Abstract
OBJECTIVES
To measure the association between proportion of children and specific pediatric age groups in a local population with the timing and rate of adult emergency department utilization for influenza and other acute respiratory infections (ARIs).
METHODS
We performed an ecological study on a time-series of adult patients presenting to Massachusetts emergency departments and residing in the Greater Boston area from October 1st 2001- September 30th 2005. Patients presenting with ARI, used as a marker for influenza, were aggregated by home address ZIP code. We measured geographic patterns of timing and rate of adult respiratory infection-related emergency department utilization. We performed correlation analysis of rates and peaks identified in this analysis with pediatric population data from the US Census (including specific pediatric age groups) by Poisson regression.
RESULTS
157,542 adult respiratory infection-related ED visits (30 visits per 1000 adults per year) were analyzed. Visits were distributed across 55 of ZIP codes, where proportions of children (0−18) ranged from 2.7%−34.9% in these communities. Proportion of children in a ZIP code is directly associated with timing of seasonal onset of ARIs among adults (univariate Poisson regression Rate Ratio [RR], 0.985; 95% confidence interval [CI], 0.977−0.993). The proportion of children also explains the patterns of adult ARI-related ED utilization rates (RR, 1.035; 95% CI, 1.024−1.047). 3−4 year olds are found to be the most significant predictors of adult illness rate (RR, 1.380; 95% CI, 1.238−1.539) and timing of onset (RR, 0.881; 95% CI, 0.816−0.952).
CONCLUSIONS
We demonstrate a positive correlation between the timing and rate of ED utilization by adults and the proportion of children in the population. These findings add to a growing body of evidence supporting a critical role played by children in community-wide transmission of ARIs.
Introduction
Influenza epidemics occur each year during the winter season in temperate areas of the world causing tens of thousands of deaths 1-3, hundreds of thousands of hospitalizations 4, 5, and having enormous economic impact.6 Other respiratory virus epidemics, including respiratory syncytial virus (RSV) and parainfluenza virus (PIV), co-occur with influenza epidemics, contributing to morbidity and mortality.7 The ready spread of acute respiratory infections (ARIs) among children in daycare, preschool and schools and household transmission to family members appears to be an important mode of transmission.8-13
There is empirical confirmation of a relationship between geography and respiratory infection risk and timing across spatial scales.14 However, spatial patterns for healthcare utilization and the contribution of population age structure are not well-characterized usually because of the paucity of healthcare datasets with high geographical resolution. In this study, we seek to measure the role of children as vectors for spread of influenza and other ARIs among adults. Specifically, we measure the association, per administrative geographic area, of the proportion children on the rate and timing of adult ED utilization for ARIs. We then break down the association by specific policy-relevant pediatric age groups.
PATIENTS AND METHODS
Study Design, Setting, Data Collection
We performed an ecologic study based on time series analyses of influenza and other ARI related adult ED visits in Eastern Massachusetts to define spatial and temporal patterns of disease in areas surrounding Boston and association with the pediatric population. These data are from the Massachusetts Division of Health Care Finance and Policy.15 This database, which began fiscal year 2002, captures data for outpatient visits to all Massachusetts acute care hospitals EDs and satellite emergency facilities (89 facilities included in total). Data elements include patient demographics, clinical characteristics, services provided, charges, and hospitals and practitioner information, as well as mode of transport. The study had Institutional Review Board approval (Protocol Number X05−09−069R).
Selection of participants
Inclusion criteria were adults age of 18 years or over, presentation to one of 89 medical institutions in Massachusetts with an ARI-related ED diagnostic code, and home address five digit ZIP code in the Greater Boston area. Diagnostic codes were based on the International Classification of Diseases, Ninth Revision (ICD-9) and included: 079.99, 382.9, 460, 461.9, 465.8, 465.9, 466.0, 486, 487.0, 487.1, 487.8, 490, 780.6, and 786.2. These ICD-9 codes have been used as markers of influenza-like illness in the absence of laboratory confirmed infection data.10 The time series of these syndromes corresponds especially tightly with time series for virological isolates of influenza. The sample spanned October 1st, 2001 to September 30th, 2005 inclusive of four winter seasons. ARI visits were aggregated by the 5-digit ZIP code of the patient's home address.
