the cells were pre-incubated with luteolin (1, 10 and 100 μM) for 15 min before stimulating hCBMCs. #P<0.05 compared with spontaneous release; *P<0.05 compared with release after MBP. hCBMCs, human umbilical cord blood-derived cultured mast cells; IL, interleukin; MBP, myelin basic protein; TGF-β1, transforming growth factor; TNF-α, tumour necrosis factor-α; VEGF, vascular endothelial growth factor.
Effect of mast cells on activated Jurkat cells
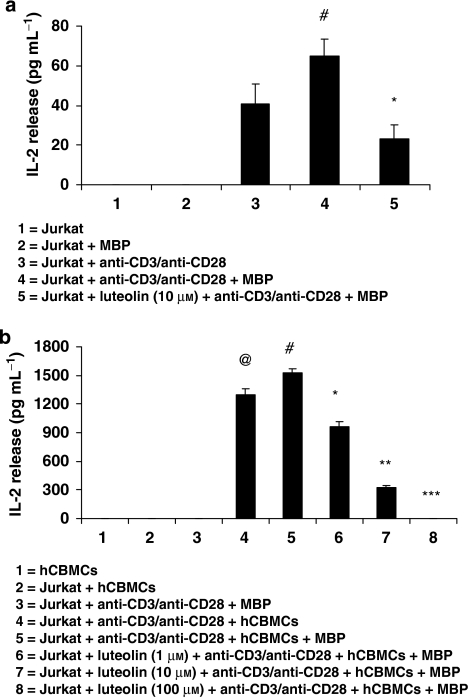
To investigate whether mast cells may affect T-cell activation, hCBMCs and Jurkat cells were co-cultured in 96-well plates and Jurkat cells were activated with anti-CD3/anti-CD28 (1 μg mL−1 each) where appropriate. Figure 2a shows the activation (as IL-2 release) of Jurkat cells alone and Figure 2b shows the activation of Jurkat cells with hCBMCs. Addition of MBP or hCBMCs to unstimulated Jurkat cells did not release any detectable IL-2 (Figures 2a and b). Jurkat cells stimulated by anti-CD3/anti-CD28 released significantly more IL-2 (P<0.05, Figure 2a), compared with control cells. Addition of MBP to activated Jurkat cells slightly increased IL-2 release, when compared with cells treated with only anti-CD3/anti-CD28 (Figure 2a). When mast cells were added to anti-CD3/anti-CD28-activated Jurkat cells, there was over 30-fold (P<0.05) increase in IL-2 release (Figure 2b); this amount of IL-2 was further significantly increased (P<0.05) when MBP (10 μM) was added to the co-cultured cells for 48 h (Figure 2b). There was no such effect when pancreatic cells (INS-1 rat insulinoma) were substituted for mast cells as control (results not shown). Pre-incubation of Jurkat cells for 15 min with luteolin at 1, 10 and 100 μM significantly inhibited (P<0.05) mast cells+MBP stimulated the release of IL-2 by 38, 78 and 100%, respectively (Figure 2b). Luteolin also inhibited (P<0.05) Jurkat cell activation by anti-CD3/anti-CD28 (Figure 2b).
Figure 2.
Mast cells and MBP enhance Jurkat cell activation. Jurkat cells were activated with anti-CD3/anti-CD28 (1 μg mL−1 each) and then incubated for 48 h with MBP or (a) without or (b) with equal number of hCBMCs in 96-well tissue culture plates at 37°C. In some samples, the cells were pre-incubated with luteolin (1, 10 and 100 μM) for 15 min before stimulating Jurkat cells or hCBMCs. After 48 h, IL-2 levels in the supernatant fluid were measured by ELISA (n=3). (a) #,*P<0.05; #=compared with Jurkat+anti-CD3/anti-CD28, *=compared with Jurkat+anti-CD3/anti-CD28+MBP; (b) @,#,*,**,***P<0.05; @=compared with Jurkat+hCBMCs, #=compared with Jurkat+anti-CD3/anti-CD28+hCBMCs, *,**,***=compared with Jurkat Jurkat+anti-CD3/anti-CD28+hCBMCs+MBP. hCBMCs, human umbilical cord blood-derived cultured mast cells; MBP, myelin basic protein.
