Abstract
Recent studies indicate that decreased central dopamine is associated with diet-induced obesity in humans and in animal models. In the current study, we assessed the hypothesis that diet-induced obesity reduces mesolimbic dopamine function. Specifically, we compared dopamine turnover in this region between rats fed a high-fat diet and those consuming a standard low-fat diet. We also assessed behavioral consequences of diet-induced obesity by testing the response of these animals in a conditioned place paradigm using amphetamine as a reinforcer and in an operant conditioning paradigm using sucrose reinforcement. Results demonstrate that animals consuming a high-fat diet, independent of the development of obesity, exhibit decreased dopamine turnover in the mesolimbic system, reduced preference for an amphetamine cue, and attenuated operant responding for sucrose. We also observed that diet induced obesity with a high fat diet attenuated mesolimbic dopamine turnover in the nucleus accumbens. These data are consistent with recent hypotheses that the hormonal signals derived from adipose tissue regulate the activity of central nervous system structures involved in reward and motivation, which may have implications for the treatment of obesity and/or addiction.
Keywords: Diet-induced obesity, high-fat diet, psychostimulants, reward, operant conditioning, conditioned place preference
Introduction
Obesity and its co-morbidities have reached epidemic proportions (Flegal et. al 2002). Consumption of high-calorie foods is proposed as a major factor contributing to the wide-spread development of obesity (Clinical Guidelines NIH 1998). Importantly, food intake is driven not only by energy demands, termed “homeostatic” feeding, but also by the hedonic gratification associated with eating a preferred food, referred to as “non-homeostatic” feeding (Berthoud H 2004). The hedonic value of food is derived from its reinforcing properties and is integral to factors that drive increased food intake, including palatability, experience, and social setting. The degree of adiposity may also influence the reinforcing properties, as obese individuals displaying a stronger preference for diets high in fat and carbohydrates, compared to non-obese individuals (Drewnowski A, & Holden-Wiltse J 1992, Drewnowski A et. al 1992).
Previous research finds several links between the hedonic value of food and dopamine levels, although the specific underlying mechanisms are not well understood. Increases in mesolimbic dopamine increase the drive to engage in non-homeostatic feeding (Pecina S et. al 2003), and decreased mesolimbic dopamine has been associated with obesity (Wang GJ et. al 2001). The evaluation of palatability is regulated, in part, by dopamine transmission from the ventral tegmental area to the nucleus accumbens (Berridge KC & Robinson TE 2003). Genetic alterations leading to reduced central dopamine levels are associated with increased consumption (Shinohara M et. al 2004) and craving (Sobik L et. al 2005) of high-fat, high-carbohydrate foods.
The mesolimbic dopamine system is known for its involvement in the reinforcing properties of natural rewards such as food (Gamabarna et. al 2003, Salamone et. al 1990, Kelley and Stinus, 1985) and sex behavior (Wenkstern et al. 1992, Damsma et al. 1992) as well as pathological behaviors associated with recreationally abused drugs (Cadoni & DiChiaria 1999, Pontieri et. al 1995). Importantly, the mutual activation of this system by ingestive behavior and drug exposure suggests that a common neural mechanism may underlie the reinforcing value of both food and drugs. Relevant to this, recent human studies suggest that obese individuals have a decreased propensity to engage in the use of recreational drugs and a decreased frequency of substance abuse disorders (Warren M et. al 2005, Simon G et. al 2006), consistent with the hypothesis that obesity is capable of altering the brain’s processing of rewarding stimuli in general.
Here, we demonstrate that chronic consumption of a high-fat diet decreases aspects of amphetamine reward and food motivation in addition to altering dopamine metabolism in the mesolimbic system. Specifically, we observe that rats maintained on a high-fat diet, independent of any change of body weight gain, are relatively insensitive to amphetamine reward and also fail to acquire lever-press responding for sucrose pellets. We further find that that both a high-fat diet and diet-induced obesity alter dopamine neurochemistry in the nucleus accumbens, suggesting a potential neurochemical mechanism for these novel behavioral findings.
Methods
Animals
Male Long-Evans rats (Harlan, IN) weighing 200-250 g were housed individually in a vivarium with a 12:12 light/dark schedule. Room temperature was maintained at 25° C. All animals had ad libitum access to one of two rodent diets: pelleted standard chow (SC) (Teklad, 3.41 kcal/gm, 0.51 kcal/gm from fat) or pelleted high-fat chow (HF) (Research Diets, New Brunswick, NJ, 4.41 kcal/gm, 1.71 kcal/gm from fat).
Apparatus
All conditioning and testing procedures were conducted in four identical conditioning chambers constructed of aluminum end walls and clear Plexiglas sides and measuring 21.6 × 21.6 × 27.9 cm. A grid of 0.48-cm diameter stainless steel bars, spaced 1.9 cm apart, served as the floor of each chamber. A food cup was located on one end wall of each chamber inside a 5 × 5-cm recessed opening. Two levers were located approximately 3 cm to the left and right of the food cup and level with the top of the opening. Only the right lever was active during this experiment. All experimental events were controlled and recorded by computers located in an adjoining room running ABET software (Lafayette Instruments; Lafayette, IN).
