Abstract
Bone loss that causes aseptic loosening of orthopaedic implants is initiated by pro-inflammatory cytokines produced by macrophages in response to implant-derived wear particles. MAPK and NF-κB signaling pathways are activated by the particles; however, it is not clear which of the signaling pathways are important for the initial response to the wear particles and which are only involved at later steps in the process, such as osteoclast differentiation. Here, we show that the ERK1/2, p38, JNK, and NF-κB pathways are rapidly activated by the wear particles but that only the ERK1/2 and NF-κB pathways are required for the initial response to the wear particles, which include increases in TNFα promoter activity, TNFα mRNA expression, and secretion of TNFα protein. Moreover, ERK1/2 activation by wear particles is also required for increased expression of the transcription factor Egr-1 as well as Egr-1’s ability to bind to and activate the TNFα romoter. These results, together with our previous studies of the PI3K/Akt pathway, demonstrate that wear particles coordinately activate multiple signaling pathways and multiple transcription factors to stimulate production of pro-inflammatory cytokines, such as TNFα. The current study also demonstrates that the signaling pathways are activated to a much greater extent by wear particles with adherent endotoxin than by “endotoxin-free” wear particles. These results, together with those demonstrating the requirement for ERK1/2/Egr-1 and NF-κB, show that activation of these signaling pathways is responsible for the ability of adherent endotoxin to potentiate cytokine production, osteoclast differentiation, and bone loss induced by wear particles.
Keywords: Implant loosening, Wear particles, Osteolysis, Endotoxin, TNFα, ERK1/2, NF-κB
INTRODUCTION
Aseptic loosening of orthopaedic implants, or loosening in the absence of any clinical signs of infection, is initiated by wear particles generated from the surface of the implant (Greenfield et al., 2005; Wilkinson et al., 2005; Wright and Goodman, 2001). Wear particles stimulate the production of pro-inflammatory cytokines, including tumor necrosis factor-α (TNFα), interleukin-1 (IL-1), and interleukin-6 (IL-6) by macrophages (Greenfield et al., 2002; Wright and Goodman, 2001). These cytokines induce bone loss adjacent to the implant by promoting the differentiation of osteoclast precursors into mature osteoclasts, the cells responsible for the degradation of bone (Bi et al., 2001b; Greenfield et al., 2002). This bone loss causes pain, loss of function, and the need for revision surgery. Thus, aseptic loosening of orthopaedic implants is a multi-step process mediated primarily by wear particle-induced activation of macrophages.
TNFα is the best characterized pro-inflammatory cytokine involved in aseptic loosening. Mice lacking both TNFα receptors are partially protected from wear particle-induced osteolysis (Merkel et al., 1999; Schwarz et al., 2000). Similarly, inhibition of TNFα activity significantly decreases wear particle-induced osteoclast differentiation (Taki et al., 2007) and osteolysis (Childs et al., 2001). This cytokine likely also contributes to osteolysis in patients since a polymorphism at position −238 in the TNFα gene promoter is associated with an increased rate of aseptic loosening (Wilkinson et al., 2003). TNFα is also the first pro-inflammatory cytokine produced in response to wear particles (Akisue et al., 2002; Blaine et al., 1996; Kim et al., 2007), and contributes to production of other pro-inflammatory cytokines in response to LPS (Covert et al., 2005; Fong et al., 1989). By focusing on early signaling events responsible for TNFα production, this study examined the primary signaling pathways induced by wear particles prior to secondary activation of other signaling pathways by TNFα and other pro-inflammatory cytokines.
A growing body of evidence is consistent with the hypothesis that bacterially-derived endotoxins adherent to the surface of orthopaedic wear particles contribute to aseptic loosening despite the absence of clinical signs of infection (Greenfield et al., 2005; Nelson et al., 2005; Sundfeldt et al., 2006; Wright and Goodman, 2001). Both LPS, the classical endotoxin produced by Gram-negative bacteria, and TLR-4, the primary LPS receptor on mammalian cells, have been found in peri-prosthetic tissue from patients with aseptic loosening (Nalepka and Greenfield, 2004; Takagi et al., 2007). Immunostimulatory molecules produced by Gram-positive bacteria such as lipoteichoic acid (LTA) and peptidoglycan have biological effects similar to endotoxin (Hauschildt et al., 2000; Wang et al., 2000). Peptidoglycan exists in synovial membranes (Schrijver et al., 2000), and therefore also likely exists in peri-prosthetic tissue from patients with aseptic loosening. There are at least three potential sources of endotoxin and other bacterial molecules during aseptic loosening of orthopaedic implants. First, a subclinical biofilm of Gram-positive bacteria exists on many implants from patients with aseptic loosening (Greenfield et al., 2005; Tunney et al., 1999). Second, circulating endotoxin (Cooperstock et al., 1975; Inada et al., 1991) derived from the gut flora, minor infections, and dental procedures may adhere to the wear particles. In support of this, we have demonstrated that circulating LPS accumulates when “endotoxin-free” wear particles are implanted on the calvaria of mice (Tatro et al., 2007). Third, the manufacturing process may result in substantial amounts of adherent endotoxin on the implants themselves (Anonymous, 2003; Ragab et al., 1999).
Adherent endotoxin substantially stimulates the biological activity of orthopaedic wear particles (Greenfield et al., 2005; Nelson et al., 2005; Sundfeldt et al., 2006; Wright and Goodman, 2001). We have shown that this stimulation is partially due to LPS since it is inhibited, both in vitro and in vivo, by an inactivating missense mutation in TLR-4 (Bi et al., 2001a). To identify potential therapeutic targets which could reduce the effects of wear particles at the implant-bone microenvironment, our approach has therefore been to examine signaling pathways known to be activated by TLR-4 in response to soluble LPS. The PI3K/Akt, MAPK (ERK1/2, p38 and JNK) and NF-κB pathways are activated by soluble LPS in a TLR-4 dependent manner (Vogel et al., 2001) and are required for stimulation of TNFα production by soluble LPS (Baeuerle and Baltimore, 1996; Baldassare et al., 1999; Ojaniemi et al., 2003; Zhu et al., 2000); however, for p38 compare (Baldassare et al., 1999; Li et al., 2003; Lu et al., 1999; Zhu et al., 2000). We have recently demonstrated that activation of the PI3K/Akt pathway by wear particles depends on adherent endotoxin and contributes to TNFα production induced by wear particles (Smith et al., 2007). Wear particles also activate the MAPK and NF-κB pathways (Abbas et al., 2003; Akisue et al., 2002; Schwarz et al., 2000), and it has been reported that NF-κB activation by wear particles is dependent on adherent endotoxin (Akisue et al., 2002). NF-κB activation by wear particles is also required for TNFα promoter activation (Soloviev et al., 2002) and contributes to inflammatory responses induced by wear particles in vivo (Schwarz et al., 2000).
The ERK1/2, p38, JNK and NF-κB pathways were also reported to mediate wear particle-induced osteoclast differentiation in vitro (Abbas et al., 2003; Clohisy et al., 2004; Yamanaka et al., 2006). However, those studies (Abbas et al., 2003; Clohisy et al., 2004; Yamanaka et al., 2006) could not distinguish between direct effects of the pathways on particle-induced cytokine production versus downstream effects on osteoclastogenesis since these pathways are known to be required for osteoclast differentiation (David et al., 2002; Franzoso et al., 1997; Hotokezaka et al., 2002; Ikeda et al., 2004; Lee et al., 2002; Li et al., 2002; Li et al., 2003; Mansky et al., 2002; Matsumoto et al., 2000; Xing et al., 2002). In this regard, Li et al. (Li et al., 2003) reported that a p38 inhibitor blocks osteoclast differentiation induced by LPS but does not affect stimulation of cytokine production by LPS.
Thus, a major goal of the current study was to examine the direct effects of the MAPK and NF-κB pathways on particle-induced TNFα production by macrophages rather than on later events such as osteoclastogenesis. The second major goal of the current study was to determine whether adherent endotoxin contributes to activation of the signaling pathways by orthopaedic wear particles.
MATERIALS AND METHODS
Orthopaedic wear particles
Commercially pure titanium particles were obtained from Johnson Matthey Cat. no. 00681, Lot G11G04; Ward Hill, MA, USA), sterilized with 95% ethanol, and stored in PBS as described previously (Ragab et al., 1999). The titanium particles mimic the size (>90% of the particles are <3.6 µm in size (Taki et al., 2005)) and biological activity of authentic wear particles (Bi et al., 2001a; Kaar et al., 2001). These particles have substantial amounts of adherent endotoxin (12–33 EU/109 particles) as measured using the high-sensitivity version of the chromogenic Limulus Amebocyte Lysate (LAL) assay (Biowhittaker, Walkersville, MD) in the presence of β-glucan blockers (Nalepka and Greenfield, 2004). “Endotoxin-free” titanium particles were prepared by removal of >99.9% of the adherent endotoxin without altering the size or shape of the particles as previously described (Ragab et al., 1999).
Cell culture reagents
All cell culture media, supplements, and inhibitors were from lots that contained the lowest amount of endotoxin available and were tested for endotoxin as described in the previous paragraph (Nalepka and Greenfield, 2004). Endotoxin levels were <0.01 EU/ml for the highest concentration of all inhibitors and their vehicles used in the experiments. All cells were maintained (5% CO2 and 37°C) in Minimum Essential Medium (MEM; HyClone, Logan, Utah) supplemented with 10% heat inactivated fetal bovine serum (FBS; HyClone), 2 mM L-glutamine (Mediatech, Herndon, VA), non-essential amino acids (Mediatech), 100 U/ml penicillin, and 100 µg/ml streptomycin (Mediatech). Serum-free media was MEM supplemented with 0.1% low endotoxin bovine serum albumin (BSA; Cat. no. 63K1249; Sigma, St. Louis, MO or SKU# 68700; Proliant Biologicals, Ankeny, IA), L-glutamine, non-essential amino acids, penicillin, and streptomycin. LPS from E. coli 055:B5 (Cat. no. L-6529; Sigma) was reconstituted in PBS (Cat no. 21-031-CV; Mediatech) to a concentration of 1 mg/ml and vortexed for 1 minute. Aliquots of LPS were stored at −20°C for up to six months prior to experiments. TNFα promoter-luciferase constructs (pTNF(−615), pTNF(−161), pTNF(−95), pTNF(Egr-1m), and pTNF(κB3m) were a kind gift from Dr. Nigel Mackman, and have been described previously (Yao et al., 1997).
RAW264.7 cells and Bone Marrow Macrophages (BMM ϕs)
RAW264.7 cells (ATCC, Mannassas, VA) were maintained in petri dishes to ensure that the majority of cells were non-adherent monocytes at the same stage of differentiation. For experiments, they were transferred to tissue culture dishes (4.1×104 cells/cm2) and cultured (5% CO2 and 37°C) in the serum-containing media described in the previous paragraph. Experiments were performed the following day when the cells were 60–70% confluent. Virtually all of the cells were adherent to the tissue culture plastic and exhibited a macrophage-like morphology with multiple cellular extensions.
Bone marrow was obtained by centrifugation of long bones from 5–12 week-old male C57BL6 mice (The Jackson Laboratory, Bar Harbor, ME) as previously described (Dobson et al., 1999) except that the centrifugation was performed at 3,800 g for 10 seconds. Bone marrow derived macrophages were then isolated by differential adherence (Clohisy et al., 1987). Thus, marrow cells (3.4×105 cells/cm2) were cultured in serum-containing media supplemented with M-CSF (10 ng/ml; Cat. no. 416-ML; R&D Systems, Minneapolis, MN). After 24 hours, non-adherent cells (2×105 cells/cm2) were transferred to fresh tissue culture dishes and cultured in serum-containing media supplemented with M-CSF. Cells that remained non-adherent after 72 hours were removed by washing the plates two times with serum-free media. The adherent cells were then cultured in fresh serum-containing media supplemented with M-CSF for 20–24 hours prior to each experiment. Virtually all of the cells were adherent to the tissue culture plastic and exhibited a macrophage-like morphology as described above.
Experimental Design
Experiments were initiated by addition of an equal volume of serum-free media containing either titanium particles (3.4×106 particles/cm2 for RAW264.7 cells or 6.6×107 particles/cm2 for BMMϕs), LPS (200 pg/ml), or vehicle controls (PBS). Thus, during the experiments, the cells were exposed to equal volumes of media containing 5% FBS and 0.05% BSA. In selected experiments, cells were pre-treated with U0126 or PD98059, compounds which inhibit MEK1/2 (the kinase upstream of ERK1/2 (Shi et al., 2002)), or U0124, a relatively inactive analogue of U0126, for 2 hours (Shi et al., 2002). Cells were pre-treated with all other inhibitors, SB220025/SB202190 (p38α/βinhibitors), SP600125 (a JNK1/2/3 inhibitor), or relatively inactive analogues, SB202474, or N1-methyl-1,9-pyrazoloanthrone (all from Calbiochem, San Diego, CA), for 1 hour (Dai et al., 2006; Lahti et al., 2003). Following the pre-treatment period, cells were treated for the indicated times with titanium particles or vehicle (PBS) in the continued presence of inhibitors, or relatively inactive analogues. SB220025, SB202190 and SB202474 were dissolved in methanol (Sigma), while all other inhibitors and analogues were dissolved in dimethylsulphoxide (DMSO; Cat no. D2650; Sigma). Groups without inhibitors or analogues received equal amounts of methanol or DMSO.