Effect of mast cell–Jurkat cell contact on IL-2 release
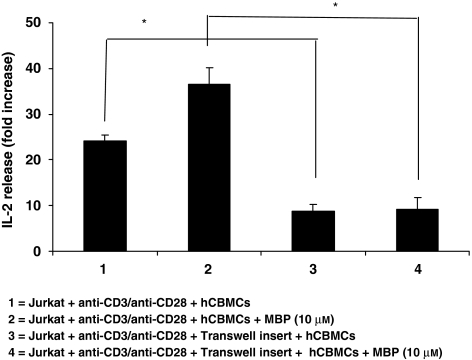
We first investigated the importance of cell-to-cell contact. Mast cells were, therefore, cultured with activated Jurkat cells separated by a Transwell permeable membrane. Under these conditions, IL-2 release from activated Jurkat cells was reduced by about 60% (Figure 3, P<0.05). In this set of experiments, the IL-2 released by activated Jurkat cells in the presence of hCBMCs was less probably because these Jurkat cells had undergone more passages during culture and released less IL-2. To normalize these data, they are presented as fold change from control; it should be noted that the IL-2 increase in these co-cultures (without the Transwell membrane) was about 25-fold (as compared with the 30-fold seen in Figure 2b).
Figure 3.
Effect of cell–cell contact on Jurkat cell activation. Mast cells were co-cultured with Jurkat cells or separated by a Transwell permeable membrane (n=6). Jurkat cells were placed in the lower well and activated with anti-CD3/anti-CD28; the transwell membrane was then inserted and an equal number of mast cells were added to the upper well and activated with MBP (10 μM). After 48 h of incubation, the supernatant fluid was collected and assayed for IL-2 levels (*P<0.05 for groups compared with as shown in parentheses). MBP, myelin basic protein.
Effect of mast cell mediators on Jurkat cell IL-2 release
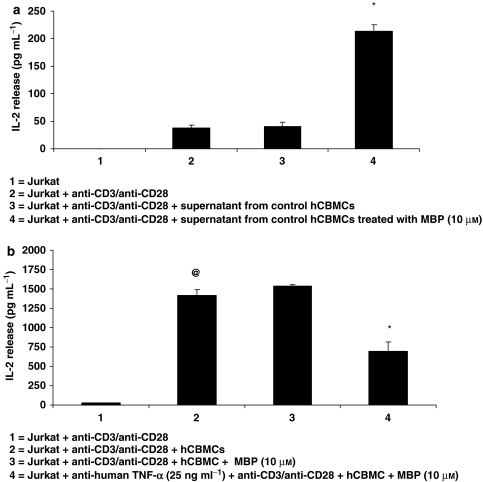
The supernatant fluid from hCBMCs stimulated with MBP (10 μM) for 24 h was collected and then added to Jurkat cells for 48 h. This supernatant fluid increased IL-2 release (Figure 4a, P<0.05). As TNF has been implicated in MS, in another set of experiments, a neutralizing anti-human TNF-α antibody was added for 1 h to the Jurkat cell–mast cell co-culture prior to stimulation with MBP. This treatment reduced (55% inhibition) IL-2 release from the MBP-stimulated mast cell-activated Jurkat cell co-culture (Figure 4b, P<0.05).
Figure 4.
Effect of mast cell mediators on Jurkat cell activation. Jurkat cells were also incubated with (a) supernatant fluid collected from mast cells treated with MBP (10 μM) and IL-2 release was measured in the supernatant fluid by ELISA (n=3); (b) with TNF-neutralizing antibody (n=3) for 1 h before activation with anti-CD3/anti-CD28 and the addition of mast cells with or without MBP. After 48 h, the supernatant fluid was collected and assayed for IL-2 level by ELISA. (a) *P<0.05 compared with condition 3; (b) @,*P<0.05, @=compared with condition 1, *=compared with condition 3.