Procedures
Experiment 1 (HF/Sucrose Operant Responding)
Two groups of rats were maintained on either SC or HF diet (n=8/group) for 12 weeks prior to the start of any behavioral experiment. A control group was pair-fed (PF) the high-fat diet in amounts that limited body weight gain to match that of the SC animals. The daily ration of HF diet was fed to the PF group in the middle of the light phase each day and each rat was given twenty four hours before being re-fed. With the exception of two animals, each rat ate their entire daily food ration within the twenty four hour period. Each group of rats SC, HF and PF were maintained on this feeding regimen for the entirety of the study and throughout each behavioral test employed. However, to ensure responding and to maintain body weight consistency across all sessions, prior to beginning operant conditioning, animals were given a modest food restriction (reduced to 85% of their adlib body weights over approximately 7 days) and were maintained at this weight throughout all operant training and testing only. The reinforcer was a single 45-mg sucrose pellet (TestDiet, Richmond, IN). Operant training was carried out over 8 consecutive days with one 1-hr trial per day. During the first two days of training, a fixed ratio (FR) 1 autoshaping procedure was employed, in which each lever press earned a single reinforcer. In addition, whenever 600 sec elapsed with no reinforcer delivery, a “free” sucrose pellet was dispensed into the food cup. This procedure was used to facilitate the acquisition of responding for the reinforcer prior to exposure to schedules with a higher work requirement. All animals were then trained for 2 days using an FR1 schedule with no autoshaping component, followed by 2 days of FR2 training, then 2 days of FR3 training. At the conclusion of the 8-day operant training regimen, animals were given a single trial to lever press for sucrose under a progressive ratio (PR) 2 schedule of reinforcement (lever press requirement for each subsequent reinforcer increased by 2, with an initial requirement of 1 lever press). The breakpoint for each animal was defined as the final completed requirement that preceded a 20-min period without earning a reinforcer.
Experiment 1.2 (DR/DIO Animals/Sucrose Reward)
To rule out any potential contributions of negative contrast between HF diet and sucrose during operant conditioning responding and to determine if obesity alone may alter the motivation to obtain a food reward, we conducted another operant experiment in rats maintained on standard rodent chow. This experiment utilized an outbred strain of Sprague Dawley rats that are genetically predisposed towards becoming obese on a high-fat diet (Levin et al 1997). Both diet resistant (DR) and diet induced obese (DIO) animals (n=8/group) were maintained on chow for 6 weeks prior to the start of the experiment. After the 6 weeks of ad libitum access to food and water animals were restricted to 85% of their daily intake for two days prior to the beginning of operant conditioning. Operant training was carried out over eight consecutive days as described for Experiment 1.
Experiment 2 (Amphetamine-Induced Locomotion)
At the conclusion of operant testing, rats used in experiment 1 were given subcutaneous injections of amphetamine to determine if there were any pre-existing differences in the efficacy of amphetamine prior to the place preference conditioning procedure. Each animal had a home cage fitted with a locomotor activity monitoring unit (Lafayette Instruments, Lafayette, IN). On the first test day, each animal was administered either 1.0 mg/kg D-amphetamine or an equivalent volume of isotonic saline (i.p.) at the midpoint of the light phase of the light/dark cycle. Two days later, each animal received the opposite injection, with the order of injections counterbalanced within and across diet conditions. Total activity for each animal was measured beginning 60 min prior to injection and continuing until 90 min subsequent to injection.
Experiment 2.1 (Amphetamine-Induced Conditioned Place Preference)
The conditioned place preference apparatus had three chambers separated by guillotine doors. One side chamber had white walls and a grid floor, and the other side chamber was black with stainless steel rods as flooring. The center chamber was grey with plexiglass flooring (Med Associates, St. Albans, VT). Rats used in this experiment were the same animals used in experiment 2. On the baseline day, all animals were placed into the center chamber with free access to all chambers for 15 min to allow each animal to habituate to the conditioning chamber. The next day, Conditioning Day 1, animals were given either a 1.0 mg/kg i.p. dose of D-amphetamine or isotonic saline. After 10 min, the animals were placed into one side of the chamber and restricted to that side for thirty min. On the subsequent day, Conditioning Day 2, animals that were given amphetamine on Day 1 received saline injections and animals given saline on Day 1 received amphetamine, and all animals were placed into the opposite side chamber from Day 1. The treatment (amphetamine or saline) and side of chamber (black or white) were counterbalanced across animals to eliminate any potential bias during the conditioning procedure. This cycle was repeated for 12 consecutive days, totaling 6 amphetamine/chamber pairings and 6 saline/chamber pairings. On the test day (1 day after the conclusion of training), animals were placed in the center chamber and allowed free access to all chambers for 5 min. During the baseline and test days, each animal’s behavior was videotaped from overhead, and a computerized tracking system (TopScan, Clever Sys, Inc., Reston, VA) was used to determine time spent in each chamber. Data are presented as preference scores, which are calculated as time in the amphetamine-paired chamber / (time in amphetamine-paired chamber + time in non-paired chamber).