Immunoblotting
Cultures were rinsed with 4°C PBS supplemented with 1 mM sodium orthovanadate, and lysed at 4°C in 1% Triton X-100, 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 µg/ml leupeptin, 1 mM PMSF, and a protease inhibitor cocktail (Complete Mini; Roche, Mannheim, Germany). Total cell lysates were sonicated (60 Sonic Dismembrator, 60% of maximum power; Fisher Scientific, Pittsburgh, PA), and centrifuged (10,700 g×10 minutes, 4°C) to remove cellular debris and titanium particles.
In selected experiments, cytosolic and nuclear fractions were isolated as described previously (Chen et al., 2005). Cells were washed twice with ice-cold PBS and scraped into lysis buffer containing 10 mM NaCl, 10 mM Tris-HCl (pH 7.4), 3 mM MgCl2, 0.3% Nonidet P-40 and a protease inhibitor cocktail. Cells were incubated on ice for 3 minutes followed by centrifugation (500 g×5 minutes, 4°C). The supernatants contained the cytosolic fraction. Pellets containing the nuclei were pipetted in buffer containing 10 mM NaCl, 10 mM Tris-HCl (pH 7.4), 3 mM MgCl2, and a protease inhibitor cocktail using a wide bore tip to ensure that pellets containing titanium particles were resuspended thoroughly. Following centrifugation (500 g×5 minutes, 4°C), the nuclear pellets were lysed in the 1% Triton X-100 buffer described in the previous paragraph. Nuclear lysates were sonicated on ice for 5 seconds three times. To remove titanium particles and cellular debris, nuclear and cytosolic lysates were centrifuged (10,700 g×10 minutes, 4°C).
Protein levels were determined in total cell lysates using the Bicinchoninic Acid assay (BCA; Pierce, Rockford, IL), and the Coomassie Blue assay (Bio-Rad; Hercules, CA) was used with cytosol and nuclear fractions. Western blots were performed on aliquots representing equal cell numbers using phospho-specific polyclonal antibodies to ERK1/2 (T202/Y204), p38 (T180/Y182), JNK (T183/Y185), c-Jun (Ser 63), MAPKAPK-2 (T334) (#9101, 9211, 9251, 9261, and 3041 respectively; Cell Signaling Technology, Beverly, MA), and a phospho-specific monoclonal antibody to JNK (T183/Y185) (# 9255; Cell Signaling Technology). Levels of total proteins were assessed by Western blotting using specific antibodies to ERK1/2, p38, JNK, c-Jun, MAPKAPK-2, p65, and Egr-1 (#9102, 9212, 9252, 9162, 3042, 3034 and 4152; Cell Signaling Technology). Antibodies specific to Akt and CREB (#9272 and 9192, respectively; Cell Signaling Technology) were used to confirm the identity and purity of the cytosolic and nuclear fractions. HRP-linked secondary antibodies (#7074 or #7076; Cell Signaling Technology) and ECL reagents (Amhersham, Buckinghamshire, England) were used to detect binding of the primary antibodies. All Western blots are representative of at least four independent experiments.
TNFα ELISAs and cytotoxicity assays
Conditioned media were harvested and centrifuged (2,500 g× 25 minutes, 4°C) to remove cellular debris and titanium particles. TNFα levels in conditioned media were measured by ELISA as previously described (Bi et al., 2001a). In experiments involving MAPK inhibitors, cytotoxicity was assessed by measuring LDH (Cytotoxicity Detection Kit, Cat. no. 1644793; Roche, Indianapolis, IN) release into the conditioned media. Percent cytotoxicity was determined by comparing the amount of LDH in conditioned media to the amount in cells lysed with 1% Triton X-100.
Real-Time Polymerase Chain Reaction (RT-PCR)
TNFα primers were described by Overbergh and colleagues (Overbergh et al., 1999). GAPDH primers were designed using Primer3 software (Rozen and Skaletsky, 2000). GAPDH primers were 5’-CAGCCTCAAGATCATCAGCAA-3’ and 5’-CCAGTAGAGGCAGGGATGAT-3’. Real-time PCR was performed as previously described (Dai et al., 2006). TNFα mRNA levels were determined from standard curves and normalized to the GAPDH levels. PCR efficiencies calculated from the standard curves were 70–101%. Melting curves and agarose gels confirmed that the PCR reactions amplified single products and the identity of those products were confirmed by sequencing. All real-time PCR assays included negative controls consisting of reverse transcriptase reactions performed in the absence of RNA.
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts were isolated as described above and DNA binding activity was assessed using an oligonucleotide corresponding to the Egr-1 binding site within the promoter region of the TNFα gene (5’-AACCCTCTGCCCCCGCGATGGAG-3’) as described previously (Shi et al., 2002).
Dual Luciferase Reporter Assays
RAW264.7 cells were transiently transfected as previously described (Shi et al., 2002). Briefly, RAW264.7 cells were cultured in tissue culture dishes at a density of 4.2×104 cells/cm2 for 72 hours until they reached 80% confluency. The cells were then washed once with PBS and transiently transfected with TNFα promoter-luciferase constructs or empty vector, along with pRLTK which expresses Renilla luciferase (Cat. no. E224A; Promega, Madison, WI) using SuperFect Transfection Reagent (Cat. no. 301305; Qiagen, Carlsbad, CA). Cells were then subcultured into 96-well tissue culture plates at 4.3×104 cells/well for 24 hours. In selected experiments, cells were pre-treated with U0126 or U0124 for 2 hours. After this period, the cells were incubated with titanium particles with adherent endotoxin (6.8×107 particles/cm2), LPS (1 ng/ml), or vehicle (PBS) in triplicate wells in the continued presence or absence of U0126 or U0124 for 4 hours.
Samples were prepared according to the Dual Luciferase Reporter Assay System (Promega). Briefly, cultures were washed twice with PBS and then lysed with buffer provided by Promega. Lysates from triplicate wells for each promoter construct incubated with the same stimuli were pooled. Firefly and Renilla luciferase were measured using the Softmax Pro software (Molecular Devices, Sunnyville, CA). TNFα promoter activity was calculated by dividing firefly luciferase activity by Renilla luciferase activity to normalize for transfection efficiency.
Statistical analyses
Quantitative data (mean ± SEM) are pooled from all available experiments of each type. The number of each type of experiment (each performed on duplicate or triplicate groups of cells) appears in the figure captions. Symbols without error bars represent standard errors smaller than the symbol. All statistical analyses used SuperANOVA software (Abacus Concepts, Berkeley, CA). Data sets that passed normality and equal variance testing were analyzed by parametric, repeated measures ANOVA (Fig. 2A, 2C, Fig 3A, Fig 4A, 4C, 4D, Fig 6A, 6C, Fig 7D, 7E, Fig 8A and 8B). Data sets that failed normality and/or equal variance testing were analyzed by non-parametric, repeated measures ANOVA on RANKS (Fig. 1A, Fig 3C, Fig 5A, 5C, and Fig 9C).
Fig. 2.
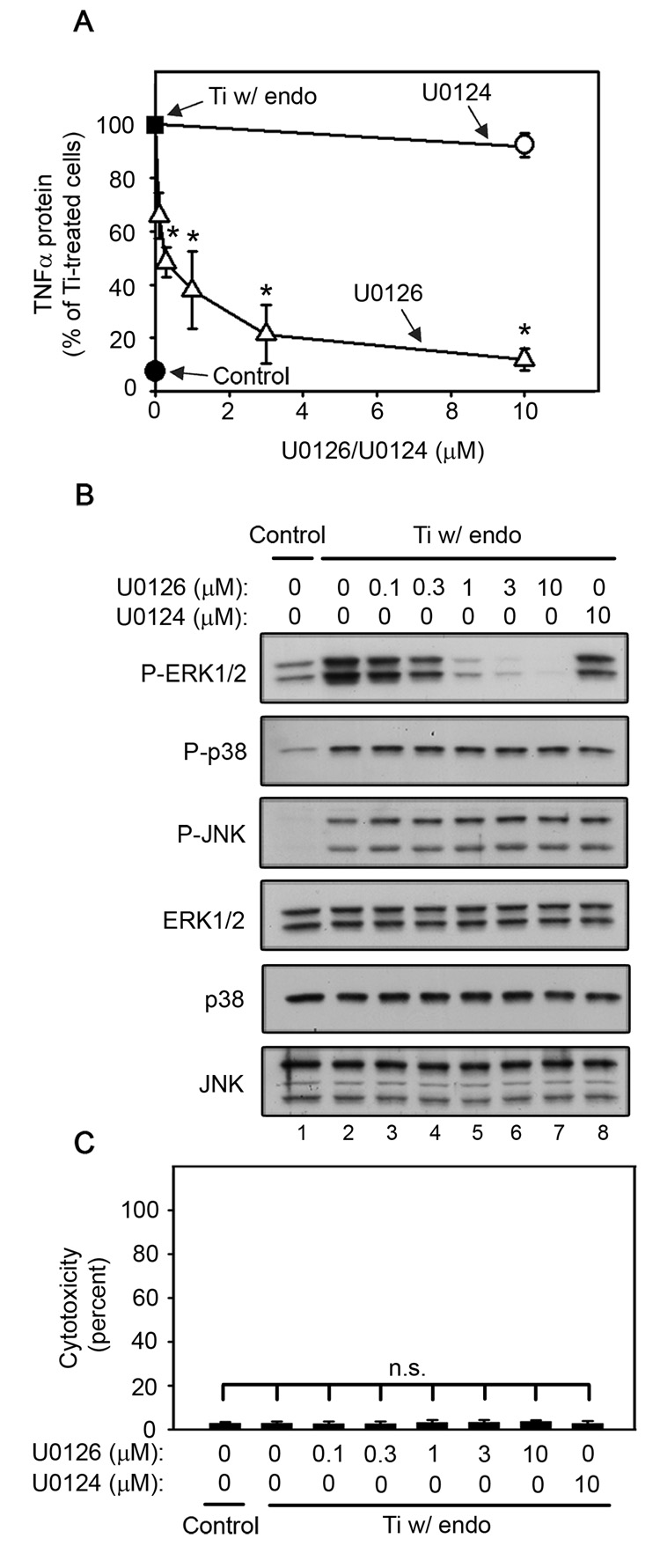
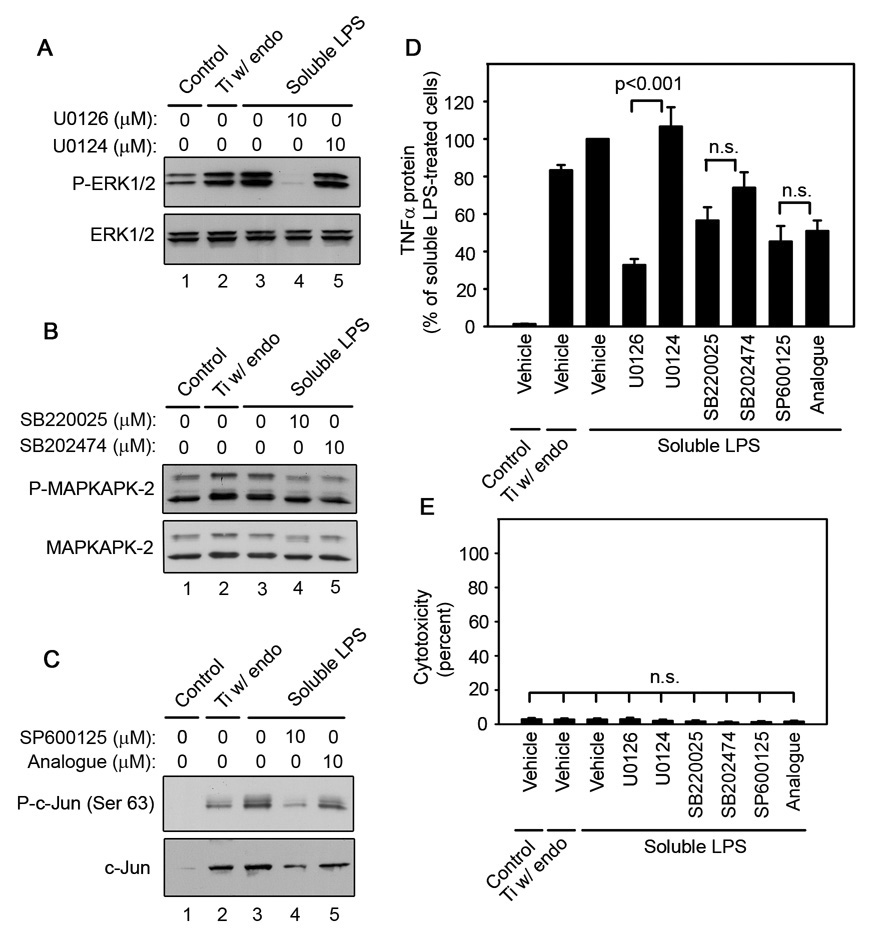
ERK1/2 activation is required for titanium particle-induced TNFα production by RAW264.7 macrophages since U0126 dose-dependently inhibits TNFα production (A) and ERK1/2 phosphorylation (B), but does not induce cytotoxicity (C) following stimulation by titanium particles with adherent endotoxin. All groups received a mixture of vehicle controls, such that all contained 0.04% PBS and 0.1% DMSO. (A) TNFα protein was measured in conditioned media by ELISA following stimulation for 90 minutes with titanium particles with adherent endotoxin in the presence of indicated concentrations of U0126 (open triangles) or U0124 (open circle), or in the absence of both (closed square). TNFα protein was also measured in conditioned media collected following culture without titanium particles or inhibitor (closed circle). Results (mean ±SEM of four experiments) are presented as a percentage of TNFα protein following stimulation with titanium particles with adherent endotoxin in the absence of inhibitors (closed square). Asterisks denote p<0.005 compared to the control group (closed square) and the group with the relatively inactive analogue (open circle). (B) MAPK activation was assessed in whole cell lysates as described in Figure 1B. Western blots are from the most representative of the four experiments pooled in (A). (C) Cytotoxicity results are presented as the mean ± SEM of the four experiments pooled in (A).