Effect of Jurkat cells on mast cell activation
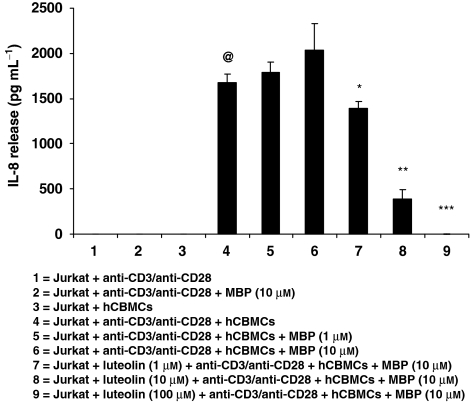
We also investigated whether Jurkat cells could affect mast cell activation (n=3). Mast cells released significantly higher amount of IL-8 (P<0.05) when they were co-cultured with activated Jurkat cells (Figure 5). Addition of MBP (10 μM) to mast cells for 48 h further increased IL-8 release (P<0.005). This IL-8 release was significantly reduced (P<0.05) when Jurkat cells and mast cells were pretreated before activation for 15 min with luteolin at concentrations of 1 μM (35% inhibition), 10 μM (83% inhibition) and 100 μM (100% inhibition) (Figure 5).
Figure 5.
IL-8 release from hCBMCs co-cultured with Jurkat cells. hCBMCs were incubated with Jurkat cells either with or without MBP or anti-CD3/anti-CD28 alone (n=3). After 48 h, IL-8 levels in the supernatant fluid were measured by ELISA. @,*,**,***P<0.05, @=compared with condition 3; *,**,***=compared with condition 6.
Discussion
Here, we show that MBP stimulated human mast cells to release the pro-inflammatory mediators, IL-6, IL-8, TGF-β1, TNF-α, VEGF, histamine and tryptase. The amounts released were less than after allergic stimulation. MBP and myelin fragment P2 had been previously shown to induce rat mast cell degranulation (Johnson et al., 1988; Theoharides et al., 1993), and led to homogeneic brain demyelination, but at millimolar concentrations (Theoharides et al., 1993). The MBP concentration stimulating human cultured mast cells were still higher than the levels expected to be present in CSF of MS patients (Sellebjerg et al., 1998; Ohta and Ohta, 2002). However, brain mast cells may respond differently to MBP compared with the fairly undifferentiated human cultured mast cells.
We also showed that activated Jurkat cells were stimulated by normal human cultured mast cells through direct contact, as well as through TNF-α released from mast cells in response to MBP. We present the IL-2 release results in Figure 3 as fold change because there is decreased IL-2 production from those Jurkat cells, as explained in the Results section. Even though the absolute amounts of IL-2 released is lower, the addition of hCBMCs to activated Jurkat cells still releases about 25 times, compared with 30 times in the other experiments, more IL-2 than Jurkat cells alone. Activated Jurkat cells also induced mast cell release of IL-8. Luteolin inhibits MBP-induced mast cell mediator release, as well as IL-2 release from Jurkat cells, whether activated only by anti-CD3/anti-CD28 or by mast cells. Our finding of the importance of cell-to-cell contact is supported by previous results using rodent mast cells. Soluble TNF increased the surface expression of OX40, ICOS, PD-1 and other co-stimulatory molecules on CD3+ T cells (Nakae et al., 2005). Expression of mast cell co-stimulatory molecules, OX40 ligand and 4-1BB ligand, was enhanced by IgE activation and promoted T-cell activation through cell–cell contact (Gregory et al., 2006). Co-culture of leukaemic mast cells with activated but not resting T cells promoted marked adhesion of mast cells to vascular adhesion molecule-1 (Brill et al., 2004).
