Abstract
Small-conductance Ca2• -activated K• (SK) channels play an important role in regulating the frequency and in shaping urinary bladder smooth muscle (UBSM) action potentials, thereby modulating contractility. Here we investigated a role for the SK2 member of the SK family (SK1−3) utilizing: 1) mice expressing • -galactosidase (• -gal) under the direction of the SK2 promoter (SK2 • -gal mice) to localize SK2 expression and 2) mice lacking SK2 gene expression (SK2• /• mice) to assess SK2 function. In SK2 • -gal mice, UBSM staining was observed, but staining was undetected in the urothelium. Consistent with this, urothelial SK2 mRNA was determined to be 4% of that in UBSM. Spontaneous phasic contractions in wild-type (SK2• /•) UBSM strips were potentiated (259% of control) by the selective SK channel blocker apamin (EC50 • 0.16 nM), whereas phasic contractions of SK2• /• strips were unaffected. Nerve-mediated contractions of SK2• /• UBSM strips were also increased by apamin, an effect absent in SK2• /• strips. Apamin increased the sensitivity of SK2• /• UBSM strips to electrical field stimulation, since pretreatment with apamin decreased the frequency required to reach a 50% maximal contraction (vehicle, 21 • 4 Hz, n • 6; apamin, 12 • 2 Hz, n • 7; P & 0.05). In contrast, the sensitivity of SK2• /• UBSM strips was unaffected by apamin. Here we provide novel insight into the molecular basis of SK channels in the urinary bladder, demonstrating that the SK2 gene is expressed in the bladder and that it is essential for the ability of SK channels to regulate UBSM contractility.
Keywords: bladder, contractility, small-conductance calcium-activated potassium channel, apamin
THE COORDINATION OF NEURONAL and smooth muscle electrical activity is critical to the maintenance of proper urinary bladder function. Micturition occurs through the initiation of action potentials within the parasympathetic nerves leading to the bladder. These neuronal action potentials on reaching efferent terminals evoke the release of the excitatory neurotransmitters, acetylcholine and ATP. Acetylcholine and ATP bind to muscarinic (M2, M3) and purinergic (P2×1) receptors, respectively, located in the urinary bladder smooth muscle (UBSM) membrane. These transmitters contract the bladder via a coordinated potentiation of UBSM action potentials, which occur “spontaneously” during bladder filling functioning to maintain an appropriate basal bladder tone (10).
Overactive bladder, a major underlying cause of urinary incontinence, is frequently caused by alterations in neuronal and/or UBSM electrical activity. Currently, the main therapy used to treat overactive bladder is muscarinic receptor antagonists that function to reduce the coupling of excitatory acetylcholine to UBSM muscarinic receptors. These agents are somewhat effective but have significant side effects, such as dry mouth, and in some cases can lead to incomplete urine voiding during micturition. Therefore, the identification of novel targets to be used for the development of better therapies for overactive bladder and urinary incontinence is of great interest.
The modulation of ion channels involved in neuronal and UBSM electrical activity has demonstrated effectiveness in altering in vivo bladder function. The A-type voltage-gated K• channel activator KW-7158 increases bladder capacity via a potentiation of neuronal A-type currents in bladder neurons. This dampens neuronal firing and reduces bladder stimulation (22, 31). Activation of UBSM ATP-sensitive K• channels hyperpolarizes the resting UBSM membrane potential and reduces the action potential frequency, resulting in a decreased contractility (3, 21, 30) and an increased bladder capacity (27). Elimination of large-conductance, Ca2•- activated K• channels, which facilitate UBSM action potential repolarization, results in overactive detrusor and incontinence (11, 25, 35). In contrast, activation of large-conductance, Ca2•- activated K• channels enhances bladder capacity (33) due to a reduction in UBSM contractility (23). In addition, UBSM cells have: 1) voltage-gated K• channels that facilitate the UBSM action potential repolarization and afterhyperpolarization phases (19, 36), 2) L-type voltage-gated Ca2• channels that are an absolute requirement for action potential initiation and depolarization (8, 9, 11, 16), and 3) spontaneously active nonselective cation channels that regulate the resting membrane potential, modulating action potential frequency and contractility (37).
Small-conductance Ca2•- activated K• (SK or KCNN) channels have long been implicated as active modulators of UBSM action potentials underlying phasic bladder contractions (10). This is based on robust effects observed with the selective SK channel peptide inhibitor apamin. Spontaneous UBSM phasic contractile activity (3, 8, 9, 13, 14, 16, 40) and nerve-mediated contractions (12) are increased by apamin. Apamin reduces the UBSM action potential afterhyperpolarization, in many cases converting isolated, single action potentials into bursts of action potentials (4, 6, 8, 9). In vivo, the SK channel activator NS-309 administered intravesicularly has been shown to increase bladder capacity and micturition volume and has demonstrated an ability to prevent bladder over-activity (29). Taken together these data strongly support a relaxant role of SK channels in urinary bladder physiology.