Experiment 3 (Dopamine Neurochemistry)
The nucleus accumbens (NAcc) and orbito-frontal cortex (OFC) was collected from each group of animals (SC, HF, and PF) under basal conditions at the conclusion of all behavioral experiments. Bilateral micropunches for each animal were dissected using a 26-gauge needle attached to a 1-ml syringe containing isotonic saline. Although substantial care was taken to minimize contamination by neighboring brain regions, due to the nature and size of each micro punch our method did not allow us to distinguish subregions (i.e. core vs. shell) within the NAcc. For high-performance liquid chromatography (HPLC) analysis, an antioxidant solution (0.4 N perchlorate, 1.343 mM ethylenediaminetetraacetic acid (EDTA) and 0.526 mM sodium metabisulfite) was added to the samples followed by homogenization using an ultrasonic tissue homogenizer (Biologics, Gainesville, VA). A small portion of the tissue homogenate was dissolved in 2% sodium dodecyl sulfate (SDS) (w/v) for protein determination (Pierce BCA Protein Reagent Kit, Rockford, IL). The remaining suspension was spun at 14,000g for 20 min in a refrigerated centrifuge. The supernatant was reserved for HPLC. Samples were separated on a Microsorb MV C-18 column (5 Am, 4.6_250 mm, Varian, Walnut Creek, CA) and simultaneously examined for DA, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), both of which are markers of dopamine degradation, 5-HT and 5-HIAA. Compounds were detected using a 12-channel coulometric array detector (CoulArray 5200, ESA, Chelmsford, MA) attached to a Waters 2695 Solvent Delivery System (Waters, Milford, MA) under the following conditions: flow rate of 1 ml/min; detection potentials of 50, 175, 350, 400 and 525 mV, and; scrubbing potential of 650 mV. The mobile phase consisted of a 10% methanol solution in distilled H2O containing 21 g/l (0.1 M) citric acid, 10.65g/l (0.075 M) Na2HPO4, 176 mg/l (0.8 M) heptanesulfonic acid and 36 mg/l (0.097 mM) EDTA at a pH of 4.1. Unknown samples were quantified against a 6-point standard curve with a minimum R2 of 0.97. Quality control samples were interspersed with each run to ensure HPLC calibration.
Statistical analysis
Data were analyzed using STATISTICA version 6.0 for PC’s. Amphetamine activity and conditioned place preference scores were analyzed using analysis of variance (ANOVA). LSD post-hoc comparisons were used to assess the source of significant main effects. Significant differences in breaking points were assessed by Fishers Planned Comparison.
Results
Diet-induced obesity attenuates operant responding for sucrose pellets
