Abstract
Therapeutic irradiation of the brain is commonly used to treat brain tumors but can induce cognitive impairments that can severely affect quality of life. The underlying mechanisms responsible for radiation-induced cognitive deficits are unknown but likely involve alterations in neuronal activity. To gain some mechanistic insight into how irradiation may affect hippocampal neurons known to be associated with cognitive function, we quantitatively assessed the molecular distribution of the behaviorally-induced immediate early gene (IEG) Arc (activity-regulated cytoskeleton-associated protein) at the level of mRNA and the protein. Young adult C57BL/6 mice received whole brain irradiation with 0 or 10 Gy, and 1 week or 2 months later, exploration of a novel environment was used to induce Arc expression. The fractions of neurons expressing Arc mRNA and Arc protein were detected using fluorescence in situ hybridization (FISH) and immunocytochemistry, respectively. Our results showed that there was a significant reduction in the percentage of neurons expressing Arc protein one week after irradiation, whereas two months after irradiation there was a reduction in the percentage of neurons expressing both Arc mRNA and Arc protein. Importantly, radiation induced changes in Arc expression were not a result of neuronal cell loss. The changes observed at 2 months were associated with a significant increase in the number of activated microglia, supporting the idea that inflammation may contribute to neuronal dysfunction. These findings are the first to demonstrate that local brain irradiation initiates changes in hippocampal neurons that disrupt the activity patterns (Arc expression) associated with neuroplasticity and memory.
Keywords: Radiation injury, brain, Arc, cognition
Introduction
The brain is exposed to ionizing irradiation during the management of brain tumors, and the dose that can be administered safely is largely dictated by the tolerance of normal tissues surrounding the tumor (1). In the US, a large number of patients (200,000 – 300,000/yr) with primary or metastatic tumors in the brain will require large volume or whole brain irradiation (2), and in at least some of these patients, there is a strong likelihood of developing adverse reactions in terms of cognitive decline (3, 4). In fact, it has been recently reported that after irradiation, neurocognitive function changes correlate with alterations in quality of life that in turn correlate with median survival (5). Currently there are no successful long-term treatments or preventive strategies for radiation-induced cognitive impairments (6-8). A better understanding of the cellular and molecular factors associated with the development of cognitive injury is essential to the management of this serious complication of cranial radiotherapy.
Radiation injury can involve multiple regions and cell/tissue types, and a large number of physical and biologic factors influence the expression and extent of damage (9, 10). In patients, overt tissue injury generally occurs after relatively high doses (>60 Gy, fractionated), and the morphologic consequences of such exposures are well documented in humans and experimental systems (1, 9, 10). In humans, less severe morphologic changes can occur after relatively lower doses (e.g. as low as 20 Gy fractionated), resulting in variable degrees of cognitive impairment (11-13). Such impairment has a diverse character, but often includes hippocampal dependent functions involving learning, memory and spatial information processing. Recently, this has prompted consideration of whether or not strategies should be implemented to specifically shield or reduce hippocampal exposure during radiotherapy (5). A number of animal studies have been performed that confirm the importance of hippocampus-related effects in the evolution of radiation-induced cognitive injury (14-17). While those laboratory studies involved varying doses, delivery schemes (e.g. fractionation), endpoints and rodents of various ages, they provide compelling evidence that relatively low radiation doses cause hippocampus-dependent cognitive impairments without necessarily inducing overt signs of tissue destruction.
The cellular and molecular mechanisms underlying radiation-induced cognitive impairments are still not known, but almost certainly involve changes associated with neuronal function, either through direct cell damage or damage mediated through factors from the irradiated microenvironment. Information is starting to appear regarding gene expression changes in brain cells after exposure to ionizing irradiation (reviewed in: (18)) but most of the available experimental data relate to very early changes (<1 – 24 hr) (19, 20), and there is only one consideration of a gene (c-fos) specifically known to be linked to learning and memory (20). Gene expression induced during learning produces proteins that alter the composition of neuronal networks and provide a mechanism for translating synaptic plasticity into changes in synaptic strength (memory); a number of activity-regulated genes have been identified for this function (21). While immediate early genes (IEG) like c-fos and zif268 are involved in mechanisms associated with the maintenance of memory (22), Arc (activity-regulated cytoskeleton-associated protein) is an activity-induced gene that correlates both temporally and spatially with the stimulus that induced its transcription (23). Arc is induced in hippocampal and parietal neurons after a behavioral experience (24), and Arc protein plays a critical role in the maintenance phase of long-term potentiation, and spatial memory consolidation (25). Further, the reduction of Arc expression either genetically (26) or with antisense oligonucleotides (25) results in cognitive impairment with respect to long term memory formation. Perhaps most importantly, Arc is the only known IEG whose mRNA moves rapidly to the dendrites closest to active synapses where it is locally translated (23). Taken together, this information provides a mechanistic link between Arc and hippocampal-dependent function. Furthermore, it provides a strong rationale for using Arc expression to assess specific neuronal activities associated with cognitive impairments and if those activities are altered by cranial irradiation. It is relevant to this argument that Alzheimer's Disease patients with pronounced deficits in hippocampus-dependent memory functions, have almost a 3.5 fold lower baseline level of Arc expression when compared to non-Alzheimer's patients (27). These data support the general hypothesis that reduced Arc expression in the hippocampus is associated with cognitive impairments.