Of the SK channel family (SK1−3), SK2 and SK3 subunit mRNA has been reported in isolated UBSM cells (28). SK3 channels have demonstrated an ability to modulate bladder function, altering the apamin sensitivity of UBSM contractions (15). Surprisingly, suppression of SK3 expression resulted in an enhanced apamin sensitivity of phasic contractions in UBSM strips, suggesting a role in UBSM for other SK iso-forms that have an enhanced apamin sensitivity, such as the SK2 channel (1, 32). However, the role and distribution of SK2 channels in UBSM is not known.
The goal of the current study was to elucidate the role of SK2 channels in UBSM contractility, using SK2 reporter mice and SK2 knock out mice. Here we demonstrate the expression of SK2 subunits in UBSM and provide novel findings that SK2 is the essential mediator of apamin sensitivity and therefore, the principal functional component of SK channels in the mouse urinary bladder.
METHODS
Strains of mice. SK2 • -galactosidase (• -gal) reporter mice were made by crossing SK2• /T mice (7) with tTA-• gal mice (18) at the Oregon Health and Sciences University. In SK2• /T mice, the expression of the tTA protein is driven by the SK2 promoter. When SK2• /T mice are crossed with tTA-• gal mice, the tTA protein binds to the tet operator driving expression of the lacZ reporter gene, thereby indicating SK2 expression. SK2 • -gal reporter mice were breed at the University of Vermont, and mice heterozygous for both the SK2 allele and the • -gal allele were used for SK2 expression studies. SK2• /• mice (2) were generated by crossing male and female SK2• /• mice. SK2• /• mice used were a mixture of littermate controls and back-ground strain C57Bl6 mice purchased from Taconic. All respective experiments received approval and were performed in accordance with the Institutional Animal Care and Use Committees of the University of Vermont, Oregon Health and Science University, and/or GlaxoSmithKline Pharmaceuticals.
X-gal staining. Urinary bladders of SK2• /• and SK2 • -gal mice were fixed for 15 min in 2% formaldehyde, 0.2% glutaraldehyde, and PBS, pH 7.4. Excess fixative was removed by three washes of PBS (20 min each). Bladders were incubated overnight at room temperature in X-gal staining solution: 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2, PBS, pH 7.4, and 1 mg/ml X-gal (5-Bromo-4-chloro-3-indolyl-•- d-galactopyranoside; Sigma) with agitation and protection from light. Bladders were washed with PBS, and images were obtained using a Leica MZ 6 microscope and Leica IM 50 Image Manager software (version 4.0).
RT-PCR. Urinary bladders were isolated from SK2• /• and SK2• /• mice, and the urothelium was removed by sharp dissection. Total RNA was purified with Trizol reagent, and RT-PCR was performed using a Mastercycler thermocycler (Eppendorf). SK2-specific primers were TTCTAACAACCTGGCGCTCT and TCCAGTCATCTGCTCCATTG. PCR products were separated on 1% agarose gels, stained with ethidium bromide, visualized, and photographed under ultraviolet light. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was routinely amplified from RT reaction products of SK2• /• and SK2• /• mRNA to confirm mRNA integrity.
Taqman. Tri reagent was used to isolate total RNA (Ambion), and cDNA was synthesized using MultiScribe Reverse Transcriptase (Applied Biosystems). All probe/primer sets were purchased as “assay on demand” (Applied Biosystems). GAPDH was used as the housekeeper. The relative expression of SK1, −2, and −3 in SK2• /• and SK2• /• urinary bladders was assessed using RNA isolated from intact bladders. Dissection of the urothelium and UBSM layers of wild-type mouse bladders was performed to compare SK2 expression between these tissues. Successful separation of these two layers was verified by Taqman, amplifying uroplakin 3A and SM22 as urothelial and SM markers, respectively.
Contractility measurements.Force measurements were performed and analyzed as reported previously (12, 15, 35). SK2• /• and SK2• /• mice were killed with an overdose of intraperitoneally injected pentobarbital sodium (150 mg/kg body wt). Bladders were cleaned free of fat and connective tissues, cut open, and pinned flat in a dissecting dish to remove the urothelium by sharp dissection. The remaining UBSM layer was cut into eight strips, and silk sutures were used to secure both ends of the bladder strips. Force production was then measured by placing strips in a MyoMed myograph (MED Associates, Georgia, VT) containing 7 ml volume of oxygenated physiological saline solution heated to 37°C. Apamin was purchased from Sigma and was dissolved/diluted with water as concentrated stocks 1,000-fold of the desired bathing concentrations. The amplitude of phasic contractions during the last 3 min of each 12-min interval was averaged. This value was then normalized to the average control amplitude in the same strip, taken as the last 3 min before the addition of 0.01 nM apamin. Controls represent strips analyzed in the same manner as apamin-treated strips, receiving vehicle in the place of apamin at 12-min intervals. EC50 (×50) values and maximum potentiation (A2) for apamin were calculated fitting average data with y • A2 • (100 • A2)/1 • (×/×50). Electrical field stimulation (EFS) parameters were as defined (12), evoking completely tetrodotoxin-sensitive contractions. In experiments using multiple-frequency EFS, strips were pretreated with apamin (1 • M) or vehicle for 12 min before stimulating strips. This 12-min pretreatment is consistent with the time between doses in the phasic contractility studies and allows for an equilibration of apamin into the UBSM tissue. Fifty percent frequency values (F50) were calculated from each strip using normalized data fit with the Boltzmann sigmoidal equation, Y • 100/[1 • e(F50 • > ×)/slope], with the slope unfixed. These individual values were then averaged for each treatment group. Boltzmann fits were also performed on the averaged curves for each treatment group, yielding almost identical F50 values.
