Abstract
Background and purpose:
Calcitonin gene-related peptide (CGRP) receptor antagonists effectively abort migraine headache and inhibit neurogenic vasodilatation in humans as well as rat models. Monoclonal antibodies typically have long half-lives, and we investigated whether or not function-blocking CGRP antibodies would inhibit neurogenic vasodilatation with a long duration of action and therefore be a possible approach to preventive therapy of migraine. During chronic treatment with anti-CGRP antibodies, we measured cardiovascular function, which might be a safety concern of CGRP inhibition.
Experimental approach:
We used two rat blood flow models that measure electrically stimulated vasodilatation in the skin or in the middle meningeal artery (MMA). These vasomotor responses are largely dependent on the neurogenic release of CGRP from sensory afferents. To assess cardiovascular function during chronic systemic anti-CGRP antibody treatment, we measured heart rate and blood pressure in conscious rats.
Key results:
Treatment with anti-CGRP antibodies inhibited skin vasodilatation or the increase in MMA diameter to a similar magnitude as treatment with CGRP receptor antagonists. Although CGRP antibody treatment had a slower onset of action than the CGRP receptor antagonists, the inhibition was still evident 1 week after dosing. Chronic treatment with anti-CGRP antibodies had no detectable effects on heart rate or blood pressure.
Conclusions and implications:
We showed for the first time that anti-CGRP antibodies exert a long lasting inhibition of neurogenic vasodilatation in two different rat models of arterial blood flow. We have provided strong preclinical evidence that anti-CGRP antibody may be a suitable drug candidate for the preventive treatment of migraine.
Keywords: antibody, CGRP, headache, migraine, neuropeptide, vasodilatation
Introduction
Calctonin gene-related peptide (CGRP) is a vasoactive neuropeptide and a key mediator in migraine headache (Arulmani et al., 2004). CGRP occurs in two isoforms, αCGRP (Amara et al., 1982; Rosenfeld et al., 1983) and βCGRP (Amara et al., 1985), and is expressed in the central and peripheral nervous system where it is localized in the majority of small- and medium-sized sensory afferents, including perivascular trigeminal afferents (Edvinsson et al., 1987b; Uddman et al., 1986, 1989). Upon afferent stimulation, CGRP is released from sensory nerve terminals (Holzer, 1998) contributing to neurogenic effects such as vasodilatation (Peroutka, 2005) and nociceptive transmission (Storer et al., 2004). The functional system between trigeminal afferents and intracranial blood vessels has been termed the trigeminovascular system (Buzzi and Moskowitz, 1992).
Many lines of evidence suggest that, as well as dilating vessels, CGRP is involved in activating the trigeminovascular system in rats (Cumberbatch et al., 1999) and humans (Lassen et al., 2002; Petersen et al., 2005). In particular, the trigeminovascular system of migraineurs is more sensitive to exogenous CGRP (Lassen et al., 2002), and that during migraine and cluster headache attacks, CGRP is increased in the venous outflow from the head, suggesting an endogenous source of CGRP (Goadsby et al., 1990; Goadsby and Edvinsson, 1994). This increased concentration of CGRP is normalized upon successful sumatriptan (5-HT1B/5-HT1D agonist) treatment of migraine symptoms (Edvinsson and Goadsby, 1994). A very important piece of evidence of the relevance of CGRP mechanisms in migraine has come from two-phase two clinical trials using different CGRP1 receptor antagonists, which showed these compounds to effectively relieve the pain of migraine in patients (Durham, 2004; Olesen et al., 2004; Ho et al., 2008). The combination of these results suggests that CGRP has an important function in migraine.
We sought to discover if CGRP function-blocking antibodies could exert an effect on physiological CGRP mechanisms by using vasodilatation as an indicator of endogenous CGRP effects. A number of studies have been performed to investigate the vasoactive effects of CGRP. Exogenous CGRP is a potent vasodilator of cranial blood vessels (Edvinsson et al., 1987a). Endogenous CGRP released from sensory afferents in their innervation target zones also causes vasodilatation. Electrical stimulation of the saphenous nerve leads to locally increased blood flow in the skin of the dorsal medial part of the rat hind paw (Escott and Brain, 1993; Tan et al., 1995). In addition, this blood flow increase can be blocked with the CGRP receptor antagonist CGRP-(8-37) (Escott and Brain, 1993) or anti-CGRP antibody Fab fragments (antigen binding fragment) (Tan et al., 1995). These investigators were unable to demonstrate any effect of the IgG form of an anti-CGRP antibody on blood flow. A CGRP-dependent vasodilator response was also demonstrated more directly in a series of experiments by Williamson et al., 1997a; Williamson and Hargreaves, 2001a). In this dural blood flow model, vasodilatation of a branch of the middle meningeal artery (MMA) can be observed visually in response to electrical field stimulation and this vasodilatation response was shown to be largely dependent on CGRP signalling (Williamson et al., 1997b). Several drugs that effectively abort or prevent migraine symptoms in humans also inhibit blood flow increase in the same or similar dural blood flow models (Williamson et al., 1997b, 2001b; Petersen et al., 2004; Akerman and Goadsby, 2005). Hence, the blood flow increases after neurogenic stimulation (Williamson and Hargreaves, 2001a) can be used as an indicator for CGRP-mediated effects and these effects can have implications for migraine therapy.
Given that CGRP is a potent vasodilator, many of the effects of CGRP are also associated with the cardiovascular system (Brain and Grant, 2004). In rats (Ando et al., 1990) and humans (Lassen et al., 2002), intravenous administration of CGRP induces hypotension and is associated with decreased blood pressure and increased heart rate. Although intravenous injection of the CGRP1 receptor antagonist BIBN4096BS into healthy pigs (Kapoor et al., 2003), primates (Doods et al., 2000) or humans (Olesen et al., 2004; Petersen et al., 2005) did not affect the heart rate or blood pressure, the long-term inhibition of CGRP, due to the long half-life of a function-blocking antibody in vivo, potentially bears a risk of cardiovascular effects.
In this study, we used a skin and a dural vasodilatation model in the rat to test the hypothesis that systemic treatment with two different CGRP function-blocking antibodies will result in reduced blood flow increases after electrical stimulation. Secondly, we used the skin vasodilatation model to test the hypothesis that a single dose of a CGRP function-blocking antibody will result in a long-lasting inhibition of blood flow increases after electrical stimulation. Thirdly, we used a telemetric rat model to test the hypothesis that chronic administration of a CGRP function-blocking antibody will not lead to a change in weight gain, motor activity, heart rate or blood pressure. Together our data provide strong preclinical evidence supporting the use of CGRP function-blocking antibodies for the preventive treatment of vascular headaches including migraine.
Methods
Animal handling
All protocols involving animal handling were reviewed and approved by an ethics committee according to IACUC guidelines.
