Abstract
Background and purpose:
The endocannabinoid virodhamine is a partial agonist at the cannabinoid CB1 receptor and a full agonist at the CB2 receptor, and relaxes rat mesenteric arteries through endothelial cannabinoid receptors. Its concentration in the periphery exceeds that of the endocannabinoid anandamide. Here, we examined the influence of virodhamine on the human pulmonary artery.
Experimental approach:
Isolated human pulmonary arteries were obtained during resections for lung carcinoma. Vasorelaxant effects of virodhamine were examined on endothelium-intact vessels precontracted with 5-HT or KCl.
Key results:
Virodhamine, unlike WIN 55,212-2, relaxed 5-HT-precontracted vessels concentration dependently. The effect of virodhamine was reduced by endothelium denudation, two antagonists of the endothelial cannabinoid receptor, cannabidiol and O-1918, and a high concentration of the CB1 receptor antagonist rimonabant (5 μM), but only slightly attenuated by the NOS inhibitor L-NAME and not affected by a lower concentration of rimonabant (100 nM) or by the CB2 and vanilloid receptor antagonists SR 144528 and capsazepine, respectively. The COX inhibitor indomethacin and the fatty acid amide hydrolase inhibitor URB597 and combined administration of selective blockers of small (apamin) and intermediate and large (charybdotoxin) conductance Ca2+-activated K+ channels attenuated virodhamine-induced relaxation. The vasorelaxant potency of virodhamine was lower in KCl- than in 5-HT-precontracted preparations.
Conclusions and implications:
Virodhamine relaxes the human pulmonary artery through the putative endothelial cannabinoid receptor and indirectly through a COX-derived vasorelaxant prostanoid formed from the virodhamine metabolite, arachidonic acid. One or both of these mechanisms may stimulate vasorelaxant Ca2+-activated K+ channels.
Keywords: endothelial cannabinoid receptor, virodhamine, cannabidiol, O-1918, KCa channels, human pulmonary artery
Introduction
Acute and chronic use of cannabis in human elicits complex cardiovascular effects (Jones, 2002). In addition, an increase in the levels of endogenously formed cannabinoids has been reported in the serum of patients with septic shock (Wang et al., 2001) and with liver cirrhosis (Fernández-Rodriguez et al., 2004). In this respect, it is important to note that endogenous cannabinoids are involved in the hypotension occurring in animal models of the latter diseases (see Pacher et al., 2006). The mechanisms underlying the cardiovascular effects of exo- and endocannabinoids have been examined mainly in experimental animals (see Pacher et al., 2006), and the effects of endocannabinoids on human vessels have been investigated only in a few studies. Thus, the endocannabinoid anandamide failed to relax myometrial arteries isolated from pregnant women (Kenny et al., 2002). The same compound increased human skin microcirculatory flow; however, this effect was obtained only if it was administered on the skin and not if it was injected intravascularly (Movahed et al., 2005). In our own hands, anandamide relaxed isolated human pulmonary artery rings (Kozłowska et al., 2007).
Anandamide exerts its cardiovascular effects in mice and rats through cannabinoid CB1 and CB2 receptors and vanilloid TRPV1 receptors (see Begg et al., 2005; Pacher et al., 2006). In addition, it relaxes isolated blood vessels of mice (Járai et al., 1999) and rats (Járai et al., 1999; Wagner et al., 1999; Ho and Hiley, 2003; Offertáler et al., 2003; Milman et al., 2006), and decreases blood pressure in mice (Járai et al., 1999; Offertáler et al., 2003) through an as yet unidentified endothelial cannabinoid receptor, which is also activated by the cannabinoid, abn-cbd. The latter receptor appears to be involved in the effect of anandamide in the human pulmonary artery, as the vasorelaxation was mimicked by abn-cbd, and the effect of the latter was counteracted by two antagonists of the endothelial cannabinoid receptor (Kozłowska et al., 2007). In recent years, anandamide and abn-cbd have been reported to activate the GPR55 receptor also, an orphan cannabinoid receptor (Pertwee, 2007; Ryberg et al., 2007). However, no differences were noticed in hypotension and vasorelaxation of isolated mesenteric arteries elicited by abn-cbd between GPR55 knockout mice and their wild-type littermate controls (Johns et al., 2007) arguing against the hypothesis that the GPR55 receptor is the endothelial cannabinoid receptor.
It is possible that the endothelial cannabinoid receptor in the human pulmonary artery could be a target for the treatment of pulmonary hypertension (Kozłowska et al., 2007). To assess further the properties of this receptor in our experimental model, we studied the effect of another endocannabinoid, virodhamine. Virodhamine (O-arachidonoyl ethanolamine) is not only a partial agonist at the CB1 and a full agonist at the CB2 receptor (Porter et al., 2002), but also a full agonist at the endothelial cannabinoid receptor (Ho and Hiley, 2004) with a potency even slightly higher than that of abn-cbd (Ho and Hiley, 2003). It also displays a markedly greater potency at GPR55 than at CB1 or CB2 receptors and a particularly high efficacy at GPR55 receptors (Pertwee, 2007; Ryberg et al., 2007; McHugh et al., 2008). The concentration of virodhamine in peripheral tissues is two- to ninefold higher than that of anandamide (Porter et al., 2002). For comparison, the effect of WIN 55,212-2, a synthetic agonist at CB1 and CB2 receptors without effect on the endothelial cannabinoid receptor (Pacher et al., 2006), was studied as well.
Methods
All protocols were approved by the Human Ethics Committee of the Medical University of Białystok.