Definition of terms, outcome of interest, and primary data analyses
The primary outcome is rate of ED utilization for ARIs among adults in each ZIP code. We calculated this rate of visits among adults (18 and over) attributed to ARIs per year using all adults living in those selected ZIP codes from 2000 US Census as the denominator. The secondary outcome was the timing or onset of the winter ARIs among these adults. To construct this outcome, we created a weekly time series of adult ARI-related ED utilization in each ZIP code. We identified the timing of winter ARIs in a given ZIP code by calculating peak week of ARI visits. We estimated phase shifts (i.e., lead time) between the underlying yearly components of the ARI time series in each ZIP code by calculating the mean peak week of activity; these estimates are measures of the extent to which one series leads the other, yielding the second outcome of relative timing of activity in each ZIP code. This approach allowed determination of the relative timing of spread across different areas.
First, our two outcomes were analyzed against the proportion of pediatric patients in each ZIP code to measure the association of the proportion children with the rate and timing of adult ED utilization due to ARIs. The 2000 US Census provided estimates for the proportion of all children (under 18 years old) in each ZIP code. We assessed the association between the proportion of all children and the timing and rate of ARI-related utilization by fitting generalized linear models to respiratory presenting complaints.16 Poisson distribution was assumed as it is appropriate for modeling counts of independent events To account for this overdispersion in the count data, extra-Poisson variability was modeled and incorporated into estimates of standard errors.17 Specifically, standard errors were multiplied by a dispersion (scale) parameter, a ratio of the deviance to its associated degrees of freedom. We built log-linear regression models of ZIP-code based counts of adult-related ARI infection using the log-transform of the underlying Census estimated population as the offset.
Because other demographic factors may influence both the rate and timing of adult ARIs and the population children, we adjusted for potential confounding of the effect of age by population density, race and socioeconomic status. For each of the study ZIP codes, we calculated the number of individuals per square mile, and proportion of the population below the poverty line per ZIP code, as provided by the US Census. We also examined confounding by race by including the proportion of non-white residents by ZIP code. Each of these factors was included as additional covariates in the model. Their interaction with the proportion of children per ZIP was also examined.
Second, we assessed association between our two outcomes and specific pediatric age groups to measure which pediatric age groups are most associated with the rate and timing of adult ED utilization due to ARIs. We obtained US Census population estimates for five policy-relevant pediatric age groups: 0−2, 4−4, 5−9, 10−14, and 15−17. For each ZIP code, we calculated the proportion of each pediatric age group relative to the estimated total population in the ZIP. We applied the same regression strategy as above but this time including each age group as a covariate. Individual age groups were included in the model both separately and in aggregate.
Overall model fit for each of the Poisson regression models was calculated by comparing deviance statistics with their asymptotic chi-square.18 Parameter estimates were transformed to rate ratios (RRs) so that they could be expressed as percentage change in respiratory presenting complaints. A significant contribution of each effect to model fit was assessed by using 2-tailed chi-square tests and α≤0.05 for rejecting the null hypothesis of no effect. All analyses were carried out using the SAS statistical software (version 9.0, SAS Institute Inc., Cary, NC).