Next, we tested the effects of diet induced obesity and exposure to dietary fat on operant responding for sucrose reward. Animals maintained on high-fat under ad libitum feeding conditions prior to training displayed significantly fewer lever presses for sucrose pellets under all schedules of reinforcement [Fs(1,21) > 5.00, p < .05] compared to animals maintained on standard chow (Fig 1 A). When tested using a progressive ratio (PR2) schedule of reinforcement, animals maintained on the HF diet exhibited decreased breakpoints relative to animals maintained on the SC diet [t(14) = 3.28, p<0.05]. Similar to the group given ad lib access to HF, PF animals exhibited decreased levels of lever-press responding throughout all schedules of reinforcement [ts(14) > 2.85, p < .05]. These data suggest that high-fat diets are capable of attenuating the reward of sucrose even without the development of obesity.
Figure 1.
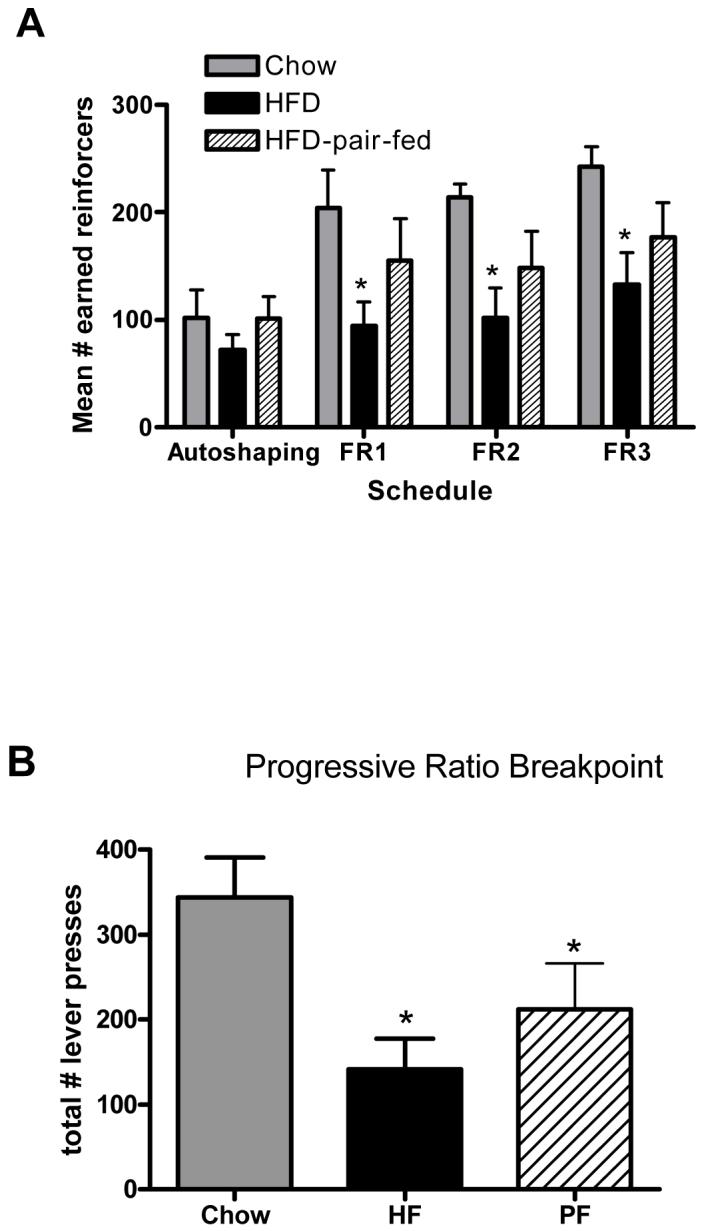
A) Acquisition of operant responding for sucrose pellets in rats maintained on chow, HFD or pair-fed HFD. B) Progressive ratio responding for sucrose pellets in rats maintained on chow, HFD or pair-fed HFD. * = p<0.05
Obesity alone attenuates operant responding for sucrose pellets
Because it is possible that rats maintained on HF diet respond less for sucrose because they find sucrose pellets less rewarding in comparison to the HF maintenance diet, we ran another operant experiment in rats maintained on standard rodent chow. DIO animals maintained on a standard chow diet were significantly heavier (DIO=376.28 ± 5.22; DR=284.09 ± 5.67g) displayed significantly fewer lever presses under all FR schedules of reinforcement relative to DR animals, and when tested under the PR schedule of reinforcement, DIO animals exhibited significantly lower breakpoints than their DR counterparts (p < 0.05, Figure 2). A possible interpretation of these data is that obesity by itself is capable of attenuating operant responding independent of diet.
Figure 2.
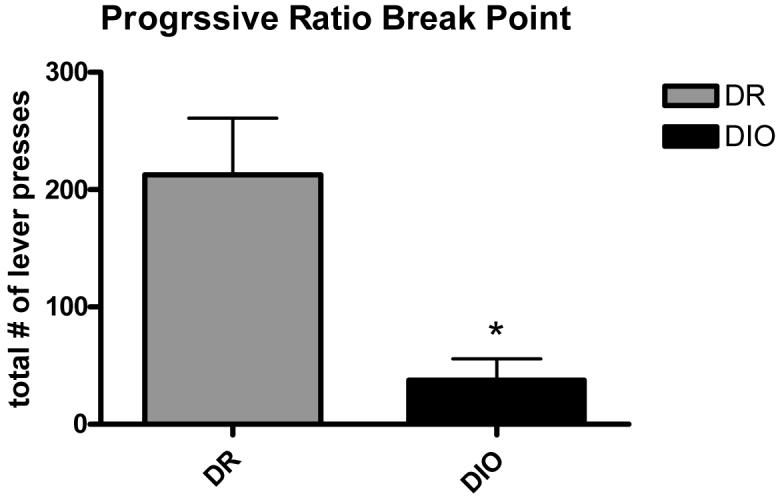
Progressive ratio responding for sucrose in DR and DIO animals in Experiment 1.2 *=p < 0.05.
Exposure to dietary fat attenuates amphetamine conditioned place preference
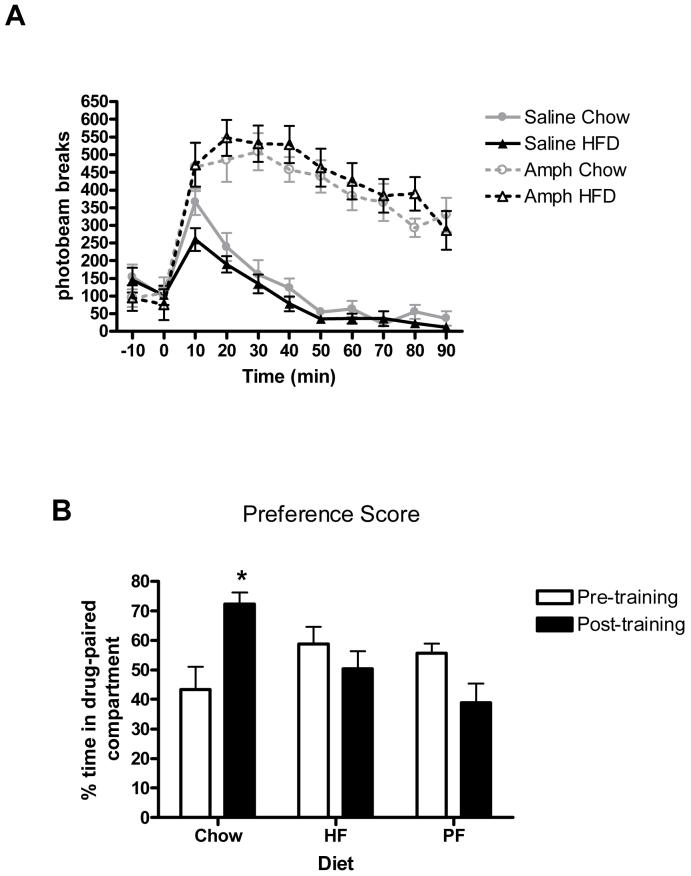
Rats maintained on a HF diet for twelve weeks were significantly (ps < .05) heavier in comparison to animals maintained on SC diet or the PF control group (HF = 455.18 +/- 13.40 g; SC = 395.34 +/- 7.94 g; PF = 378.45 +/- 9.09 g). Importantly, however, animals maintained on HFD or standard chow did not differ significantly in baseline locomotor activity [F(1,14) = 0.26, p > 0.05] and a 1.0 mg/kg dose of amphetamine significantly increased locomotor activity in both groups compared to saline injection (Fig. 3A) (main effect of Drug [F(1,14) = 91.47, p < 0.01]; main effect of Time [F(8,112) = 42.40, p < 0.01]; Drug x Time interaction [F(8,112) = 10.38, p < 0.01]). There was no effect of diet on the level of amphetamine-induced locomotion [largest F = 1.26, p = 0.27], which we interpreted as evidence that amphetamine is equally efficacious in both lean and obese animals. Thus, any difference in the capacity of amphetamine to support reward-related learning cannot be accounted for by general physiological actions of amphetamine between these groups.