We contend that a better understanding of the cellular and molecular mechanisms underling radiation-induced cognitive impairment is critical for the development of approaches to manage this potentially serious effect. Thus, the present study was done to provide novel insight into how ionizing irradiation affects a specific neuronal function (Arc expression) that is known to be associated with cognitive performance. This type of information is currently unavailable and is essential when trying to ascertain risks of specific CNS-related effects and develop potential strategies to manage radiation-induced cognitive dysfunction.
Methods
Animals
A total of 56 two month old male C57BL/6 mice were used in these studies; 12 for a pilot study of the characterization of Arc expression, and 48 for the study of radiation effects. Animals were purchased from a commercial vendor (Jackson Laboratory, Bar Harbor, ME), and all animal care and use was conducted in accordance with the United States Department of Health and Human Services Guide for the Care and Use of Laboratory Animals, and institutional guidelines for care and handling of laboratory animals. To minimize any stress associated with group housing that could affect our endpoints, all animals were housed singly in Plexiglas cages with free access to food and water and maintained on a 12/12-h light-dark cycle in a temperature-controlled room (22°C). Animals were handled for 5 minutes once a day for 10 days before the behavioral experiment to familiarize them to the experimenter and to the handling procedures.
Pilot Study
To characterize the molecular distribution of the IEG Arc at the level of mRNA and protein in dentate granule neurons (Fig. 1), a group of 12 mice were randomly separated into 2 groups of 6; caged controls and animals that were allowed to explore a novel environment (23). The behavioral procedure involved an initial 5 minute exploration of a novel environment, a 25 minute rest period, and then a second 5 minute exploration of the same environment. During the interval between the 2 exploration sessions, animals remained undisturbed in their own cages. The novel environment was an open white acrylic box measuring 61cm × 61cm with 20cm high walls that contain plastic chews and a polycarbonate house. To exclude the possibility that deficits in IEG expression resulted from altered exploratory behavior and consequent lack of sensory stimulation, motor activity of the mice was visually monitored by an operator blind to animal identity. Light intensity and distal and local spatial cues were maintained throughout both behavioral explorations. Immediately after the second 5 minute exploration, mice were deeply anesthetized and killed by decapitation. The brain was quickly removed (within 60 seconds) and frozen in −70°C isopentane. Mice from the caged control group remained undisturbed in their cage until euthanasia.
Fig. 1.
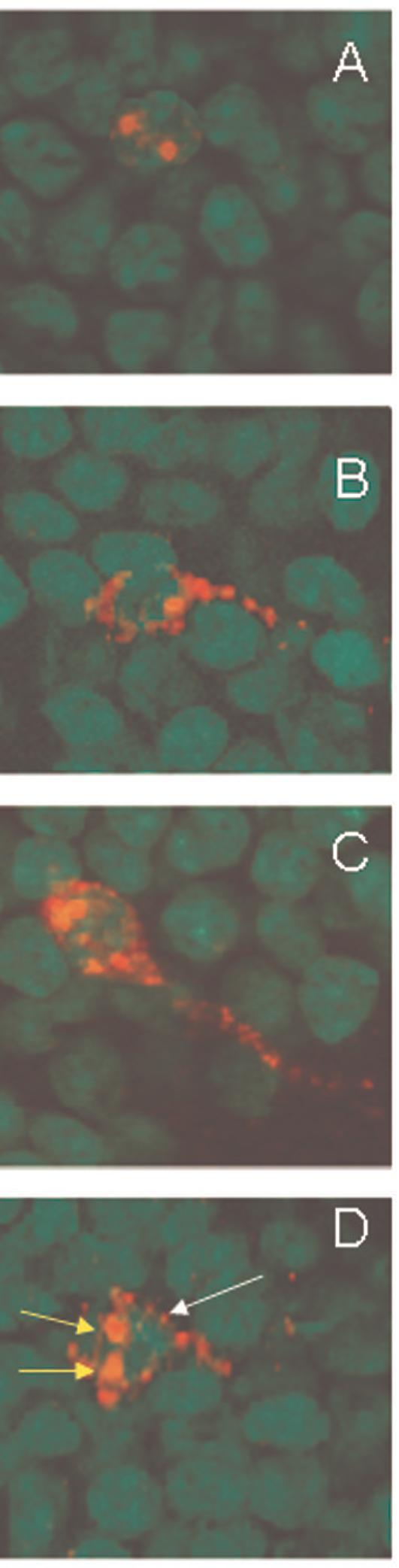
Qualitative characterization of Arc expression in granule cell neurons of the dentate gyrus. Arc was induced by 2 five minute behavioral explorations of a novel environment that were separated by 25 minutes. Intranuclear foci of Arc mRNA were induced by the second exploration, ∼ 5 minutes before tissue collection, and were detected using fluorescent in situ hybridization (A). Cytoplasmic Arc mRNA (B) or Arc protein (C) were detected in neurons activated by the first exploration. Both nuclear foci (second exploration, short yellow arrows) and cytoplasmic Arc mRNA (first exploration, long white arrow) were seen in ∼ 90% of cells immunoreactive for Arc (D). Digoxigenine labeled Arc antisense probe was detected with Cy3 (red, A, B, D), and immunofluorescence staining detected Arc protein (red, C). Cell nuclei were counterstained green and the magnification for all the images was 63×.