Data analysis and statistics. Statistical comparisons were made using paired or unpaired t-tests or two-way repeated-measures ANOVA, as applicable, and data are expressed with SEs. A P • 0.05 was deemed significant. Data were analyzed and presented using a combination of MyoMed (MED Associates), MiniAnalysis (Synaptosoft), Origin (OriginLab), Prism (GraphPad), and CorelDraw (Corel) software.
RESULTS
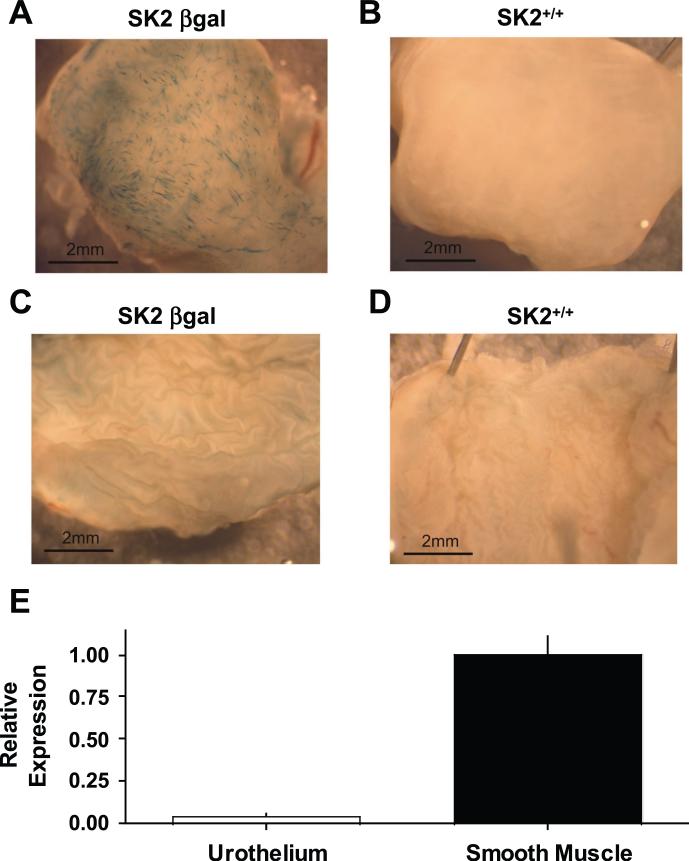
Expression of SK2 in mouse UBSM. To determine the expression pattern of the SK2 gene, homologous recombination was used to construct an SK2 • -gal reporter mouse (SK2 • -gal). Urinary bladders from SK2 • -gal mice (Fig. 1A) demonstrated staining in the UBSM layer, staining that was absent in UBSM of wild-type SK2• /• mice (Fig. 1B). In contrast to the staining in the SK2 • -gal UBSM, the urothelium from SK2 • -gal mice did not produce detectable • -gal expression (Fig. 1C). Taqman for SK2 in wild-type bladders confirmed significantly higher expression levels of SK2 mRNA in the UBSM compared with the urothelium, with urothelial SK2 expression determined to be only 4% of that in UBSM (Fig. 1E). These data are consistent with the reported SK2 mRNA expression in isolated mouse UBSM cells (28). By conventional RT-PCR, SK2 mRNA was amplified from the UBSM layer of SK2• /• mice and was confirmed absent in UBSM of SK2• /• mice (Fig. 2A). The expression levels of the other SK isoforms, SK1 and SK3, in SK2• /• bladders were evaluated by Taqman. SK1 and SK3 levels in SK2• /• bladders were determined to be 1.02 and 2.64 times those in SK2• /• bladders (Fig. 2B).
Fig. 1.
Small-conductance Ca2•- activated K• channel (SK2) expression in the mouse urinary bladder. A: external view of urinary bladder wall demonstrating • -galactosidase (• -gal) expression (blue) under SK2 promoter control in SK2 • -gal mice. B: staining was absent in negative control SK2• /• mice. C and D: luminal bladder view of the urothelium demonstrating a lack of • -gal staining in the urothelium of SK2 • -gal (C) and SK2• /• mice (D). E: SK2 mRNA levels in urothelium and smooth muscle of wild-type mouse bladders (n • 3). SK2 mRNA is presented relative to the average in smooth muscle.
Fig. 2.