Figure 3.
A) Mean number of activity counts over 90 minutes in rats maintained on chow or HFD after an acute 1.0 mg/kg dose of D-amphetamine or saline. B) Conditioned place preference score (time in amphetamine-paired compartment/total time spent in amphetamine- and saline-paired compartments) after 6 conditioning sessions with D-amphetamine in male rats fed chow, HFD or pair-fed HFD * = p<0.05.
Next, we measured the effect of HF consumption and diet-induced obesity on the acquisition of an amphetamine based conditioned place preference (CPP). Figure 3B indicates that animals in the SC group increased their preference for the compartment in which they received amphetamine, while animals consuming HF did not appear to alter their preference based on experience with amphetamine. An ANOVA using preference for the amphetamine-paired compartment as the dependent variable confirms this observation, yielding a significant interaction between diet condition and pre-training vs. post-training [F(2,21) = 7.10, p<0.01]. One-way repeated measures analysis of each diet condition yielded a significant training effect for the animals consuming standard chow [F(1,7) = 8.25, p<0.05], but not for the ad lib high-fat [F(1,7) = 1.23, p<0.3] or pair-fed high-fat [F(1,7) = 3.12, p< 0.1] groups.
Exposure to dietary fat and obesity decrease DA transmission in the NAcc
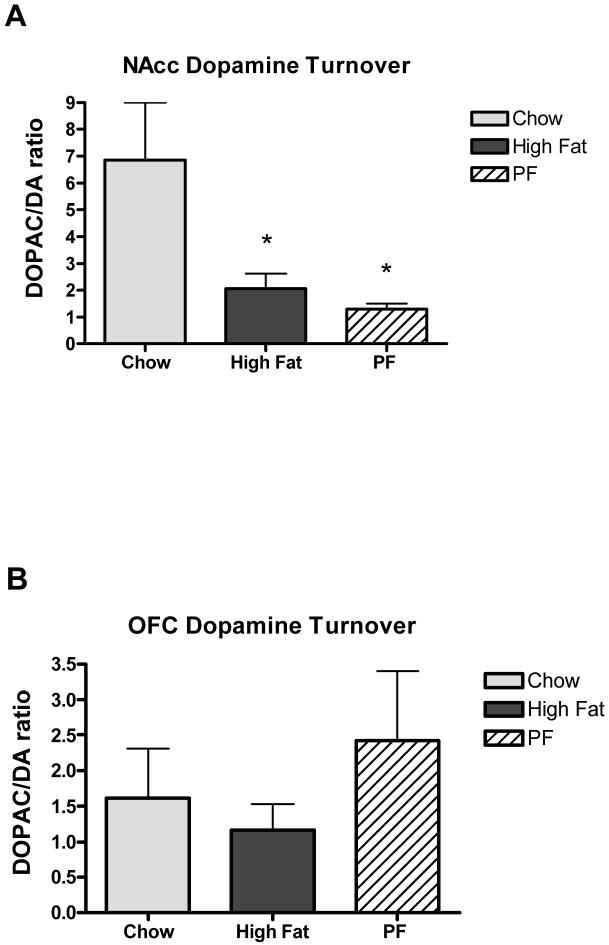
Mesolimbic dopamine flux is associated with the ability of animals to respond to cues that predict the delivery of a variety of different types of reward including ingestive (food or liquid), drug, and sex related rewards (Shultz W 1997, 2007). A large body of evidence supports a role for mesolimbic dopamine signaling in food reward paradigms (Bassareo V & Di Chiara G 1999, Sotak B et. al 2005), therefore, we sought to determine if a high-fat diet was capable of altering mesolimbic dopamine metabolism. ANOVA revealed a main effect of group on NAcc dopamine turnover [F(1, 18) = 4.163; p<0.05]. Animals given ad libitum access to the HF diet displayed decreased dopamine turnover in the NAcc relative to SC control animals (p<0.05), as did animals exposed to HF diet without becoming obese (p<0.05, Fig. 4A). There were no differences in dopamine concentration in the NAcc between any of the groups tested. Additionally, there were no differences (ps > .05) in dopamine concentration or turnover in the orbitofrontal cortex (OFC) between any of the three groups (Fig. 4B), nor did we observe differences in catecholamine metabolites (DA, 5HT, HVA) in either brain region, suggesting a specific effect of a high-fat diet on dopamine neurochemistry that is restricted to the mesolimbic system.