Saphenous nerve assay
Experiments were carried out as described earlier (Escott and Brain, 1993) with the following modifications. Sprague Dawley rats (200–400 g) were anaesthetized with 2% isoflurane and treated with bretylium tosylate (10 mg kg−1, i.v.) to block sympathetic activity. The saphenous nerve of the left hind limb was exposed surgically, cut proximally and placed over platinum bipolar electrodes for stimulation. In between stimulations, the nerve was removed from the electrodes and was covered with plastic wrap to prevent it from drying. Skin blood flow was measured on the mediodorsal side of the hind paw using a skin probe connected to a laser Doppler flow metre. After a stable baseline flux (less than 5% variation) had been established, the distal end of the saphenous nerve was electrically stimulated with 60 pulses (2 Hz, 10 V, 1 ms, for 30 s) and repeated at 30 min intervals. All data were recorded using chart software. All compounds and controls were injected intravenously through the right femoral vein except in animals injected 1 and 7 days prior to nerve stimulation where test compounds were injected through the tail vein. CGRP-(8-37) (400 nmol kg−1, 1520 μg kg−1), a CGRP receptor antagonist, was injected 3–5 min before nerve stimulation in one group as a positive control for blocking CGRP-induced vasodilatation. Intra-group comparison was performed for each group using a one-way ANOVA (time) with repeated measures at each time point, followed by Dunnett's multiple comparison test in case of significant time effect to compare each time value with the T0 value (Figures 2a and b). Inter-group comparison was performed with a non-parametric Kruskal–Wallis test followed by Dunn's multiple comparison test (Figures 2c).
Closed cranial window model
Experiments were carried out as described earlier (Williamson et al., 1997a, 1997b) with the following modifications: Sprague Dawley rats (300–400 g) were anaesthetized with pentobarbital (70 mg kg−1, i.p.). Anaesthesia was maintained with i.v. pentobarbital (20 mg kg−1 h−1). The rats were tracheotomized and breathing rate was maintained at 75 breaths per minute at a volume of 3.5 mL. The jugular vein of each rat was cannulated for delivery of all drugs. Blood pressure was monitored with a probe (Mikro-Tip catheter) threaded through the femoral artery into the abdominal aorta. After the head had been immobilized in a stereotactic apparatus, an incision along the midline was made and the skull was exposed. A 2 × 6 mm window in the left parietal area just lateral to the sagittal suture was made by thinning the bone with a dental drill and a 0.9 mm steel burr bit. Using a micromanipulator, a platinum bipolar electrode was lowered onto the surface and covered with heavy mineral oil. Lateral to the electrode window another window of 5 × 6 mm was created and filled with heavy mineral oil through which the diameter of a branch of the MMA was continuously monitored with a charge coupled device camera and a video dimension analyser. The rats were rested for no less than 45 min after the preparation. Stimulations of 15 V, 10 Hz, 0.5 ms and 30 s were performed every 30 min. All data were recorded using chart software. Intra-group comparison was performed using a one-way ANOVA (time) with repeated measures at each time point, followed by Dunnett's multiple comparison test in case of significant time effect to compare each time value with the T0 value (Figures 2d).
Rat telemetry
Male Wistar rats were anaesthetized (sodium pentobarbital, 50 mg kg−1, i.p.). After a 5-cm midline incision on the abdomen, a DSI TA11PA-C40 (Data Sciences International, St Paul, MN, USA) implantable telemetric device was inserted into the peritoneal cavity with the catheter facing upstream into the descending aorta, below the renal arteries. The abdominal and skin incisions were closed. The animals were given an injection of carprofen (7.5 mg kg−1, s.c.) and an injection of amoxicillin (100 mg kg−1, i.m.) and returned individually to their cages. After 24 h, animals were again given amoxicillin (100 mg kg−1, s.c.). One week later, the animals were placed individually within their cage on a RPC-1 or a RLA 2000 receiver (Data Sciences International) to record on computer mean, systolic and diastolic arterial blood pressure (BP, mm Hg), heart rate (HR, beats per minute), respiratory rate (r.p.m.), which is derived from pulse blood pressure, and motor activity (c.p.m.). All generated data were acquired using Data Sciences International software. Recordings were taken in blocks of 2 min for 2 h the day prior to the first administration (day 0). Then, recordings were taken in blocks of 2 min 1 h before and for 48 h after test substance or vehicle administration on days 1, 8 and 22. Cardiovascular effects were measured at time points 0, 30, 60, 90, 120, 150 min and 3, 4, 8, 12, 24, 36 and 48 h after antibody treatment. Motor activity effects are presented per blocks of 1 h for the first 12 h and then for 1 h between the 23rd and 24th hour, between the 35th and 36th hour and between the 47th and 48th hour after administration. Baseline recordings were taken again on day 35 (blocks of 2 min for 2 h). To validate the model, on day 36, all animals received an oral administration of sibutramine (15 mg kg−1 base) to induce hypertension. The absence of heterogeneity between groups before the drug administration of each 48-h test session (T0 values) was verified using a one-way ANOVA (group). Intra-group comparison was performed for the control group (days 1, 8 and 22) using a one-way ANOVA (time) with repeated measures at each time, followed by Dunnett's t-tests in case of significant time effect to compare each time value with the T0 value (basal value before administration). Inter-group comparison was performed using a two-way ANOVA (group, time) with repeated measures at each time, followed by a one-way ANOVA (group) at each time in case of significant group × time interaction, on days 1, 8 and 22. Intra-group comparison was also performed for the sibutramine-treated groups (day 36) using a one-way ANOVA (time) with repeated measures at each time point, followed by Dunnett's t-tests in case of significant time effect to compare each time value with the T0 value (that is, basal value before administration). To confirm that anti-CGRP antibody was not eliminated and present throughout the study, blood samples from all animals were taken before the first dosing and at the end of each study period (days 0, 3, 10, 17, 24 and 36). The presence of free CGRP antibody in the serum was then determined as described below under serum analysis. Statistical analysis was performed on GraphPad Prism.
Cell-based cAMP assay
A volume of 5 μL of 100 nM human or rat αCGRP in the presence of anti-CGRP antibody (0.5-3000 nM) was dispensed into a 384-well plate at 25 °C and incubated for 15 min. Human SK-N-MC or rat L6 cells from ATCC were dissociated with 2.5 mM EDTA, washed once and resuspended in stimulation buffer (20 mM HEPES, pH 7.4, 146 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 500 μM 3-isobutyl-1-methylxanthine); 30 000 cells in 10 μL stimulation buffer were added to each well. The plate was then incubated at 37 °C for 30 min before cells were lysed. A HitHunter cAMP XS assay kit was used to measure cAMP. Lysis buffer, enzyme donor (ED), enzyme acceptor (EA) reagents and substrate were added according to the manufacturer's instructions. Plates were analysed on a plate reader (Tecan, Männedorf, Switzerland) detecting chemiluminescence (1 s per well).