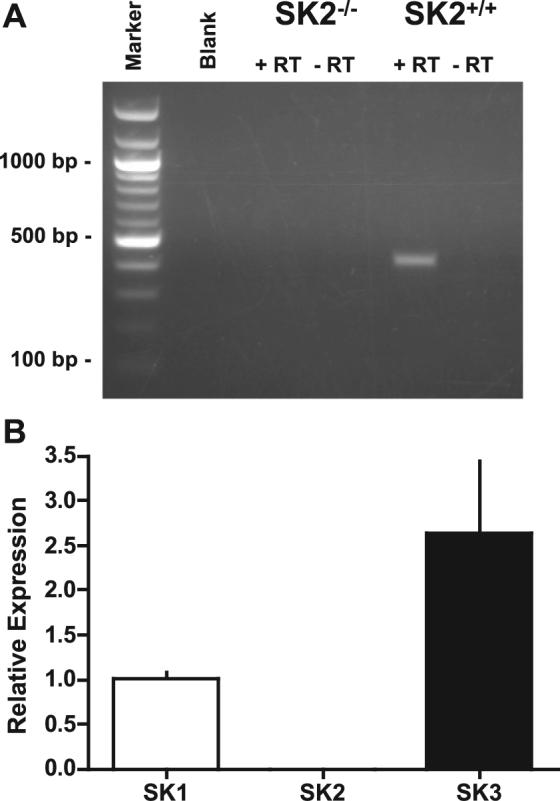
SK1−3 expression in SK2• /• and SK2• /• bladders. A: RT-PCR using RNA isolated from SK2• /• and SK2• /• UBSM, with (• ) and without (• ) inclusion of the RT enzyme. Amplified product was sequenced to confirm SK2 specificity. B: Taqman quantifying SK1−3 mRNA expression in SK2• /• (n • 4) relative to SK2• /• bladders (n • 6). SK1 expression is unaltered, whereas SK3 expression is increased in SK2• /• bladders.
Role of SK2 channels in regulating phasic contractility. To investigate the functional role of SK2 gene expression in UBSM, we compared basal phasic contractility, and the effects of apamin, in urothelium-denuded UBSM strips. The baseline amplitude of spontaneous phasic contractions was not different between SK2• /• and SK2• /• UBSM strips [SK2• /• 0.049 • 0.007 mN (n • 40); SK2• /• 0.053 • 0.008 mN (n • 79)], and depolarization-mediated contractions of strips elicited by 60 mM external K• [SK2• /• 6.6 • 0.5 mN (n • 40); SK2• /• 5.6 • 0.3 mN (n • 79)] were not significantly different.
To measure the sensitivity of phasic contractions to apamin in SK2• /• and SK2• /• UBSM strips, a cumulative apamin dose-response curve with escalating doses (0.01−1,000 nM) every 12 min was performed, along with vehicle/time controls for each genotype. This broad range of apamin concentrations was used because of the reported differences in apamin sensitivity between heterologously expressed SK isoforms (1, 32) and the wide range of apamin sensitivity reported previously in UBSM (14, 15). Apamin caused a robust increase in SK2• /• phasic contraction amplitudes (Fig. 3A). On average, the maximal effect of apamin in SK2• /• strips was 259% of control and was reached at • 1 nM apamin in the bath (EC50 • 0.16 • 0.03 nM; Fig. 3C). In contrast, apamin did not alter phasic contractions of UBSM strips from SK2• /• mice (Fig. 3, B and C). These results support the central role of SK2 channels in SK channel-mediated modulation of UBSM contractility in the absence of nerve activity.
Fig. 3.
Apamin potentiates phasic contractions in SK2• /• but not in SK2• /• mice. A: representative dose response to apamin on phasic contractions in a SK2• /• strip, demonstrating a robust increase in contractile activity. B: lack of effect of apamin on phasic contractions of SK2• /• strips. C: average dose-response curve with phasic contraction amplitudes expressed as a percentage of control (SK2• /• apamin EC50 • 0.16 • 0.03 nM, n • 15; SK2• /• vehicle n • 15; SK2• /• apamin n • 11; SK2• /• vehicle n • 10).
Role of SK2 channels in regulating nerve-mediated contractions. To investigate the role of SK2 channels in modulating nerve-mediated contractility, we used EFS of urothelium-denuded UBSM strips. The EFS conditions used selectively stimulate neurotransmitter release from nerve terminals present within the strips, without a direct stimulation of UBSM cells (12). On stimulation of the bladder strips with a repeated 20-Hz stimulation, a time-dependent decline in the response was observed in both SK2• /• and SK2• /• strips because of a desensitization of the response (Fig. 4). Application of 1 • M apamin to repeated 20-Hz EFS contractions resulted in a rapid and significant increase in SK2• /• contraction amplitudes (n • 14; Fig. 4, A and C). With the use of this protocol, 1 nM apamin also potentiated nerve-mediated contractions of SK2• /• strips (n • 13). As observed for spontaneous phasic contractions of SK2• /• strips, nerve-mediated contractions of SK2• /• strips were insensitive to apamin at concentrations up to 1 • M(n • 15; Fig. 4, B and C).