Fig. 3.
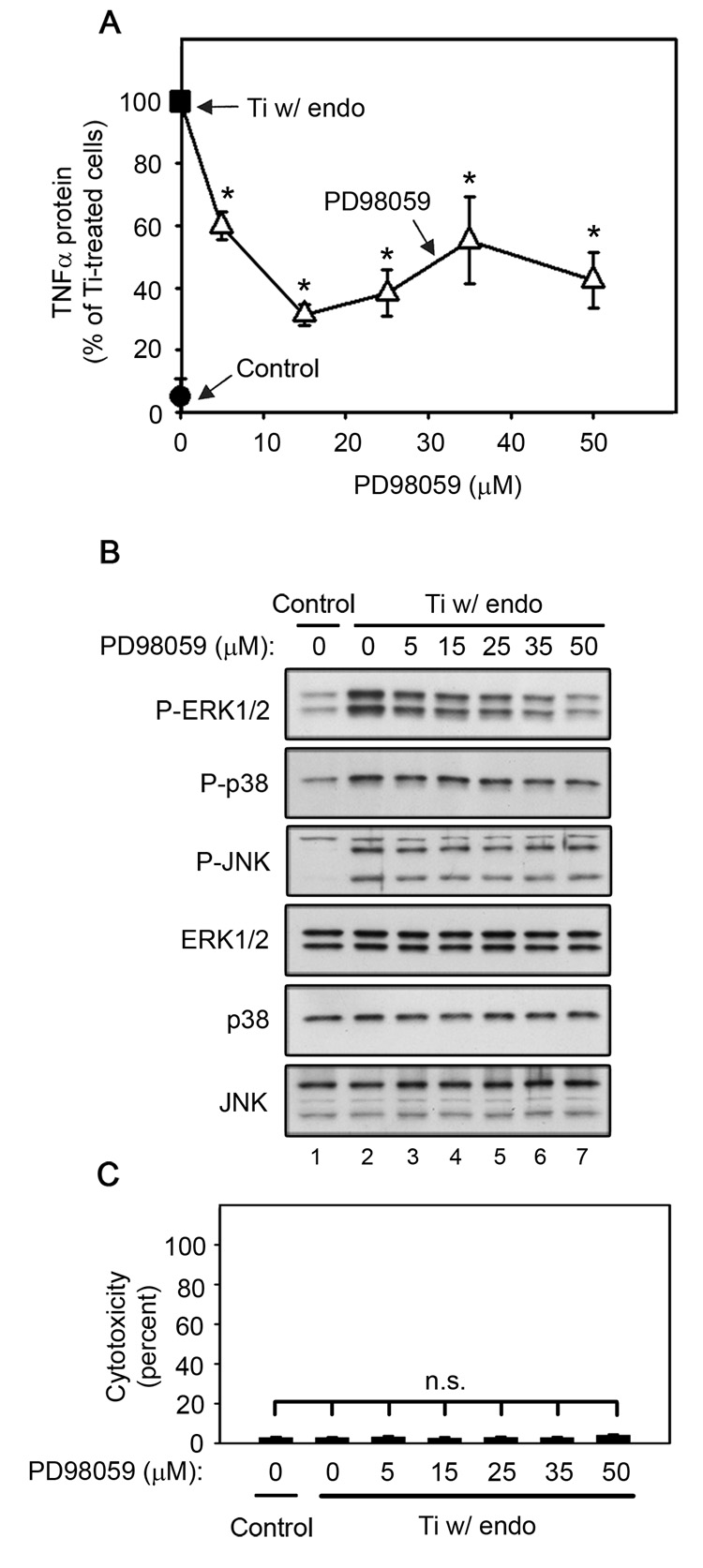
ERK1/2 activation is required for titanium particle-induced TNFα production by RAW264.7 macrophages since PD98059 dose-dependently inhibits TNFα production (A) and ERK1/2 phosphorylation (B), but does not induce cytotoxicity (C) following stimulation by titanium particles with adherent endotoxin. All groups received a mixture of vehicle controls, such that all contained 0.04% PBS and 0.1% DMSO. (A) TNFα protein was measured in conditioned media by ELISA following stimulation for 90 minutes with titanium particles with adherent endotoxin in the presence (open triangles) or absence (closed square) of indicated concentrations of PD98059. TNFα protein was also measured in conditioned media collected following culture without titanium particles or inhibitor (closed circle). Results (mean ± SEM of five experiments) are presented as a percentage of TNFα protein following stimulation with titanium particles with adherent endotoxin in the absence of inhibitors (closed square). Asterisks denote p<0.005 compared to the control group (closed square). (B) MAPK activation was assessed in whole cell lysates as described in Figure 1B. Western blots are from the most representative of the five experiments pooled in (A). (C) Cytotoxicity results are presented as the mean ± SEM of the five experiments pooled in (A).
Fig. 4.
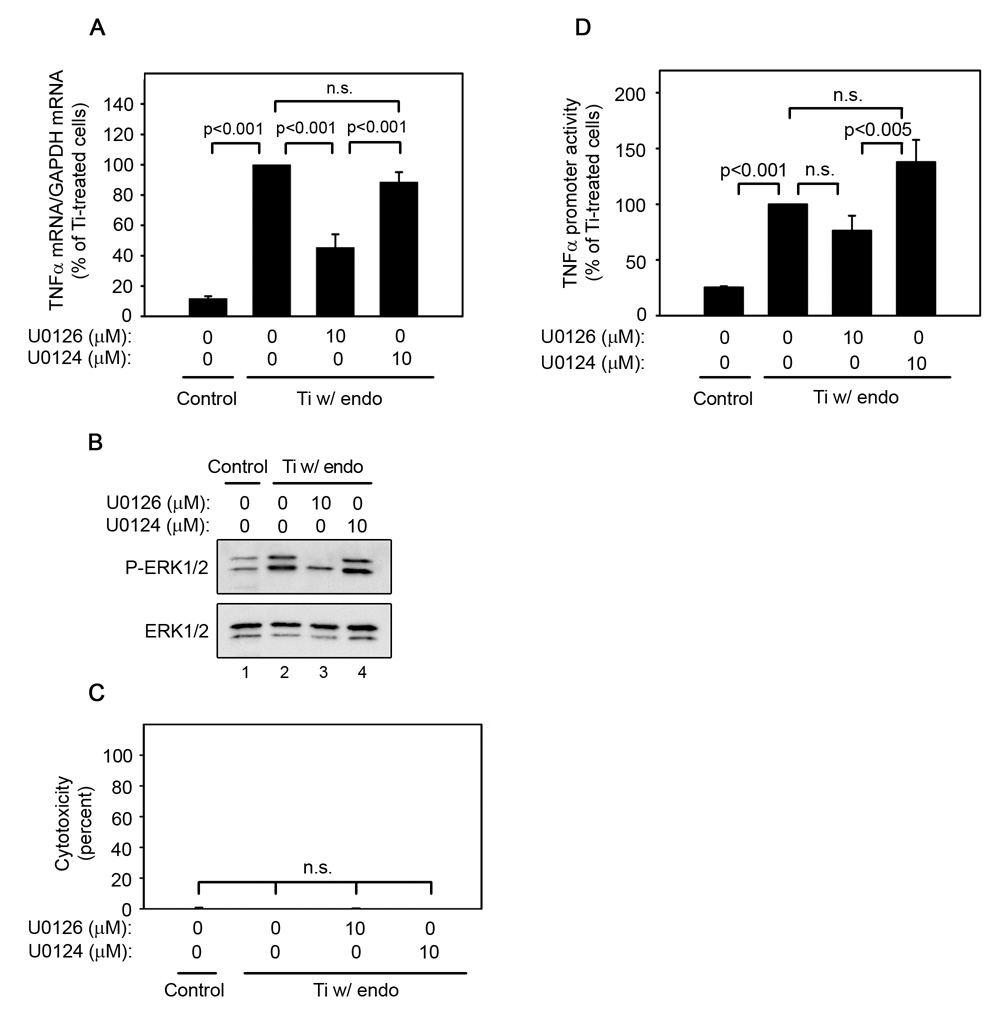
ERK1/2 activation is required for titanium particle-induced TNFα mRNA expression and promoter activity since U0126 inhibits TNFα mRNA (A), promoter activity (D), and ERK1/2 phosphorylation (B), but does not induce cytotoxicity (C) following stimulation by titanium particles with adherent endotoxin. All groups received a mixture of vehicle controls, such that all contained 0.04% PBS and 0.1% DMSO. (A) TNFα mRNA was measured by real-time PCR following stimulation of bone marrow-derived macrophages for 30 minutes with or without titanium particles with adherent endotoxin in the presence or absence of indicated concentrations of U0126 or U0124. Results (mean ± SEM of four experiments) are presented as a percentage of TNFα mRNA following stimulation with titanium particles with adherent endotoxin in the absence of inhibitors. (B) MAPK activation was assessed in whole cell lysates as described in Figure 1B. Western blots are from the most representative of the four experiments pooled in (A). (C) Cytotoxicity results are presented as the mean ± SEM of the four experiments pooled in (A). (D) TNFα promoter activity was measured using a luciferase reporter construct following stimulation of RAW264.7 cells with or without titanium particles with adherent endotoxin in the presence or absence of U0126 or U0124 for 4 hours. Results (mean ± SEM of eight experiments) are presented as a percentage of promoter activity following stimulation with titanium particles with adherent endotoxin in the absence of inhibitors.
Fig. 6.
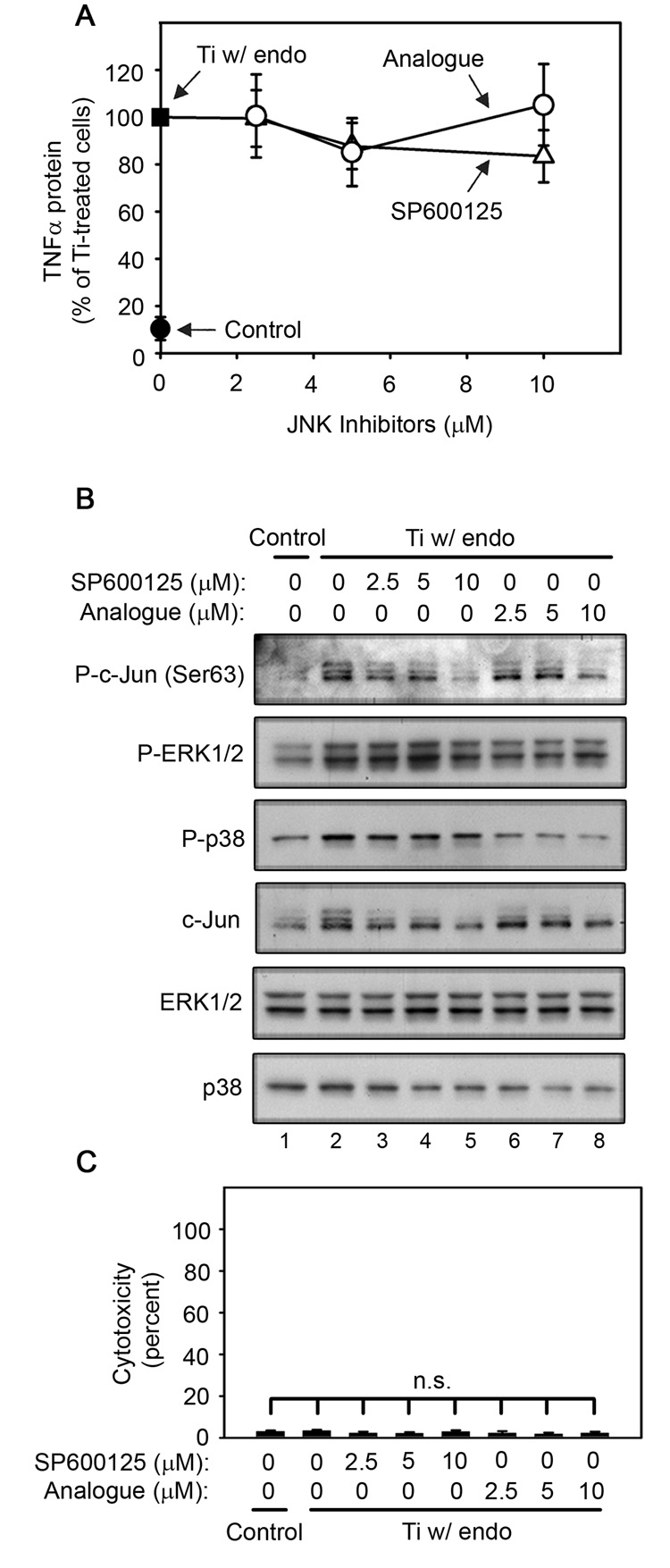
JNK activation is not required for titanium particle-induced TNFα production by RAW264.7 macrophages since SP600125 and its relatively inactive analogue, N1-methyl-1,9-pyrazoloanthrone, have no detectable effect on TNFα production (A) following stimulation with titanium particles with adherent endotoxin. Both SP600125 and its relatively inactive analogue inhibit c-Jun phosphorylation and c-Jun expression with the expected differences in potency (B), and do not induce cytotoxicity (C) following stimulation by titanium particles with adherent endotoxin. All groups received a mixture of vehicle controls, such that all contained 0.04% PBS and 0.2% DMSO. (A) TNFα protein was measured in conditioned media by ELISA following stimulation for 90 minutes with titanium particles with adherent endotoxin in the absence (closed square) or presence of indicated concentrations of SP600125 (open triangles), or analogue (open circles). TNFα protein was also measured in conditioned media collected following culture without titanium particles or inhibitor (closed circle). Results (mean ± SEM of five experiments) are presented as a percentage of TNFα protein following stimulation with titanium particles with adherent endotoxin in the absence of inhibitors (closed square). There is no statistical difference between the two analogues at any of the concentrations. Signal transduction (B) was assessed at 90 minutes since preliminary experiments showed that c-Jun phosphorylation was maximal at this time (data not shown). (B) MAPK activation was assessed in whole cell lysates as described in Figure 1B. Western blots are from the most representative of the five experiments pooled in (A). (C) Cytotoxicity results are presented as the mean ±SEM of four of the five experiments pooled in (A) since LDH activity was not measured in the fifth experiment.