Radioligand binding assay
A binding assay was performed to measure the IC50 of anti-CGRP antibody in blocking human αCGRP from binding to the receptor as described earlier (Zimmermann et al., 1995; Doods et al., 2000). Membranes (25 μg) from SK-N-MC cells were incubated for 90 min at 25 °C in incubation buffer (50 mM Tris-HCl, pH 7.4, 5 mM MgCl2, 0.1% BSA) containing 10 pM human [125I]αCGRP in a total volume of 1 mL. To determine IC50, antibodies or unlabelled human αCGRP was added at varying concentrations in the incubation buffer and incubated at the same time with membranes and 10 pM human [125I]αCGRP. Incubation was terminated by filtration through a glass microfibre filter (GF/B, 1 μm), which had been blocked with 0.5% polyethylenimine. The protein-bound radioactivity was determined in a γ-counter. Dose–response curves were plotted, and Ki values were determined using the following equation: Ki=IC50(1+([ligand]KD−1)−1, where the equilibrium dissociation constant KD=8 pM for human αCGRP to CGRP1 receptor as present in SK-N-MC cells. The reported IC50 value (in terms of IgG molecules) was converted into binding sites (by multiplying it by 2) so that it could be compared with the affinities (KD) determined by Biacore/GE Healthcare, Piscataway, NJ, USA (see Table 1).
Table 1.
In vitro characterization of CGRP function blocking antibodies by Biacore (KD), cell-based cAMP blocking and radioligand binding
| Name | KD rat 25 °C (nM) | cAMP blocking assay rat αCGRP | KD human 25 °C (nM) | Binding assay, IC50 (nM), (in anti-CGRP binding sites) | cAMP blocking assay human αCGRP |
|---|---|---|---|---|---|
| MuMab 4901a | 1.0 | Yes | 17 | 125.9 | Yes |
| MuMab 7E9 | 58 | Yes | 1.0 | 10.9 | Yes |
Abbreviation: CGRP, calcitonin gene-related peptide.
muMab 4901 was identified earlier (Wong et al., 1993).
Immunization and antibody screening, anti-CGRP antibodies
Balb/C mice were first immunized with 50 μg of human αCGRP or βCGRP conjugated to KLH (keyhole limpet haemocyanin) in CFA (complete Freund's adjuvant). After 21 days, mice were boosted with 25 μg of human βCGRP (for mice first immunized with human αCGRP) or αCGRP (for mice first immunized with human βCGRP) conjugated to KLH in IFA (incomplete Freund's adjuvant). Twenty-three days after the second immunization, a third immunization was performed with 25 μg of rat αCGRP conjugated to KLH in IFA. Ten days later, antibody titres were estimated using ELISA. Twenty-four days after the third immunization, a fourth immunization was performed with 25 μg of rat αCGRP conjugated to KLH in IFA. A final booster was performed with 100 μg soluble peptide (rat αCGRP) 32 days after the fourth immunization. Hybridomas were gained as described earlier (Kohler and Milstein, 1975, 1976) with the following modifications: splenocytes were obtained from the immunized mouse and fused with SP2 myeloma cells at a ratio of 10:1, with polyethylene glycol 1500 (PEG-1500). The hybrids were plated out into 96-well plates in DMEM (Dulbecco's modified Eagle's medium) containing 15% foetal bovine serum, 10% hybridoma cloning factor, penicillin/streptomycin and grown for 2 days before selection was begun by adding hypoxanthine/aminopterin/thymidine (HAT). On day 14 after fusion, 100 μL supernatant from each well was transferred to a CGRP-coated ELISA plate, which had been coated overnight at 4 °C with 100 μL human or rat αCGRP diluted in phosphate-buffered saline (PBS; final 1 μg mL−1), blocked with 0.5% albumin in PBS for 1 h at room temperature and washed four times with 200 μL per well of 0.1% Tween 20 in PBS. After the application of cell supernatant or detection, antibody plates were incubated for 1 h and washing was repeated as above. Determination of antibody subtype class was done with subtype-specific secondary HRP rabbit anti-mouse IgG/γ-specific or rabbit anti-mouse IgM.
Control antibody
Histidine-tagged Drosophila amnesiac was transiently expressed in HEK293 cells and purified from supernatants by affinity chromatography according to the manufacturer's instructions (Qiagen, Valencia, CA, USA). Balb/C mice were first immunized with 50 μg of Drosophila amnesiac coupled to KLH in CFA. As described for anti-CGRP antibodies, mice were boosted with KLH coupled in IFA. Splenocytes were fused and antibody-secreting clones were identified by ELISA as described above using Drosophila amnesiac-coated plates.
Antibody production and purification
Hybridoma cells were cultured in DMEM, 10% foetal bovine serum containing penicillin/streptomycin, harvested and washed with DMEM and then injected intraperitoneally into pristane-primed balb/C mice at 8 × 106 cells per mL in 0.5 mL. After 8–10 days, injected mice were anaesthetized and asphyxiated with CO2 and ascites fluid was removed with an 18-gauge needle connected to a syringe. Ascites fluid was diluted 1:2 with PBS, filtered and bound in batch mode to protein A resin before washing with PBS (10 times resin volume) and eluting with 0.1 M citric acid (pH 3). The eluate was neutralized with 1:10 volume 0.1 M Tris (pH 8.5) and dialyzed overnight in PBS 0.01% Tween 20.
Analysis to determine anti-CGRP antibody concentration in serum samples
Nunc Maxisorp plates were coated overnight at 4 °C with 100 μL of rat αCGRP diluted in PBS (final 1 μg mL−1) and processed as described above under anti-CGRP-antibody screening. Antibody standard (muMab 4901) or rat serum samples were diluted appropriately in 0.5% albumin in PBS and applied in duplicates. An HRP-conjugated goat anti-mouse IgG (H+L) (dilution: 1:10 000) was used for detection.
Epitope mapping of anti-CGRP antibodies
Nunc Maxisorp plates were coated overnight at 4 °C with 100 μL of rat αCGRP, human αCGRP or human βCGRP fragments 1-13-COOH, 1-19-COOH, 19-27-COOH, 8-37-COOH, 1-36-COOH and 19-37-CONH2 diluted in PBS (final 1 μg mL−1) and processed as described above under anti-CGRP antibody screening. A constant concentration of 111 ng mL−1 (100 μL per well) murine monoclonal antibodies (muMab) 4901 or muMab 7E9 was applied. An HRP-conjugated goat anti-mouse IgG (H+L) (dilution: 1:10 000) was used for detection.
Biacore assay
Interaction analysis was conducted at 25 °C on a Biacore 3000 system equipped with streptavidin-coated sensor chips using a standard Biacore running buffer. N-biotinylated human and rat αCGRPs were captured on individual flow cells at low levels (typically 100 response units) to provide the reaction surfaces, whereas an unmodified flow cell served as a reference channel. Purified Fab fragments of anti-CGRP antibodies were generated by digesting full-length IgGs with papain using an ImmunoPure Fab preparation kit according to the manufacturer's instructions. Fabs were titrated over the chip using 1 μM as the top concentration of a two-fold dilution series. Association and dissociation phases were monitored at 100 μL min−1 for 1 min and 5 min, respectively. Surfaces were regenerated with a mixture of 35% ethanol+25 mM NaOH+0.5 M NaCl. Injections were duplicated to demonstrate that the assay was reproducible. The binding responses were double-referenced and fit globally to a simple model using BiaEvaluation v. 4.0 software. Affinities were deduced from the quotient of the kinetic rate constants (KD=koff × kon−1).