Tissue preparation
Human lung tissue was obtained from 54 patients (46 men and 8 women, mean age 58.2±0.9 years; including 78.4% smokers) undergoing lobectomy or pneumonectomy during resection of lung carcinoma. Preoperative echocardiography revealed normal left and right ventricular function in each case. Patients did not have any clinical evidence of pulmonary hypertension nor did they receive β-blockers. Before the operation, all patients received cephalosporins and low-molecular-weight heparin as anti-infection and antithrombotic prophylaxis, respectively. The tissue was transported to the laboratory within half an hour in cold (4 °C), pregassed Tyrode's bicarbonate solution (for composition, see below). Lobar and segmental pulmonary artery branches were cleaned from lung parenchyma and cut into rings (from the middle portion of each artery; 3–5 mm length and 2–4 mm outer diameter).
Organ bath technique
The arterial rings were suspended on stainless steel wires in 10 mL organ baths containing Tyrode's solution (concentration in mM: NaCl, 139.2; KCl, 2.7; CaCl2, 1.8; MgCl2, 0.49; NaHCO3, 11.9; NaH2PO4, 0.4; glucose, 5.5 and pH 7.4), gassed continuously with 95% O2 and 5% CO2, at 37 °C. Pulmonary artery rings were allowed to equilibrate for 90 min; during this time period, the bath fluid was changed every 10 min with fresh Tyrode's solution. The optimal resting tension was 2–2.5 g (depending on the rings' internal diameter), which ensured that responses to agonists were maximal. Muscle tension was recorded by a force displacement transducer (PIM 100RE, BIO-SYS-TECH, Białystok, Poland) and displayed on a computer.
Concentration–response curves
After the equilibration period, all rings were constricted submaximally with phenylephrine (10 μM) to prime the tissues and to check the functionality of the endothelium (at least 80% relaxation in response to acetylcholine 1 μM). When the endothelium was not required, vessels were denuded by rubbing the intimal surface mechanically, and successful endothelial removal was confirmed by a lack of vasorelaxant response to acetylcholine. To examine the vasorelaxant effect of virodhamine, WIN 55,212-2 and iloprost, arteries were constricted submaximally with 5-HT (creatinine sulphate complex; 0.5–1.2 μM; for details see Table 1) or high K+ (60 mM) Tyrode's solution, which was prepared by equimolar substitution of NaCl by KCl. Then cumulative concentration–response curves to virodhamine were constructed.
Table 1.
Contractile responses induced by 5-HT or KCl and pEC25 values for the vasorelaxant effects of virodhamine in isolated human pulmonary arteries
| Vasoconstrictor agents | Concentration | Group | Endothelium | n | Tension (mN) | pEC25 |
|---|---|---|---|---|---|---|
| 5-HT | 1 μM | Control rings | Intact | 37 | 7.85±0.49 | 5.67±0.08 |
| Rimonabant (100 nM) | 5 | 7.55±0.68 | 5.35±0.10 | |||
| Rimonabant (5 μM) | 5 | 8.63±1.17 | 4.93±0.22*** | |||
| O-1918 (10 μM) | 5 | 6.87±1.47 | 4.58±0.11*** | |||
| SR 144528 (1 μM) | 6 | 7.45±0.68 | 5.32±0.05 | |||
| Capsazepine (5 μM) | 6 | 7.65±0.98 | 5.68±0.17 | |||
| URB597 (1 μM) | 7 | 9.81±0.86 | 4.87±0.21*** | |||
| 1–1.2 μM | Cannabidiol (3 μM) | 5 | 7.84±0.69 | 4.50±0.15*** | ||
| 0.5–1 μM | Apa (0.3 μM)+Chtx (0.3 μM) | 5 | 9.81±1.56 | 4.88±0.13*** | ||
| 0.1–1 μM | L-NAME (300 μM) | 7 | 9.81±1.37 | 5.67±0.16 | ||
| 0.1–1 μM | Indomethacin (10 μM) | 7 | 9.50±1.08 | 4.60±0.15*** | ||
| 1 μM | Control rings | Denuded | 11 | 7.85±0.59 | 4.51±0.13*** | |
| O-1918 (10 μM)+indomethacin (10 μM)+URB597 (1 μM) | 5 | 8.33±0.9 | 4.50±0.14*** | |||
| KCl | 60 mM | Control rings | Intact | 4 | 10.70±3.90 | ND |
Abbreviations: Apa, apamin; Chtx, charybdotoxin; L-NAME, NG-nitro-L-arginine methyl ester, ND, not determined because the extent of vasorelaxant effect of virodhamine is very low under this condition.
Data are expressed as means±s.e.mean.
***P<0.001, compared with control rings (with intact endothelium; as determined by one-way ANOVA followed by Dunnett's post hoc test) precontracted with 5-HT (1 μM).
To examine the mechanisms involved in the vasorelaxant effect of virodhamine, rings were treated for 30 min with the following antagonists and enzyme or ion channel inhibitors: cannabidiol 3 μM and O-1918 10 μM (putative endothelial cannabinoid receptor; Kozłowska et al., 2007); and rimonabant 100 nM and 5 μM (CB1 receptor and putative endothelial cannabinoid receptor, respectively; Járai et al., 1999; O'Sullivan et al., 2004); SR 144528 1 μM (CB2 receptor; Zoratti et al., 2003), capsazepine 5 μM (vanilloid TRPV1 receptor; Járai et al., 1999); indomethacin 10 μM (COX; Kozłowska et al., 2007); L-NAME 300 μM (NOS; Kozłowska et al., 2007); URB597 1 μM (FAAH; Di Marzo et al., 2005) and apamin 0.3 μM given together with charybdotoxin 0.3 μM (blockers of Ca2+-sensitive K+ channels; Kozłowska et al., 2007). In control tissues, the respective vehicles were used instead. In each individual preparation, only one experimental curve was determined.
To examine the antagonistic properties of virodhamine against 5-HT-induced contraction, some rings were treated for 30 min with one concentration (10 μM) of virodhamine or its vehicle (control tissues), and then a concentration–response curve for 5-HT (0.01–100 μM) was constructed.