Irradiation
For irradiation, mice were anesthetized using an i.p injection of ketamine (60mg/kg) and medetomidine (0.25mg/kg). Irradiation with a Phillips orthovoltage x-ray system was done as previously described (15, 28). Briefly, a special positioning jig was used so 4 animals could be irradiated simultaneously; the heads were centered in a 5 χ 6 cm2 field. The incident beam was directed down onto the head and a special lead shield was used to cover the eyes, nose, cerebellum and the body. Dosimetry was done using a Keithley electrometer ionization chamber as previously described (28). Unirradiated mice were anesthetized at the same time as those who received a single dose of 10 Gy.
Behavioral Procedures
At specified times after irradiation (7 days or 2 months), mice from each treatment cohort (0 Gy, 10 Gy) were randomly assigned to 2 groups of 6 mice; exploration and caged controls. The animals were treated exactly as described above for the pilot study.
Histological Procedures
Frozen brains (kept in −70°C) were divided at the midline, and one hemisphere from each of the 6 animals from a given experimental group were blocked together, cryosectioned and placed on a glass microscopic slide (29). Brain sections were taken from the medial portion of the dorsal hippocampus (anteroposterior ∼2.92-4.0 mm from bregma). Thus, tissues from all the individuals in a given group were processed and stained simultaneously. All slides were stored at −70°C until processed for immunocytochemistry or in situ hybridization
Fluorescence in situ hybridization (FISH)
Four to 6 slides from each treatment group, each containing sections from all the mice in that group, were prepared for FISH. Arc mRNA (Fig. 1 A,B,D; 2 B), and zif268 and c-fos mRNA (Fig. 2 C,D) were detected as previously reported in detail (23, 29). Briefly, hapten-labeled antisense riboprobes were hybridized together with the tissues overnight. The digoxigenin-labeled Arc full probe riboprobe was detected with anti-digoxigenin-HRP conjugate (Roche, Alameda, CA) and revealed with a CY3 substrate kit. Nuclei were counterstained with sytox-green (Molecular Probes, Eugene, OR).
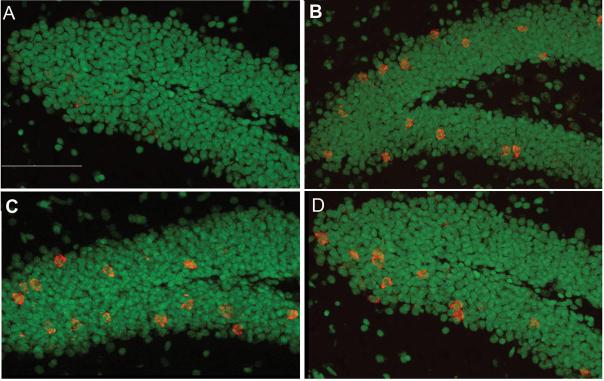
Fig. 2.
mRNA expression (red) of the immediate early genes Arc (B), zif268 (C) and c-fos (D) within the granule cell layer of the dentate gyrus after behavioral exploration. Caged control animals showed low expression of immediate early genes (A). The scale bar represents 100µm.
Immunofluorescence staining
Four to 6 slides from each treatment group were also used for immunohistochemical staining of Arc protein; the sections used were adjacent to those used for FISH. Quantitative assessment was done using methods previously reported by us in detail (29, 30). Briefly, tissues were fixed in 2% paraformaldehyde, and after blocking with a tyramide signal amplification kit (TSA; PerkinElmer Life Science, Emeryville, CA), were incubated in polyclonal rabbit anti-Arc antibody for 48 h at 4°C, (rabbit anti-Arc a gift from S. Chowdhury and P.F. Worley, Johns Hopkins University School of Medicine, Baltimore, MD). Sections were then incubated with an anti-rabbit biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) for 2 h at room temperature, followed by amplification with an avidin-biotin system for 45 minutes. Arc protein staining was reveled using a cyanine-3 (CY3) TSA fluorescence system (PerkinElmer Life Science) (Fig. 1C). For staining of activated microglia, after fixation in paraformaldehyde and the subsequent blocking step (29-31) the primary monoclonal rat antimouse CD68 antibody (Serotec, Inc. Raleigh, NC) was applied overnight followed by a secondary biotinylated anti-mouse antibody (Vector Laboratories). Activated microglia were labeled with CY3 and nuclei were counterstainded with sytox-green (Molecular Probes).
Microscopy, Image Acquisition, and Analysis
Microscopic imaging for Arc mRNA, Arc protein and activated microglia was performed using an Zeiss AXIO IMAGER Z1 microscope with motorized Z-drive for transmitted light and epi-fluorescence (31). For each endpoint, the 4-6 coronal sections per mouse were used to reconstruct mosaics of the entire hippocampal dentate gyrus (DG) (30). Each mosaic was formed from 4-5 Z-stack (1.0 µm optical thickness/plane) images. Contrast and intensity parameters for Arc, zif268 and c-fos were set using the tissue sections from the caged only controls. For consistency, these parameters were kept constant for the rest of the sections on the slide.