Materials
Isoflurane (Aerrane) was obtained from Baxter (Deerfield, IL, USA); the laser Doppler flow meter from Moor Instruments (Axminster, UK); pentobarbital from Ovation Pharmaceuticals (Deerfield, IL, USA); the Mikro-Tip catheter from Millar Instruments (Houston, TX, USA); the video dimension analyser from Living Systems (Burlington, VT, USA); chart software from AD Instruments (Colorado Springs, CO, USA); carprofen, Rimadyl; amoxicillin, Clamoxyl LA; sibutramine from Chempacific (Baltimore, MD, USA); the 384-well plate from Nunc (Rochester, NY, USA); EDTA and HEPES from Gibco BRL (Gaithersburg MD, USA). The following chemicals were all obtained from Sigma (St Louis, MO, USA): NaCl, KCl, CaCl2, MgCl2, KLH, 3-isobutyl-1-methylxanthine, Tris-HCl, MgCl2, polyethylenimine, NaOH, BSA, HAT, Tween 20, albumin, citric acid, Tris, pristine and bretylium tosylate. HitHunter cAMP XS assay kit was from DiscoveRx/GE Healthcare (Fremont, CA, USA) and the plate reader from Tecan (Männedorf, Switzerland). Human [125I]αCGRP was from MDS Pharma Services (King of Prussia, PA, USA) and the glass microfibre filter from Perkin Elmer (Waltham, MA, USA). CGRP-(8-37), BCGRP, rat αCGRP, human αCGRP and human βCGRP fragments and N-biotinylated human and rat αCGRPs were all obtained from Global Peptide Services (Huntsville, AL, USA); CFA and incomplete Freund's adjuvant form Difco (Franklin Lakes, NJ, USA); DMEM, PBS and penicillin/streptomycin from Gibco BRL; foetal bovine serum from Hyclone (Waltham, MA, USA); hybridoma cloning factor from Bioveris Corporation (Gaithersburg, MD, USA); CGRP-coated ELISA plate from Nunc. Subtype-specific secondary HRP-rabbit anti-mouse IgG/γ-specific and rabbit anti-mouse IgM were obtained from Zymed/Invitrogen (Gaithersburg, MD, USA); HRP-conjugated goat anti-mouse IgG from Jackson ImmunoResearch (West Grove, PA, USA); protein A resin from Mabselect/GE Healthcare (Piscataway, NJ, USA); HBS-P and Biacore 3000 system from GE Healthcare (Piscataway, NJ, USA); streptavidin-coated sensor chips from Biacore AB (Uppsala, Sweden); ImmunoPure Fab preparation kit from Pierce (Rockford, IL, USA).
MuMab 4901 anti-CGRP antibody was identified previously (Wong et al., 1993) and received through a licensing agreement from UCLA (CA, USA).
BIBN4096BS (C38H47Br2N9O5) was synthesized by standard techniques. The purity and molecular weight of the compound were determined by LC-MS (liquid chromatography-mass spectrometry). BIBN4096BS was dissolved in 0.1 N HCl at 10 mg mL−1 and then diluted in PBS at 1 mg mL−1. Aliquots were stored at −20 °C. CGRP1 receptor-specific antagonistic activity was determined experimentally (Ki=34 pM) by CGRP binding assay as described above.
All drug/molecular target nomenclature (for example, receptors and ion channels) conform with BJP's Guide to Receptors and Channels (Alexander et al., 2008).
Results
Identification of anti-CGRP antibodies
Previous work identified an anti-CGRP antibody (muMab 4901) that blocks the function of rat αCGRP with high affinity (Plourde et al., 1993; Wong et al., 1993). To develop a CGRP function-blocking antibody for the preventive treatment of migraine, we intended to identify an anti-CGRP antibody that blocks the function of human CGRP with high affinity. Therefore, we fused spleen cells prepared from a mouse immunized with human and rat CGRPs with murine plasmacytoma cells and screened the supernatants of the resulting hybridoma cells by CGRP ELISA. We identified a hybridoma cell clone that secreted an antibody (muMab 7E9) that bound αCGRP and βCGRP derived from human and rat. Affinities of muMab 7E9 and muMab 4901 were determined on a Biacore 3000 (Figures 1a–d and Table 1). Their affinities against rat (Figures 1a and c and Table 1) and human αCGRP (Figures 1b and d and Table 1) displayed species selectivity, in that muMab 4901 had the highest affinity to rat αCGRP (Figure 1a and Table 1), whereas muMab 7E9 had the highest affinity to human αCGRP (Figure 1d and Table 1). Both antibodies bound to a C-terminal epitope of CGRP as determined by CGRP-ELISA (Figures 1e and f).
Figure 1.
Antibody characterization of muMab 4901 and muMab 7E9 by Biacore, CGRP-ELISA, cell-based cAMP assay and CGRP binding assay. (a–d) Binding kinetics of muMab 4901 and muMab 7E9 to human and rat αCGRP was determined by surface plasmon resonance using Biacore. The coloured lines represent the measured data and the black lines show the simulated global fits. (e, f) Epitope mapping of muMab 4901 and muMab 7E9 by CGRP-ELISA. Both antibodies bind to a C-terminal epitope. Full-length human αCGRP, full-length rat αCGRP and CGRP fragments were used for binding. (g) Cell-based cAMP inhibition assay using 17 nM rat αCGRP with various concentrations of muMab 4901 and muMab 7E9. (h) CGRP binding assay using 10 pM human [125I]-αCGRP with various concentrations of muMab 4901 and muMab 7E9.
Identification of CGRP function-blocking antibodies
To test both antibodies (muMab 7E9 and muMab 4901) for their function-blocking effect, we used cell lines expressing either rat (L6 cells) or human (SK-N-MC cells) CGRP1 receptor. Rat or human αCGRP dose-dependently increased intracellular cAMP levels (data not shown). The blocking effect was determined using anti-CGRP antibodies at various concentrations (3000–0.5 nM) to antagonize the response to 17 nM of rat or human αCGRP. We observed that both antibodies blocked the increase of cAMP induced by human (Table 1; data not shown) and rat αCGRP (Figure 1g and Table 1) in a dose-dependent manner.
To characterize this biological inhibition further, both antibodies were tested in a sensitive-binding assay using human CGRP1 receptors from cell membrane extracts (SK-N-MC cells). The inhibition concentrations (IC50) were determined using 10 pM human [125I]αCGRP and varying concentrations of anti-CGRP antibody. Both antibodies blocked the binding of human [125I]αCGRP in a dose-dependent manner (Figure 1h and Table 1). This shows that binding of these monoclonal antibodies (Mabs) to CGRP prevents its interaction with the CGRP1 receptor.
Anti-CGRP antibody inhibits neurogenic vasodilatation
Antidromic stimulation of the saphenous nerve produces a transient and significant increase in blood flow in the dorsomedial skin of the rat hind paw as measured by laser Doppler. This has been demonstrated to be largely a CGRP-dependent effect (Escott and Brain, 1993; Tan et al., 1995). We used this skin blood flow model to test the efficacy of muMab 4901 and muMab 7E9 to block endogenously released CGRP in vivo. Changes in blood flow parameters were expressed as the area under the curve (change in arbitrary Doppler flux units multiplied by time). CGRP receptor antagonist CGRP-(8-37) (400 nmol kg−1, i.v.) was used as a positive control. To determine the effect of CGRP-(8-37) or anti-CGRP antibody, prior to dosing for each animal, the baseline blood flow response was established with two saphenous nerve stimulations 30 min apart. On account its short half-life in vivo, rats were treated with CGRP-(8-37) 5 min before a third stimulation. In this paradigm, CGRP-(8-37) significantly blocked the blood flow response to electrical stimulation (Figure 2a). The inhibitory effect of CGRP-(8-37) had disappeared at the second stimulation 35 min after dosing.