Fig. 4.
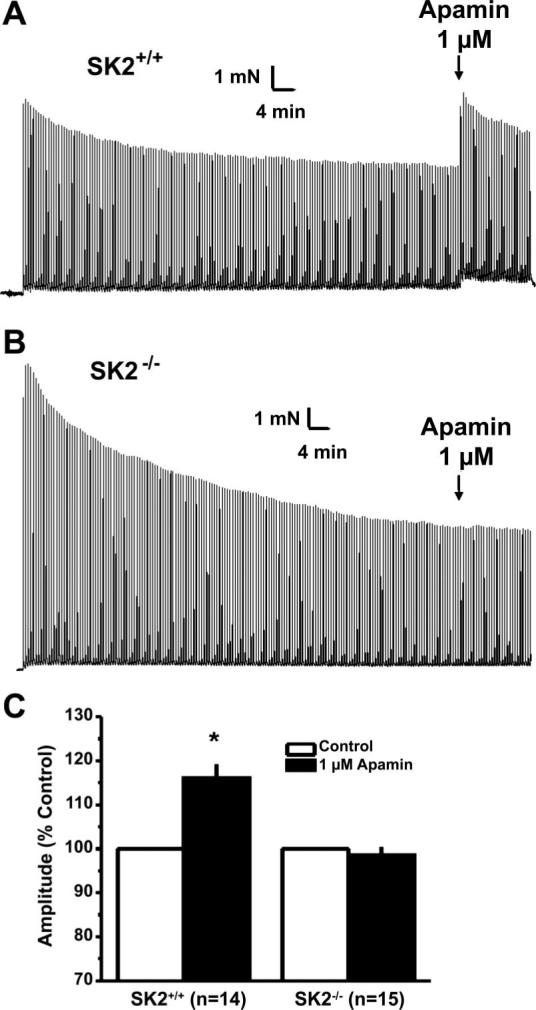
Apamin potentiation of nerve-mediated contractions is lost in SK2• /• UBSM strips. A: effect of a maximal dose of apamin on 20-Hz evoked electrical field stimulation contractions in a SK2• /• strip. B: lack of effect apamin in a SK2• /• UBSM strip. C: average contraction amplitudes expressed as a percentage of control.
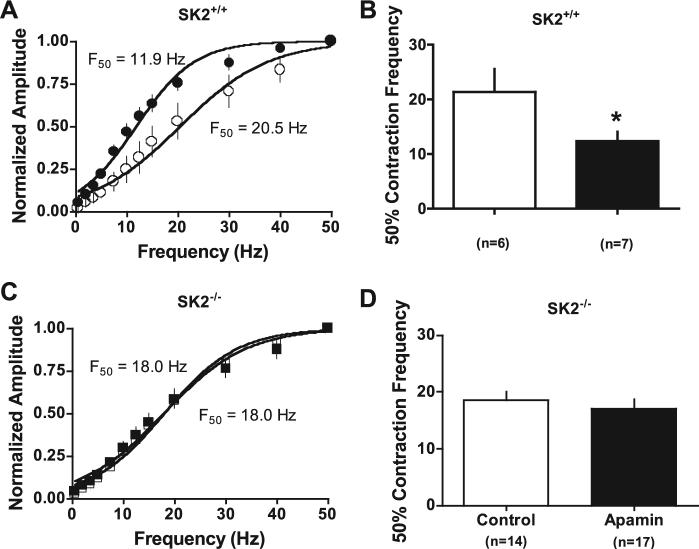
EFS at frequencies from 0.5 to 50 Hz demonstrated no difference in the raw amplitude of contractions between SK2• /• (n • 13) and SK2• /• (n • 31) UBSM strips. With the use of this same protocol, SK2• /• strips pretreated with apamin were compared with vehicle-treated SK2• /• strips. Apamin-treated SK2• /• strips demonstrated a leftward shift in the frequency dependence of contractility compared with vehicle-treated controls, shifting the 50% contraction frequency from 21 • 4 Hz (n • 6) for vehicle to 12 • 2 Hz for apamin (n • 7, P > • 0.05; Fig. 5). With the sue of the identical protocol, apamin did not alter the force-frequency relationship of UBSM strips from SK2• /• mice (50% contraction frequency: vehicle 18 • 1 Hz, n • 14; apamin 17 • 2 Hz, n • 16; Fig. 5). Fifty percent contraction frequencies under vehicle treatment were not significantly different between genotypes.
Fig. 5.
Effect of apamin on the frequency dependence of nerve-evoked contractions. A: the frequency response is significantly different in the presence of apamin (1 • M, shaded symbols) than in the presence of vehicle (open symbols) in SK2• /• strips (P • 0.03, 2-way repeated-measures ANOVA). B: apamin increases the sensitivity of SK2• /• strips to electrical field stimulation as demonstrated by a reduction in the 50% contraction frequency (F50, *P • 0.05). C and D: no effect of apamin compared with vehicle on the sensitivity of SK2• /• strips to electrical field stimulation.