Image analysis
Manual cell counts of cells expressing Arc mRNA and Arc protein were performed by an experimenter blind to the relationship between the experimental conditions they represented (i.e., sham, irradiated, caged control, exploration) (29, 30). Arc mRNA positive and Arc protein positive neurons were identified when the staining constituted at least 60% of the cell body (Fig. 1) and was detectable throughout 3 planes across the Z-stack. To avoid classification errors, we carefully verified that the staining belonged to the cell of interest by checking the nuclear counterstaining. The numbers of cells/mm2 characterized by these criteria were determined. The total number of neurons per DG was estimated using a correction factor which was derived from the 24 different z-stacks (20× magnification) from representative mice from both irradiated and non-irradiated treatment groups. The total number of neurons per stack was counted and the area in the granule cell layer (in mm2) from the middle plane was calculated. Using this factor we calculated the percentage of neurons expressing Arc mRNA and Arc protein for each mouse (29, 30). The total number of neurons analyzed for each mouse averaged ∼2000. The total numbers of activated microglia (CD68 positive) within the DG and hilar region of the hippocampus was manually counted and divided for the area in mm2, as reported previously (31).
Statistical methods and Sample Size
StatView software (version 5.0.1, Cary, NC) was used to perform one-way ANOVA tests. The caged control (0 Gy, 10 Gy) and behaviorally tested groups (0 Gy, 10 Gy) were the independent variables, and the percentages of positive cells, from various categories described above, were the dependent variables. When an overall ANOVA was significant (p< 0.05), individual between-group comparisons were performed with Bonferroni post hoc tests to correct for multiple comparisons.
Results
The aim of the pilot study was the characterization of the molecular distribution of the IEG Arc at the level of mRNA and protein in the dentate granule neurons. Similar to what was observed previously in rats (23), neurons expressing only intranuclear foci Arc (Fig. 1A) represented cells activated within ∼ 5 minutes of euthanasia (i.e. second exploration). Neurons showing only cytoplasmic Arc mRNA (Fig. 1B) or Arc protein (Fig. 1D) were active for ∼30 min before the animal was euthanized (i.e. first exploration). Cells showing both Arc foci and cytoplasmic Arc mRNA (Fig. 1D) represented neurons that were activated during both explorations, and constituted approximately 90% of the Arc positive neurons in the granule cell layer of the DG. In caged control animals there were few neurons expressing Arc mRNA and Arc protein. Exploration of a novel environment also resulted in the concomitant expression of the IEGs zif268 and c-fos (Fig. 2). Overall, given the well-described characterization of Arc expression (23, 24, 29) and its relationship to learning and memory (see introduction), we focused our radiation studies solely on the expression of the IEG Arc.
Whole brain irradiation with 10 Gy was tolerated by all animals; none were lost and all mice gained weight normally over the duration of the study. All mice that explored the novel environment (irradiated and non-irradiated) did so in a similar manner (data not shown), indicating that there were no deficits in motor or exploratory behavior as a result of brain irradiation. To assure that the measures of Arc mRNA and Arc protein were not influenced by radiation-induced decreases in neuronal cell number, we quantified neurons in the dentate granule cell layer 2 months after irradiation. In non-irradiated mice there were 1502 ± 351 (mean ± SEM) neurons/mm2, while after 10 Gy there were 1227 ± 191; the difference was not significant.
Caged control non-irradiated mice showed only few granule cells expressing Arc mRNA (Fig. 3A) and Arc protein (not shown). Quantitatively in these mice, the fractions of neurons expressing Arc mRNA averaged < 1% at both post-irradiation time points (Fig. 4 A, C). The fractions of neurons expressing Arc protein were slightly higher (Fig. 4 A, C). In non-irradiated mice that did explore the novel environment, there were more neurons expressing Arc mRNA (Fig. 3B) and protein (not shown). In those mice, the percentages of neurons that expressed Arc mRNA and protein were similar and were increased 5-6 fold relative to caged only controls at both post-irradiation time points (Fig. 4 A, C); (ANOVA, Arc mRNA: F (1,10) = 54.8, p<0.001, 1 week; F (1,10) = 69.24; p<0.001, 2 months; ANOVA, Arc protein: (F (1,10) = 42.5; p<0.001, 1 week; F (1,10) = 23; p<0.001, 2 months).
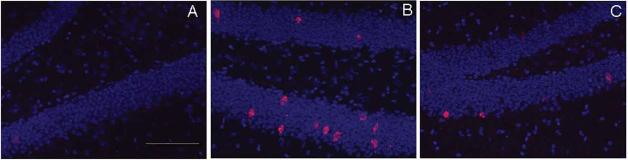
Fig. 3.
Arc mRNA expression (red), in caged control animals (A), non irradiated animals that explored a novel environment (B), and in irradiated animals that explored a novel environment (C). Nuclei were counterstained blue in the fluorescent in situ hybridization analysis. The scale bar represents 100µm..
Fig.4.