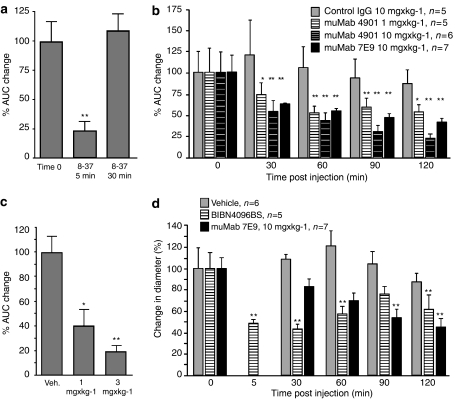
Figure 2.
Anti-CGRP antibodies block neurogenic vasodilatation in vivo. (a) CGRP-(8-37) (400 nmol kg−1, i.v.) blocked neurogenic vasodilatation in the dorsomedial skin of the rat hind paw 5 min after electrical stimulation (**P=0.01, n=10). (b) muMab 7E9 and muMab 4901 inhibited neurogenic vasodilatation in the dorsomedial skin of the rat hind paw as early as 30 min after injection (*P=0.05; **P=0.01). (c) muMab 4901 blocked neurogenic vasodilatation in the dorsomedial skin of the rat hind paw 7 days after treatment (*P=0.05; **P=0.01). (d) BIBN4096BS and muMab 7E9 blocked neurogenic vasodilatation of the middle meningeal artery (**P=0.01).
In a separate group, rats were treated with control IgG, muMab 7E9 or muMab 4901 after the blood flow response of the second stimulation had returned to baseline levels (approximately 10 min post-stimulation) and an additional four stimulations at 30 min intervals were performed. In control IgG (10 mg kg−1)-treated animals, no significant change in blood flow response was observed (Figure 2b and Table 2). Rats treated with muMab 7E9 (10 mg kg−1) showed a significant decrease in blood flow response starting at 30 min after dosing, and reached a maximal effect at 120 min after dosing (Figure 2b and Table 2). Rats treated with muMab 4901 (1 and 10 mg kg−1) also showed a significant decrease in blood flow response starting at 30 min and reached a maximal effect 120 min after dosing (Figure 2b and Table 2). The maximal change in blood flow response with muMab 4901 (10 mg kg−1) after electrical stimulation was comparable to that observed at 5 min after administration of CGRP-(8-37). This shows that muMab 7E9 and muMab 4901 block neurogenic vasodilatation in the rat skin to a similar magnitude to that seen with the well-characterized receptor antagonist CGRP-(8-37).
Table 2.
Anti-CGRP antibody treatment inhibited neurogenic vasodilatation in the skin
| Treatment | Pre-Mab | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|
| Control IgG (10 mg kg−1, i.v.) | 100±26.1 | 121.6±34.1 | 106.5±24.6 | 94.6±22.5 | 87.5±15 |
| muMab 4901 (10 mg kg−1, i.v.) | 100±23.7 | 54.3±11.8 | 44±10.2 | 31.1±7.2 | 23±5 |
| muMab 4901 (1 mg kg−1, i.v.) | 100±30.3 | 73.7±14 | 53.3±8 | 59.7±10 | 54.4±7 |
| muMab 7E9 (10 mg kg−1, i.v.) | 100±20.5 | 62.4±2 | 55.4±4.2 | 47.2±5.4 | 42.8±3.9 |
Abbreviation: CGRP, calcitonin gene-related peptide.
Values are mean±s.e.mean and presented as percentage change from normalized preinjection values.
The inhibition seen after anti-CGRP antibody treatment clearly had a slower onset of action than CGRP-(8-37) in this model, but had a dramatically longer duration of action; at least 120 min. To investigate how long lasting a single treatment with anti-CGRP antibody might be, longer time course experiments were undertaken.
Anti-CGRP antibody exerts a long-lasting blocking effect
To determine whether CGRP function-blocking antibodies have a long-lasting effect, we treated rats with a single dose of muMab 4901 or vehicle control and measured skin vasodilatation after saphenous nerve stimulation 7 days later. In control-treated animals, the average blood flow response from two stimulations was set to 100% (100±13%, n=32; Figure 2c). In muMab 4901 (1 and 3 mg kg−1)-treated animals, the average blood flow response was significantly decreased from that seen in control animals (Figure 2c). We determined by anti-CGRP ELISA the concentration of free anti-CGRP antibody in the serum of rats dosed with 1 mg kg−1 muMab 4901. We found that after 7 days, free anti-CGRP antibody (6.3±0.3 μg mL−1; n=6) was present in serum. This shows that function-blocking CGRP antibodies have a long half-life consistent with the long-lasting effects we observed. To summarize, a single administration of a CGRP function-blocking antibody can inhibit neurogenic vasodilatation in the rat skin for at least 1 week after dosing.
Anti-CGRP antibody blocks neurogenic vasodilatation of meningeal blood vessels
The results from the skin vasodilatation model established, using both muMab 4901 and muMab 7E9, that it is possible for anti-CGRP antibodies to inhibit neurogenic vasodilatation for fairly long periods of time with a single dose. To have confidence in a migraine prevention approach in humans, we wanted to establish efficacy in a more disease relevant animal model. In migraine, head pain originates in the region of the meninges and is thought to arise in part through mechanisms related to the distension of dural blood vessels. We therefore chose to examine whether muMab 7E9 can inhibit neurogenic vasodilatation of meningeal blood vessels. Prior to dosing with the test substance, the dilatation response of the MMA to electrical field stimulation was established with two stimulations 30 min apart. The average percentage increase in blood vessel diameter obtained in response to these two stimulations was defined as the value at time zero. After the average percentage increase of the MMA after electrical field stimulation had been established for each animal, the test substance was injected intravenously. Treatment with vehicle, the clinically validated CGRP1 receptor antagonist BIBN4096BS (300 μg kg−1, i.v.) or muMab 7E9 (10 mg kg−1) did not change the baseline dural blood vessel diameter or mean arterial blood pressure (MABP) (data not shown). In vehicle-treated animals, electrical stimulation of the cranial window at 30 min intervals resulted in a reproducible increase in dural blood vessel diameter (Figure 2d and Table 3). Injection of BIBN4096BS (300 μg kg−1, i.v.) led to a significant inhibition of MMA diameter increase (starting 5 min post-injection, Figure 2d and Table 3) after stimulation. This inhibition was fairly well maintained 30 min post-injection but decreased thereafter (Figure 2d and Table 3). In rats treated with muMab 7E9 (10 mg kg−1, i.v.), blood vessel diameter increases after electrical stimulation was significantly smaller at 90 and 120 min after antibody injection (Figure 2d and Table 3). Hence, anti-CGRP muMab 7E9 inhibits neurogenic vasodilatation of meningeal blood vessels and has an effect that is comparable to BIBN4096BS in magnitude. As was seen in the saphenous stimulation assay, the antibody had a slower onset of action than a low-molecular-weight receptor antagonist but with longer lasting efficacy.