Figure 4.
Dopamine turnover (the ratio of the dopamine metabolite DOPAC to DA) in A) Nucleus accumbens (NAcc) and B) Orbitofrontal cortex (OFC) * = p<0.05
Discussion
It has been well documented that caloric status regulates reward-related behavior. In particular, food deprivation has been reported to increase drug-reinforced behavior in self-administration models, as well as the cellular activating effects of psychostimulant drugs including cocaine and amphetamine (Carr K & Kutchukhidze N 2000, Carrol M & Meish R 1984). Because it is unclear if weight gain or specific dietary components that lead to obesity might also be capable of eliciting functional changes within the mesolimbic system, we tested the ability of two independent caloric regimens to attenuate reward-related behavior, 1) consumption of a high fat diet, without consequent obesity (PF group) and 2) dietary-induced hyperphagia, leading to obesity (HF group). These studies examined the consequences of a high-fat diet and diet-induced obesity on the behavioral responses to two types of rewarding stimuli, as well as its effect on mesolimbic dopamine metabolism. Findings suggest that both consumption of a diet high in fat and obesity resulting from a high-fat diet attenuate amphetamine-induced conditioned place preference, operant responding for sucrose, and decreased dopamine turnover in the mesolimbic system.
Diet-induced obesity and operant responding for sucrose
We report that consumption of a HF diet is capable of attenuating responding to obtain sucrose pellets. Rats maintained on HF diet exhibit attenuated responding for sucrose pellets under a variety of reinforcement schedules, even though they are food deprived at the time of training. These effects of diet-induced obesity on responding are consistent with previous reports using a “supermarket” diet to induce obesity (Sclafani A & Springer D 1976). Importantly, the decreased operant responding reported here was present in obese animals as well as animals fed the HF diet without becoming obese. It is possible that the effects of HF diet exposure and/or obesity could be accounted for by a contrast effect. That is, the reinforcing efficacy of the sucrose pellets is reduced due to being negatively compared with the HF diet available in the home cage, whereas the pellets compared favorably with the SC diet. To address this issue, in a separate experiment rats maintained on standard rodent chow were tested for their ability to acquire operant responding for sucrose. In this experiment, DIO rats were also significantly heavier than the DR control rats at the time of operant testing. Subsequently, DIO rats displayed fewer operant responses for sucrose under every reinforcement schedule tested compared to DR control rats. Although exposure to HF diet without the development of obesity is sufficient to attenuate operant responding, these data support the notion that obesity alone produces similar effects. Collectively these data suggest that both exposure to HF diet and obesity are capable of attenuating the motivation to obtain sucrose reward, an effect independent from negative contrast between maintenance diet and sucrose.
Diet-induced obesity and amphetamine-induced CPP
In addition to the effects on operant responding, we report that consumption of dietary fat and obesity attenuates the rewarding properties of psychostimulants. In the initial set of experiments designed to test locomotor activating effects of amphetamine, all animals displayed similar locomotor responses to amphetamine, regardless of their diet condition. However, only chow-fed animals formed a conditioned place preference to amphetamine. Animals consuming the HF diet failed to show any difference between an amphetamine-paired and a saline-paired context. These results are consistent with recent reports in which exposure to a high-fat diet attenuates cocaine self-administration in rats (Wellman PJ et. al 2007) and suggest that the rewarding properties of psychostimulants are significantly altered by high-fat diet consumption. Further, the results of the Wellman et al study and the findings from the present study suggest that consumption of high-fat diet is a critical factor that alters the rewarding aspects of psychostimulants and that this effect occurs independent of body weight gain.