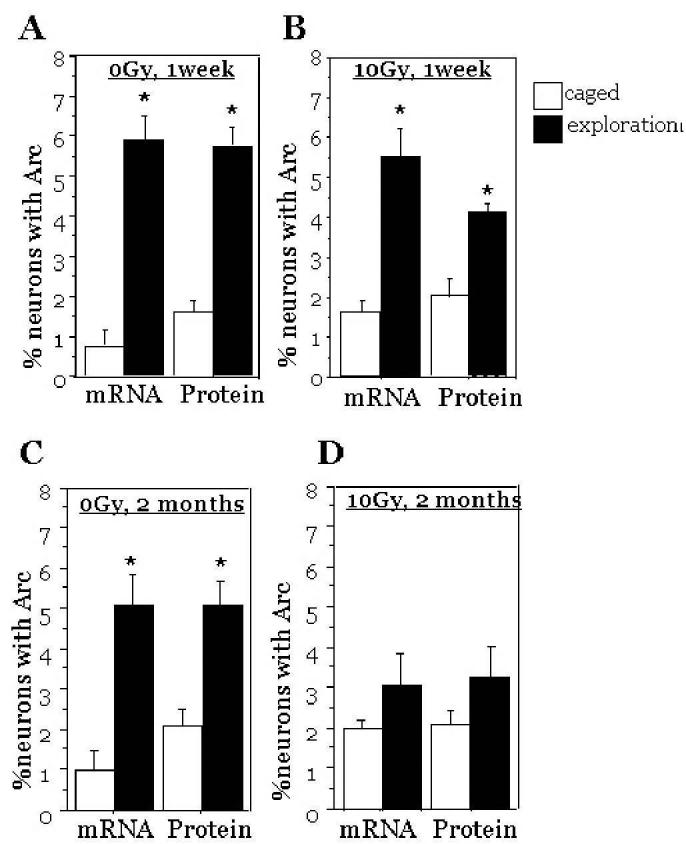
Percentages of granule cell neurons expressing Arc mRNA and Arc protein after exploration of a novel environment. The data represent analyses of tissues from non-irradiated (A, C) and irradiated (B, D) mice either one week (A, B) or two months (C, D) after treatment. After exploration of a novel environment, the fraction of neurons expressing Arc mRNA and Arc protein were significantly higher than caged control animals except for 2 months after irradiation. Each symbol represents an average of 6 mice and error bars are SEM. * p<0.001
At both times after irradiation, the fractions of neurons expressing Arc mRNA and Arc protein in caged control mice were similar to non-irradiated animals (Fig. 4 B, D), indicating that radiation alone did not affect the basal expression levels of Arc. In irradiated animals that did explore the novel environment, there were significant increases in the percentages of neurons expressing Arc mRNA and Arc protein relative to irradiated caged controls at 1 wk after irradiation (ANOVA, Arc mRNA: F (1, 10) = 22.5; p<0.001; ANOVA, Arc protein: F (1, 10) = 40.5; p<0.001), but not at 2 months (ANOVA: F (1,10) = 2.76; p<0.12, Arc mRNA; ANOVA: F (1,10) = 1.23; p<0.29, Arc protein; Fig. 4 B, D).
When irradiated and non-irradiated mice that explored the novel environment were compared at 1 week, there was no significant difference in the fraction of neurons expressing Arc mRNA (Fig. 5 A), but there was a significant decrease in the fraction of neurons expressing Arc protein (ANOVA: F (1, 10) = 9.08; p<0.013, for Arc Protein; Fig. 5 B). Two months following brain irradiation, there were clear qualitative reductions in the numbers of neurons expressing Arc mRNA (Fig. 3 C) and Arc protein (not shown), and the fractions of neurons expressing Arc mRNA (Fig. 5 C) or Arc protein (Fig. 5 D) were significantly reduced compared to non-irradiated animals (ANOVA, Arc mRNA: F (1, 10) = 7.8; p<0.019; and ANOVA, Arc protein: F (1,10) =6.42; p<0.029).
Fig.5.
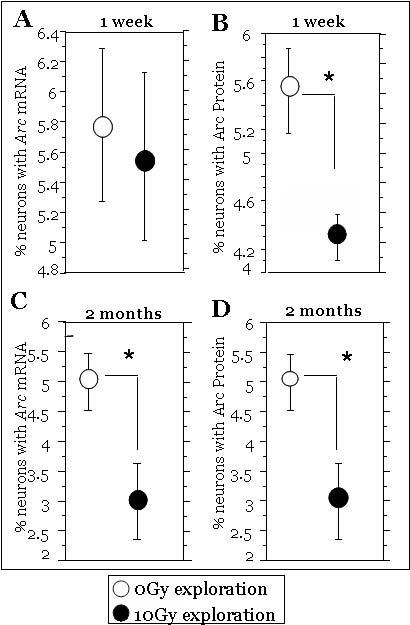
The effects of irradiation on the fractions of neurons expressing Arc mRNA (A, C) and Arc protein (B, D) in mice allowed to explore a novel environment. One week after irradiation there was no effect on Arc mRNA (A) but a significant decrease in Arc protein (B). Two months after irradiation there were significant decreases in both the fractions of neurons expressing Arc mRNA and Arc protein (C, D) when compared to non-irradiated mice. Each symbol represents an average of 6 mice and error bars are SEM. * p < 0.05.
The expression of behaviorally-induced Arc mRNA and Arc protein have been shown by us to be influenced by the presence of activated microglia (29, 30), the intrinsic brain inflammatory cells that are increased after irradiation (15, 28, 31, 32). Thus, we quantified total numbers of activated microglia in the DG of mice that received 0 Gy or 10 Gy. Immunofluorescent staining for CD68 showed that 1 week after irradiation there was no significant increase in the total number of activated microglia/mm2 in the DG compared to non irradiated animals (data not shown). In contrast, at 2 months after irradiation there were significantly more activated microglia as compared to non irradiated animals (ANOVA: F (1, 22)= 4.56; p<0.04; Fig. 6).