Table 3.
Anti-CGRP antibody treatment inhibited neurogenic vasodilatation of the middle meningeal artery
| Treatment | Pre-Mab | 5 min | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|---|
| Vehicle | 100±18.7 | ND | 108.4±4.9 | 120±13.9 | 103.8±12.5 | 87.4±8.4 |
| BIBN4096BS (300 μg kg−1, i.v.) | 100±14.3 | 47.8±3.9 | 42.9±4.7 | 56.9±7.1 | 76.1±6.7 | 61.6±12.9 |
| muMab 7E9 (10 mg kg−1, i.v.) | 100±11.2 | ND | 82.8±7.1 | 69.6±7 | 53.8±7.2 | 44.3±8.5 |
Abbreviation: CGRP, calcitonin gene-related peptide.
Values are mean±s.e.m. and presented as percentage change from normalized preinjection values.
Blood pressure, heart rate, weight and motor activity in chronic anti-CGRP antibody-treated rats is indistinguishable from controls
Although we did not observe acute changes in MABP in rats after treatment with the CGRP inhibitor BIBN4096BS or anti-CGRP antibody injection, there may be cardiovascular effects with chronic treatment. We therefore recorded respiratory rate, MABP and heart rate from conscious rats treated with anti-CGRP antibody or control IgG. Two groups of rats were dosed every week for 4 weeks with a relatively high dose of either control IgG (30 mg kg−1, n=5) or muMab 4901 (30 mg kg−1, n=6). Cardiovascular parameters were recorded 1 h prior to dosing and 48 h after dosing on three study days (days 1, 8 and 22). Both in control IgG- and muMab 4901-treated rats, a transient increase in arterial blood pressure, heart rate and motor activity was observed following each administration and during the dark period (that is, time points 12 and 36 h) (Figures 3a and b). Comparison between control IgG (30 mg kg−1, i.v.)- and muMab 4901 (30 mg kg−1, i.v.)-treated animals showed no significant change in respiratory rate, weight, motor activity (all data not shown), MABP (Figure 3a) and heart rate (Figure 3b) on all study days (data for days 1 and 8; data not shown). To summarize, a 4-week systemic application of a function-blocking CGRP antibody did not alter motor activity, weight gain or cardiovascular function in the conscious rat.
Figure 3.
Mean arterial blood pressure (MABP) and heart rate in a conscious telemetric rat model after 4 weekly treatments with control IgG (30 mg kg−1, n=5) or muMab 4901 (30 mg kg−1, n=6). (a) Mean arterial blood pressure and (b) heart rate were unchanged between control IgG- and muMab 4901-treated rats.
Discussion
This study shows that function-blocking CGRP antibodies inhibit neurogenic vasodilatation without affecting heart rate and arterial blood pressure in the rat. The two blood flow models we used involve endogenous, neurogenic CGRP-dependent vasodilatation. Neurogenic CGRP is believed to be an important mediator in migraine headache. Existing migraine drugs, such as triptans (Williamson et al., 1997b), morphine (Williamson et al., 2001b) and topiramate (Akerman and Goadsby, 2005), inhibit neurogenic vasodilatation in the same or similar intravital microscopy models to the one used here. We show for the first time that function-blocking CGRP antibodies block neurogenic vasodilatation of subcutaneous blood vessels and meningeal blood vessels with an effect that is comparable in magnitude to a single maximally effective dose of CGRP-(8-37) (400 nmol kg−1, 1520 μg kg−1) or BIBN4096BS (300 μg kg−1). The blocking effect after a single dose of anti-CGRP antibody was achieved within 1–2 h after systemic injection and lasted for at least 7 days.
During migraine attacks, there is a significant increase in CGRP in the venous outflow from the head (Goadsby et al., 1990). This observation led to the hypothesis that CGRP released from perivascular nerve endings is causally related to the pain experienced during migraine attack. This view is supported by the finding that the CGRP1 receptor antagonist BIBN4096BS is effective in treating migraine (Olesen et al., 2004). In animal models, it was shown previously that the CGRP receptor antagonist CGRP-(8-37) blocks neurogenic vasodilatation in subcutaneous blood vessels of the rat paw (Escott and Brain, 1993) and the CGRP receptor antagonist BIBN4096BS blocks neurogenic vasodilatation in intracranial blood vessels (Petersen et al., 2004). In contrast to inhibiting the CGRP1 receptor, a recent study used systemic injection of an αCGRP-specific Spiegelmer to block the blood flow increase in the dura after electrical stimulation (Denekas et al., 2006). This Spiegelmer directly interferes with rat αCGRP and blocks its function (Vater et al., 2003). In separate studies in a rat dural blood flow model, the Spiegelmer (Denekas et al., 2006) showed an effect comparable in magnitude to the CGRP1 receptor antagonist BIBN4096BS (Troltzsch et al., 2007). In contrast to the small-molecule CGRP1 receptor antagonists and the CGRP–Spiegelmer that transiently inhibit CGRP-mediated vasodilatation in the rat, we showed that a single dose of CGRP function-blocking antibodies exerts an effect with similar magnitude, a slower onset, but much longer duration, up to 7 days.
Function-blocking CGRP antibodies have been used previously to block endogenous CGRP in the rat. In the rodent stomach, sensory afferents activate gastroprotective cues in part through CGRP (Holzer, 2007). In two studies, muMab 4901 has been shown to abolish capsaicin-induced gastroprotection after ethanol-induced injury (Lambrecht et al., 1993; Peskar et al., 1993). This shows that function-blocking CGRP antibodies can block endogenous CGRP in the gastric mucosa. Furthermore, Fab fragments (antigen binding fragment of antibody, 50 kDa) of an anti-CGRP antibody have been used previously to block neurogenic vasodilatation in the skin of the rat paw. Interestingly, in the same study, treatment with the IgG form of the anti-CGRP antibody (10 mg kg−1, i.v.) did not significantly block the blood flow response 2 h after dosing (Tan et al., 1995), suggesting that the difference in mass between the IgG form (150 kDa) and the Fab fragment (50 kDa) might be important. We show here that the IgG form of at least two different anti-CGRP antibodies is, in fact, able to inhibit neurogenic vasodilatation in a similar model. Further work will be required to understand the difference between the work presented here and previous findings.