DISCUSSION
Here we demonstrate that SK2 is the key molecular component of SK channels in mouse UBSM, since UBSM apamin sensitivity requires SK2 expression. With the use of SK2 • -gal reporter mice, SK2 expression was visualized in UBSM (Fig. 1) but was not observed in the urothelial layer of the bladder (Fig. 1). SK2 Taqman supported this finding, consistent with the reported detection of SK2 mRNA in isolated UBSM cells via conventional RT-PCR (28). The well-documented procontractile effect of SK inhibition by apamin in UBSM was reproduced in SK2• /• and absent in SK2• /• UBSM. This was observed in studies on both spontaneous phasic (Fig. 3) and nerve-mediated contractions (Figs. 4 and 5).
In this study, SK2 expression was localized to the UBSM layer as observed for SK3 channels by immunohistochemistry (15), but, unlike the additional expression of SK3 channels in the urothelium, SK2 was very low or undetectable in the urothelium. This suggests potential functional differences for SK2 and SK3 family members in urinary bladder physiology. The role of SK3 in bladder function was previously assessed using mice overexpressing SK3 in a doxycycline-suppressible manner (15). These results showed a reduction in spontaneous phasic contraction frequency with SK3 overexpression and enhancement by SK3 suppression. SK3 suppression was also shown to increase the apamin sensitivity of phasic contractions, implicating a role for another SK family member with greater apamin sensitivity in regulating UBSM contractility, such as SK2 (reported apamin IC50 values: SK1 0.7−12 nM; SK2 0.027−0.140 nM; SK3 0.6−4 nM; see Refs. 1 and 32).
Apamin increases spontaneous phasic contractions, indicating an active basal role of SK channels in the regulation of UBSM excitability and contractility in the absence of nerve activity. Furthermore, SK channel activation can enhance bladder relaxation, as supported by the observation that the SK channel activator NS-309 increases bladder capacity and micturition volume in normal, conscious rats when infused intravesicularly into the bladder (29). However, in the present study, elimination of SK2 channels did not result in an increase in basal phasic contractility or nerve-mediated contractions as observed in mice lacking the large-conductance Ca2•- activated K• channel (25, 35). This lack of an increase in basal and nerve-mediated contractions in SK2• /• UBSM suggests that compensation mechanisms may play a role during the development of SK2• /• mice, for example, an altered expression/function of other UBSM ion channels. Evidence for this is supported by the upregulation of SK3 mRNA expression in the SK2• /• bladders. Nonetheless, the dramatic loss of apamin sensitivity in SK2• /• mice on spontaneous phasic and nerve-mediated contractions demonstrates the key role of SK2 channel gene expression in the mediation of SK channel function in UBSM.
SK channels are present within UBSM cells functioning to hyperpolarize the membrane potential in response to increases in cytoplasmic Ca2•. SK channels are gated by increases in Ca2• at the intracellular side of the channel through calmodulin bound to the COOH terminus of the subunits (39), thereby providing a brake on Ca2• -induced contraction. SK channel activation hyperpolarizes the UBSM membrane potential, reducing the activity of voltage-gated Ca2• channels, which are the key source of Ca2• -eliciting UBSM contraction. Although multiple cell types, in addition to UBSM cells, are present within the UBSM layer of the bladder, including nerves, fibroblasts, and interstitial-like cells (5, 25), it seems likely that at least part of the apamin effects observed in SK2• /• (and lost in SK2• /•) are due to the expression of SK2 channels in UBSM cells, since SK2 is expressed in isolated mouse UBSM cells (28) and SK whole cell currents have been recorded from UBSM cells (14, 15). Ohya et al. (28), using single channel recording techniques, were unable to detect charbydotoxin-insensitive K• channels, consistent with SK channels. Their lack of observation of single SK channels could be attributed to recording conditions and/or a low SK channel density, since UBSM SK whole cell currents are relatively small (14). Furthermore, phasic contractions, which apamin potentiates, are thought to be myogenic in origin. Consistent with this, inhibiting neuronal function by pretreating SK2• /• UBSM strips with the sodium channel blocker tetrodotoxin (1 • M) did not alter basal SK2• /• spontaneous phasic contraction amplitude/frequency or apamin sensitivity (n • 7). Although the evidence reported here supports a central role of SK2 channels in UBSM cells, we cannot exclude a contribution of SK2 expressed in other cell types within these UBSM strips.
Four SK channel subunits, each with a six-transmembrane topology, must coassemble to form a functional SK channel. As a result of the multimeric assembly nature of SK channels, this leads to the possibility of heteromultimeric assembly in which more than one SK family member (SK1−3) may assemble into a given channel complex. This has been demonstrated in heterologous expression systems (1, 17, 20, 26) and directly demonstrated in native tissue using immunoprecipitation (33). In addition, the transgenic expression of a dominant negative SK subunit supports the heteromultimeric assembly of SK channels in vivo (38). Based on the required role for SK2 reported here in mediating UBSM apamin effects, and the documented ability of SK3 expression to alter apamin sensitivity (15), this suggests that SK2 subunits are the core SK subunit in UBSM and that the expression of other SK subunits, such as SK3 (15, 28) and/or SK1, may result in the formation of a population of heteromultimeric SK2-SK3 channels. However, this study does not exclude the presence of a population of SK2 homomultimers in mouse UBSM. Overall, it is tempting to speculate that SK2 subunits may be required for the functional localization of mouse UBSM SK channels to the plasma membrane.