Dietary fat attenuates mesolimbic dopamine turnover
In the present study, both PF and ad libitum HF groups displayed decreased dopamine turnover in the nucleus accumbens (NAcc). Previous studies in animals suggest that central dopamine agonists may normalize body weight in a genetic model of obesity (Bina KG, & Cincotta AH 2000). Furthermore, genetic studies reveal that polymorphisms linked to decreased central dopamine are also associated with increased craving for palatable foods (Sobik L et. al 2005). Additionally, previous work links mesolimbic dopamine flux and, more specifically, changes in NAcc dopamine, with feeding and ingestion of palatable foods. In particular, consumption of a 0.3M sucrose solution leads to substantial increases in NAcc dopamine flux, and predicted delivery of sucrose produces increases in NAcc dopamine turnover suggesting that both acute and repeated exposure to palatable foods can alter NAcc dopamine neurochemistry (Hajnal A & Norgen R 2001, 2002). Our data suggest that chronic consumption of a calorically dense diet is capable of modulating this process. Specifically, these data support the contention that altered mesolimbic dopamine metabolism is a consequence of high levels of dietary fat. Dopamine increases upon initial exposure to the HF diet, and dopamine signaling through the D2 and D3 receptor subtypes is necessary for the positive contrast hyperphagia associated with ingestion of the HF diet itself (Roitman et. al 2001, Davis et. al 2006). Therefore, it is possible that chronic exposure to palatable foods, and/or the hyperphagia in response to this exposure, leads to decreases in dopamine turnover in response to repeated release of dopamine within the NAcc. . In fact, cues associated with a preferred saccharin solution alone are capable of evoking dopamine release in the NAcc (Grigson P & Hajnal A 2007) suggesting that anticipation of palatable meals is capable of activating the mesolimbic system. Additionally, when given simultaneous access to a “preferred food” like saccharin and to an abused drug, animals display impaired acquisition and expression of drug self administration behavior (Campbell et. al. 1998, Carroll 1995) suggesting that reinforcing strength of palatable foods prior to changes in caloric status is capable of altering the motivation to engage in drug self administration.
It is also possible that the effects reported here on both reward-related behavior and dopamine turnover are caused by increased levels of the metabolic hormone leptin. Previous data demonstrate that icv leptin attenuates operant responding and place preferences for sucrose pellets (Figlewicz et. al 2004, 2006) and that leptin may directly target the VTA to regulate preference for palatable foods as well as the integration of behavioral responses to psychostimulant drugs (Hommel et. al 2006; Fulton et. al 2006). Diet-induced obesity and the consumption of dietary fats increase circulating plasma leptin levels. Thus it is possible that leptin, either through a reduction in dopamine turnover or by altering mesolimbic function in some other way, could be a plausible mechanism contributing to our present results.
Conclusions
Overall, the data presented here are consistent with the hypothesis that diets high in fat attenuate rewarding aspects of multiple types of stimuli, including palatable foods and psychostimulants. An important point raised by these data is that chronic consumption of a diet high in saturated fats, even without the development of obesity, is capable of attenuating dopamine turnover within mesolimbic areas associated with reward-related behaviors. Changes in mesolimbic circuitry are hypothesized to contribute to the onset of addictive behavior (Nestler E 2005) and non-homeostatic feeding behavior has recently begun to be studied in the context of addiction (Corwin R & Hajnal A 2005). In that regard, therapeutic interventions aimed at treating obesity through blockade of neural signals which regulate reward (dopamine, cannabinoids) are beginning to emerge (Goldfield GS et. al 2007, Van Gaal LF et. al 2005). The results presented here support the notion of a critical interaction between diet and the physiological processes that regulate reward and reward-related behaviors, including non-homeostatic feeding.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/bne/
Disclosure/Conflict of Interest
The authors declare that no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest for the current manuscript.
References
- Balfour ME, Yu L, Coolen LM. Sex behavior and sex associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology. 2004;4:718–30. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiology and Behav. 2004;81:781–793. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosci. 2003;26:507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci. 1999;11:4389–97. doi: 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- Bina KG, Cincotta AH. Dopaminergic agonists normalize elevated hypothalamic neuropeptide Y and corticotropin-releasing hormone, body weight gain, and hyperglycemia in ob/ob mice. Neuroendocrinology. 2000;71:68–78. doi: 10.1159/000054522. [DOI] [PubMed] [Google Scholar]
- Cadoni C, DiChiara Differential changes in accumbens core and shell dopamine in behavioral sensitization to nicotine. European Journal of Pharmacology. 1999;387:R23–25. doi: 10.1016/s0014-2999(99)00843-2. [DOI] [PubMed] [Google Scholar]
- Carr K, Kutchukhidze N. Chronic food restriction increases fos-like immunoreactivity (FLI) induced in rat forebrain by amphetamine. Brain Res. 2000;861(1):88–96. doi: 10.1016/s0006-8993(00)02018-7. [DOI] [PubMed] [Google Scholar]
- Carroll M, Meisch R. Increased drug reinforced behavior due to food deprivation. Adv in Behav Pharmacol. 1984;4:47–88. [Google Scholar]
- Cincotta AH, Tozzo E, Scislowski PW. Bromocriptine/ SKF38393 treatment ameliorates obesity and associated metabolic dysfunctions in obese (ob/ob) mice. Life Sci. 1997;61:951–6. doi: 10.1016/s0024-3205(97)00599-7. [DOI] [PubMed] [Google Scholar]
- Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Institutes of Health, National Heart, Lung, and Blood Institute; Jun, 1998. [Google Scholar]