Fig. 7.
ERK1/2 is required for LPS-induced TNFα production since U0126 inhibits ERK1/2 phosphorylation (A) and TNFα production (D), but does not inhibit activity of p38 (B) or JNK (C) and does not induce cytotoxicity (E). RAW264.7 macrophages were stimulated with titanium particles with adherent endotoxin or soluble LPS in the presence or absence of the indicated concentrations of inhibitors or vehicle controls. All measurements were made after 90 minutes of stimulation, except for p38 activity (B), which was measured after 30 minutes. All groups received a mixture of vehicle controls, such that all contained 0.04% PBS, 0.1% DMSO and 0.2% methanol. MAPK activation (A–C) was assessed in whole cell lysates as described in Figure 1B. Western blots are from the most representative of the seven experiments pooled in (D). TNFα protein was measured by ELISA in conditioned media collected following stimulation with titanium particles with adherent endotoxin or soluble LPS in the absence or presence of inhibitors. (D) Results (mean ± SEM of seven experiments) are presented as a percentage of the amount measured following stimulation with soluble LPS in the absence of inhibitors. (E) Cytotoxicity results are presented as the mean ± SEM of six of the seven experiments pooled in (D) since LDH activity was not measured in the seventh experiment.
Fig. 8.
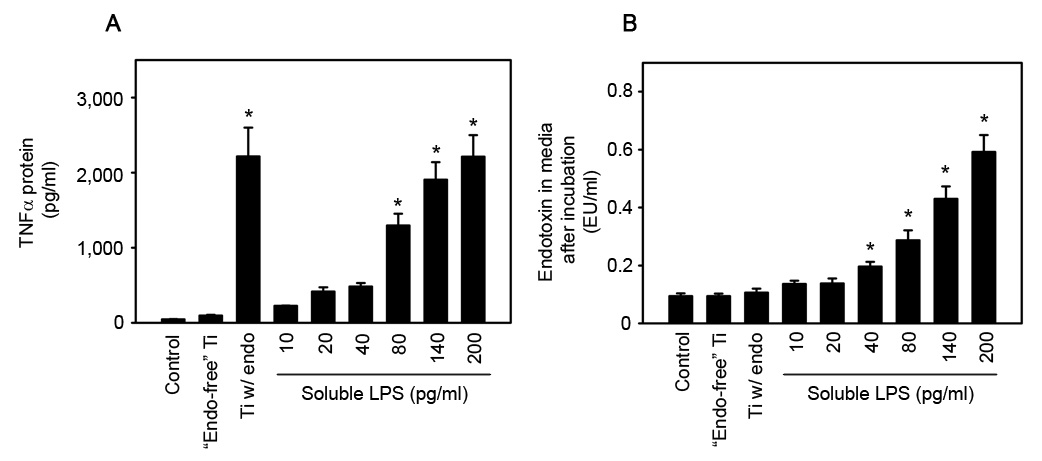
The biological activity of titanium particles with adherent endotoxin is not due to the release of soluble LPS. RAW264.7 macrophages were stimulated with “endotoxin-free” titanium particles, titanium particles with adherent endotoxin, or soluble LPS for 90 minutes. Following this incubation period, TNFα protein (A) was measured by ELISA and endotoxin levels (B) were measured by the LAL assay. Results are presented as (mean ± SEM of three experiments). Asterisks denote p<0.05 compared to the PBS control group.
Fig. 1.
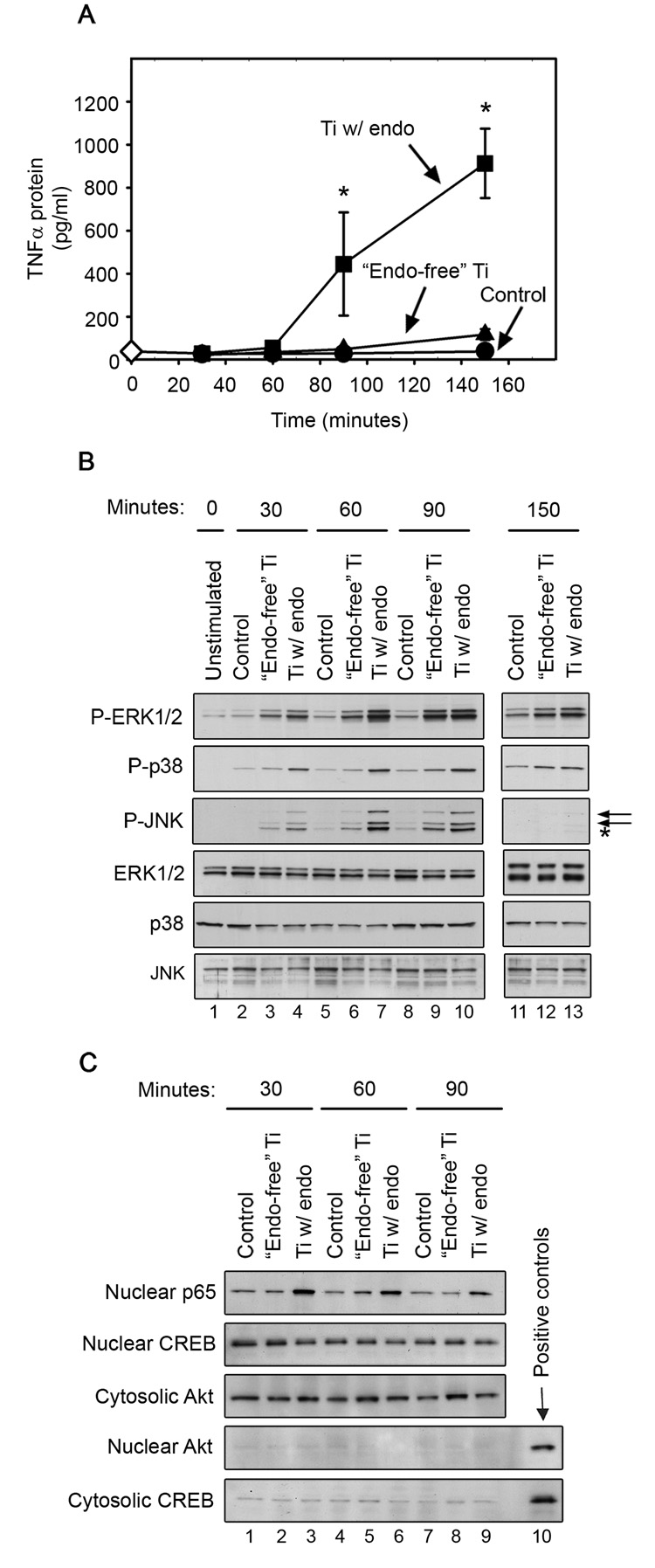
Titanium particles with adherent endotoxin induce significantly more TNFα protein (A), MAPK activation (B), and NF-κB p65 nuclear translocation (C) than “endotoxin-free” titanium particles. (A) TNFα (mean ± SEM of four experiments) was measured by ELISA in conditioned media collected at each time point following stimulation of RAW264.7 macrophages with titanium particles with adherent endotoxin (squares), “endotoxin-free” titanium particles (triangles), or PBS (Control; circles). As an additional control, media was collected at time zero under unstimulated conditions to measure basal TNFα (open diamond). Asterisks denote p<0.05 compared to groups incubated with “endotoxin-free” titanium particles and PBS control groups at the same time point. (B) MAPK activation was assessed in whole cell lysates by Western blotting using phospho-specific antibodies (first-third panels). Levels of total MAPKs were assessed as loading controls (fourth-sixth panels). Western blots are from the most representative of the four experiments pooled in (A). In the third panel, the upper and lower arrows identify JNK2/3 and JNK1, respectively while the asterisk indicates ERK1 (see text for explanation). (C) NF-κB translocation was assessed by examining levels of p65 in nuclear fractions by Western blotting (first panel). Purity of the nuclear and cytosolic fractions was confirmed by assessing CREB and Akt levels (second–fifth panels). Nuclear and cytosolic aliquots were used respectively as positive controls for CREB and Akt. Western blots are from the most representative of six experiments.
Fig. 5.
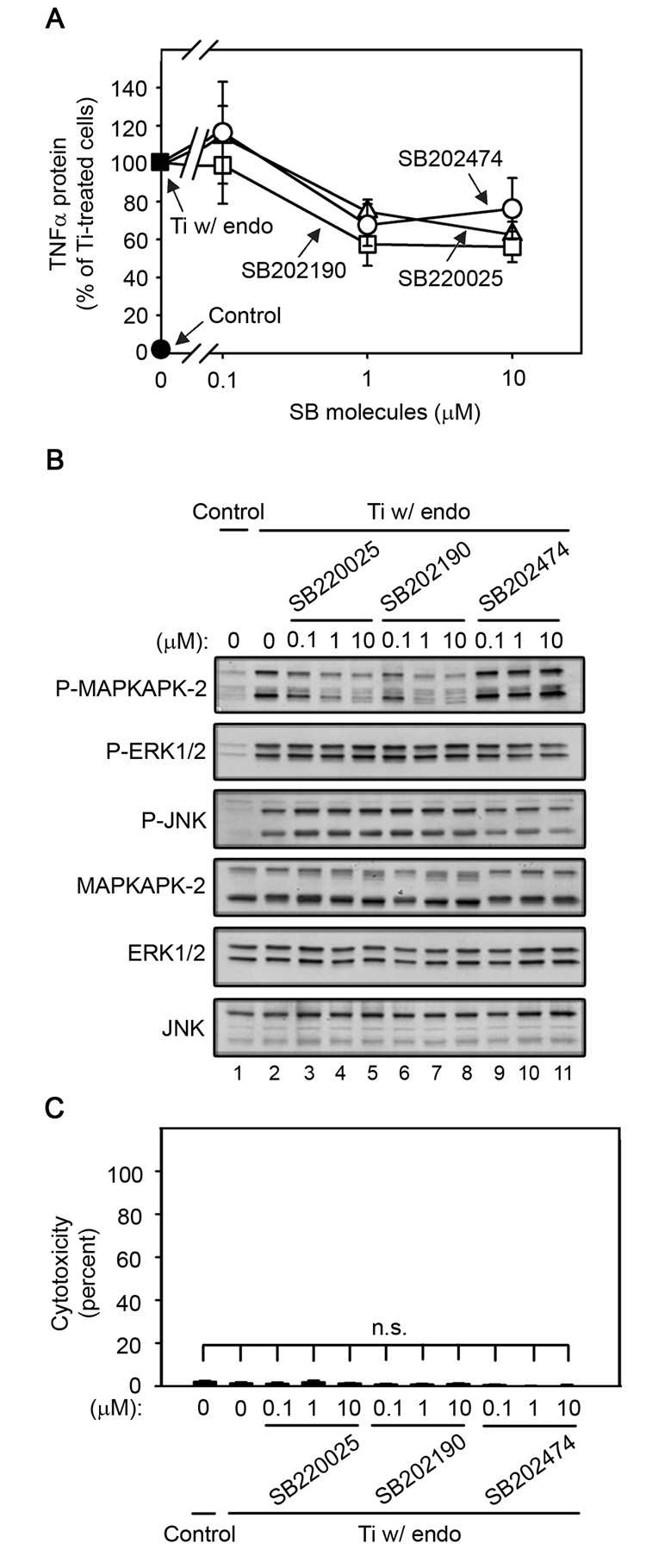
p38α/β activation is not required for titanium particle-induced TNFα production by RAW264.7 macrophages since SB220025 and SB202190 inhibit TNFα production with dose-dependencies similar to the relatively inactive analogue, SB202474 (A), while only SB220025 and SB202190 inhibit p38 activity as assessed by (B) following stimulation by titanium particles with adherent endotoxin. All three analogues do not induce cytotoxicity (C). TNFα protein (A) and cytotoxicity (C) were measured at 90 minutes, while signal transduction (B) was assessed at 30 minutes since preliminary experiments showed that MAPKAPK-2 phosphorylation was maximal at this time (data not shown). All groups received a mixture of vehicle controls, such that all contained 0.04% PBS and 0.2% methanol. (A) TNFα protein was measured in conditioned media by ELISA following stimulation with titanium particles with adherent endotoxin in the absence (closed square) or presence of indicated concentrations of SB220025 (open triangles), SB202190 (open squares), or SB202474 (open circles). TNFα protein was also measured in conditioned media collected following culture without titanium particles or inhibitor (closed circle). Results (mean ± SEM of four experiments) are presented as a percentage of TNFα protein following stimulation with titanium particles with adherent endotoxin in the absence of inhibitors (closed square). There is no statistical difference between the three analogues at any of the concentrations. (B) MAPK activation was assessed in whole cell lysates as described in Figure 1B. Western blots are from the most representative of the four experiments pooled in (A). (C) Cytotoxicity results are presented as the mean ± SEM of the four experiments pooled in (A).