RESULTS
Rate of ARI visits and onset of winter ARI season
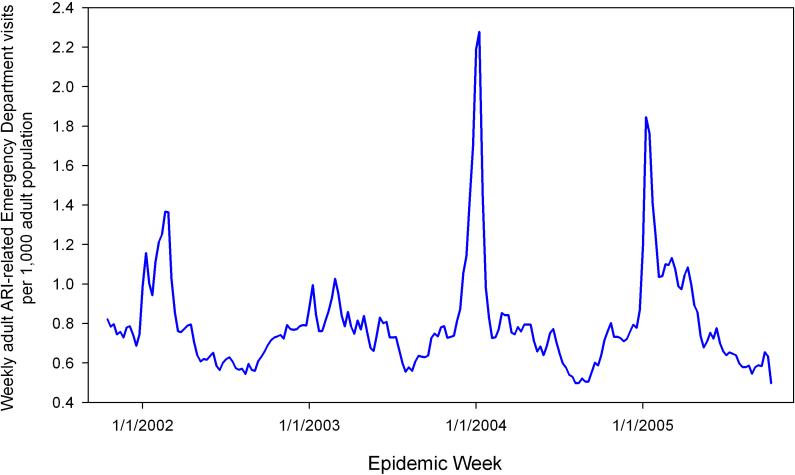
Our dataset included 18,710,970 visits over the four influenza seasons (October 1st, 2001-September 30th, 2004) across 89 healthcare institutions (Figure 1). Of those, 247,843 were classified as ARI in the 55 ZIP codes comprising the greater Boston area. 157,542 of the visits (63.7%) were by adults (age 18 and over), which comprised our study sample. The rate of visits attributed to ARI ranged from 2.94 to 87.67 per 1,000 adult population per year with median of 31.93 per 1,000 adults (Figure 2b). Each ZIP code population had a highly seasonal cycle where onset of illness occurs from the beginning of December to the end of April (Figure 2c). The majority of ZIP codes had onset within a much narrower time frame at the end of January/beginning of February; the 95% confidence interval ranged 3.2 weeks.
Figure 1.
Weekly rate of adult acute respiratory infection-related emergency department visits per 1,000 adult population, Eastern Massachusetts, USA, 2001−2005.
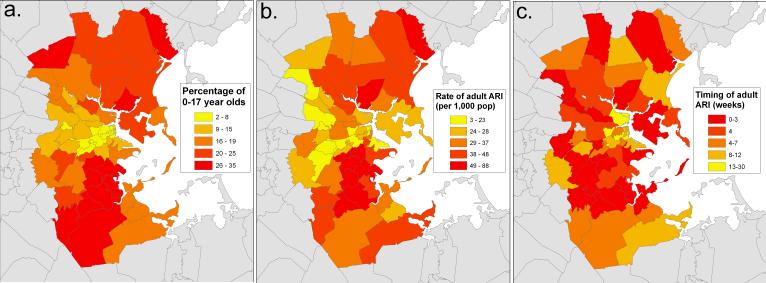
Figure 2. Comparison of spatial distribution of children and overall population-wide timing and incidence of ARI-related Emergency Department visits, Eastern Massachusetts, USA, 2001−2005.
a) Proportion of children under 18 in the population across according to the 2000 US Census in 55 ZIP codes surrounding Boston, MA. b) Rate of adult visits related due to ARI per 1,000 adult population per year by ZIP code. c) Timing of influenza activity as determining by peak analysis performed on each ZIP code time series data. The phase shift (in weeks) between each data stream represents the timeliness of the data stream, where lower values indicates earlier activity and week 1 represents the first week of the year.
Distribution of the pediatric population
We found wide variation in population age structure across our study area in Eastern Massachusetts. Based on the Census 2000 data, the proportion of children under 18 ranged from 2.69% to 34.91% in the study ZIP codes (Figure 2a). ZIP codes with relatively higher numbers of children were identified in outlying areas primarily south and north of Boston.
Impact of the pediatric population on timing and onset of the ARI season
Onset of the winter ARI season among the adult population was associated with the proportion of children in each ZIP code (Table 1). Univariate Poisson regression reveals that increase in the proportion of children in a ZIP code is significantly associated with an earlier than average onset of ARIs (RR, 0.985; 95% confidence interval [CI], 0.977−0.993) (Figure 3b). Further Poisson regression analyses were conducted to examine the influence of specific pediatric age groups. (Table 1) All pediatric age groups were predictors of ARI timing among adults, with the 3−4 year olds having the strongest association (RR, 0.881; 95% CI, 0.816−0.952).
Table 1.