Luteolin effectively inhibited MBP-induced mast cell mediator release at 10 and 100 μM, whereas it exhibited a dose-dependent inhibition (1–100 μM) of mast cell-induced IL-2 release. Flavonoids comprise a group of polyphenolic compounds naturally occurring in fruits, vegetables, nuts, seeds, herbs, spices and red wine with antioxidant properties (Middleton et al., 2000). Flavonoids are potent scavengers of reactive oxygen species that also has a prominent function in MS (Lu et al., 2000) and EAE (Ruuls et al., 1995). Luteolin, a flavone analogue of quercetin, inhibits IgE-mediated release of histamine, leukotrienes, prostaglandin D2 and granulocyte-macrophage colony-stimulating factor from hCBMCs (Kimata et al., 2000). Luteolin also reduces inflammation and axonal damage in the CNS by preventing monocyte migration across the brain endothelium (Hendriks et al., 2004) and inhibits clinical symptoms of EAE through the inhibition of macrophage myelin phagocytosis (Hendriks et al., 2004). Luteolin also inhibits in vitro antigen-specific proliferation and interferon-γ production by murine and human autoimmune T cells (Verbeek et al., 2004). Regarding its mechanism of action, luteolin may inhibit mast cell activation through the inhibition of Ca2+ influx and PKC activation, as histamine and cytokines production are regulated by intracellular Ca2+ levels (Kimata et al., 2000). It was also shown that quercetin inhibited the activation of PKC-θ involved in IL-1-induced IL-6 release from hCBMCs (Kempuraj et al., 2005). Flavonoids, including luteolin, also inhibit 15-lipoxygenase-1, which may contribute to their antioxidant and anti-inflammatory activity (Sadik et al., 2003).
Mast cells secrete a wide variety of potent chemical mediators that can initiate and modulate several inflammatory pathways (Galli et al., 2005; Theoharides and Kalogeromitros, 2006), including T-cell activation (Mekori and Metcalfe, 2000). In fact, EAE, an animal model of MS, was reduced and delayed in mast cell-deficient W/Wv mice (Brown et al., 2002) and mast cells were required for optimal T-cell responses in this model (Gregory et al., 2005). Mast cells stimulated by FcɛRI aggregation release TNF-α (Nakae et al., 2006) and could activate T cells (Bongioanni et al., 2000a; Gregory et al., 2006). Mast cells represent a major potential source of TNF-α, which influences T-cell recruitment and activation (Tartaglia et al., 1993) in MS. Moreover, mast cell-derived TNF-α can promote neutrophil recruitment (Nakae et al., 2007). Mast cell-derived histamine and TNF-α increase microvascular permeability, leukocyte rolling and adhesion, thus contributing to the infiltration of T cells and monocytes into the CNS in MS.
The sites crucial in antigen entry are trafficked by CD4+ T cells. Mast cells can interact with T cells to amplify the magnitude of immune responses elicited in sensitized hosts at sites of antigen challenge. In terms of MS, the most likely place for contact, especially because mast cells do not circulate like T cells, would be at the BBB around which mast cells are critically located (Theoharides et al., 1993). In the CNS, mast cells are mainly found in the leptomeninges, choroid plexus and the median eminence (Silver et al., 1996), In fact, the mast cell had been proposed to act as ‘the immune gate to the brain' (Theoharides, 1990), by regulating the permeability of the BBB (Esposito et al., 2001), through the activation of corticotrophin-releasing factor receptors (Esposito et al., 2002). Disruption of the BBB precedes any clinical or pathological signs of MS (Stone et al., 1995; Minagar and Alexander, 2003). Mast cells express corticotrophin-releasing factor receptors, activation of which leads to selective release of VEGF (Cao et al., 2005). This fact led to the premise that corticotrophin-releasing factor released under stress regulates BBB permeability (Theoharides and Konstantinidou, 2007). In this study, we report that MBP induces VEGF release from mast cells. VEGF was upregulated in MS plaques (Prescholdt et al., 2002) and in serum of MS patients (Su et al., 2006). In fact, serum levels of VEGF correlated with disease activity in autoimmune diseases (Carvalho et al., 2007).