Fig. 9.
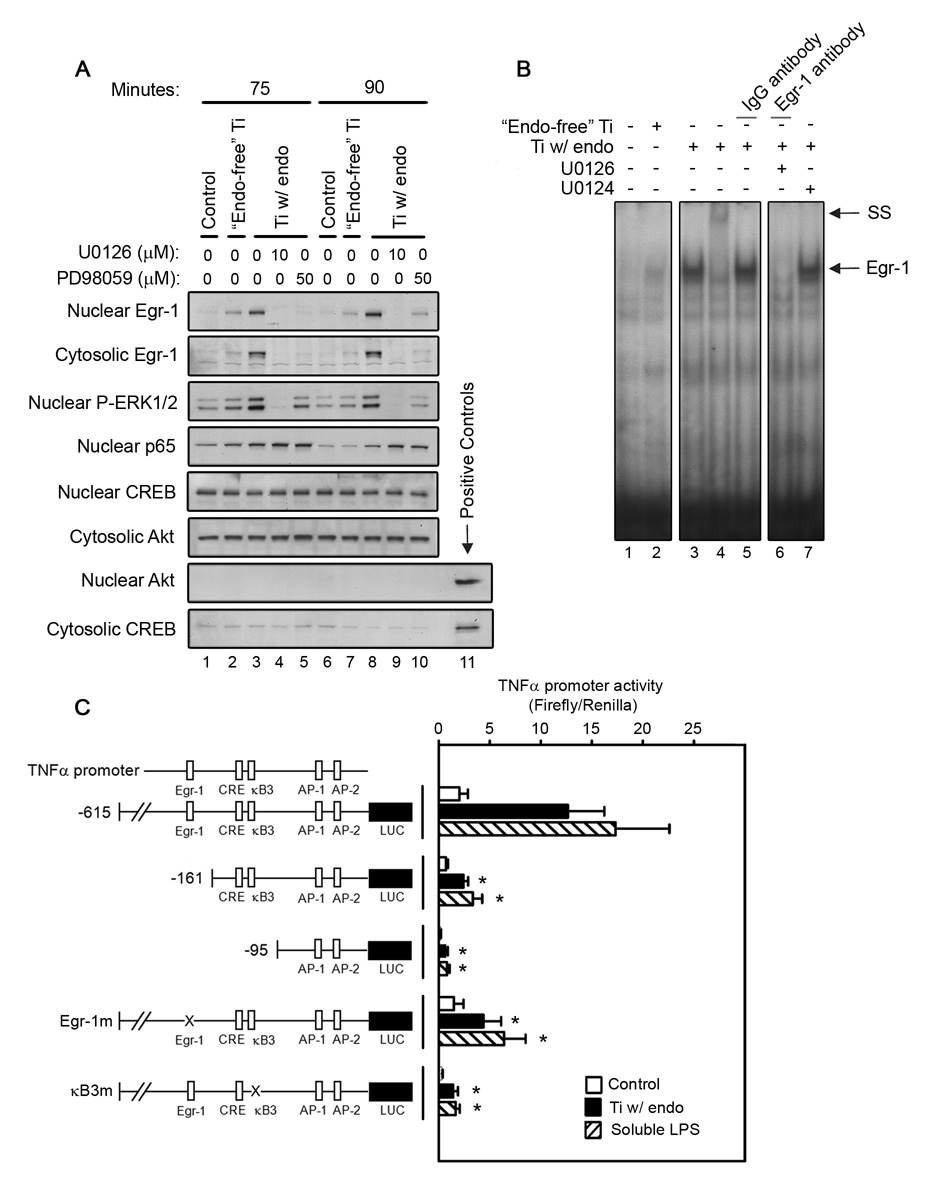
Egr-1 expression is stimulated by titanium particles, potentiated by adherent endotoxin, dependent on ERK1/2 activation, and required for activation of the TNFα promoter. RAW264.7 macrophages were stimulated with either “endotoxin-free” titanium particles or titanium particles with adherent endotoxin for the indicated times in the presence of absence of either U0126 or PD98059. As a control, cells were cultured without titanium particles or inhibitors (PBS; vehicle). All groups received a mixture of vehicle controls, such that all contained 0.04% PBS and 0.1% DMSO. Cytosolic and nuclear fractions were isolated as described in Materials and Methods. (A) The presence of Egr-1, phospho-ERK1/2, and NF-κB in the cytosol or nucleus was assessed by Western blotting (first–fourth panels). Purity of the fractions was confirmed by assessing CREB and Akt levels (fifth–eighth panels). Nuclear and cytosolic aliquots were used respectively as positive control for CREB and Akt. Western blots are from the most representative of four experiments. (B) Binding to an Egr-1 specific region within the TNFα promoter was assessed by EMSA following stimulation for 90 minutes. Supershift analysis compared an Egr-1 antibody and an IgG non-immune control (panel 2, lanes 4–5). EMSA results are representative of three experiments. (C) TNFα promoter activity (mean ± SEM of six experiments) was measured using luciferase reporter constructs following stimulation for 4 hours. The deletions and mutations in the TNFα promoter constructs are shown. Asterisks denote p<0.05 compared to groups with the same stimuli and full-length promoter.
RESULTS
Adherent endotoxin strongly stimulates MAPK and NF-κB activation induced by titanium particles
We first determined the time course of TNFα production in RAW264.7 cells, as well as MAPK and NF-κB activation. The initial significant increase in TNFα production occurred at 90 minutes following stimulation of cells with titanium particles with adherent endotoxin and increased further at 150 minutes (Fig. 1A; squares). Titanium particles with adherent endotoxin also strongly and rapidly induced activation of all three MAPKs and NF-κB. Thus, MAPK phosphorylation was increased at 30 minutes and peaked at 60–90 minutes (Fig. 1B; first-third panels, lanes 4, 7, and 10). NF-κB p65 translocation to the nucleus peaked at 30 minutes after stimulation by titanium particles with adherent endotoxin (Fig. 1C; first panel, lane 3).
In contrast to titanium particles with adherent endotoxin, “endotoxin-free” titanium particles did not significantly induce TNFα production at any of the time points (Fig. 1A; triangles). Activation of the MAPK and NF-κB pathways by “endotoxin-free” titanium particles was substantially weaker and slower than activation by titanium particles with adherent endotoxin. Thus, “endotoxin-free” titanium particles induced less phosphorylation of all three MAPKs at 30 and 60 minutes than did titanium particles with adherent endotoxin, and peak phosphorylation was delayed until 90 minutes (Fig. 1B; first-third panels, compare lanes 3, 6 and 9 with lanes 4, 7, and 10). “Endotoxin-free” titanium particles also stimulated much less NF-κB p65 translocation and peak translocation was delayed until 60 minutes (Fig. 1C; first panel, compare lanes 2, 5 and 8 with lanes 3, 6, and 9). Purity of cytosolic and nuclear fractions was demonstrated using nuclear and cytosolic markers, CREB and Akt, respectively (Fig. 1C; second-fifth panels). As expected, the levels of total MAPKs were not affected by either type of titanium particle (Fig. 1B; bottom 3 panels).
It is important to note that in Figure 1B, JNK phosphorylation was assessed with a polyclonal antibody that detects the expected 54 and 46 kDa bands (JNK2/3 and JNK1, respectively; see arrows) as well as a 44 kDa band (see asterisk) that according to Cell Signaling Technical Service is ERK1 and that, as expected, is not detected with a phospho-JNK monoclonal antibody (Fig. 2, Fig 3, and Fig 5). All subsequent experiments were therefore performed using the phospho-JNK monoclonal antibody.
Activation of ERK1/2 is required for TNFα production induced by titanium particles with adherent endotoxin
U0126 and PD98059, which inhibit MEK1/2 (English and Cobb, 2002; Hotokezaka et al., 2002), the kinase upstream of ERK1/2, were used to examine the role of the ERK1/2 pathway in titanium particle-induced TNFα production. Both U0126 and PD98059 are fairly specific for the MEK1/2/ERK1/2 pathway (Bain et al., 2007; Davies et al., 2000). The primary other target of both U0126 and PD98059 is MEK5; however, both inhibitors are significantly more effective at blocking MEK1/2 (Bain et al., 2007; Mody et al., 2001). Concentrations of both U0126 and PD98059 that strongly inhibit MEK1/2 but not MEK5 (Bain et al., 2007; Mody et al., 2001) dose-dependently decreased both TNFα production (Fig. 2A and Fig 3A; triangles) and ERK1/2 phosphorylation (Fig. 2B and Fig 3B; first panel, lanes 3–7) in RAW264.7 macrophages following stimulation with titanium particles with adherent endotoxin. As expected (Bain et al., 2007; Mody et al., 2001), U0126 inhibited both TNFα production and ERK1/2 phosphorylation more completely and at lower concentrations than PD98059 (Fig. 2A–B and Fig 3A–B). Specificity of the MEK1/2 inhibitors was further demonstrated by showing that U0124, a less active analogue of U0126, had no significant effect on TNFα production (Fig. 2A; open circle), and only modestly inhibited ERK1/2 phosphorylation (Fig. 2B; first panel, lane 8). Moreover, the MEK1/2 inhibitors did not affect phosphorylation of the other MAPK pathways, JNK and p38 (Fig. 2B and Fig 3B; second-third panels), did not affect total MAPK protein levels (Fig. 2B and Fig 3B; fourth-sixth panels), and did not induce cytotoxicity (Fig. 2C and Fig 3C).
To determine whether activation of ERK1/2 is required for TNFα production in authentic macrophages as well as in the RAW264.7 cell line, bone marrow-derived macrophages were cultured in the presence or absence of titanium particles with adherent endotoxin and/or the MEK1/2 inhibitors. These experiments also measured TNFα mRNA expression to determine whether the response to the particles occurs at this level. Titanium particles potently increased both TNFα mRNA (Fig. 4A) and phosphorylation of ERK1/2 (Fig. 4B; first panel, lane 2). Moreover, U0126 significantly inhibited TNFα expression (Fig. 4A) and ERK1/2 phosphorylation (Fig. 4B; first panel, lane3), while U0124 had no detectable effects (Fig. 4A, 4B; first panel, lane 4). Neither U0126 nor U0124 affected total ERK1/2 protein levels (Fig. 4B; second panel) or cytotoxicity (Fig. 4C). All of these effects are similar to those observed at the TNFα protein level in the RAW264.7 cell line.
Having demonstrated that the ERK1/2 pathway contributes to stimulation of TNFα mRNA and protein levels by titanium particles with adherent endotoxin, we asked whether the ERK1/2 pathway also contributes to TNFα transcription. For this purpose, RAW264.7 cells were transiently transfected with the full-length TNFα promoter and treated with titanium particles with adherent endotoxin in the presence or absence of U0126. Titanium particles with adherent endotoxin significantly increased TNFα promoter activity (Fig. 4D; second bar). TNFα promoter activity was significantly less in the presence of U0126 than in the presence of the inactive analogue, U0124 (Fig. 4D; compare third and fourth bars). This partial inhibition of TNFα promoter activity by U0126 did not reach significance when compared to promoter activity in the absence of inhibitor (Fig. 4D; compare second and third bars).