Timing and rate of acute respiratory infection-related emergency department utilization among adults per percent increase in the proportion of pediatric age groups in Eastern Massachusetts, USA, 2001−2005.
| Population |
Regression Models* |
|
|---|---|---|
| Effect of percent increase in pediatric population on timing of adult ARI visits (by week of year) |
Effect of percent increase in pediatric population on rate of adult ARIs visits (per 1,000 population) |
|
| RR (95% confidence interval) | RR (95% confidence interval) | |
| All children | 0.98 (0.98, 0.99) | 1.04 (1.02, 1.05) |
| Age 0−2 | 0.98 (0.92, 1.05) | 1.24 (1.14, 1.37) |
| Age 3−4 | 0.88 (0.81, 0.96) | 1.38 (1.24, 1.54) |
| Age 5−9 | 0.95 (0.92, 0.97) | 1.12 (1.08, 1.16) |
| Age 10−14 | 0.999 (0.9998, 1.0) | 1.0002 (1.0001, 1.0003) |
| Age 15−17 | 0.91 (0.87, 0.95) | 1.23 (1.15, 1.32) |
Results are based on univariate Poisson regression analyses.
Figure 3. Association between pediatric populations, and timing and rate of acute respiratory infection-related emergency department utilization among adults, Eastern Massachusetts, US, 2001−2005.
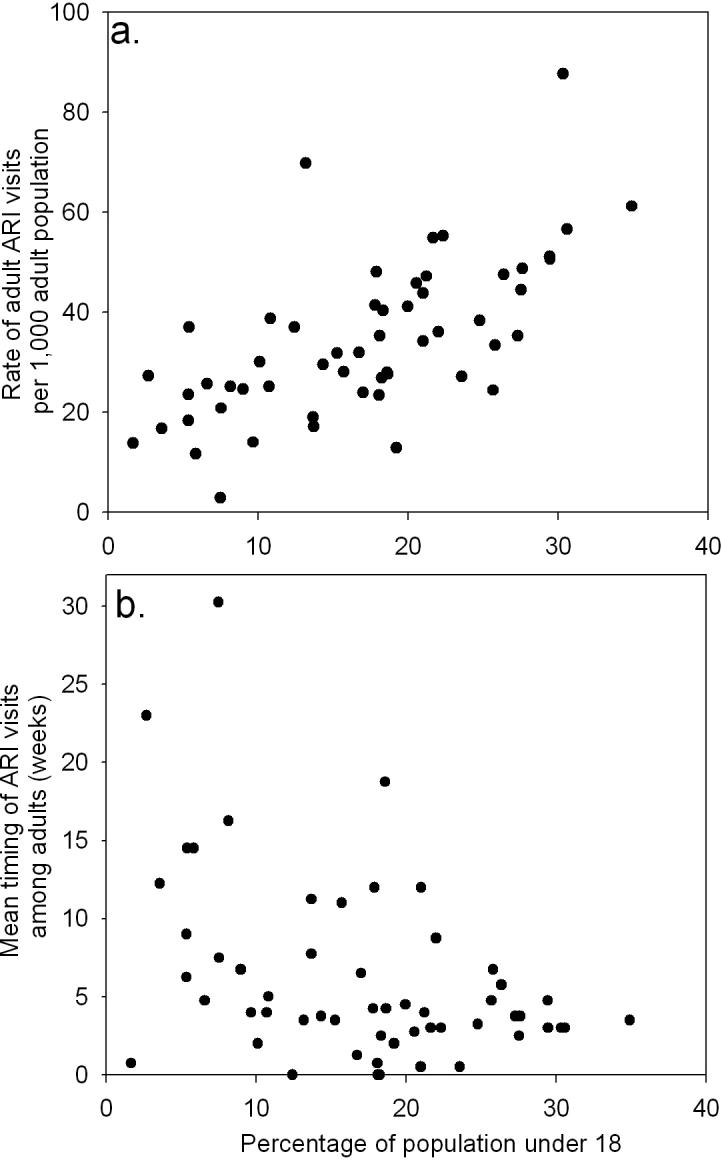
Figures depict the influence of the proportion of all children living in each of 55 ZIP codes on (a) rate (per 1000 adult population per year) and (b) timing of visits due to acute respiratory infection by adults (over 18 years of age) where week 1 is the first week of the year.