We also showed that MBP induced histamine and tryptase release. CSF levels of histamine were elevated in MS patients with remitting and progressive disease (Tuomisto et al., 1983). Mast cell proteases are potent myelinolytic agents (Dietsch and Hinrichs, 1991) and can cause direct myelin damage (Johnson et al., 1988). Tryptase is elevated in CSF of MS patients (Rozniecki et al., 1995), and could activate peripheral blood mononuclear cells isolated from MS patients to synthesize and release IL-1β, IL-6 and TNF-α (Malamud et al., 2003), both involved in the pathogenesis of MS. Tryptase could also activate protease-activated receptor-2, leading to widespread inflammation (Malamud et al., 2003). MBP also induces TGF-β release from mast cells. TGF-β1 and TGF-β3 were present in leukocytes found in active MS lesions (De Groot et al., 1999). Mast cell-derived IL-6 and TGF-β1 could participate in maturation/proliferation of Th17 cells, recently shown to be critical in MS and EAE (Weaver et al., 2006). IL-6 is upregulated in MS patients (Bongioanni et al., 2000b), and T cells from MS patients expressed significantly more IL-6 and TNF-α receptors compared with healthy controls (Bongioanni et al., 2000b). It is of interest that human mast cells release IL-6 selectively, without degranulation in response to IL-1 (Kandere-Grzybowska et al., 2003). Critical to mast cell involvement in MS is the mast cell's ability to secrete some mediators selectively without degranulation (Cao et al., 2005), as also suggested by ultrastructural observations in monkey demyelination (Letourneau et al., 2003).
We report here that MBP released high amounts of IL-8 from mast cells that are also stimulated to release IL-8 by Jurkat cells. IL-8 is elevated in serum and CSF of MS patients (Lund et al., 2004), and is a chemoattractant for neutrophils and monocytes triggering their adhesion to the endothelium (Salamon et al., 2005). Moreover, there is elevated expression of the IL-8 receptors CXCR1 and CXCR2 on macrophages, astrocytes, microglia and oligodendrocytes in MS lesions (Muller-Ladner et al., 1996). CSF IL-8 decreased when relapsing–remitting MS patients were treated with cladribine (Bartosik-Psujek et al., 2004); moreover, high doses of glucocorticoids decreased IL-8 production by monocytes in MS patients during relapse (Mirowska-Guzel et al., 2006). Interferon-β used to treat MS is also associated with the reduction of serum IL-8 levels (Lund et al., 2004).
There are no curative therapies presently available for MS (Fox and Ransohoff, 2004). Mast cells could serve as a therapeutic target for MS (Zappulla et al., 2002). The ability of the naturally occurring flavonoid luteolin to inhibit the processes described therein suggests that it may be useful in MS, alone or in combination with other treatments.
Acknowledgments
This work was supported in part by Theta Biomedical Consulting and Development Co., Inc. (Brookline, MA, USA). We thank Amgen (Thousand Oaks, CA, USA) for their generous gift of hrSCF and Ms Jessica Christian for her patience and word processing skills.
Abbreviations
- BBB
blood–brain barrier
- EAE
experimental autoimmune encephalomyelitis
- hCBMCs
human umbilical cord blood-derived cultured mast cells
- IL
interleukin
- MBP
myelin basic protein
- MS
multiple sclerosis
- TGF-β
transforming growth factor-β
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
Conflict of interest
TC Theoharides has filed an US patent application on the use of flavonoids, alone or together with interferon-β, for the treatment of MS.
References
- Bartosik-Psujek H, Belniak E, Mitosek-Szewcryk K, Dobosz B, Stelmasiak Z. Interleukin-8 and RANTES levels in patients with relapsing–remitting multiple sclerosis (RR-MS) treated with cladribine. Acta Neurol Scand. 2004;109:390–392. doi: 10.1111/j.1600-0404.2004.00259.x. [DOI] [PubMed] [Google Scholar]
- Benoist C, Mathis D. Mast cells in autoimmune disease. Nature. 2002;420:875–878. doi: 10.1038/nature01324. [DOI] [PubMed] [Google Scholar]