The slower onset of action of CGRP antibodies compared with the CGRP receptor antagonist we observed is consistent with the idea of a slower penetration into the interstitial space of the vascular smooth muscle tissue. A slower tissue penetration of the IgG form of an antibody compared with smaller antigen-binding fragments in tumour tissue has been shown previously (Holliger and Hudson, 2005). Our results are also consistent with a rat middle cerebral artery explant assay that demonstrated that a CGRP function-blocking antibody or a CGRP–Spiegelmer has to act on the extra-luminal side to inhibit vasodilatation (Edvinsson et al., 2007). Similarly, in a rat dural blood flow model, a pegylated CGRP–Spiegelmer (40 kDa pegylation tag) or an anti-CGRP antibody blocked systemically injected CGRP (10 mg kg−1, i.v.) but did not inhibit neurogenic vasodilatation 20–30 min after treatment (Juhl et al., 2007), suggesting that this time interval is insufficient for a pegylated Spiegelmer or an antibody to penetrate into the interstitial space of the vessel wall.
The normal physiological function of CGRP is not completely understood. However, cardiovascular effects are a potential concern when inhibiting CGRP. Intravenous injection of human αCGRP leads to a significant decrease in MABP (−12.2±2.9%), when compared with placebo-infused patients (Lassen et al., 2002). In the same study, CGRP also caused a significant increase in heart rate (+59.9±7.7%). This shows that systemic administration of CGRP has a hypotensive effect in humans. In contrast, injection of the receptor antagonist CGRP-(8-37) shows no effect on systemic blood pressure in rats and dogs (Shen et al., 2001). Similarly, our data show that weekly injection of anti-CGRP antibody into rodents monitored over a 36-day period did not result in changes in heart rate or blood pressure. In addition, the injection of BIBN4096BS into healthy pigs (Kapoor et al., 2003), primates (Doods et al., 2000) or humans (Olesen et al., 2004; Petersen et al., 2005) did not affect the heart rate or blood pressure. This suggests that systemic CGRP in the normal state has a minimal physiological role in cardiovascular homoeostasis.
The constitutive genetic deletion of CGRP in mice cannot distinguish either between developmental and adult function or between peripheral and central effects. One study reported normal haemodynamics (Lu et al., 1999), whereas two other studies, using different knockout mice of which one also deletes the calcitonin gene, reported an increase in MABP and heart rate (Gangula et al., 2000; Oh-hashi et al., 2001). When injected intracerbroventricularly, CGRP can increase blood pressure because of sympathetic nerve stimulation and the release of the vasoconstrictor noradrenaline (Fischer and Born, 1985). Hence, studies using constitutive deletion of CGRP or the CGRP receptor (Tsujikawa et al., 2007) may not be relevant for the treatment with CGRP function-blocking antibodies, which would act on peripheral but not central CGRP in the adult.
Taken together, our findings demonstrate for the first time that function-blocking CGRP antibodies block neurogenic vasodilatation without affecting heart rate and arterial blood pressure in the rat. This effect can last for several days after a single injection. Therefore, function-blocking CGRP antibodies potentially provide a novel approach for the preventive treatment of vascular headaches including migraine.
Acknowledgments
We thank Davide Foletti and Marina Pasca for comments on the manuscript and Ray Liu for help with statistical analysis.
Abbreviations
- CGRP
calcitonin gene-related peptide
- Fab
antigen binding fragment
- MABP
mean arterial blood pressure
- MMA
middle meningeal artery
- muMab
murine monoclonal antibody
Conflict of interest
JZ, KTP, JES, YNA, SC, CAG, RC, JP, AR and DLS are or were employees of Rinat Neuroscience Inc. and/or Pfizer Inc.
References
- Akerman S, Goadsby PJ. Topiramate inhibits trigeminovascular activation: an intravital microscopy study. Br J Pharmacol. 2005;146:7–14. doi: 10.1038/sj.bjp.0706290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara SG, Arriza JL, Leff SE, Swanson LW, Evans RM, Rosenfeld MG. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science. 1985;229:1094–1097. doi: 10.1126/science.2994212. [DOI] [PubMed] [Google Scholar]
- Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Ando K, Pegram BL, Frohlich ED. Hemodynamic effects of calcitonin gene-related peptide in spontaneously hypertensive rats. Am J Physiol. 1990;258 2 Part 2:R425–R429. doi: 10.1152/ajpregu.1990.258.2.R425. [DOI] [PubMed] [Google Scholar]
- Arulmani U, Maassenvandenbrink A, Villalon CM, Saxena PR. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur J Pharmacol. 2004;500:315–330. doi: 10.1016/j.ejphar.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Buzzi MG, Moskowitz MA. The trigemino-vascular system and migraine. Pathol Biol (Paris) 1992;40:313–317. [PubMed] [Google Scholar]
- Cumberbatch MJ, Williamson DJ, Mason GS, Hill RG, Hargreaves RJ. Dural vasodilation causes a sensitization of rat caudal trigeminal neurones in vivo that is blocked by a 5-HT1B/1D agonist. Br J Pharmacol. 1999;126:1478–1486. doi: 10.1038/sj.bjp.0702444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denekas T, Troltzsch M, Vater A, Klussmann S, Messlinger K. Inhibition of stimulated meningeal blood flow by a calcitonin gene-related peptide binding mirror-image RNA oligonucleotide. Br J Pharmacol. 2006;148:536–543. doi: 10.1038/sj.bjp.0706742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, et al. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham PL. CGRP-receptor antagonists–a fresh approach to migraine therapy. N Engl J Med. 2004;350:1073–1075. doi: 10.1056/NEJMp048016. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Ekman R, Jansen I, McCulloch J, Uddman R. Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J Cereb Blood Flow Metab. 1987a;7:720–728. doi: 10.1038/jcbfm.1987.126. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Ekman R, Jansen I, Ottosson A, Uddman R. Peptide-containing nerve fibers in human cerebral arteries: immunocytochemistry, radioimmunoassay, and in vitro pharmacology. Ann Neurol. 1987b;21:431–437. doi: 10.1002/ana.410210503. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Goadsby PJ. Neuropeptides in migraine and cluster headache. Cephalalgia. 1994;14:320–327. doi: 10.1046/j.1468-2982.1994.1405320.x. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Nilsson E, Jansen-Olesen I. Inhibitory effect of BIBN4096BS, CGRP(8-37), a CGRP antibody and an RNA-Spiegelmer on CGRP induced vasodilatation in the perfused and non-perfused rat middle cerebral artery. Br J Pharmacol. 2007;150:633–640. doi: 10.1038/sj.bjp.0707134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escott KJ, Brain SD. Effect of a calcitonin gene-related peptide antagonist (CGRP8-37) on skin vasodilatation and oedema induced by stimulation of the rat saphenous nerve. Br J Pharmacol. 1993;110:772–776. doi: 10.1111/j.1476-5381.1993.tb13878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JA, Born W. Novel peptides from the calcitonin gene: expression, receptors and biological function. Peptides. 1985;6 Suppl 3:265–271. doi: 10.1016/0196-9781(85)90384-5. [DOI] [PubMed] [Google Scholar]