Activation of p38 is not required for TNFα production induced by titanium particles with adherent endotoxin
The pyridinyl imidazole inhibitors of p38α/β have potent non-specific effects (Bain et al., 2007; Huang et al., 2002; Karaman et al., 2008; Montero et al., 2002). Therefore, three structurally related analogues were used to determine the role of p38 in titanium particle-induced TNFα production: SB202190, a commonly used inhibitor of p38α/β (Davies et al., 2000; Lee et al., 1994), SB220025, a newly described analogue that inhibits p38α/β with similar potency to SB202190, but is more specific (Huang et al., 2002; Montero et al., 2002), and SB202474, a relatively inactive analogue (Lee et al., 1994). The three analogues had indistinguishable effects on TNFα production induced by titanium particles with adherent endotoxin (Fig. 5A; open symbols) in RAW264.7 macrophages, indicating that p38α/β activation is not required. In agreement with this conclusion, the three analogues had the expected divergent effects on p38 activity, as assessed by measuring phosphorylation of MAPKAPK-2 (Fig. 5B; top panel, lanes 3–11). MAPKAPK-2 is a specific p38 substrate that is essential for induction of TNFα by LPS (Kotlyarov et al., 1999). As expected, SB202474 had no detectable effect on phosphorylation of MAPKAPK-2 at any of the concentrations tested (Fig. 5B; first panel, lanes 9–11). Also as expected, the p38 inhibitors and inactive analogue did not affect phosphorylation of the other MAPK pathways, ERK1/2 and JNK (Fig. 5B; second-third panels), did not affect total MAPK protein levels (Fig. 5B; fourth-sixth panels), and did not induce cytotoxicity (Fig. 5C).
Activation of JNK is not required for TNFα production induced by titanium particles with adherent endotoxin, but is required for upregulation of c-Jun protein levels
SP600125, a JNK1/2/3 inhibitor (Bennett et al., 2001; Han et al., 2001; Lahti et al., 2003) was used to determine whether JNK is required for TNFα production induced by titanium particles with adherent endotoxin in RAW264.7 cells. Since SP600125 has potent non-specific effects (Bain et al., 2007), its relatively inactive analogue, N1-methyl-1,9-pyrazoloanthrone (Bennett et al., 2001; Lahti et al., 2003), was also examined. Neither SP600125, nor N1-methyl-1,9-pyrazoloanthrone, detectably affected titanium particle-induced TNFα production (Fig. 6A; open symbols), indicating that JNK1/2/3 activation is not required for titanium particle-induced TNFα production. As expected, both SP600125 and N1-methyl-1,9-pyrazoloanthrone dose-dependently inhibited JNK activity, but N1-methyl-1,9-pyrazoloanthrone was substantially less potent (Fig. 6C; first panel, lanes 3–8). JNK activity was assessed by measuring phosphorylation of c-Jun. Interestingly, titanium particles upregulated total c-Jun protein levels and this required activation of the JNK pathway since it is blocked completely by SP600125 and partially by N1-methyl-1,9-pyrazoloanthrone (Fig. 6B; fourth panel, lanes 2–8). As expected, SP600125 and its relatively inactive analogue did not affect phosphorylation of the other MAPK pathways, ERK1/2 and p38 (Fig. 6B; second-third panels), did not affect total ERK1/2 or p38 protein levels (Fig. 6B; fifth-sixth panels), and did not induce cytotoxicity (Fig. 6C).
ERK1/2 also mediates TNFα production induced by soluble LPS
Similar to our findings with titanium particles with adherent endotoxin, soluble LPS strongly induced activation of all three MAPKs (Fig. 7A–C; first panels, lane 2) in RAW264.7 macrophages. In addition, the ERK1/2 pathway is also required for LPS-induced TNFα production since U0126 significantly decreased both ERK1/2 phosphorylation (Fig. 7A; first panel, lane 4) and TNFα production (Fig. 7D). Neither the p38 nor the JNK pathways were required for LPS-induced TNFα production since their relatively inactive analogues had effects similar to the active inhibitors (Fig. 7D). As expected, the inhibitors did not affect total protein levels (Fig. 7A–C; second panels, lanes 1–5), and did not induce cytotoxicity (Fig. 7E).
The biological activity of wear particles with adherent endotoxin is not due to release of soluble endotoxin
The similarities in mechanisms responsible for stimulation of TNFα production by titanium particles with adherent endotoxin and by soluble LPS raised the possibility that endotoxin is released from the particles into the culture media and, therefore, acts similarly to soluble LPS. To test this possibility, TNFα and endotoxin levels were measured in the culture media following incubation of RAW264.7 cells with titanium particles with adherent endotoxin or soluble LPS. Titanium particles with adherent endotoxin significantly stimulated TNFα production (Fig. 8A; compare first and third bars) but did not detectably release endotoxin into the culture media (Fig. 8B; compare first and third bars). Soluble LPS dose-dependently stimulated TNFα production (Fig. 8A; fourth–ninth bars) and, as expected, there was a corresponding dose-dependent increase in endotoxin levels in the culture media (Fig. 8B; fourth–ninth bars). These results provide strong evidence that stimulation of TNFα production by titanium particles with adherent endotoxin is not due to release of endotoxin from the surface of the titanium particles.
Egr-1 expression is stimulated by titanium particles, potentiated by adherent endotoxin, dependent on ERK1/2 activation, and required for TNFα promoter activation
Activation of ERK1/2 by LPS induces Egr-1 expression, and subsequent binding of Egr-1 to the TNFα promoter is required for full transcriptional activation (Guha et al., 2001; Shi et al., 2002; Yao et al., 1997). We therefore examined whether titanium particles with adherent endotoxin regulate Egr-1 expression and binding to the TNFα promoter in RAW264.7 macrophages and if this process is dependent on adherent endotoxin and ERK1/2. Both nuclear and cytosolic Egr-1 expression was substantially upregulated at 75 and 90 minutes following stimulation with titanium particles with adherent endotoxin (Fig. 9A; first-second panels, lanes 3 and 8). Egr-1 binding activity to the TNFα promoter was also substantially increased by titanium particles with adherent endotoxin (Fig. 9B; lane 3). Supershift analysis confirmed the presence of Egr-1 in the complex (compare lanes 4 and 5 in Fig. 9B). In contrast, “endotoxin-free” titanium particles only modestly increased Egr-1 expression (Fig. 9A; first-second panels, lanes 2 and 7) and binding to the TNFα promoter (Fig. 9B, lane 2). ERK1/2 pathway inhibitors blocked Egr-1 expression and binding to the TNFα promoter following stimulation with titanium particles with adherent endotoxin (Fig. 9A; first-second panels, lanes 4, 5, 9 and 10, and Fig. 9B, lane 6). We also measured the amount of phosphorylated ERK1/2 in the nucleus since ERK1/2 activation triggers its translocation (Khokhlatchev et al., 1998). Titanium particles with adherent endotoxin substantially increased the level of nuclear phosphorylated ERK1/2 and the effect of the particles was blocked by either U0126 or PD98059 (Fig. 9A; third panel). In some systems, but not all, the ERK1/2 pathway also stimulates NF-κB translocation to the nucleus (Briant et al., 1998; Ghoda et al., 1997; Tuyt et al., 1999). However, neither U0126 nor PD98059 detectably affected NF-κB p65 translocation induced by titanium particles with adherent endotoxin (Fig. 9A; third panel, lanes 4, 5, 9 and 10). Thus, similar to findings with soluble LPS (Guha et al., 2001), p65 translocation induced by titanium particles with adherent endotoxin is not regulated by the ERK1/2 pathway. Purity of cytosolic and nuclear fractions was confirmed with nuclear and cytosolic markers, CREB and Akt, respectively (Fig. 9A, fifth–eighth panels).
To determine whether Egr-1 activation is required for TNFα expression induced by titanium particles with adherent endotoxin, we utilized TNFα promoter constructs which have either deleted or mutated binding sites for the transcription factor. Titanium particles with adherent endotoxin increased the activity of the full-length promoter by 6.3-fold (Fig. 9C; second bar). Deletion of the Egr-1 binding site decreased titanium particle-induced promoter activity by 75–80% (Fig. 9C; fifth bar). Deletion of both the Egr-1 and NF-κB binding sites further decreased titanium particle-induced promoter activity by 90–95% (Fig. 9C; eighth bar). To more specifically assess the roles of Egr-1 and NF-κB, promoter constructs with specific mutations in their binding sites were examined (Yao et al., 1997). Mutation of either the Egr-1 or NF-κB binding sites decreased titanium particle-induced promoter activity by 70–75% or 85–90%, respectively (Fig. 9C; eleventh and fourteenth bars). All of these effects are similar to those observed when TNFα promoter activity was stimulated by soluble LPS rather than by titanium particles with adherent endotoxin (Fig. 9C; compare closed and cross-hatched bars and (Shi et al., 2002)).
DISCUSSION
Wear particles generated from the surface of orthopaedic implants induce bone loss at the implant-bone interface through macrophage production of pro-inflammatory cytokines which stimulate osteoclast differentiation (Greenfield et al., 2002; Wright and Goodman, 2001). The current study was designed to identify signaling pathways which are important for the initial response to wear particles and to determine whether adherent endotoxin contributes to these initial responses. To examine signaling pathways that are directly activated by wear particles, experiments focused on early time points prior to production of pro-inflammatory cytokines that would themselves activate signaling pathways in an autocrine fashion (Covert et al., 2005; Fong et al., 1989). This study led to three major conclusions. First, activation of the ERK1/2/Egr-1 and NF-κB pathways is required for initial responses to wear particles which include increases in TNFα promoter activity, TNFα mRNA expression, and secretion of TNFα protein. Second, activation of the p38α/β and JNK1/2/3 pathways is not required for TNFα production induced by wear particles; however, JNK is required for upregulation of c-Jun protein levels. Third, adherent endotoxin strongly stimulates MAPK and NF-κB activation induced by wear particles. These conclusions are illustrated in Figure 10 and discussed below.
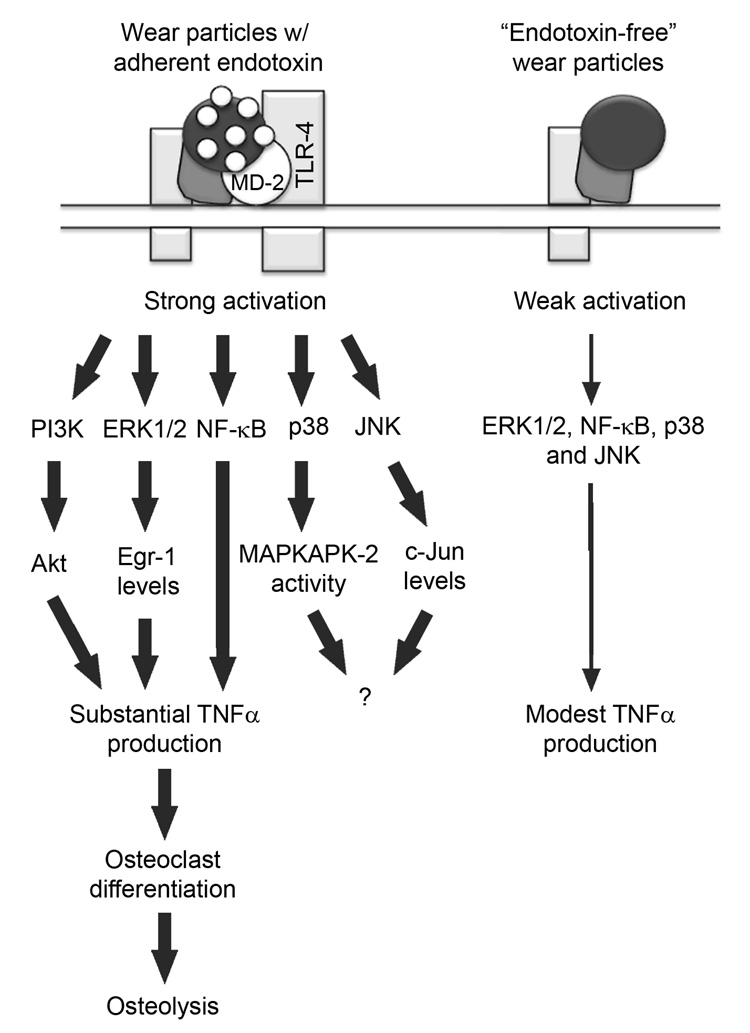
Fig. 10.
Model of orthopaedic wear particle-induced responses. See Discussion for details.
The first major conclusion of our study is that activation of the ERK1/2/Egr-1 and NF-κB pathways is required for TNFα production induced by wear particles with adherent endotoxin (Fig. 10). Thus, ERK1/2 pathway inhibitors, U0126 and PD98059, decreased TNFα production induced by wear particles with adherent endotoxin with dose dependencies similar to those required for inhibition of wear particle-induced ERK1/2 activation. Moreover, U0124, a relatively inactive analogue, had little effect on ERK1/2 activation or TNFα production. In addition, the ERK1/2 pathway inhibitors blocked wear particle-induced expression of Egr-1, Egr-1 binding to the TNFα promoter, and TNFα promoter activity. Moreover, mutations or deletions in the Egr-1 and NF-κB binding sites blocked TNFα promoter activation induced by wear particles with adherent endotoxin. Stimulation of TNFα production by wear particles is therefore due, in part, to increased TNFα transcription regulated by the ERK1/2/Egr-1 pathway. These results do not exclude the possibility that wear particles also increase TNFα production through post-transcriptional mechanisms in response to activation of various signaling pathways including the ERK1/2 pathway. In this regard, soluble LPS increases TNFα mRNA stability and translation, in part, through activation of the ERK1/2 and p38 pathways (Anderson, 2008; Deleault et al., 2008; Rutault et al., 2001). Indeed, our results together with previous studies on NF-κB (Schwarz et al., 2000; Soloviev et al., 2002) and PI3K/Akt (Smith et al., 2007) show that multiple signaling pathways act together to increase TNFα production in response to wear particles with adherent endotoxin. Thus, our model (Fig. 10) proposes that strong activation of multiple pathways by wear particles induces substantial TNFα production.