- Bongioanni P, Lombardo F, Moscato G, Mosti S, Meucci G. T-cell interleukin-6 receptor binding in interferon-b-1b-treated multiple sclerosis patients. Eur J Neurol. 2000a;7:647–653. doi: 10.1046/j.1468-1331.2000.00139.x. [DOI] [PubMed] [Google Scholar]
- Bongioanni P, Mosti S, Romano MR, Lombardo F, Moscato G, Meucci G. Increased T-lymphocyte interleukin-6 binding in patients with multiple sclerosis. Eur J Neurol. 2000b;7:291–297. doi: 10.1046/j.1468-1331.2000.00075.x. [DOI] [PubMed] [Google Scholar]
- Brill A, Baram D, Sela U, Salamon P, Mekori YA, Hershkoviz R. Induction of mast cell interactions with blood vessel wall components by direct contact with intact T cells or T cell membranes in vitro. Clin Exp Allergy. 2004;34:1725–1731. doi: 10.1111/j.1365-2222.2004.02093.x. [DOI] [PubMed] [Google Scholar]
- Brown MA, Tanzola M, Robbie-Ryan M. Mechanisms underlying mast cell influence on EAE disease course. Mol Immunol. 2002;38:1373–1378. doi: 10.1016/s0161-5890(02)00091-3. [DOI] [PubMed] [Google Scholar]
- Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- Carvalho JF, Blank M, Shoenfeld Y. Vascular endothelial growth factor (VEGF) in autoimmune diseases. J Clin Immunol. 2007;27:246–256. doi: 10.1007/s10875-007-9083-1. [DOI] [PubMed] [Google Scholar]
- De Groot CJ, Montagne L, Barten AD, Sminia P, Van der Valk P. Expression of transforming growth factor (TGF)-beta1, -beta2, and -beta3 isoforms and TGF-beta type 1 and II receptors in multiple sclerosis lesions and human adult astrocyte cultures. J Neuropathol Exp Neurol. 1999;58:174–187. doi: 10.1097/00005072-199902000-00007. [DOI] [PubMed] [Google Scholar]
- Dietsch GN, Hinrichs DJ. Mast cell proteases liberate stable encephalitogenic fragments from intact myelin. Cell Immunol. 1991;135:541–548. doi: 10.1016/0008-8749(91)90297-o. [DOI] [PubMed] [Google Scholar]
- Esposito P, Chandler N, Kandere-Grzybowska K, Basu S, Jacobson S, Connolly R, et al. Corticotropin-releasing hormone (CRH) and brain mast cells regulate blood–brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002;303:1061–1066. doi: 10.1124/jpet.102.038497. [DOI] [PubMed] [Google Scholar]
- Esposito P, Gheorghe D, Kandere K, Pang X, Conally R, Jacobson S, et al. Acute stress increases permeability of the blood–brain-barrier through activation of brain mast cells. Brain Res. 2001;888:117–127. doi: 10.1016/s0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- Fox RJ, Ransohoff RM. New directions in MS therapeutics: vehicles of hope. Trends Immunol. 2004;25:632–636. doi: 10.1016/j.it.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke M, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Eng J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- Gregory GD, Raju SS, Winandy S, Brown MA. Mast cell IL-4 expression is regulated by Ikaros and influences encephalitogenic Th1 responses in EAE. J Clin Invest. 2006;116:1327–1336. doi: 10.1172/JCI27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory GD, Robbie-Ryan M, Secor VH, Sabatino JJ, Jr, Brown MA. Mast cells are required for optimal autoreactive T cell responses in a murine model of multiple sclerosis. Eur J Immunol. 2005;35:3478–3486. doi: 10.1002/eji.200535271. [DOI] [PubMed] [Google Scholar]
- Hendriks JJ, Alblas J, van der Pol SM, van Tol EA, Dijkstra CD, de Vries HE. Flavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitis. J Exp Med. 2004;200:1667–1672. doi: 10.1084/jem.20040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MZ, Reder AT, Lawand R, Takash W, Sallouh-Khatib S. The mast cells of the multiple sclerosis brain. J Neuroimmunol. 1996;70:131–138. doi: 10.1016/s0165-5728(96)00102-6. [DOI] [PubMed] [Google Scholar]
- Johnson D, Seeldrayers PA, Weiner HL. The role of mast cells in demyelination. 1. Myelin proteins are degraded by mast cell proteases and myelin basic protein and P2 can stimulate mast cell degranulation. Brain Res. 1988;444:195–198. doi: 10.1016/0006-8993(88)90929-8. [DOI] [PubMed] [Google Scholar]