- Coons EE, Levak M, Miller NE. Lateral hypothalamus: learning of food-seeking response motivated by electrical stimulation. Science. 1965;701:1320–1. doi: 10.1126/science.150.3701.1320. [DOI] [PubMed] [Google Scholar]
- Corwin R, Hanjal A. Too much of a good thing: Neurobiology of non-homeostatic eating and drug abuse. Physiol Behav. 2005;86(12):5–8. doi: 10.1016/j.physbeh.2005.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106:181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- Dougherty J, Pickens R. Fixed-interval schedules of intravenous cocaine presentation in rats. Journal of Experimental Analysis of Behavior. 1973;20:111–18. doi: 10.1901/jeab.1973.20-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Kurth C, Holden-Wiltse J, Saari J. Food preferences in human obesity: carbohydrates versus fats. Appetite. 1992;18:207–21. doi: 10.1016/0195-6663(92)90198-f. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Taste responses and preferences for sweet high-fat foods: evidence for opioid involvement. Physiol Behav. 1992;51:371–9. doi: 10.1016/0031-9384(92)90155-u. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:678–80. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Figelwicz DP, Bennet JL, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioning for high fat diet in rats. Behav Neurosci. 2004;3:479–87. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- Figelwicz DP, Bennet JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiology and Behav. 2006;4:611–16. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Gambarna C, Masi F, Leggio B, Gappi S, Nanni G, Scheggi S, DeMontis MG, Tagliamonte A. Acquisition of a palatable-food-sustained appetitive behavior in saited rats is dependent on the dopaminergic response to this food in limbic areas. Neuroscience. 2003;121:179–87. doi: 10.1016/s0306-4522(03)00383-x. [DOI] [PubMed] [Google Scholar]
- Goldfield GS, Lorello C, Doucet E. Methylphenidate reduces energy intake and dietary fat intake in adults: a mechanism of reducing the reinforcing values of food? Am J Clin Nutr. 2007;86:308–15. doi: 10.1093/ajcn/86.2.308. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain Research. 2001;904:76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Norgren R. Repeated access to sucrose augments dopamine turnover in the nucleus accumbens. Neuroreport. 2002;17:2213–6. doi: 10.1097/00001756-200212030-00010. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;6:801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Stinus L. Disappearance of hoarding behavior after 6-hydroxydopamine lesions of the mesolimbic dopamine neurons and its reinstatement with L-Dopa. Behav Neurosci. 1985;99:531–45. doi: 10.1037//0735-7044.99.3.531. [DOI] [PubMed] [Google Scholar]
- McTigue K, Garret J, Popkin B. The natural history of the development of obesity in a cohort of young US adults between 1981 and 1998. Annals of Inter Med. 2002;136:857–64. doi: 10.7326/0003-4819-136-12-200206180-00006. [DOI] [PubMed] [Google Scholar]
- Mokdad A, Ford E, Bowman B, Dietz W, Vinicor F, Bales V, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors. J Am Med Assoc. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Nestler E. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8(11):1445–9. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- O’Brien P, Dixon J. Laparoscopic adjustable gastric banding in the treatment of morbid obesity. Arch Surg. 2003;138(4):376–82. doi: 10.1001/archsurg.138.4.376. [DOI] [PubMed] [Google Scholar]
- Olshansky S, Passaro D, Hershow R, Layden J, Carnes B, Bordy J, Hawflick L, Butler R, Allison D, Ludwig D. A potential decline in life expectancy in the United States in the 21 century. N Eng J Med. 2005;352:1138–45. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyper-domaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Neurosci. 2003;23:9395–402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci. 1995;26:12304–8. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, van Dijk G, Thiele TE, Bernstein IL. Dopamine mediation of the feeding response to violations of spatial and temporal expectancies. Behav Brain Res. 2001;122:193–199. doi: 10.1016/s0166-4328(01)00189-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Zigmond MJ, Stricker EM. Characterization of impaired feeding behavior in rats given haloperidol or dopmaine-depleting brain lesions. 1990;39:17–24. doi: 10.1016/0306-4522(90)90218-s. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annual Rev Neuroscience. 2007;30:259–88. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Springer D. Dietary Obesity in Adult Rats: Similarities to Hypothalamic and Human Obesity Syndromes. Physiol Behav. 1976;17:461–477. doi: 10.1016/0031-9384(76)90109-8. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Mizushima H, Hirano M, et al. Eating disorders with binge-eating behaviour are associated with the s allele of the 3′-UTR VNTR polymorphism of the dopamine transporter gene. J Psychiatry Neurosci. 2004;29:134–7. [PMC free article] [PubMed] [Google Scholar]
- Simon G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psych. 2006;63:824–30. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: a fresh approach to the study of binge eating. Appetite. 2005;44:253–61. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Sotak B, Hnasko T, Robinson S, Kremer E, Palmiter R. Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res. 2005;1061:88–96. doi: 10.1016/j.brainres.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–97. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357:354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Warren M, Frost-Pineda K, Gold M. Body Mass Index and Marijuana use. J Addict Dis. 2005;24(3):95–100. doi: 10.1300/J069v24n03_08. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Nation JR, Davis KW. Impairment of cocaine self-administration in rats maintained on a high fat diet. Pharmacol Biochem Behav. 2007;88:89–93. doi: 10.1016/j.pbb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenkstern D, Pfaus JG, Fibiger HC. Dopamine transmission increases in the nucleus accumbens of male rats during their first exposure to sexually receptive female rats. Brain Res. 1993;618:41–46. doi: 10.1016/0006-8993(93)90426-n. [DOI] [PubMed] [Google Scholar]
- Woods S, Seeley R, Rushing P, D’Alessio D, Tso P. A controlled high fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]