The second major conclusion of this study is that activation of the p38α/β and JNK1/2/3 pathways is not required for TNFα production induced by wear particles with adherent endotoxin (Fig. 10). Thus, p38α/β and JNK1/2/3 inhibitors had only modest effects on TNFα production that were indistinguishable from those induced by the relatively inactive analogues. In contrast, p38 and JNK pathway inhibitors block osteoclast differentiation induced by wear particles (Abbas et al., 2003; Yamanaka et al., 2006). However, our study suggests two possible interpretations for these results. First, the effects observed in those studies may have been non-specific since they did not examine the relatively inactive analogues. Alternatively, the effects may be due to the known requirement for p38α/β and JNK1/2/3 during osteoclast differentiation rather than direct wear particle-mediated events (see Introduction for details). Nonetheless, our results do not rule out the possibility that p38α/β or JNK1/2/3 may be involved in cellular processes other than production of TNFα following stimulation with wear particles with adherent endotoxin. An example of this is described in the next paragraph.
Although JNK1/2/3 activation is not required for TNFα production induced by wear particles with adherent endotoxin, it is required for upregulation of c-Jun protein levels by wear particles (Fig. 10). Thus, phosphorylation of c-Jun and upregulation of total c-Jun protein levels by wear particles with adherent endotoxin are blocked completely by SP600125, but substantially less potently by N1-methyl-1,9-pyrazoloanthrone. These results are consistent with the ability of phosphorylated c-Jun to upregulate its own transcription and downregulate its degradation (Angel et al., 1988). Future studies will be needed to determine the physiological significance of increased c-Jun protein levels during wear particle-induced osteolysis. SP600125 inhibits JNK1, JNK2, and JNK3 with similar potencies (Bennett et al., 2001). However, JNK1 or JNK2 are likely responsible for the effects observed in our study since JNK3 is primarily restricted to the nervous system (Mohit et al., 1995). The relative roles of JNK1 and JNK2 appear to be dependent on cell-type and stimulus. For example, JNK1 is responsible for osteoclastogenesis induced by receptor activator of NF-κB ligand (RANKL) (David et al., 2002; Ikeda et al., 2004), and for regulation of the cell cycle in fibroblasts (Sabapathy et al., 2004). JNK2, on the other hand, is responsible for induction of inflammatory arthritis by TNFα overexpression (Koller et al., 2005).
The third major conclusion of this study is that adherent endotoxin strongly stimulates MAPK and NF-κB activation induced by wear particles (Fig. 10). These findings establish a cellular mechanism for previous results which demonstrate that adherent endotoxin substantially stimulates the effects of wear particles on pro-inflammatory cytokine production, osteoclast differentiation, and osteolysis (Greenfield et al., 2005). The model in Fig. 10 is similar to one comparing the effects of fully active LPS and LPS analogues (Triantafilou et al., 2004). The fully active form of LPS induced strong activation of MAPK and NF-κB pathways leading to substantial TNFα production, while the LPS analogues induced weak activation of the signaling pathways and modest TNFα production (Triantafilou et al., 2004).
The current study is the first demonstration that wear particles with adherent endotoxin strongly and rapidly induce activation of the three MAPK pathways (ERK1/2, p38, and JNK), while “endotoxin-free” wear particles induce substantially weaker and slower activation. Our results also confirm and extend the report that NF-κB p65 translocation is stimulated by adherent endotoxin on wear particles (Akisue et al., 2002). That study used immunofluorescence and did not detect NF-κB p65 translocation in response to wear particles without adherent endotoxin. In contrast, our biochemical study found that “endotoxin-free” wear particles induce low, but reproducible, NF-κB p65 translocation while wear particles with adherent endotoxin induce substantially stronger and faster NF-κB p65 translocation. Moreover, in contrast to our results and those in Akisue et al. (2002), it has been reported that polymethylmethacrylate particles induce translocation of NF-κB to the nucleus of macrophages independently of TLR-4 (Clohisy et al., 2002). However, that study did not include either the quantitative analysis or the dose-response experiments that are necessary to evaluate the role of TLR-4 (Bi et al., 2001a; Majde, 1992; Morrison and Curry, 1979).
In summary, this study demonstrated that the ERK1/2/Egr-1 and NF-κB pathways are required mediators of the initial response by macrophages to orthopaedic wear particles, which causes increases in TNFα promoter activity, TNFα mRNA expression, and secretion of TNFα protein. In contrast, the p38 and JNK pathways are rapidly activated by orthopaedic wear particles, but their activity is not required for TNFα production. Moreover, the increases in activation of all of the signaling pathways, and in TNFα production, are substantially potentiated by adherent endotoxin on the orthopaedic wear particles.
ACKNOWLEDGMENTS
We would like to thank Joscelyn Tatro for the particle preparations, Jennifer Nalepka for the endotoxin measurements, and Robert Brookover for designing GAPDH PCR primers.
Contract grant sponsor: NIH (to EMG); Contract grant number: RO1 AR43769
Contract grant sponsor: Sulzer Settlement Trust Fund; Contract grant number: n/a
Contract grant sponsor: NIH (to MAB); Contract grant number: T32 AR07505
Contract grant sponsor: Allen Resident Research Fellowships (to MVS and ASI); Contract grant number: n/a
REFERENCES
- Abbas S, Clohisy JC, Abu-Amer Y. Mitogen-activated protein (MAP) kinases mediate PMMA-induction of osteoclasts. J Orthop Res. 2003;21:1041–1048. doi: 10.1016/S0736-0266(03)00081-0. [DOI] [PubMed] [Google Scholar]
- Akisue T, Bauer TW, Farver CF, Mochida Y. The effect of particle wear debris on NFkappaB activation and pro-inflammatory cytokine release in differentiated THP-1 cells. J Biomed Mater Res. 2002;259:507–515. doi: 10.1002/jbm.1264. [DOI] [PubMed] [Google Scholar]
- Anderson P. Post-transcriptional control of cytokine production. Nature Immunology. 2008;9(4):353–359. doi: 10.1038/ni1584. [DOI] [PubMed] [Google Scholar]
- Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Anonymous. Orthopaedic Device Forum Meeting Summation Report. 2003. [Google Scholar]
- Baeuerle P, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87(1):13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408(3):297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassare J, Bi Y, Bellone C. The role of p38 mitogen-activated protein kinase in IL-1beta transcription. J Immunol. 1999;162:5367–5373. [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proceedings of the National Academy of Science, USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Seabold JM, Kaar SC, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin on orthopaedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001a;16:2082–2091. doi: 10.1359/jbmr.2001.16.11.2082. [DOI] [PubMed] [Google Scholar]
- Bi Y, VanDeMotter RR, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Titanium particles stimulate bone resorption by inducing differentiation of murine osteoclasts. J Bone Joint Surg Am. 2001b;83A:501–508. doi: 10.2106/00004623-200104000-00004. [DOI] [PubMed] [Google Scholar]
- Blaine TA, Rosier RN, Puzas JE, Looney RJ, Reynolds PR, Reynolds SD, O'Keefe RJ. Increased levels of tumor necrosis factor-alpha and interleukin-6 protein and messenger RNA in human peripheral blood monocytes due to titanium particles. J Bone Joint Surg Am. 1996;78A:1181–1192. doi: 10.2106/00004623-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Briant L, Robert-Hebmann V, Sivan V, Brunet A, Pouyssegur J, Devaux C. Involvement of extracellular signal-regulated kinase module in HIV-mediated CD4 signals controlling activation of nuclear factor-kappaB and AP-1 transcription factors. J Immunol. 1998;160:1875–1885. [PubMed] [Google Scholar]
- Chen X, Dai JC, Orellana SA, Greenfield EM. Endogenous protein kinase inhibitorg terminates immediate-early gene expression induced by cAMP-dependent protein kinase (PKA) signaling: Termination depends on PKA inactivation rather than PKA export from the nucleus. J Biol Chem. 2005;280:2700–2707. doi: 10.1074/jbc.M412558200. [DOI] [PubMed] [Google Scholar]
- Childs L, Goater J, O'Keefe R, Schwarz E. Effect of anti-tumor necrosis factor-alpha gene therapy on wear debris-induced osteolysis. J Bone Joint Surg Am. 2001;83A:1789–1797. doi: 10.2106/00004623-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Clohisy D, Bar-Shavit Z, Chappel J, Teitelbaum S. 1,25-Dihydroxyvitamin D3 modulates bone marrow macrophage precursor proliferation and differentiation. Up-regulation of the mannose receptor. J Biol Chem. 1987;262:15922–15929. [PubMed] [Google Scholar]
- Clohisy JC, Hirayama T, Frazier E, Han SK, Abu-Amer Y. NF-kB signaling blockade abolishes implant particle-induced osteoclastogenesis. J Orthop Res. 2004;22(1):13–20. doi: 10.1016/S0736-0266(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Clohisy JC, Teitelbaum S, Chen S, Erdmann JM, Abu-Amer Y. Tumor necrosis factor-a mediates polymethylmethacrylate particle-induced NF-kB activation in osteoclast precursor cells. J Orthop Res. 2002;20:174–181. doi: 10.1016/S0736-0266(01)00088-2. [DOI] [PubMed] [Google Scholar]
- Cooperstock MS, Tucker RP, Baublis JV. Possible pathogenic role of endotoxin in Reye's Syndrome. Lancet. 1975;1:1272–1274. doi: 10.1016/s0140-6736(75)92553-2. [DOI] [PubMed] [Google Scholar]
- Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- Dai JC, He P, Chen X, Greenfield EM. TNFa and PTH utilize distinct mechanisms to induce IL-6 and RANKL expression with markedly different kinetics. Bone. 2006;38:509–520. doi: 10.1016/j.bone.2005.10.007. [DOI] [PubMed] [Google Scholar]
- David JP, Sabapathy K, Hoffmann O, Idarraga MH, Wagner EF. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J Cell Sci. 2002;115:4317–4325. doi: 10.1242/jcs.00082. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleault KM, Skinner SJ, Brooks SA. Tristetraprolin regulates TNF TNF-alpha mRNA stability via a proteasome dependent mechanism involving the combined action of the ERK and p38 pathways. Mol Immunol. 2008;45:13–24. doi: 10.1016/j.molimm.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Dobson K, Reading L, Haberey M, Marine X, Scutt A. Centrifugal isolation of bone marrow from bone: an improved method for the recovery and quantitation of bone marrow osteoprogenitor cells from rat tibiae and femurae. Calcif Tissue Int. 1999;65:411–413. doi: 10.1007/s002239900723. [DOI] [PubMed] [Google Scholar]
- English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci. 2002;23(1):40–45. doi: 10.1016/s0165-6147(00)01865-4. [DOI] [PubMed] [Google Scholar]
- Fong Y, Tracey K, Moldawer L, Hesse D, Manogue K, Kenney J, Lee A, Kuo G, Allison A, Lowry S, Cerami A. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989;170(5):1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Xing L, Poljak L, Shores E, Brown K, Leonardi A, Tran T, Boyce B, Siebenlist U. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11(24):3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoda L, Lin X, Greene WC. The 90-kDa ribosomal S6 kinase (pp90rsk) phosphorylates the N-terminal regulatory domain of IkappaBalpha and stimulates its degradation in vitro. J Biol Chem. 1997;272:21281–21288. doi: 10.1074/jbc.272.34.21281. [DOI] [PubMed] [Google Scholar]
- Greenfield EM, Bi Y, Ragab AA, Goldberg VM, Nalepka JL, Seabold JM. Does endotoxin contribute to aseptic loosening of orthopaedic implants? J Biomed Mater Res B Appl Biomater. 2005;72B:179–185. doi: 10.1002/jbm.b.30150. [DOI] [PubMed] [Google Scholar]
- Greenfield EM, Bi Y, Ragab AA, Goldberg VM, VanDeMotter RR. The role of osteoclast differentiation in aseptic loosening. J Orthop Res. 2002;20:1–8. doi: 10.1016/S0736-0266(01)00070-5. [DOI] [PubMed] [Google Scholar]
- Guha M, O'Connell MA, Pawlinski R, Hollis A, McGovern P, Yan SF, Stern D, Mackman N. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor-alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood. 2001;98:1429–1439. doi: 10.1182/blood.v98.5.1429. [DOI] [PubMed] [Google Scholar]
- Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108(1):73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschildt S, Brabetz W, Schromm AB, Hamann L, Zabel P, Rietschel ET, Muller-Loennies S. Structure and activity of endotoxins. In: Aktories K, Just I, editors. Handbook of Experiental Pharmacology. Berlin: Springer; 2000. pp. 619–659. [Google Scholar]