- Kandere-Grzybowska K, Letourneau R, Kempuraj D, Donelan J, Poplawski S, Boucher W, et al. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J Immunol. 2003;171:4830–4836. doi: 10.4049/jimmunol.171.9.4830. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Madhappan B, Christodoulou S, Boucher W, Cao J, Papadopoulou N, et al. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol. 2005;145:934–944. doi: 10.1038/sj.bjp.0706246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempuraj D, Saito H, Kaneko A, Fukagawa K, Nakayama M, Toru H, et al. Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999;93:3338–3346. [PubMed] [Google Scholar]
- Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy. 2000;30:501–508. doi: 10.1046/j.1365-2222.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- Krüger PG. Mast cells and multiple sclerosis: a quantitative analysis. Neuropathol Appl Neurobiol. 2001;27:275–280. doi: 10.1046/j.0305-1846.2001.00331.x. [DOI] [PubMed] [Google Scholar]
- Kumazawa Y, Kawaguchi K, Takimoto H. Immunomodulating effects of flavonoids on acute and chronic inflammatory responses caused by tumor necrosis factor alpha. Curr Pharm Des. 2006;12:4271–4279. doi: 10.2174/138161206778743565. [DOI] [PubMed] [Google Scholar]
- Letourneau R, Rozniecki JJ, Dimitriadou V, Theoharides TC. Ultrastructural evidence of brain mast cell activation without degranulation in monkey experimental allergic encephalomyelitis. J Neuroimmunol. 2003;145:18–26. doi: 10.1016/j.jneuroim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Lu F, Selak M, O'Connor J, Croul S, Lorenzana C, Butunoi C, et al. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci. 2000;177:95–103. doi: 10.1016/s0022-510x(00)00343-9. [DOI] [PubMed] [Google Scholar]
- Lund BT, Ashikian N, Ta HQ, Chakryan Y, Manoukian K, Groshen S, et al. Increased CXCL8 (IL-8) expression in multiple sclerosis. J Neuroimmunol. 2004;155:161–171. doi: 10.1016/j.jneuroim.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Malamud V, Vaaknin A, Abramsky O, Mor M, Burgess LE, Ben-Yehudah A, et al. Tryptase activates peripheral blood mononuclear cells causing the synthesis and release of TNF-alpha, IL-6 and IL-1 beta: possible relevance to multiple sclerosis. J Neuroimmunol. 2003;138:115–122. doi: 10.1016/s0165-5728(03)00090-0. [DOI] [PubMed] [Google Scholar]
- Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Minagar A, Alexander JS. Blood–brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- Mirowska-Guzel DM, Kurowska K, Skierski J, Koronkiewicz M, Wicha W, Kruszewski J, et al. High dose of intravenously given glucocorticoids decrease IL-8 production by monocytes in multiple sclerosis patients treated during relapse. J Neuroimmunol. 2006;176:134–140. doi: 10.1016/j.jneuroim.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, et al. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- Muller-Ladner U, Jones JL, Wetsel RA, Gay S, Raine CS, Barnum SR. Enhanced expression of chemotactic receptors in multiple sclerosis. J Neurol Sci. 1996;144:135–141. doi: 10.1016/s0022-510x(96)00217-1. [DOI] [PubMed] [Google Scholar]
- Nakae S, Suto H, Berry GJ, Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;109:3640–3648. doi: 10.1182/blood-2006-09-046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci USA. 2005;102:6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Eng J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Ohta M, Ohta K. Detection of myelin basic protein in cerebrospinal fluid. Expert Rev Mol Diagn. 2002;2:627–633. doi: 10.1586/14737159.2.6.627. [DOI] [PubMed] [Google Scholar]
- Pedotti R, De Voss JJ, Steinman L, Galli SJ. Involvement of both ‘allergic' and ‘autoimmune' mechanisms in EAE, MS and other autoimmune diseases. Trends Immunol. 2003;24:479–484. doi: 10.1016/s1471-4906(03)00233-3. [DOI] [PubMed] [Google Scholar]