In conclusion, we have provided novel genetic insight into the molecular basis of SK channels in the urinary bladder and the well-documented procontractile effects of apamin. SK2 is expressed in the mouse urinary bladder as the essential functional component of SK channels and the key mediator of apamin contractile effects observed in this tissue.
ACKNOWLEDGMENTS
We thank Dr. Jonathan Ledoux, Dr. Nicholas Laping, and Bernhard Nausch for comments on the manuscript.
The work was supported by National Institutes of Health Grants DK-5R01, DK-053832, HL-44455, and 1R01DK-065947, through Canadian Institutes of Health Research (CIHR) and Alberta Heritage Foundation for Medical Research (AHFMR) fellowships, and GlaxoSmithKline.
REFERENCES
- 1.Benton DC, Monaghan AS, Hosseini R, Bahia PK, Haylett DG, Moss GW. Small conductance Ca2·-activated K· channels formed by the expression of rat SK1 and SK2 genes in HEK 293 cells. J Physiol. 2003;553:13–19. doi: 10.1113/jphysiol.2003.054551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP. Small conductance Ca2·-activated K· channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci. 2004;24:5301–5306. doi: 10.1523/JNEUROSCI.0182-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckner SA, Milicic I, Daza AV, Coghlan MJ, Gopalakrishnan M. Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br J Pharmacol. 2002;135:639–648. doi: 10.1038/sj.bjp.0704499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creed KE, Ishikawa S, Ito Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol. 1983;338:149–164. doi: 10.1113/jphysiol.1983.sp014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol. 2005;173:1385–1390. doi: 10.1097/01.ju.0000146272.80848.37. [DOI] [PubMed] [Google Scholar]
- 6.Fujii K, Foster CD, Brading AF, Parekh AB. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. Br J Pharmacol. 1990;99:779–785. doi: 10.1111/j.1476-5381.1990.tb13006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW. Small-conductance Ca2·-activated K· channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci. 2006;26:1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol. 2003;140:146–158. doi: 10.1038/sj.bjp.0705319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol. 2003;140:159–169. doi: 10.1038/sj.bjp.0705320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004;141:183–193. doi: 10.1038/sj.bjp.0705602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heppner TJ, Bonev AD, Nelson MT. Ca2·-activated K· channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 1997;273:C110–C117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- 12.Herrera GM, Etherton B, Nausch B, Nelson MT. Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R402–R409. doi: 10.1152/ajpregu.00488.2004. [DOI] [PubMed] [Google Scholar]
- 13.Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol. 2000;279:R60–R68. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- 14.Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2· signals from Ca2· channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol. 2003;551:893–903. doi: 10.1113/jphysiol.2003.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai T, Okamoto T, Yamamoto Y, Tanaka H, Koike K, Shigenobu K, Tanaka Y. Effects of different types of K· channel modulators on the spontaneous myogenic contraction of guinea-pig urinary bladder smooth muscle. Acta Physiol Scand. 2001;173:323–333. doi: 10.1046/j.1365-201X.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 17.Ishii TM, Maylie J, Adelman JP. Determinants of apamin and D-tubocurarine block in SK potassium channels. J Biol Chem. 1997;272:23195–23200. doi: 10.1074/jbc.272.37.23195. [DOI] [PubMed] [Google Scholar]
- 18.Jerecic J, Single F, Kruth U, Krestel H, Kolhekar R, Storck T, Kask K, Higuchi M, Sprengel R, Seeburg PH. Studies on conditional gene expression in the brain. Ann NY Acad Sci. 1999;868:27–37. doi: 10.1111/j.1749-6632.1999.tb11271.x. [DOI] [PubMed] [Google Scholar]
- 19.Klockner U, Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985;405:329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- 20.Kolski-Andreaco A, Tomita H, Shakkottai VG, Gutman GA, Cahalan MD, Gargus JJ, Chandy KG. SK3-1C, a dominant-negative suppressor of SKCa and IKCa channels. J Biol Chem. 2004;279:6893–6904. doi: 10.1074/jbc.M311725200. [DOI] [PubMed] [Google Scholar]
- 21.Li JH, Yasay GD, Zografos P, Kau ST, Ohnmacht CJ, Russell K, Empfield JR, Brown FJ, Trainor DA, Bonev AD, Heppner TJ, Nelson MT. Zeneca ZD6169 and its analogs from a novel series of anilide tertiary carbinols: in vitro KATP channel opening activity in bladder detrusor. Pharmacology. 1995;51:33–42. doi: 10.1159/000139314. [DOI] [PubMed] [Google Scholar]