Calculations and statistical analysis
The relaxation produced with virodhamine, WIN 55,212-2 and iloprost or their solvents was expressed as the percentage relaxation of the precontraction induced by 5-HT or KCl. To determine the potency of agonists, EC50 values (that is, the concentrations causing the half maximum effect) were determined from the individual concentration–response curves. A series of experimental procedures markedly attenuated the vasodilator effect of virodhamine. To have a rough measure for its potency under such conditions, the concentration causing a relaxation of 25% of the precontracted vessel was determined (EC25). The EC50 and EC25 values were transformed into pEC50 and pEC25 values, that is, the negative logarithms of EC50 and EC25 values, respectively.
The antagonistic potency (estimated pKb) of cannabidiol, O-1918 and rimonabant against virodhamine (vasorelaxation) was calculated from the equation:
where (B) is the molar concentration of the antagonist and CR is the concentration ratio of the EC25 values of virodhamine in the presence and absence of the antagonist.
Results are expressed as means±s.e.mean of n experiments. Statistical analyses were performed using the t-test for unpaired data. When two or more treatment groups were compared with the same control, ANOVA followed by Dunnett's test was used. Differences were considered as significant when P<0.05.
Drugs used
(−)-Phenylephrine hydrochloride, acetylcholine chloride, 5-HT, apamin, charybdotoxin, L-NAME, indomethacin (Sigma, Munich, Germany) were dissolved in deionized water with the exception of 5-HT (dissolved in 0.1 N HCl) and indomethacin (dissolved in 0.5 M NaHCO3). Iloprost (Schering AG, Berlin, Germany) was dissolved in 0.9% NaCl. Virodhamine, (−)-cannabidiol, capsazepine (Tocris, Bristol, UK), O-1918 (1,3-dimethoxy-5-methyl-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-benzene), URB597 (3′-(aminocarbonyl)[1,1′-biphenyl]-3-yl)-cyclohexylcarbamate; Cayman Chemicals, Ann Arbor, MI, USA); rimonabant and SR 144528 (Sanofi-Aventis, Montpellier, France) were supplied in 100% ethanol. WIN 55,212-2 (R(+) [2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)-methanone mesylate) (RBI, Natick, MA, USA) was dissolved in a mixture of water and DMSO (4:6).
Results
General
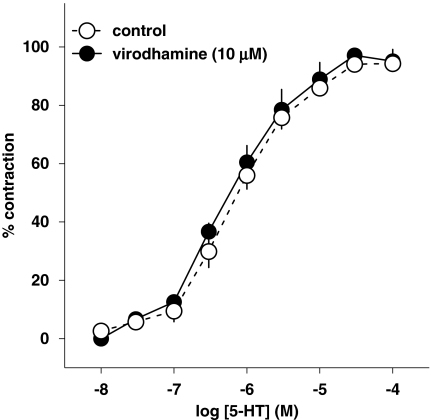
5-HT (0.01–100 μM) induced a concentration-dependent contraction of human pulmonary artery rings (pEC50=5.98±0.26; Figure 1). Virodhamine (10 μM) failed to modify the concentration–response curve for 5-HT (pEC50=6.24±0.21; Figure 1).
Figure 1.
Concentration–response curve of 5-HT for its contractile effect on endothelium-intact human pulmonary arteries and its interaction with virodhamine. Results are expressed as percentage of maximal contraction. Means±s.e.mean of five arteries for each curve. In many cases, s.e.mean is smaller than or equal to the size of symbols.
The vasorelaxant effects of virodhamine, WIN 55,212-2 and iloprost on the human pulmonary artery were examined in vascular rings precontracted with 5-HT using a concentration of 1 μM (that is, approximately equivalent to its EC60) or with 60 mM KCl (virodhamine only); the majority of the experiments was carried out on preparations precontracted with 5-HT. As L-NAME, indomethacin and K+ channel blockers slightly increased and cannabidiol slightly decreased the resting tension of pulmonary rings by themselves, a reduced (0.5–1 μM) and higher (1–1.2 μM) concentration of 5-HT was used, respectively (for details, see Table 1). Virodhamine (0.1–100 μM) and the other drugs under study did not affect the tone of vessels not precontracted with 5-HT, when given alone (results not shown).
Vasorelaxant effect of virodhamine and WIN 55,212-2
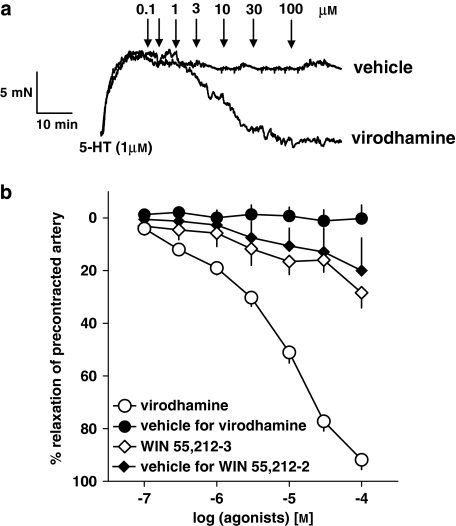
As shown in Figure 2, the endocannabinoid virodhamine (0.1–100 μM), but not its vehicle (0.01–1% v/v; ethanol), produced a concentration-dependent relaxation of endothelium-intact isolated human pulmonary arteries precontracted with 5-HT (for original traces, see Figure 2a). A full relaxation (96.2±3.1%, n=37; expressed as percentage of relaxation of the tone induced by 5-HT 1 μM) was induced by the highest concentration of the agonist. The pEC50 value for the vasorelaxant effect of virodhamine in vessels precontracted with 5-HT was 5.07±0.07 (n=37). We did not observe any differences in virodhamine-elicited relaxation of 5-HT-precontracted human pulmonary arteries isolated from smokers and non-smokers; this refers to the potency (pEC50 of 5.08±0.09 (n=29) and 5.14±0.10 (n=8), respectively) and the maximum effect (results not shown). The vasorelaxant effect of virodhamine was gradual and it took 46.5±4.1 min (n=37) to construct complete concentration–response curves.