- Prescholdt MA, Jacobson S, Tresser N, Oldfield EH, Merill MJ. Vascular endothelial growth factor is expressed in multiple sclerosis plaques and can induce inflammatory lesions in experimental allergic encephalomyelitis rats. J Neuropathol Exp Neurol. 2002;61:914–925. doi: 10.1093/jnen/61.10.914. [DOI] [PubMed] [Google Scholar]
- Rozniecki JJ, Hauser SL, Stein M, Lincoln R, Theoharides TC. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- Ruuls SR, Bauer J, Sontrop K, Huitinga I, 't Hart BA, Dijkstra CD. Reactive oxygen species are involved in the pathogenesis of experimental allergic encephalomyelitis in Lewis rats. J Neuroimmunol. 1995;56:207–217. doi: 10.1016/0165-5728(94)00154-g. [DOI] [PubMed] [Google Scholar]
- Sadik CD, Sies H, Schewe T. Inhibition of 15-lipoxygenases by flavonoids: structure–activity relations and mode of action. Biochem Pharmacol. 2003;65:773–781. doi: 10.1016/s0006-2952(02)01621-0. [DOI] [PubMed] [Google Scholar]
- Salamon P, Shoham NG, Gavrieli R, Wolach B, Mekori YA. Human mast cells release interleukin-8 and induce neutrophil chemotaxis on contact with activated T cells. Allergy. 2005;60:1316–1319. doi: 10.1111/j.1398-9995.2005.00886.x. [DOI] [PubMed] [Google Scholar]
- Sellebjerg F, Christiansen M, Nielsen PM, Frederiksen JL. Cerebrospinal fluid measures of disease activity in patients with multiple sclerosis. Mult Scler. 1998;4:475–479. doi: 10.1177/135245859800400603. [DOI] [PubMed] [Google Scholar]
- Silver R, Silverman A-J, Vitkovic L, Lederhendler II. Mast cells in the brain: evidence and functional significance. Trends Neurosci. 1996;19:25–31. doi: 10.1016/0166-2236(96)81863-7. [DOI] [PubMed] [Google Scholar]
- Stone LA, Smith ME, Albert PS, Bash CN, Maloni H, Frank JA, et al. Blood–brain barrier disruption on contrast-enhanced MRI in patients with mild relapsing–remitting multiple sclerosis: relationship to course, gender, and age. Neurology. 1995;45:1122–1126. doi: 10.1212/wnl.45.6.1122. [DOI] [PubMed] [Google Scholar]
- Su JJ, Osoegawa M, Matsuoka T, Minohara M, Tanaka M, Ishizu T, et al. Upregulation of vascular growth factors in multiple sclerosis: correlation with MRI findings. J Neurol Sci. 2006;243:21–30. doi: 10.1016/j.jns.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Goeddel DV, Reynolds C, Figari IS, Weber RF, Fendly BM, et al. Stimulation of human T-cell proliferation by specific activation of the 75-kDa tumor necrosis factor receptor. J Immunol. 1993;151:4637–4641. [PubMed] [Google Scholar]
- Theoharides TC. Mast cells: the immune gate to the brain. Life Sci. 1990;46:607–617. doi: 10.1016/0024-3205(90)90129-f. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Dimitriadou V, Letourneau RJ, Rozniecki JJ, Vliagoftis H, Boucher WS. Synergistic action of estradiol and myelin basic protein on mast cell secretion and brain demyelination: changes resembling early stages of demyelination. Neuroscience. 1993;57:861–871. doi: 10.1016/0306-4522(93)90030-j. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Kalogeromitros D. The critical role of mast cell in allergy and inflammation. Ann NY Acad Sci. 2006;1088:78–99. doi: 10.1196/annals.1366.025. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Konstantinidou A. Corticotropin-releasing hormone and the blood–brain-barrier. Front Biosci. 2007;12:1615–1628. doi: 10.2741/2174. [DOI] [PubMed] [Google Scholar]
- Tuomisto L, Kilpelainen H, Riekkinen P. Histamine and histamine-N-methyltransferase in the CSF of patients with multiple sclerosis. Agents Action. 1983;13:255–257. doi: 10.1007/BF01967346. [DOI] [PubMed] [Google Scholar]
- Verbeek R, Plomp AC, van Tol EA, van Noort JM. The flavones luteolin and apigenin inhibit in vitro antigen-specific proliferation and interferon-gamma production by murine and human autoimmune T cells. Biochem Pharmacol. 2004;68:621–629. doi: 10.1016/j.bcp.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Zappulla JP, Arock M, Mars LT, Liblau RS. Mast cells: new targets for multiple sclerosis therapy. J Neuroimmunol. 2002;131:5–20. doi: 10.1016/s0165-5728(02)00250-3. [DOI] [PubMed] [Google Scholar]