Impact of the pediatric population on rate of adult infection
The pediatric population also significantly explains patterns of adult ARI-related ED utilization, with increases in the proportion of children in a ZIP code significantly associated with higher utilization rates (RR, 1.035; 95% CI, 1.024−1.047) (Figure 3a). Although all proportions of each of the pediatric age groups were significant predictors of ARI utilization rate, the preschool age children (3−4) were the best predictors of utilization among adults (RR, 1.380; 95% CI, 1.238−1.539).
We examined potential confounding of the effect of age on timing and rate of ED utilization by population density, race/ethnicity and poverty. We did not find a significant effect nor did inclusion in the model including interaction terms influence prediction by age.(Table 2) Notably, however, in the geographic region studied the population was relatively homogeneous with respect to these factors.
Table 2.
Timing and rate of acute respiratory infection-related emergency department utilization among adults per percent increase in the proportion of children after accounting for potential confounders in Eastern Massachusetts, USA, 2001−2005.
| Variables* |
Regression Models |
|
|---|---|---|
| Effect of percent increase in pediatric population on timing of adult ARI visits (by week of year) |
Effect of percent increase in pediatric population on rate of adult ARIs visits (per 1,000 population) |
|
| RR (95% confidence interval) | RR (95% confidence interval) | |
| Children alone (Univariate) | 0.98 (0.98, 0.99) | 1.04 (1.02, 1.05) |
| + Poverty | 0.98 (0.98, 0.99) | 1.03 (1.02, 1.05) |
| + Density | 0.98 (0.97, 0.99) | 1.04 (1.02.,1.05) |
| + Race/Ethnicity | 0.99 (0.98, 1.00) | 1.03 (1.01, 1.04) |
| + Poverty, Density, Race/Ethnicity | 0.98 (0.97, 0.99) | 1.02 (1.00, 1.04) |
Poverty was defined as percent population per ZIP code below poverty level. Population density was defined as total population per square mile by ZIP code. Race was defined as proportion of non-white residents by ZIP code.
LIMITATIONS
Our study is based on a number of assumptions. The indirect approach to defining the association between ED visits and census level characteristics may result in ecological fallacy. While we identify a strong correlation, the study design prevents us from making inference about causation. We did not measure the contact network between individual pediatric ARI cases and infection in adults. Furthermore, our use of patient residence address ZIP code may incorrectly infer that infection took place in the home. Other unmeasured variables related to changes in the pediatric population may explain the geographic differences in adult ARI. For instance, the Boston city center, with the smallest pediatric populations, is populated by college students who may not receive care from the ED or may report a home address outside of the state. This confounder would tend to artificially diminish the rates of adult ARI illness. Furthermore, given our relatively small RR values, we expect that other unmeasured individual factors may play an important role in the observed spatial patterns of utilization.
The study may be also limited by the broad case definition for ARI and the lack of viral confirmation of cases, though this method of identifying cases of influenza has been validated in multiple previous studies.19, 20 Children in particular have different clinical presentations which may lead to misclassification. Thus influenza-like illness surveillance is not entirely specific to influenza. Nonetheless, previous studies have shown that this subset of ICD-9 codes data are accurate measures of ARI burden in the absence of viral data and that ED data do track closely with viral isolates and with traditional CDC influenza surveillance data.8, 21 Because primary uses of these data are for non-research purposes and healthcare utilization encompasses a complex behavioral component, caution should be applied to any conclusions drawn from these primarily administrative datasets. Finally, since our analysis was based on population data from the 2000 U.S. Census, and the study is from 2001−2004, potential population shifts in the pediatric population could not be accounted for.