- 22.Lu SH, Yamagata T, Atsuki K, Sun L, Smith CP, Yoshimura N, Chancellor MB, de Groat WC. Effect of KW-7158, a putative afferent nerve inhibitor, on bladder and vesico-vascular reflexes in rats. Brain Res. 2002;946:72–78. doi: 10.1016/s0006-8993(02)02828-7. [DOI] [PubMed] [Google Scholar]
- 23.Malysz J, Buckner SA, Daza AV, Milicic I, Perez-Medrano A, Gopalakrishnan M. Functional characterization of large conductance calcium-activated K· channel openers in bladder and vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:481–489. doi: 10.1007/s00210-004-0920-y. [DOI] [PubMed] [Google Scholar]
- 24.McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168:832–836. [PubMed] [Google Scholar]
- 25.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive urinary bladder and incontinence in the absence of the BK Ca2· activated K· channel. J Biol Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- 26.Monaghan AS, Benton DC, Bahia PK, Hosseini R, Shah YA, Haylett DG, Moss GW. The SK3 subunit of small conductance Ca2·-activated K· channels interacts with both SK1 and SK2 subunits in a heterologous expression system. J Biol Chem. 2004;279:1003–1009. doi: 10.1074/jbc.M308070200. [DOI] [PubMed] [Google Scholar]
- 27.Myers RA, Plym MJ, Signor LJ, Lodge NJ. 1-(2-Pyrimidinyl)-piperazine, a buspirone metabolite, modulates bladder function in the anesthetized rat. Neurourol Urodyn. 2004;23:709–715. doi: 10.1002/nau.20037. [DOI] [PubMed] [Google Scholar]
- 28.Ohya S, Kimura S, Kitsukawa M, Muraki K, Watanabe M, Imaizumi Y. SK4 encodes intermediate conductance Ca2·-activated K· channels in mouse urinary bladder smooth muscle cells. Jpn J Pharmacol. 2000;84:97–100. doi: 10.1254/jjp.84.97. [DOI] [PubMed] [Google Scholar]
- 29.Pandita RK, Ronn LCB, Jensen BS, Andersson KE. Urodynamic effects of intravesical administration of the new small/intermediate conductance calcim activated potassium channel activator NS309 in freely moving, conscious rats. J Urol. 2006;176:1220–1224. doi: 10.1016/j.juro.2006.04.081. [DOI] [PubMed] [Google Scholar]
- 30.Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of K(ATP) channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1427–R1433. doi: 10.1152/ajpregu.2001.280.5.R1427. [DOI] [PubMed] [Google Scholar]
- 31.Sculptoreanu A, Yoshimura N, de Groat WC. KW-7158 [(2S)-(·)-3,3,3-trifluoro-2-hydroxy-2-methyl-N-(5,5,10-trioxo-4,10-dihydrothieno [3,2-c][1]benzothiepin-9-yl)propanamide] enhances A-type K· currents in neurons of the dorsal root ganglion of the adult rat. J Pharmacol Exp Ther. 2004;310:159–168. doi: 10.1124/jpet.104.065409. [DOI] [PubMed] [Google Scholar]
- 32.Stocker M. Ca(2·)-activated K· channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5(10):758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- 33.Strassmaier T, Bond CT, Sailer CA, Knaus HG, Maylie J, Adelman JP. A novel isoform of SK2 assembles with other SK subunits in mouse brain. J Biol Chem. 2005;280:21231–21236. doi: 10.1074/jbc.M413125200. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M, Sasaki Y, Kimura Y, Fukui T, Hamada K, Ukai Y. A novel pyrrole derivative, NS-8, suppresses the rat micturition reflex by inhibiting afferent pelvic nerve activity. BJU Int. 2003;92:1031–1036. doi: 10.1111/j.1464-410x.2003.04512.x. [DOI] [PubMed] [Google Scholar]
- 35.Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol. 2005;289:F604–F610. doi: 10.1152/ajprenal.00060.2005. [DOI] [PubMed] [Google Scholar]
- 36.Thorneloe KS, Nelson MT. Properties and molecular basis of the mouse urinary bladder voltage-gated K· current. J Physiol. 2003;549:65–74. doi: 10.1113/jphysiol.2003.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorneloe KS, Nelson MT. Properties of a tonically active, sodium-permeable current in mouse urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2004;286:C1246–C1257. doi: 10.1152/ajpcell.00501.2003. [DOI] [PubMed] [Google Scholar]
- 38.Villalobos C, Shakkottai VG, Chandy KG, Michelhaugh SK, Andrade R. SKCa channels mediate the medium but not the slow calcium-activated afterhyperpolarization in cortical neurons. J Neurosci. 2004;24:3537–3542. doi: 10.1523/JNEUROSCI.0380-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- 40.Zografos P, Li JH, Kau ST. Comparison of the in vitro effects of K· channel modulators on detrusor and portal vein strips from guinea pigs. Pharmacology. 1992;45:216–230. doi: 10.1159/000139000. [DOI] [PubMed] [Google Scholar]