- Gangula PR, Zhao H, Supowit SC, Wimalawansa SJ, Dipette DJ, Westlund KN, et al. Increased blood pressure in alpha-calcitonin gene-related peptide/calcitonin gene knockout mice. Hypertension. 2000;35 1 Part 2:470–475. doi: 10.1161/01.hyp.35.1.470. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117 Part 3:427–434. doi: 10.1093/brain/117.3.427. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, et al. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70:1304–1312. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- Holzer P. Role of visceral afferent neurons in mucosal inflammation and defense. Curr Opin Pharmacol. 2007;7:563–569. doi: 10.1016/j.coph.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhl L, Edvinsson L, Olesen J, Jansen-Olesen I. Effect of two novel CGRP-binding compounds in a closed cranial window rat model. Eur J Pharmacol. 2007;567:117–124. doi: 10.1016/j.ejphar.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Kapoor K, Arulmani U, Heiligers JP, Willems EW, Doods H, Villalon CM, et al. Effects of BIBN4096BS on cardiac output distribution and on CGRP-induced carotid haemodynamic responses in the pig. Eur J Pharmacol. 2003;475:69–77. doi: 10.1016/s0014-2999(03)02082-x. [DOI] [PubMed] [Google Scholar]
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Kohler G, Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976;6:511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Lambrecht N, Burchert M, Respondek M, Muller KM, Peskar BM. Role of calcitonin gene-related peptide and nitric oxide in the gastroprotective effect of capsaicin in the rat. Gastroenterology. 1993;104:1371–1380. doi: 10.1016/0016-5085(93)90345-d. [DOI] [PubMed] [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- Lu JT, Son YJ, Lee J, Jetton TL, Shiota M, Moscoso L, et al. Mice lacking alpha-calcitonin gene-related peptide exhibit normal cardiovascular regulation and neuromuscular development. Mol Cell Neurosci. 1999;14:99–120. doi: 10.1006/mcne.1999.0767. [DOI] [PubMed] [Google Scholar]
- Oh-hashi Y, Shindo T, Kurihara Y, Imai T, Wang Y, Morita H, et al. Elevated sympathetic nervous activity in mice deficient in alphaCGRP. Circ Res. 2001;89:983–990. doi: 10.1161/hh2301.100812. [DOI] [PubMed] [Google Scholar]
- Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ. Neurogenic inflammation and migraine: implications for the therapeutics. Mol Interv. 2005;5:304–311. doi: 10.1124/mi.5.5.10. [DOI] [PubMed] [Google Scholar]
- Peskar BM, Wong HC, Walsh JH, Holzer P. A monoclonal antibody to calcitonin gene-related peptide abolishes capsaicin-induced gastroprotection. Eur J Pharmacol. 1993;250:201–203. doi: 10.1016/0014-2999(93)90645-x. [DOI] [PubMed] [Google Scholar]
- Petersen KA, Birk S, Doods H, Edvinsson L, Olesen J. Inhibitory effect of BIBN4096BS on cephalic vasodilatation induced by CGRP or transcranial electrical stimulation in the rat. Br J Pharmacol. 2004;143:697–704. doi: 10.1038/sj.bjp.0705966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Ther. 2005;77:202–213. doi: 10.1016/j.clpt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Plourde V, Wong HC, Walsh JH, Raybould HE, Tache Y. CGRP antagonists and capsaicin on celiac ganglia partly prevent postoperative gastric ileus. Peptides. 1993;14:1225–1229. doi: 10.1016/0196-9781(93)90180-o. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Mermod J, Amara SG, Swanson LW, Sawchenko PE, Rivier J, et al. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304:129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Shen YT, Pittman TJ, Buie PS, Bolduc DL, Kane SA, Koblan KS, et al. Functional role of alpha-calcitonin gene-related peptide in the regulation of the cardiovascular system. J Pharmacol Exp Ther. 2001;298:551–558. [PubMed] [Google Scholar]
- Storer RJ, Akerman S, Goadsby PJ. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol. 2004;142:1171–1181. doi: 10.1038/sj.bjp.0705807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KK, Brown MJ, Hargreaves RJ, Shepheard SL, Cook DA, Hill RG. Calcitonin gene-related peptide as an endogenous vasodilator: immunoblockade studies in vivo with an anti-calcitonin gene-related peptide monoclonal antibody and its Fab' fragment. Clin Sci (Lond) 1995;89:565–573. doi: 10.1042/cs0890565. [DOI] [PubMed] [Google Scholar]
- Troltzsch M, Denekas T, Messlinger K. The calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS reduces neurogenic increases in dural blood flow. Eur J Pharmacol. 2007;562:103–110. doi: 10.1016/j.ejphar.2007.01.058. [DOI] [PubMed] [Google Scholar]
- Tsujikawa K, Yayama K, Hayashi T, Matsushita H, Yamaguchi T, Shigeno T, et al. Hypertension and dysregulated proinflammatory cytokine production in receptor activity-modifying protein 1-deficient mice. Proc Natl Acad Sci USA. 2007;104:16702–16707. doi: 10.1073/pnas.0705974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddman R, Edvinsson L, Ekblad E, Hakanson R, Sundler F. Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Regul Pept. 1986;15:1–23. doi: 10.1016/0167-0115(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Uddman R, Hara H, Edvinsson L. Neuronal pathways to the rat middle meningeal artery revealed by retrograde tracing and immunocytochemistry. J Auton Nerv Syst. 1989;26:69–75. doi: 10.1016/0165-1838(89)90109-4. [DOI] [PubMed] [Google Scholar]
- Vater A, Jarosch F, Buchner K, Klussmann S. Short bioactive Spiegelmers to migraine-associated calcitonin gene-related peptide rapidly identified by a novel approach: tailored-SELEX. Nucleic Acids Res. 2003;31:e130. doi: 10.1093/nar/gng130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DJ, Hargreaves RJ. Neurogenic inflammation in the context of migraine. Microsc Res Tech. 2001a;53:167–178.. doi: 10.1002/jemt.1081. [DOI] [PubMed] [Google Scholar]
- Williamson DJ, Hargreaves RJ, Hill RG, Shepheard SL. Intravital microscope studies on the effects of neurokinin agonists and calcitonin gene-related peptide on dural vessel diameter in the anaesthetized rat. Cephalalgia. 1997a;17:518–524. doi: 10.1046/j.1468-2982.1997.1704518.x. [DOI] [PubMed] [Google Scholar]
- Williamson DJ, Hargreaves RJ, Hill RG, Shepheard SL. Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat—intravital microscope studies. Cephalalgia. 1997b;17:525–531. doi: 10.1046/j.1468-2982.1997.1704525.x. [DOI] [PubMed] [Google Scholar]
- Williamson DJ, Shepheard SL, Cook DA, Hargreaves RJ, Hill RG, Cumberbatch MJ. Role of opioid receptors in neurogenic dural vasodilation and sensitization of trigeminal neurones in anaesthetized rats. Br J Pharmacol. 2001b;133:807–814. doi: 10.1038/sj.bjp.0704136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HC, Tache Y, Lloyd KC, Yang H, Sternini C, Holzer P, et al. Monoclonal antibody to rat alpha-CGRP: production, characterization, and in vivo immunoneutralization activity. Hybridoma. 1993;12:93–106. doi: 10.1089/hyb.1993.12.93. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Fischer JA, Muff R. Adrenomedullin and calcitonin gene-related peptide interact with the same receptor in cultured human neuroblastoma SK-N-MC cells. Peptides. 1995;16:421–424. doi: 10.1016/0196-9781(94)00195-c. [DOI] [PubMed] [Google Scholar]