Figure 2.
Representative original traces of vasorelaxant effects of virodhamine and its vehicle (0.01–1% v/v; ethanol; a) and concentration–response curves of virodhamine and WIN 55,212-2 for their effects on endothelium-intact rings of human pulmonary artery, precontracted with 5-HT (b). (a) Arrows show the moment of application of the particular concentrations of virodhamine or of vehicle. (b) Results are expressed as percentage relaxation of the isometric contraction induced by 5-HT. Means±s.e.mean of 5–37 tissues for each curve. In few cases, s.e.mean is smaller than or equal to the size of symbols.
Figure 2b also shows the effect of the synthetic cannabinoid receptor agonist WIN 55,212-2 (0.1–100 μM) and of its vehicle DMSO (0.06–0.92% v/v) on the rings of human pulmonary arteries precontracted with 5-HT. Under both experimental conditions, a slight and virtually identical vasorelaxant effect was obtained (Figure 2b).
Influence of cannabinoid and TRPV1 receptor antagonists on the virodhamine-induced relaxation
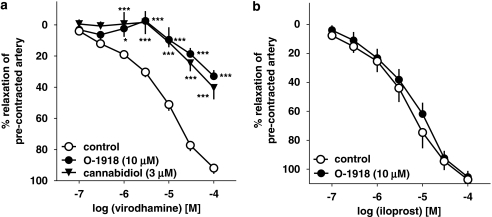
Two antagonists of the putative endothelial cannabinoid receptor, cannabidiol (3 μM) and O-1918 (10 μM), shifted the concentration–response curve for virodhamine to the right by factors of 13.8 and 11.5 (Figure 3a), yielding estimated pKb values of 6.65±0.17 (n=5) and 6.26±0.17 (n=5), respectively (for the respective pEC25 values, see Table 1). In contrast, O-1918 (10 μM) did not affect the relaxant effect of the stable analogue of prostacyclin (PGI2), iloprost (0.1–100 μM; mean pEC50 values for iloprost were 5.43±0.14 (n=5) and 5.24±0.14 (n=5) in control and O-1918-treated rings (Figure 3b)).
Figure 3.
Influence of cannabidiol and O-1918 on the concentration–response curve of virodhamine (a) or iloprost (b) for their relaxant effects on 5-HT-precontracted endothelium-intact human pulmonary arteries. Results are expressed as percentage relaxation of the isometric contraction induced by 5-HT. Means±s.e.mean of 5–37 tissues for each curve. *P<0.05, ***P<0.001 compared with the respective control (virodhamine alone; as determined by one-way ANOVA followed by Dunnett's post hoc test). In few cases, s.e.mean is smaller than or equal to the size of symbols.
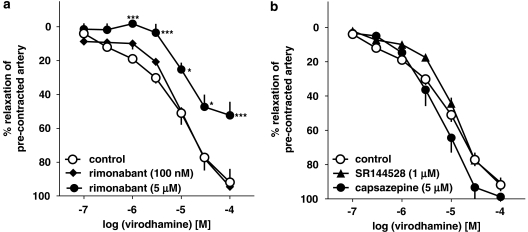
The vasorelaxant effect of virodhamine was not modified by the CB1 receptor antagonist rimonabant given at a concentration of 100 nM, known to block CB1 receptors. However, a higher concentration of this antagonist (5 μM), which also antagonizes endothelial cannabinoid receptors, produced a 5.1-fold shift to the right of the concentration–response curve of virodhamine (Figure 4a). Its estimated pKb value was 5.87±0.28 (n=5; for the respective pEC25 value, see Table 1). The CB2 receptor antagonist SR144528 (1 μM) and the TRPV1 receptor antagonist capsazepine (5 μM) failed to affect virodhamine-induced relaxations (Figure 4b).
Figure 4.
Influence of rimonabant (a), SR144528 and capsazepine (b) on the concentration–response curve of virodhamine for its relaxant effect on 5-HT-precontracted endothelium-intact human pulmonary arteries. Results are expressed as percentage relaxation of the isometric contraction induced by 5-HT. Means±s.e.mean of 5–37 tissues for each curve. *P<0.05, ***P<0.001 compared with the respective control (virodhamine alone; as determined by one-way ANOVA followed by Dunnett's post hoc test). In few cases, s.e.mean is smaller than or equal to the size of symbols.
Influence of endothelium removal, L-NAME, indomethacin and URB597 on the virodhamine-induced relaxation
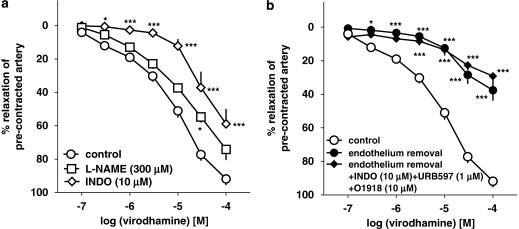
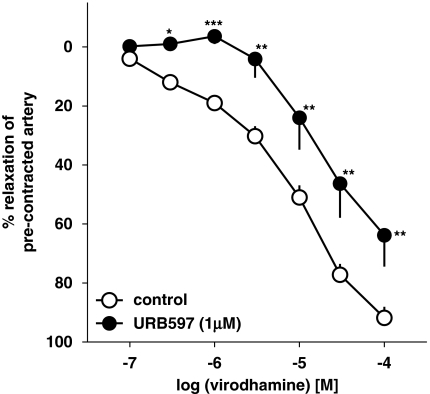
The virodhamine-induced vasorelaxation of endothelium-intact human pulmonary arteries was attenuated by the COX inhibitor indomethacin (10 μM), which shifted its concentration–response curve to the right by a factor of 9.1 (Figure 5a). The NOS inhibitor L-NAME (300 μM) only slightly attenuated the vasorelaxant effect of the three highest concentrations of virodhamine; a statistically significant influence was noticed for virodhamine 30 μM only (Figure 5a; see Table 1 for pEC25 values). The FAAH inhibitor URB597 (1 μM) produced a 5.9-fold rightward shift of the concentration–response curve for virodhamine (Figure 6; for pEC25 values, see Table 1).