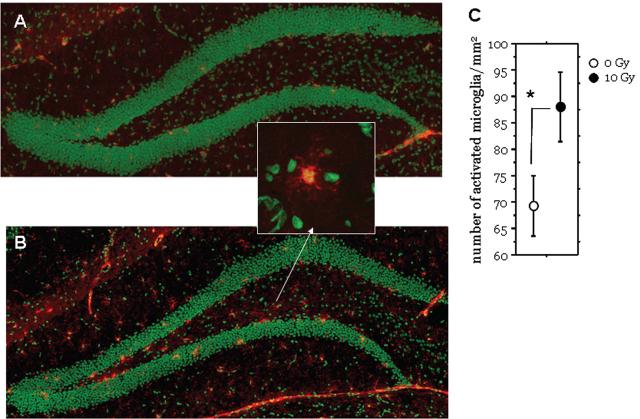
Fig.6.
Representative images of the reconstructed dentate gyrus of a mouse 2 months after irradiation with 0 Gy (A) or 10 Gy (B); nuclei were counterstained in green and activated microglia (CD68+) in red. A typical activated microglial cell is shown in the insert. Irradiation is associated with increased numbers of activated microglia. Irradiated animals exhibited a significant increase in the average number of activated microglia cell/mm2 (C). Each symbol represents an average of 6 mice and error bars are SEM. * p < 0.05. The scale bar represents 100μm.
Discussion
Therapeutic irradiation can induce cognitive impairments without necessarily causing the gross histologic disruption classically associated with exposure to high radiation doses (1). Given that post-mitotic neurons are generally considered to be relatively radioresistant, new approaches/techniques have been used to identify other ‘targets’ that may ultimately contribute to the pathogenesis of radiation-induced cognitive injury. Data now exist regarding neurogenesis (14, 15), specific genetic factors (33) or receptor expression (34), and show that changes in these endpoints can be associated with subsequent cognitive impairments. Still, there is considerable uncertainty regarding how molecular and cellular events within specific neuronal populations are translated into changes that affect behavioral performance. Understanding such changes will be critical to the development of strategies or approaches necessary to prevent or treat the cognitive changes induced by therapeutic irradiation of the brain. To our knowledge, the data shown here are the first to demonstrate that ionizing irradiation initiates changes in hippocampal neurons that disrupt the activity patterns (i.e. Arc expression) associated with neuroplasticity and memory.
In the present study we were interested in assessing the molecular distribution of Arc at the level of mRNA and protein after a single x-ray dose that is known to induce hippocampus-dependent cognitive impairments. While cognitive impairments were not directly assessed in this study, we have considerable data in our mouse model showing that 3 months after single doses of 5-10 Gy there are significant alterations in hippocampus-dependent spatial learning and memory but not in non-spatial learning and memory (15, 35) We have also reported similar finding in a gerbil model (14, 36) and other investigators have also shown cognitive impairments in rodent models using doses and post-treatment follow-up times generally similar to ours (6, 7).
The hippocampus is critical for the acquisition (learning), consolidation and retrieval of declarative memories (reviewed in (37)). Given the physiological properties of Arc as they relate to hippocampal-dependent functions (23, 24, 29), disruption of the expression of this IEG within hippocampal neurons possibly provides a mechanistic link between Arc expression and altered cognition. In fact, several lines of investigation clearly show that if Arc is reduced using either a genetic approach (26) or with antisense technology (25), animals fail to form long-term memories. The hippocampus has been shown to be sensitive to therapeutic irradiation (11), and while such exposure has been shown to acutely affect the expression of a number of genes in the hippocampal formation (20), in our study, basal (caged controls) levels of Arc mRNA or Arc protein were unchanged by a dose of 10 Gy (Fig. 4). Regardless of radiation treatment, increased Arc expression at the level of mRNA and protein was only elevated when animals were engaged in a learning experience induced by exploration, thus confirming that Arc is rapidly induced by neuronal activity associated with learning and memory. The magnitudes of behaviorally induced Arc expression were 5-6 fold higher in non-irradiated animals compared to caged controls and were still 3-5 fold higher in irradiated mice (Fig. 4). Other IEGs can also be induced by a learning experience (21) (Fig. 2), but Arc is unique given its well described temporal dynamics (24), because it is the most responsive IEG to specific behavioral demands (23) and because its mRNA moves to the site of active synapses where it is locally translated (38).
Although the percentages of Arc-expressing neurons in the DG are fairly low (i.e., < 10%), they represent a relatively large number of cells, given that the total number of granule cell neurons is about 240,000 in young adult C57BL/6 mice (39). The maintenance of a small fraction of Arc activity during a behavioral experience is critical for proper hippocampal function, and is consistent with electrophysiological recordings showing sparse activity in the DG during behavior (40), and with the principle of sparse distributed coding (41). This principle suggests that the maximally efficient storage/function requires only a fraction of the total population of cells in the DG (42). Thus, a modest reduction in the number of cells expressing Arc in the hippocampal DG may be sufficient to disrupt the finely regulated sparse coding, and thereby decrease the memory capacity of the system.
A moderate dose of x-rays, as used here, did not affect the percentage of granule cell neurons that expressed behaviorally-induced Arc mRNA 1 week after exposure (Fig. 5A), but was sufficient to significantly reduce the percentage of neurons expressing Arc protein (Fig. 5B). This could represent an interference of intracellular trafficking or involve disruption of translation in the dendrites where Arc mRNA is regulated by synaptic signals such as BDNF and reelin (43, 44). The early effects seen here could also be due to alterations in turnover and/or translation regulatory RNA binding proteins (45). Alternatively, because many radiation-induced IEG effects are impacted by alterations in proteosome processing (46), our findings could represent a faster degradation of Arc protein after irradiation. Regardless of the mechanism(s) involved, there appears to be a clear disconnect between changes related to transcription and translation seen 1 week after irradiation. This finding is consistent with a previous in vitro report demonstrating that ionizing irradiation modifies gene transcription and translation activity through different mechanisms (47).