DISCUSSION
We find for the first time that spatial patterns of ED utilization for respiratory viruses among adults are associated with the population age structure. We demonstrate a positive correlation between the rate of ARI-related ED utilization by adults and the proportion of children in the population. Further, the proportion of children in a local population is significantly associated with the timing of the winter respiratory viral season among adults. Areas with high proportion of children tend to have early and greater adult presentations for influenza and other ARIs. These findings add to a growing body of evidence supporting a critical role played by children in community-wide transmission of ARIs.10-13
Our findings are consistent with previous research showing preschool age children to be the early sentinels of activity and significantly associated with population wide morbidity and mortality due to influenza.8 The US Advisory Committee on Immunization Practices (ACIP) recently recommended vaccinating three and four year olds, extending the 2004 recommendation for vaccination of 6−24 month month-olds.22 Our findings lend support to the 2006 ACIP recommendation that expanded universal vaccination to include preschool children but further suggests that expansion of these recommendations to include all children may be the most effective strategy may yield substantial benefit. While preschool children may be the earliest sentinels of infection, schoolchildren represent a larger segment of the population and thus may contribute more substantially to overall transmission intensity.21, 23 Our findings suggest that there may be community-wide benefits from routinely vaccinating older children against influenza as well. Whereas previous recommendations were based on protecting vulnerable individuals from severe complications of influenza; such a policy would shift toward a public health orientation, vaccinating children in part may protect others by reducing the overall community burden of influenza.24
Our findings may also have application for the control and prevention of other ARIs. Development of vaccines for RSV and PIV is currently underway. Understanding understanding the impact of different pediatric age groups on community-wide ARI transmission can help define control strategies including priority groups for targeted disease control and prevention.25, 26
While we can not make claims about causality, this spatial analysis adds to a growing body of literature on the importance of the pediatric population in community-wide respiratory infection. As this study is based on patient populations in the Boston area only, a larger US sample would more accurately reflect the variability of population characteristics including age distribution, ethnicity, race, and socioeconomic status, and serve to bolster this relationship.
Funding/Support
This work was supported by R21AI073591-01 from the National Institute Allergy and Infectious Diseases and R21LM009263-01 and R01 LM007677-01 from the National Library of Medicine, National Institutes of Health, the Canadian Institutes of Health Research, and by contract 52253337HAR from the Massachusetts Department of Public Health.
Footnotes
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2−3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
REFERENCES
- 1.Simonsen L, Clarke MJ, Williamson GD, et al. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health. 1997 Dec;87(12):1944–1950. doi: 10.2105/ajph.87.12.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003 Jan 8;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Glezen WP, Couch RB. Estimating deaths due to influenza and respiratory syncytial virus. JAMA. 2003 May 21;289(19):2500. doi: 10.1001/jama.289.19.2500-a. author reply 2500−2502. [DOI] [PubMed] [Google Scholar]
- 4.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004 Sep 15;292(11):1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 5.Simonsen L, Fukuda K, Schonberger LB, et al. The impact of influenza epidemics on hospitalizations. J Infect Dis. 2000 Mar;181(3):831–837. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- 6.Schoenbaum SC. Economic impact of influenza. The individual's perspective. Am J Med. 1987 Jun 19;82(6A):26–30. doi: 10.1016/0002-9343(87)90557-2. [DOI] [PubMed] [Google Scholar]