Figure 5.
Influence of NG-nitro-L-arginine methyl ester (L-NAME) and indomethacin (INDO; a), endothelium removal and endothelium removal plus combined administration of O-1918, INDO and URB597 (b) on the concentration–response curve of virodhamine for its relaxant effect on human pulmonary arteries. Results are expressed as percentage relaxation of the isometric contraction elicited by 5-HT. Means±s.e.mean of 5–37 arteries for each curve. *P<0.05, ***P<0.001 compared with the respective control (virodhamine alone in endothelium-intact rings; as determined by one-way ANOVA followed by Dunnett's post hoc test). In few cases, s.e.mean is smaller than or equal to the size of symbols.
Figure 6.
Influence of URB597 on the concentration–response curve of virodhamine for its relaxant effect on 5-HT-precontracted endothelium-intact rings of human pulmonary arteries. Results are expressed as percentage relaxation of the isometric contraction induced by 5-HT. Means±s.e.mean of 7–37 arteries for each curve. *P<0.05, **P<0.01, ***P<0.001 compared with the respective control (virodhamine alone; as determined by the t-test for unpaired data). In many cases, s.e.mean is smaller than or equal to the size of symbols.
Removal of endothelium (which completely abolished the relaxation induced by acetylcholine 1 μM; data not shown) strongly reduced the vasorelaxant effect of virodhamine (Figure 5b), which amounted to 37.6±6.2% of relaxation of the precontracted vessels at 100 μM (n=11). Pretreatment of endothelium-denuded rings with the combination of O-1918 (10 μM), indomethacin (10 μM) and the FAAH inhibitor URB597 (1 μM) did not further amplify the inhibitory effect of endothelium removal on the vasorelaxant response to virodhamine (Figure 5b).
Influence of KCl (60 mM) and of K+ channel blockers on the virodhamine-induced relaxation
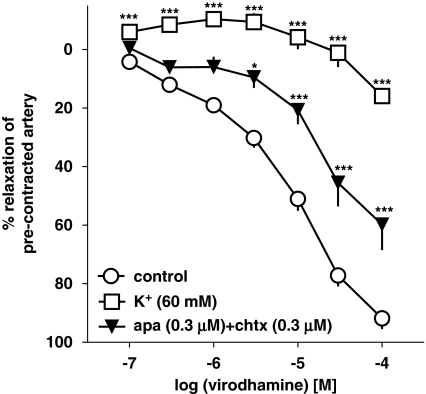
Next, the vasodilator effect of virodhamine was studied in endothelium-intact human pulmonary arteries precontracted with KCl (60 mM) and its effect in 5-HT-precontracted vessels was compared. KCl (60 mM) induced an increase in vessel tone comparable to that elicited by 5-HT (1 μM; see Table 1). However, it markedly attenuated the virodhamine-induced relaxation, reducing its effect obtained for the highest dose of the agonist to 15.9±2.2% (n=4; Figure 7). For this reason, we were not able to determine the pEC25 value for the vasorelaxant effect of virodhamine in KCl-precontracted vessels. Figure 7 also shows that combination of apamin (selective blocker of small conductance Ca2+-activated K+ channels SKCa; 0.3 μM) and charybdotoxin (selective blocker of intermediate conductance Ca2+-activated K+ channels IKCa and large conductance Ca2+-activated K+ channels BKCa; 0.3 μM) diminished the virodhamine-induced relaxation by shifting its concentration–response curve to the right by a factor of 5.8 (for pEC25 values, see Table 1).
Figure 7.
Concentration–response curves for virodhamine-induced relaxation of 5-HT and of KCl (60 mM) precontracted endothelium-intact human pulmonary arteries and interaction of virodhamine with a combination of the K+ channel blockers, apamin (apa) and charybdotoxin (chtx). Results are expressed as percentage relaxation of the isometric contraction induced by KCl (open squares) or 5-HT (other experiments). Means±s.e.mean of 4–37 arteries for each curve. *P<0.05, ***P<0.001 compared with the respective control (that is, 5-HT-precontracted arteries not exposed to K+ channel blockers; as determined by one-way ANOVA followed by Dunnett's post hoc test). In many cases, s.e.mean is smaller than or equal to the size of symbols.
Discussion
This study was carried out to further characterize the cannabinoid-induced vasorelaxation of the human pulmonary artery described by Kozłowska et al. (2007) and is based on experiments with virodhamine, an endocannabinoid and WIN 55,212, a synthetic cannabinoid receptor agonist. To precontract the human pulmonary artery rings, we used 5-HT because of the extensive physiological function of endothelium in this circulatory bed and also because of the fact that 5-HT has a major function as vasoconstrictor in the pulmonary circulation (Dempsie and MacLean, 2008). The 5-HT-induced contraction of the human pulmonary artery is predominantly, if not exclusively, related to the activation of 5-HT2A receptors (Kozłowska et al., 2007).