In contrast to what was seen one week after irradiation, there was a significant (∼ 40%) reduction in the percentage of granule cell neurons that expressed behaviorally-induced Arc mRNA 2 months after radiation treatment (Fig. 5C). The similar reduction in behaviorally-induced Arc protein (Fig. 5D) was more substantial than that seen at the earlier time point (Fig. 5B). The more robust changes in the expression of Arc protein may be responsible for the corresponding changes in Arc mRNA through a complex feedback mechanism, and may suggest that the effect of irradiation on translational control may serve as a regulator of transcription (47, 48); however this idea is speculative at this time and a more comprehensive study is required. While the molecular mechanisms responsible for radiation-induced cognitive injury are not yet known, the 40% reductions in Arc mRNA and Arc protein seen here may be involved. For instance, recent studies have reported that Arc is involved in the trafficking of glutamate (AMPA) receptors in hippocampal neurons (49) and that AMPA receptors regulate Arc (50). Based on those data, it was proposed that changes in the NMDA/AMPA receptor ratio might enhance negative-feedback control of Arc expression (50), thus influencing the transcription of Arc. Because changes in NMDA receptors in the hippocampal formation are associated with cognitive impairment after irradiation (34), one possible mechanistic explanation is that irradiation may lead to alteration in the AMPA/NMDA receptor ratio resulting in a reduction of Arc expression. Another explanation may involve reduced neurogenesis which we previously have shown to be associated with radiation-induced cognitive impairment (14, 15). It is particularly germane to this idea that the fraction of newly born neurons in the dentate subgranular zone expressing Arc is almost 2 fold higher than what is seen in more mature cells (51). These and/or other possibilities need to be further explored and may provide the mechanistic link between Arc expression and neuronal dysfunctions that ultimately result in altered cognitive function after brain irradiation.
The data shown here suggest that a dose of radiation known to induce cognitive impairments (14-17) initiates early changes that perturb neuronal activity without affecting neuronal survival. This may be due, in part, to radiation-induced changes in the microenvironment such as oxidative stress or neuronflammation, which have been shown to be impact the cells in the hippocampus (15, 28, 31, 32, 52). In our study we hypothesized that radiation-induced changes in the fraction of Arc expressing cells could involve neuroinflammation, and quantified the numbers of activated microglia in and around the DG (Fig. 6). While there was some indication of increased numbers of activated microglia at 1 week, this change became significant at 2 months following exposure (Fig. 6). Because the fraction of cells expressing Arc protein was significantly reduced at both time points while the numbers of activated microglia were only significantly elevated at 2 months, this suggested that the presence of inflammatory cells per se was probably not linked to the reduction in the numbers of neurons expressing Arc protein. On the other hand there may be an association between increased numbers of activated microglia and the later (2 months) changes in Arc mRNA; data do exist in other models of brain injury supporting a link between Arc expression and neuroinflammation (29). However, at this time it is not known if the changes observed in Arc expression after irradiation are related to the number of activated microglia or if the coincident changes are independent.
Currently, we do not understand how ionizing radiation affects neuronal function. The present study provides novel information relevant to this topic and provides insight into new approaches to address a clinically significant problem. While Arc expression has been a topic of considerable recent interest in the context of neural networks, memory consolidation and synaptic plasticity (for rev see: (53)), this is the first report of the characterization of Arc expression in a mouse model of radiation injury. Given the current availability of mutant mouse models, it will now be possible to fully address more specific questions regarding the cascade of molecular events associated with Arc expression and if and how those events affect the development of cognitive changes after brain irradiation. Such information is presently unavailable but is essential for determining the risks of specific central nervous system related effects and for the development of potential strategies to manage radiation-mediated brain injury.