- 7.Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993 Feb;110(1):145–160. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brownstein JS, Kleinman KP, Mandl KD. Identifying Pediatric Age Groups for Influenza Vaccination Using a Real-Time Regional Surveillance System. Am J Epidemiol. 2005 Aug 17;162(7):686–693. doi: 10.1093/aje/kwi257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longini IM, Jr., Halloran ME. Strategy for distribution of influenza vaccine to high-risk groups and children. Am J Epidemiol. 2005 Feb 15;161(4):303–306. doi: 10.1093/aje/kwi053. [DOI] [PubMed] [Google Scholar]
- 10.Monto AS, Koopman JS, Longini IM., Jr Tecumseh study of illness. XIII. Influenza infection and disease, 1976−1981. Am J Epidemiol. 1985 Jun;121(6):811–822. doi: 10.1093/oxfordjournals.aje.a114052. [DOI] [PubMed] [Google Scholar]
- 11.Hurwitz ES, Haber M, Chang A, et al. Effectiveness of influenza vaccination of day care children in reducing influenza-related morbidity among household contacts. JAMA. 2000 Oct 4;284(13):1677–1682. doi: 10.1001/jama.284.13.1677. [DOI] [PubMed] [Google Scholar]
- 12.Longini IM, Jr, Koopman JS, Monto AS, et al. Estimating household and community transmission parameters for influenza. Am J Epidemiol. 1982 May;115(5):736–751. doi: 10.1093/oxfordjournals.aje.a113356. [DOI] [PubMed] [Google Scholar]
- 13.Reichert TA, Sugaya N, Fedson DS, et al. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med. 2001 Mar 22;344(12):889–896. doi: 10.1056/NEJM200103223441204. [DOI] [PubMed] [Google Scholar]
- 14.Viboud C, Bjornstad ON, Smith DL, et al. Synchrony, Waves, and Spatial Hierarchies in the Spread of Influenza. Science. 2006 Mar 30; doi: 10.1126/science.1125237. [DOI] [PubMed] [Google Scholar]
- 15.Massachusetts Health and Human Services Division of Health Care Finance and Policy [January 28, 2008];Emergency Department Data. Available at: http://www.mass.gov/?pageID=eohhs2subtopic&L=6&L0=Home&L1=Researcher&L2=Physical+Health+and+Treatment&L3=Health+Care+Delivery+System&L4=Hospitals&L5=Emergency+Department+Data&sid=Eeohhs2.
- 16.Nelder JA, Wedderburn RW. Generalized linear models. Journal of the Royal Statistical Society, Series A. 1972;175(3):370–384. [Google Scholar]
- 17.McCullagh P, Nelder JA. Generalized Linear Models. 2 ed Vol. 37. Chapman and Hall; London: 1989. [Google Scholar]
- 18.Williams DA. Generalized linear model diagnostics using the deviance and single case deletions. Applied Statistics. 1987;36(2):181–191. [Google Scholar]
- 19.Marsden-Haug N, Foster VB, Gould PL, et al. Code-based syndromic surveillance for influenzalike illness by International Classification of Diseases, Ninth Revision. Emerg Infect Dis. 2007 Feb;13(2):207–216. doi: 10.3201/eid1302.060557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourgeois FT, Olson KL, Brownstein JS, et al. Validation of syndromic surveillance for respiratory infections. Ann Emerg Med. 2006 Mar;47(3):265 e261. doi: 10.1016/j.annemergmed.2005.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson DR, Heffernan RT, Paladini M, et al. Monitoring the impact of influenza by age: emergency department fever and respiratory complaint surveillance in New York City. PLoS Med. 2007 Aug 7;4(8):e247. doi: 10.1371/journal.pmed.0040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Advisory Committee on Immunization Practices Vaccines for Children Program Resolution No. 2/06−3. Eligible Groups for Inactivated Influenza Vaccine. Available at: http://www.cdc.gov/nip/vfc/acip_resolutions/0206influenza.pdf.
- 23.Weycker D, Edelsberg J, Halloran ME, et al. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005 Jan 26;23(10):1284–1293. doi: 10.1016/j.vaccine.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 24.Abramson JS, Neuzil KM, Tamblyn SE. Annual universal influenza vaccination: ready or not? Clin Infect Dis. 2006 Jan 1;42(1):132–135. doi: 10.1086/498514. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg HB, Piedra PA. Immunization against viral respiratory disease: a review. Pediatr Infect Dis J. 2004 Nov;23(11 Suppl):S254–261. doi: 10.1097/01.inf.0000144756.69887.f8. [DOI] [PubMed] [Google Scholar]
- 26.Polack FP, Karron RA. The future of respiratory syncytial virus vaccine development. Pediatr Infect Dis J. 2004 Jan;23(1 Suppl):S65–73. doi: 10.1097/01.inf.0000108194.71892.95. [DOI] [PubMed] [Google Scholar]