Receptor involved in the virodhamine-induced relaxation
Virodhamine caused full relaxation of the endothelium-intact isolated human pulmonary artery. The majority (80%) of the pulmonary arteries used in our study were obtained from smokers and this might mean that the effect of virodhamine represents an abnormal rather than the real physiological response. This possibility, however, is very unlikely as there was no difference in the response to this agonist in tissue taken from smoking and non-smoking patients. As observed in abn-cbd (Kozłowska et al., 2007), the relaxation elicited by virodhamine developed slowly and, for both of these agonists, the construction of the complete concentration–response curves took about 50 min. However, in comparison with abn-cbd, virodhamine exhibited a higher potency (pEC50 4.80±0.06; n=23, and 5.07±0.07; n=37, respectively; P<0.01). Similar differences in the potencies of abn-cbd and virodhamine were also observed in the rat mesenteric artery (pEC50 6.2 and 6.4; Ho and Hiley, 2003, 2004). Note that the level of potencies of the two agonists is higher in the rat mesenteric artery (Ho and Hiley, 2003, 2004) than in the human pulmonary artery (Kozłowska et al., 2007; this study). Importantly, the two endocannabinoids studied so far in the human pulmonary artery, anandamide (Kozłowska et al., 2007) and virodhamine (this study), exhibited higher potencies than the synthetic cannabinoid abn-cbd and, in contrast with abn-cbd, did not relax the basal tone by themselves (Kozłowska et al., 2007; this study).
Which type of receptor was involved in the vasorelaxant effect of virodhamine? First, the most trivial explanation, namely that this effect is because of antagonism of the 5-HT used to precontract the vessels, can be excluded as virodhamine (10 μM) did not modify the contractor response of the pulmonary artery rings to 5-HT. Second, the lack of a vasorelaxant effect of WIN 55,212-2 argues against the involvement of CB1 and CB2 receptors in the effect of virodhamine. The involvement of CB1 receptors can also be excluded as the selective CB1 receptor antagonist rimonabant did not influence the effect of virodhamine at a concentration of 100 nM at which it antagonizes CB1 receptor-mediated effects in rat resistance and conduit arteries (O'Sullivan et al., 2004). Moreover, the CB2 receptor antagonist SR 144528, used at a concentration of 1 μM known to block CB2 receptor-mediated response in calf pulmonary endothelial cells (Zoratti et al., 2003), failed to modify the response to virodhamine. Third, the possibility that virodhamine acts through TRPV1 receptors (at which it exhibits a modest effect; Ho and Hiley, 2004) can be excluded because the selective TRPV1 receptor antagonist capsazepine did not affect the virodhamine-induced relaxation in a concentration range at which it antagonizes TRPV1 receptor-mediated effects, for instance, in the rat mesenteric artery (Járai et al., 1999). Fourth, the involvement of the recently characterized GPR55 receptor in the effect of virodhamine had also to be considered, as it is an agonist at this receptor (Pertwee, 2007; Ryberg et al., 2007; McHugh et al., 2008). However, this possibility is very unlikely as recent experiments with a GPR55 receptor knockout mouse clearly showed that this receptor does not lead to a vasodilator response (Johns et al., 2007). Nevertheless, the GPR55 receptor might occur in the pulmonary, as opposed to the systemic, circulation or in human, as opposed to rodent, blood vessels.
Experiments with three antagonists at the endothelial cannabinoid receptor suggest that this receptor was involved in the vasorelaxant effect of virodhamine. Cannabidiol and O-1918 antagonized the effect of virodhamine at concentrations at which they had been previously shown to antagonize the endothelial cannabinoid receptor-mediated vasodilatation in the rat mesenteric (Ho and Hiley, 2003) or human pulmonary artery (Kozłowska et al., 2007). Cannabidiol has a low affinity for cannabinoid CB1 and CB2 receptors in binding studies, but a moderate potency as a non-competitive antagonist against CB1 and CB2 receptor agonists (Thomas et al., 2007). O-1918 does not bind to CB1 or CB2 receptors and does not induce relaxation up to 30 μM (Offertáler et al., 2003) and may be superior to cannabidiol as an antagonist of endothelial cannabinoid receptors. Interestingly enough, both antagonists exhibited a higher potency in the human pulmonary artery towards virodhamine (this study) when compared with abn-cbd (Kozłowska et al., 2007) The respective pA2 values were 6.6 and 5.9 for cannabidiol and 6.3 and 5.1 for O-1918 (for the possible explanation of this discrepancy, see below). The inhibitory effect of the higher concentration of rimonabant (5 μM) against the vasorelaxant effect of virodhamine in the human pulmonary artery is also compatible with the involvement of the endothelial cannabinoid receptor in this effect (Járai et al., 1999; Wagner et al., 1999; Ho and Hiley, 2003).
Endothelial mechanisms involved in the virodhamine-induced relaxation
The vasorelaxant effect of virodhamine (this study), like that of abn-cbd (Kozłowska et al., 2007), is endothelium-dependent, that is, removal of the endothelium reduces the vasorelaxant effect of both agonists by about 50% in the human pulmonary artery. In contrast, the vasorelaxant effect of virodhamine was reduced by about 80% in the endothelium-denuded rat mesenteric artery (Ho and Hiley, 2004). Thus, the vasorelaxant action of virodhamine, similar to that of abn-cbd, consists of an endothelium-dependent and endothelium-independent component. In this study, we focused on the endothelium-dependent action of virodhamine.
The next question addressed was which endothelium-derived vasodilator(s) is (are) involved in the virodhamine-induced vasodilator effect. The endothelial cannabinoid receptor is usually connected to the release of endothelium-derived hyperpolarizing factor, as shown in animal models (see Randall et al., 2004) and in the human pulmonary artery (Kozłowska et al. 2007). Two sets of experiments show that this mechanism is also implicated in the vasorelaxant effect of virodhamine in this study. First, raising the extracellular concentration of K+ (60 mM KCl) markedly attenuated the virodhamine-induced relaxation compared with preparations precontracted with 5-HT. This indicates that the virodhamine-stimulated relaxation is partially mediated by the activation of K+ channels, as a high extracellular K+ concentration abolishes the electrochemical gradient for the K+ efflux. Second, the vasorelaxant effect in our model was markedly attenuated by a combination of apamin (a selective blocker of small conductance (SKCa) Ca2+-activated K+ channels) and charybdotoxin (an inhibitor of intermediate (IKCa) and large conductance (BKCa) Ca2+-activated K+ channels).