Acknowledgments
Support: RO1 NS46051
References
- 1.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–28. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 2.Richards GM, Khuntia D, Mehta MP. Therapeutic management of metastatic brain tumors. Crit Rev Oncol Hematol. 2007;61:70–8. doi: 10.1016/j.critrevonc.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–9. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 4.Butler JM, Rapp SR, Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options Oncol. 2006;7:517–23. doi: 10.1007/s11864-006-0026-5. [DOI] [PubMed] [Google Scholar]
- 5.Ghia A, Tome WA, Thomas S, et al. Distribution of brain metastases in relation to the hippocampus: implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys. 2007;68:971–7. doi: 10.1016/j.ijrobp.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Yazlovitskaya EM, Edwards E, Thotala D, et al. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res. 2006;66:11179–86. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- 7.Zhao W, Payne V, Tommasi L, Diz DI, Hsu FC, Robbins ME. Administration of the peroxisomal proliferator-activated receptor gamma agonist pioglitazone during fractionated brain irradiation prevents radiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2007;67:6–9. doi: 10.1016/j.ijrobp.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 8.Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 9.Hopewell JW. Radiation injury to the central nervous system. Med Pediatr Oncol. 1998;(Suppl 1):1–9. doi: 10.1002/(sici)1096-911x(1998)30:1+<1::aid-mpo1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 10.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: A dynamic process. Radiat Res. 2000;153:357–70. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35:659–63. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 12.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31:983–98. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 13.Surma-aho O, Niemela M, Vilkki J, et al. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology. 2001;56:1285–90. doi: 10.1212/wnl.56.10.1285. [DOI] [PubMed] [Google Scholar]
- 14.Raber J, Fan Y, Matsumori Y, et al. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol. 2004;55:381–9. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- 15.Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–30. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–42. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 17.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 18.Verheyde J, Benotmane MA. Unraveling the fundamental molecular mechanisms of morphological and cognitive defects in the irradiated brain. Brain Res Rev. 2007;53:312–20. doi: 10.1016/j.brainresrev.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoud-Ahmed AS, Atkinson S, Wong CS. Early gene expression profile in mouse brain after exposure to ionizing radiation. Radiat Res. 2006;165:142–54. doi: 10.1667/rr3485.1. [DOI] [PubMed] [Google Scholar]
- 20.Achanta P, Thompson KJ, Fuss M, Martinez JL., Jr Gene expression changes in the rodent hippocampus following whole brain irradiation. Neurosci Lett. 2007;418:143–48. doi: 10.1016/j.neulet.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993;363:718–22. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- 22.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Imaging neural activity with temporal and cellular resolution using FISH. Curr Opin Neurobiol. 2001;11:579–84. doi: 10.1016/s0959-4388(00)00252-x. [DOI] [PubMed] [Google Scholar]
- 23.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–24. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25:1761–68. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzowski JF, Lyford GL, Stevenson GD, et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plath N, Ohana O, Dammermann B, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–44. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Ginsberg SD, Hemby SE, Lee VM, Eberwine JH, Trojanowski JQ. Expression profile of transcripts in Alzheimer's disease tangle-bearing CA1 neurons. Ann Neurol. 2000;48:77–87. [PubMed] [Google Scholar]
- 28.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of x-irradiation. Can Res. 2003;63:4021–27. [PubMed] [Google Scholar]
- 29.Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005;25:723–31. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosi S, Vazdarjanova A, Ramirez-Amaya V, Worley PF, Barnes CA, Wenk GL. Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience. 2006;142:1303–15. doi: 10.1016/j.neuroscience.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Rola R, Zou Z, Huang T-T, et al. Lack of EC-SOD in the microenvironment impacts radiation-induced changes in neurogenesis. Free Rad Biol & Med. 2007;42:1133–45. doi: 10.1016/j.freeradbiomed.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–62. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 33.Villasana L, Acevedo S, Poage C, Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res. 2006;166:883–91. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- 34.Shi L, Adams MM, Long A, et al. Spatial Learning and Memory Deficits after Whole-Brain Irradiation are Associated with Changes in NMDA Receptor Subunits in the Hippocampus. Radiat Res. 2006;166:892–99. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 35.Raber J, Rola R, LeFevour A, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 36.Fan Y, Liu Z, Weinstein PR, Fike JR, Liu J. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur J Neurosci. 2007;25:38–46. doi: 10.1111/j.1460-9568.2006.05269.x. [DOI] [PubMed] [Google Scholar]
- 37.Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 38.Lyford GL, Yamagata K, Kaufmann WE, et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–45. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 39.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997;94:10409–14. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–82. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 41.McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within distributed memory system. Trends in Neurosci. 1987;10:408–15. [Google Scholar]
- 42.McNaughton BL, Barnes CA, Gerrard JL, et al. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol. 1996;199:173–85. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- 43.Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci USA. 2002;99:2368–73. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong E, Caruncho H, Liu WS, et al. A reelin-integrin receptor interaction regulates Arc mRNA translation in synaptoneurosomes. Proc Natl Acad Sci USA. 2003;100:5479–84. doi: 10.1073/pnas.1031602100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pullmann R, Jr, Kim HH, Abdelmohsen K, et al. Analysis of turnover and translation regulatory RNA-binding protein expression through binding to cognate mRNAs. Mol Cell Biol. 2007;27:6265–78. doi: 10.1128/MCB.00500-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pajonk F, McBride WH. Ionizing radiation affects 26s proteasome function and associated molecular responses, even at low doses. Radiother Oncol. 2001;59:203–12. doi: 10.1016/s0167-8140(01)00311-5. [DOI] [PubMed] [Google Scholar]
- 47.Lu X, de la Pena L, Barker C, Camphausen K, Tofilon PJ. Radiation-induced changes in gene expression involve recruitment of existing messenger RNAs to and away from polysomes. Cancer Res. 2006;66:1052–61. doi: 10.1158/0008-5472.CAN-05-3459. [DOI] [PubMed] [Google Scholar]
- 48.Keene JD, Tenenbaum SA. Eukaryotic mRNPs may represent posttranscriptional operons. Mol Cell. 2002;9:1161–67. doi: 10.1016/s1097-2765(02)00559-2. [DOI] [PubMed] [Google Scholar]
- 49.Chowdhury S, Shepherd JD, Okuno H, et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–59. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao VR, Pintchovski SA, Chin J, Peebles CL, Mitra S, Finkbeiner S. AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat Neurosci. 2006;9:887–95. doi: 10.1038/nn1708. [DOI] [PubMed] [Google Scholar]
- 51.Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–41. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Limoli CL, Giedzinski E, Rola R, Otsuka S, Palmer TD, Fike JR. Radiation response of neural precursor cells: linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiat Res. 2004;161:17–27. doi: 10.1667/rr3112. [DOI] [PubMed] [Google Scholar]
- 53.Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, Barnes CA. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol. 2005;15:599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]