- Hotokezaka H, Sakai E, Kanaoka K, Saito K, Matsuo K, Kitaura H, Yoshida N, Nakayama K. U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast-like cells. J Biol Chem. 2002;277(49):47366–47372. doi: 10.1074/jbc.M208284200. [DOI] [PubMed] [Google Scholar]
- Huang M, Wang Y, Collins M, Gu JJ, Mitchell BS, Graves LM. Inhibition of nucleoside transport by p38 MAPK inhibitors. J Biol Chem. 2002;277:28364–28367. doi: 10.1074/jbc.C200321200. [DOI] [PubMed] [Google Scholar]
- Ikeda F, Nishimura R, Matsubara T, Tanaka S, Inoue J, Reddy SV, Hata K, Yamashita K, Hiraga T, Watanabe T, Kukita T, Yoshioka K, Rao A, Yoneda T. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J Clin Invest. 2004;114(4):475–484. doi: 10.1172/JCI19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada K, Endo S, Takahashi K, Suzuki M, Narita T, Yoshida T, Suda H, Komuro T, Yoshida M. Establishment of a new perchloric acid treatment method to allow determination of the total endotoxin content in human plasma by the limulus test and clinical application. Microbiol Immunol. 1991;35:303–314. doi: 10.1111/j.1348-0421.1991.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Kaar SG, Ragab AA, Kaye SJ, Kilic BA, Jinno T, Goldberg VM, Bi Y, Stewart MC, Carter JR, Greenfield EM. Rapid repair of titanium particle-induced osteolysis is dramatically reduced in aged mice. J Orthop Res. 2001;19:171–178. doi: 10.1016/S0736-0266(00)00033-4. [DOI] [PubMed] [Google Scholar]
- Karaman M, Herrgard S, Treiber D, Gallant P, Atteridge C, Campbell B, Chan K, Ciceri P, Davis M, Edeen P, Faraoni R, Floyd M, Hunt J, Lockhart D, Milano Z, Morrison M, Pallares G, Patel H, Pritchard S, Wodicka L, Zarrinkar P. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb MH. Phosphorylation of the MAP kinase promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- Kim D, Novak M, Wilkins J, Kim M, Sawyer A, Reichert W. Response of monocytes exposed to phagocytosable particles and discs of comparable surface roughness. Biomaterials. 2007;28(29):4231–4239. doi: 10.1016/j.biomaterials.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller M, Hayer S, Redlich K, Ricci R, David JP, Steiner G, Smolen JS, Wagner EF, Schett G. JNK1 is not essential for TNF-mediated joint disease. Arthritis Research and Therapy. 2005;7(1):R166–R173. doi: 10.1186/ar1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk H, Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nature Cell Biology. 1999;1(2):94–97. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- Lahti A, Jalonen U, Kankaanranta H, Moilanen E. c-Jun NH2-terminal kinase inhibitor anthra(1,9-cd)pyrazol-6(2H)-one reduces inducible nitric-oxide synthase expression by destabilizing mRNA in activated macrophages. Mol Pharmacol. 2003;64(2):308–315. doi: 10.1124/mol.64.2.308. [DOI] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, Strickler JE, McLaughlin MM, Siemens IR, Fisher SM, Livi GP, White JR, Adams JL, Young PR. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–745. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Lee S, Woo K, Kim S, Kim H, Kwack K, Lee Z, Kim H. The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone. 2002;30(1):71–77. doi: 10.1016/s8756-3282(01)00657-3. [DOI] [PubMed] [Google Scholar]
- Li X, Udagawa N, Itoh K, Suda K, Murase Y, Nishihara T, Suda T, Takahashi N. p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology. 2002;143(8):3105–3113. doi: 10.1210/endo.143.8.8954. [DOI] [PubMed] [Google Scholar]
- Li X, Udagawa N, Takami M, Sato N, Kobayashi Y, Takahashi N. p38 Mitogen-activated protein kinase is crucially involved in osteoclast differentiation but not in cytokine production, phagocytosis, or dendritic cell differentiation of bone marrow macrophages. Endocrinology. 2003;144(11):4999–5005. doi: 10.1210/en.2003-0166. [DOI] [PubMed] [Google Scholar]
- Lu H, Yang D, Wysk M, Gatti E, Mellman I, Davis R, Flavell R. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999;18:1845–1857. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majde JA. Endotoxin detection. Immunol Today. 1992;13:328–329. doi: 10.1016/0167-5699(92)90047-B. [DOI] [PubMed] [Google Scholar]
- Mansky KC, Sankar U, Han J, Ostrowski MC. Microphthalmia transcription factor (MITF) is a target of the p38 MAPK pathway in response to Receptor Activator of NF-KB ligand signaling. J Biol Chem. 2002;277(13):11077–11083. doi: 10.1074/jbc.M111696200. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL) J Biol Chem. 2000;275(40):31155–31161. doi: 10.1074/jbc.M001229200. [DOI] [PubMed] [Google Scholar]
- Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Amer J Pathol. 1999;154:203–210. doi: 10.1016/s0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody N, Leitch J, Armstrong C, Dixon J, Cohen P. Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett. 2001;502(1–2):21–24. doi: 10.1016/s0014-5793(01)02651-5. [DOI] [PubMed] [Google Scholar]
- Mohit AA, Martin JH, Miller CA. p493F12 kinase: a novel MAP kinase expressed in a subset of neurons in the human nervous system. Neuron. 1995;14(1):67–78. doi: 10.1016/0896-6273(95)90241-4. [DOI] [PubMed] [Google Scholar]
- Montero M, Lobaton CD, Moreno A, Alvarez J. A novel regulatory mechanism of the mitochondrial Ca2+uniporter revealed by the p38 mitogen-activated protein kinase inhibitor SB202190. FASEB J. 2002;16:1955–1957. doi: 10.1096/fj.02-0553fje. [DOI] [PubMed] [Google Scholar]
- Morrison DC, Curry BJ. The use of polymyxin B and C3H/HeJ mouse spleen cells as criteria for endotoxin contamination. J Immunol Methods. 1979;27:83–92. doi: 10.1016/0022-1759(79)90241-2. [DOI] [PubMed] [Google Scholar]
- Nalepka JL, Greenfield EM. Detection of bacterial endotoxin in human tissues. Biotechniques. 2004;37:413–417. doi: 10.2144/04373ST06. [DOI] [PubMed] [Google Scholar]
- Nelson CL, McLaren AC, McLaren SG, Johnson JJ, Smeltzer MS. Is aseptic loosening truly aseptic? Clinical Orthopaedics. 2005;437:25–30. doi: 10.1097/01.blo.0000175715.68624.3d. [DOI] [PubMed] [Google Scholar]
- Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- Ragab AA, VanDeMotter R, Lavish SA, Goldberg VM, Ninomiya JT, Carlin CR, Greenfield EM. Measurement and removal of adherent endotoxin from titanium particles and implant surfaces. J Orthop Res. 1999;17:803–809. doi: 10.1002/jor.1100170603. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Rutault K, Hazzalin CA, Mahadevan LC. Combinations of ERK and p38 MAPK inhibitors ablate tumor necrosis factor-alpha (TNF-alpha) mRNA induction. Evidence for selective destabilization of TNF-alpha transcripts. J Biol Chem. 2001;276(9):6666–6674. doi: 10.1074/jbc.M005486200. [DOI] [PubMed] [Google Scholar]
- Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol Cell. 2004;15(5):713–725. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Schrijver IA, Melief MJ, Tak PP, Hazenberg MP, Laman JD. Antigen-presenting cells containing bacterial peptidoglycan in synovial tissues of rheumatoid arthritis patients coexpress costimulatory molecules and cytokines. Arthritis Rheum. 2000;43:2160–2168. doi: 10.1002/1529-0131(200010)43:10<2160::AID-ANR3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Schwarz EM, Lu AP, Goater JJ, Benz EB, Kollias G, Rosier RN, Puzas JE, O'Keefe RJ. Tumor necrosis factor-alpha/nuclear transcription factor-kappaB signaling in periprosthetic osteolysis. J Orthop Res. 2000;18:472–480. doi: 10.1002/jor.1100180321. [DOI] [PubMed] [Google Scholar]
- Shi L, Kishore R, McMullen MR, Nagy LE. Lipopolysaccharide stimulation of ERK1/2 increases TNF-alpha production via Egr-1. American Journal of Physiology Cellular Physiology. 2002;282:C1205–C1211. doi: 10.1152/ajpcell.00511.2001. [DOI] [PubMed] [Google Scholar]
- Smith M, Lee M, Islam A, Rohrer J, Goldberg V, Beidelschies M, Greenfield E. The PI3K/Akt pathway mediates the biological response to titanium particles. J Bone Joint Surg. 2007;89A:1019–1027. doi: 10.2106/JBJS.F.00615. [DOI] [PubMed] [Google Scholar]
- Soloviev A, Schwarz EM, Kuprash DV, Nedospasov SA, Puzas JE, Rosier RN, O'Keefe RJ. The role of p105 protein in NFkappaB activation in ANA-1 murine macrophages following stimulation with titanium particles. J Orthop Res. 2002;20:714–722. doi: 10.1016/S0736-0266(01)00180-2. [DOI] [PubMed] [Google Scholar]
- Sundfeldt M, Carlsson L, Johansson C, Thomsen P, Gretzer C. Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop. 2006;77:177–197. doi: 10.1080/17453670610045902. [DOI] [PubMed] [Google Scholar]
- Takagi M, Tamaki Y, Hasegawa H, Takakubo Y, Konttinen L, Tiainen V, Lappalainen R, Konttinen Y, Salo J. Toll-like receptors in the interface membrane around loosening total hip replacement implants. J Biomed Mater Res. 2007;81A:1017–1026. doi: 10.1002/jbm.a.31235. [DOI] [PubMed] [Google Scholar]
- Taki N, Tatro J, Lowe R, Goldberg V, Greenfield E. Comparison of the roles of IL-1, IL-6, and TNFa in cell culture and murine models of aseptic loosening. Bone. 2007;40:1276–1283. doi: 10.1016/j.bone.2006.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki N, Tatro JM, Nalepka JL, Togawa D, Goldberg VM, Rimnac CM, Greenfield EM. Polyethylene and titanium particles induce osteolysis by similar, lymphocyte-independent, mechanisms. J Orthop Res. 2005;23:376–383. doi: 10.1016/j.orthres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Tatro J, Taki N, Islam A, Goldberg V, Rimnac C, Doerschuk C, Stewart M, Greenfield E. The balance between endotoxin accumulation and clearance during particle-induced osteolysis in murine calvaria. J Orthop Res. 2007;25:361–369. doi: 10.1002/jor.20289. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Brandenburg K, Kusumoto S, Fukase K, Mackie A, Seydel U, Triantafilou K. Combinational clustering of receptors following stimulation by bacterial products determines lipopolysaccharide responses. Biochem J. 2004;381:527–536. doi: 10.1042/BJ20040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunney MM, Patrick S, Curran MD, Ramage G, Hanna D, Nixon JR, Gorman SP, Davis RI, Andersonc N. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol. 1999;37:3281–3290. doi: 10.1128/jcm.37.10.3281-3290.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuyt L, Dokter W, Birkenkamp K, Koopmans SB, Lummen C, Kruijer W, Vellenga E. Extracellular-regulated kinase 1/2, Jun N-terminal kinase, and c-Jun are involved in NF-kappa B-dependent IL-6 expression in human monocytes. J Immunol. 1999;162:4893–4902. [PubMed] [Google Scholar]
- Vogel S, Hirschfeld MJ, Perera PY. Signal integration in lipopolysaccharide (LPS)-stimulated murine macrophages. J Endo Res. 2001;7:237–241. [PubMed] [Google Scholar]
- Wang ZM, Liu C, Dziarski R. Chemokines are the main proinflammatory mediators in human monocytes activated by Staphylococcus aureus, peptidoglycan, and endotoxin. J Biol Chem. 2000;275:20260–20267. doi: 10.1074/jbc.M909168199. [DOI] [PubMed] [Google Scholar]
- Wilkinson JM, Hamer AJ, Stockley I, Eastell R. Polyethylene wear rate and osteolysis: critical threshold versus continuous dose-response relationship. J Orthop Res. 2005;23:520–525. doi: 10.1016/j.orthres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Wilkinson JM, Wilson A, Stockley I, Scott I, Macdonald D, Hamer A, Duff G, Eastell R. Variation in the TNF gene promoter and risk of osteolysis after total hip arthroplasty. J Bone Miner Res. 2003;18:1995–2001. doi: 10.1359/jbmr.2003.18.11.1995. [DOI] [PubMed] [Google Scholar]
- Wright TM, Goodman SB, editors. Implant Wear in Total Joint Replacement. Rosemount, Ill: American Academy of Orthopaedic Surgeons; 2001. p. 224. [Google Scholar]
- Xing L, Bushnell T, Carlson L, Tai Z, Tondravi M, Siebenlist U, Young F, Boyce B. NF-kappaB p50 and p52 expression is not required for RANK-expressing osteoclast progenitor formation but is essential for RANK- and cytokine-mediated osteoclastogenesis. J Bone Miner Res. 2002;17(7):1200–1210. doi: 10.1359/jbmr.2002.17.7.1200. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Abu-Amer Y, Faccio R, Clohisy J. Map kinase c-Jun N-terminal kinase mediates PMMA induction of osteoclasts. J Orthop Res. 2006;24(7):1349–1357. doi: 10.1002/jor.20199. [DOI] [PubMed] [Google Scholar]
- Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. J Biol Chem. 1997;272(28):17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- Zhu W, Downey JS, Gu J, Padova FD, Gram H, Han J. Regulation of TNF expression by multiple mitogen-activated protein kinase pathways. J Immunol. 2000;164:6349–6358. doi: 10.4049/jimmunol.164.12.6349. [DOI] [PubMed] [Google Scholar]