The effect of virodhamine might also be related to nitric oxide, one of the main endothelial vasodilators (Moncada and Higgs, 2006). However, in our hands, L-NAME, an inhibitor of the biosynthesis of nitric oxide by endothelial cells, caused only a modest inhibition of the vasorelaxant effect of the highest concentrations of this endocannabinoid, suggesting that nitric oxide has only a minor function in the effect of virodhamine. Nitric oxide is not involved at all in the vasorelaxant effects of virodhamine in the rat mesenteric artery (Ho and Hiley, 2004) and of abn-cbd in the human pulmonary artery (Kozłowska et al. 2007).
An additional mechanism involved in the virodhamine-induced relaxation
The possibility that the effect of virodhamine is related to the increased formation of PGI2 (or another vasorelaxant product of COX activity) had also to be considered. This was likely as the virodhamine-induced vasorelaxation was attenuated by indomethacin, an inhibitor of COX. This effect might be because of an increase in COX activity following the activation of the endothelial cannabinoid receptor. There is, however, an entirely different possibility to account for this phenomenon, that is, that arachidonic acid formed from virodhamine is converted to PGI2. This latter possibility is suggested by a series of our results.
The endocannabinoids known so far are derivatives of, and are degraded to, arachidonic acid from which vasorelaxant metabolites may be formed. The vasodilator effect of anandamide in bovine (Pratt et al., 1998) and sheep coronary arteries (Grainger and Boachie-Ansah, 2001) or in rabbit mesenteric arteries (Fleming et al., 1999) is related to this mechanism. Unfortunately, very little is known, at present, of the degradation pathways for virodhamine (Porter et al., 2002; De Petrocellis et al., 2004). It has been reported that, in human neocortex, this endocannabinoid can interact with FAAH (Steffens et al., 2005), an enzyme known to hydrolyse anandamide to arachidonic acid. In our studies, we used the selective and potent FAAH inhibitor URB597 (Di Marzo et al., 2005) at a concentration of 1 μM, which has been shown to amplify the relaxation of the rat small mesenteric arteries to anandamide, but not that to 2-arachidonoylglycerol, another endocannabinoid (Ho and Randall, 2007). We found that blockade of FAAH by URB597 attenuated the vasorelaxant effect of virodhamine. This finding is in harmony with the concept of the involvement of this enzyme in the metabolism of virodhamine. However, the effect of URB597 was unexpected at first glance. We would have even expected an increase in the vasorelaxant effect of virodhamine because of its impaired degradation and increased availability in the biophase of the endothelial cannabinoid receptor.
The formation of a vasorelaxant COX product may also explain why the effect of virodhamine is attenuated by indomethacin, whereas that of abn-cbd is not. Abn-cbd, unlike virodhamine, is chemically not related to arachidonic acid. Moreover, the differential potency of the two antagonists, cannabidiol and O-1918, against virodhamine and abn-cbd may be caused by the fact that in the case of virodhamine, the antagonist under study is competing with a much lower concentration of the endocannabinoids, as a considerable amount of the compound has already been degraded. This phenomenon will lead to an erroneous increase of the estimated pKb value. Finally, the fact that either enzyme inhibitor produced a similar effect on the virodhamine-related vasorelaxation may support the view that the two enzymes affect two subsequent steps of a metabolic pathway (although this phenomenon might be accidental as well). To our knowledge, this is the first report to demonstrate that the effects of virodhamine are sensitive to inhibition of FAAH or COX. We can exclude the possibility that virodhamine acts only through its vasorelaxant metabolite as O-1918, the antagonist of the endothelial cannabinoid receptor, inhibited the relaxation induced by virodhamine but not that elicited by iloprost, a stable PGI2 analogue. Interestingly, when endothelium-denuded human pulmonary arteries were exposed to O-1918, indomethacin plus URB597, the vasorelaxant effect of virodhamine was not attenuated suggesting that both its direct vasorelaxant effect through the endothelial cannabinoid receptor and its indirect effect through an endogenously formed prostanoid are connected with intact endothelium.
We have already suggested (Kozłowska et al. 2007) that the endothelial cannabinoid receptor may be a target for the treatment of pulmonary hypertension. A drug like virodhamine may offer a particular advantage over abn-cbd, as it has a dual action. It is of interest in this context that PGI2 receptor agonists are already in use for the treatment of this disorder in humans (Naeije and Huez, 2007).
Conclusions
Virodhamine causes a full, slowly developing relaxation of the isolated human pulmonary artery. This effect is partially dependent on vascular endothelium and results from two mechanisms, such as the activation of the putative endothelial cannabinoid receptor (but not of cannabinoid CB1, CB2 or vanilloid receptors and probably not of the GPR55 receptor) and the hydrolysis of virodhamine to arachidonic acid and subsequent production of a vasorelaxant prostanoid through COX. One or both of these mechanisms may stimulate vasorelaxant Ca2+-activated K+ channels. In the periphery, the concentration of virodhamine is higher than that of anandamide. Thus, its ability to relax human pulmonary arteries suggests that it may be a candidate for controlling vascular tone under physiological or pathophysiological conditions or even for therapeutic purposes.
Acknowledgments
This study was supported by grants from the Medical University of Białystok (3-13671 F and 3-13549 F). B Malinowska and E Schlicker were granted by the Foundation for Polish Science (FNP; Warsaw, Poland) and the German Research Foundation (DFG; Bonn, Germany) with the Copernicus Award. We thank Sanofi-Aventis for a gift of rimonabant and SR 144528.
Abbreviations
- abn-cbd
abnormal cannabidiol
- Apa
apamin
- Chtx
charybdotoxin
- DMSO
dimethyl sulphoxide
- FAAH
fatty acid amide hydrolase
- L-NAME
NG-nitro-L-arginine methyl ester
- O-1918
1,3-dimethoxy-5-methyl-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-benzene
- SR 144528
N-[(1S)-endo-1,3,3-trimethyl bicyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide
- URB597
3′-(aminocarbonyl)[1,1′-biphenyl]-3-yl)-cyclohexylcarbamate
- WIN 55,212-2
R(+) [2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)-methanone mesylate
Conflict of interest
The authors state no conflict of interest.
References
- Begg M, Pacher P, Bátkai S, Osei-Hyiaman D, Offertáler L, Mo FM, et al. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106:133–145. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsie Y, MacLean MR.Pulmonary hypertension: therapeutic targets within the serotonin system Br J Pharmacol 2008 10.1038/bjp.2008.241e-pub ahead of print: advance online 9 June 2008, doi [DOI] [PMC free article] [PubMed]
- Di Marzo V, De Petrocellis L, Bisogno T.The biosynthesis, fate and pharmacological properties of endocannabinoids Cannabinoids, Handbook of Experimental Pharmacology 2005Springer: Berlin, Heidelberg, New York; 147–185.In: Pertwee RG (ed)vol 168 [DOI] [PubMed] [Google Scholar]
- Fernández-Rodriguez CM, Romero J, Petros TJ, Bradshaw H, Gasalla JM, Gutiérrez ML, et al. Circulating endogenous cannabinoid anandamide and portal, systemic and renal hemodynamics in cirrhosis. Liver Int. 2004;24:477–483. doi: 10.1111/j.1478-3231.2004.0945.x. [DOI] [PubMed] [Google Scholar]
- Fleming I, Schermer B, Popp R, Busse R. Inhibition of the production of endothelium-derived hyperpolarizing factor by cannabinoid receptor agonists. Br J Pharmacol. 1999;126:949–960. doi: 10.1038/sj.bjp.0702381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger J, Boachie-Ansah G. Anandamide-induced relaxation of sheep coronary arteries: the role of the vascular endothelium, arachidonic acid metabolites and potassium channels. Br J Pharmacol. 2001;134:1003–1012. doi: 10.1038/sj.bjp.0704340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol. 2003;138:1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasorelaxant activities of the putative endocannabinoid virodhamine in rat isolated small mesenteric artery. J Pharm Pharmacol. 2004;56:869–875. doi: 10.1211/0022357023682. [DOI] [PubMed] [Google Scholar]
- Ho WS, Randall MD. Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br J Pharmacol. 2007;150:641–651. doi: 10.1038/sj.bjp.0707141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, et al. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol. 2007;152:825–831. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42 11 Suppl:58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- Kenny LC, Baker PN, Kendall DA, Randall MD, Dunn WR. The role of gap junctions in mediating endothelium-dependent responses to bradykinin in myometrial small arteries isolated from pregnant women. Br J Pharmacol. 2002;136:1085–1088. doi: 10.1038/sj.bjp.0704817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozłowska H, Baranowska M, Schlicker E, Kozłowski M, Laudañski J, Malinowska B. Identification of the vasodilatory endothelial cannabinoid receptor in the human pulmonary artery. J Hypertens. 2007;25:2240–2248. doi: 10.1097/HJH.0b013e3282ef7a0a. [DOI] [PubMed] [Google Scholar]
- McHugh D, Tanner C, Mechoulam R, Pertwee RG, Ross RA. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: evidence for a site distinct from CB1 and CB2. Mol Pharmacol. 2008;73:441–450. doi: 10.1124/mol.107.041863. [DOI] [PubMed] [Google Scholar]
- Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Bátkai S, et al. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci USA. 2006;103:2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147:S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahed P, Evilevitch V, Andersson TL, Jonsson BA, Wollmer P, Zygmunt PM, et al. Vascular effects of anandamide and N-acylvanillylamines in the human and skin microcirculation. Br J Pharmacol. 2005;146:171–179. doi: 10.1038/sj.bjp.0706313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeije R, Huez S. Expert opinion on available options treating pulmonary arterial hypertension. Expert Opin Pharmacother. 2007;8:2247–2265. doi: 10.1517/14656566.8.14.2247. [DOI] [PubMed] [Google Scholar]
- Offertáler L, Mo FM, Bátkai S, Begg M, Razdan RK, Martin BR, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. Heterogeneity in the mechanisms of vasorelaxation to anandamide in conduit rat mesenteric arteries. Br J Pharmacol. 2004;142:435–442. doi: 10.1038/sj.bjp.0705810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. GPR55: a new member of the cannabinoid receptor clan. Br J Pharmacol. 2007;152:984–986. doi: 10.1038/sj.bjp.0707464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- Pratt PF, Hillard CJ, Edgemond WS, Campbell WB. N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am J Physiol. 1998;274:H375–H381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA, O'Sullivan S. The complexities of the cardiovascular actions of cannabinoids. Br J Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens M, Schulze-Bonhage A, Surgers R, Feuerstein TJ. Fatty acid amidohydrolase in human neocortex-activity in epileptic and non-epileptic brain tissue and inhibition by putative endocannabinoids. Neurosci Lett. 2005;385:13–17. doi: 10.1016/j.neulet.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Járai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Ito Y, Hashiguchi T, Kitajima I, Yamakuchi M, et al. Simultaneous measurement of anandamide and 2-arachidonoylglycerol by polymyxin B-selective adsorption and subsequent high-performance liquid chromatography analysis: increase in endogenous cannabinoids in the sera of patients with endotoxic shock. Anal Biochem. 2001;294:73–82. doi: 10.1006/abio.2001.5015. [DOI] [PubMed] [Google Scholar]
- Zoratti C, Kipmen-Korgun D, Osibow K, Malli R, Graier WF. Anandamide initiates Ca2+ signaling via CB2 receptor linked to phosholipase C in calf pulmonary endothelial cells. Br J Pharmacol. 2003;140:1351–1362. doi: 10.1038/sj.bjp.0705529. [DOI] [PMC free article] [PubMed] [Google Scholar]