Abstract
Background:
Left Ventricular Hypertrophy (LVH) is associated with end-stage renal disease and chronic kidney disease, but the association of LVH with mild impairment in kidney function is not known. We hypothesized that mild and moderate reductions in kidney function, reflected in higher serum cystatin C concentrations, would be linearly associated with a higher prevalence of LVH.
Study Design:
Cross-sectional observational study.
Settings and Participants:
4,971 participants participating in baseline examinations in the Multi-Ethnic Study of Atherosclerosis (MESA), a population-based study with several sites in the U.S.
Predictor:
Cystatin C-based estimated glomerular filtration rate (eGFRcysC)
Outcomes:
LVH and left ventricular (LV) mass index.
Measurements:
Serum cystatin C and creatinine, LV mass obtained by magnetic resonance imaging (MRI). LVH cutoffs for males and females were defined by the upper 95th percentile of LV mass index of all MESA participants without hypertension.
Results:
LVH was distinctly more prevalent (>12%) only in the lowest two deciles of eGFRcysC (<75 ml/min/1.73 m2). When participants with stage III or higher chronic kidney disease (creatinine eGFR <60 ml/min/1.73 m2) were excluded, the odds for LVH increased for each lower category of eGFRcysC below 75 ml/min/1.73 m2: 1.6 the odds for LVH with an eGFRcysC between 60-75 ml/min/1.73 m2 (95% confidence interval 1.20-2.07, P = 0.001), and 2.0 the odds for an eGFRcysC <60 ml/min/1.73 m2 (1.03-3.75, P = 0.04), after adjustment for demographic factors, study site, diabetes, and smoking. The association of the a lower eGFRcysC with LVH was attenuated after further adjustment for hypertension.
Limitations:
Cross-sectional, rather than longitudinal design, lack of participants with more advanced kidney disease, lack of a direct measurement of glomerular filtration rate.
Conclusions:
Among subjects without CKD, eGFRcysC ≤ 75 ml/min/1.73 m2 was associated with a higher odds of LVH.
Keywords: kidney disease, cystatin C, glomerular filtration rate, left ventricular hypertrophy, left ventricular mass, magnetic resonance imaging (MRI)
Introduction
Left ventricular hypertrophy (LVH) is an early, subclinical marker of cardiovascular disease and heart failure risk.(1-5) In patients with end-stage renal disease, LVH is common,(6) and is an independent predictor of cardiovascular disease and mortality.(7-9) LVH is also an independent predictor of cardiovascular disease mortality and heart failure in patients with ≥ stage three chronic kidney disease,(10-12) defined as a creatinine-based estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2.(13) Among patients with chronic kidney disease, increased LV mass is correlated with severity of GFR impairment.(14-17) In estimating LV mass, MRI may be superior to echocardiography because MRI estimates are independent of geometric assumptions and use higher-resolution imaging.(18, 19)
Serum cystatin C concentration is an alternative measure of kidney function that is less affected by age, sex, or muscle mass, and is a more sensitive indicator of early renal dysfunction than creatinine-based estimations of GFR. Cystatin C identifies impaired kidney function at an earlier stage, when the creatinine-based eGFR remains ≥60 ml/min/1.73 m2.(20, 21) Cystatin C has been shown to be a linear predictor of risk for cardiovascular death,(22, 23) cardiovascular events,(22) and heart failure in older subjects,(24) and extends the association between kidney dysfunction and increased cardiovascular disease risk to patients with a normal or mildly decreased creatinine-based eGFR (eGFR ≥60 ml/min/1.73 m2 and cystatin C ≥ 1.0 mg/L).(25) Recently, cystatin C-based GFR estimating equations have been developed from and validated in chronic kidney disease cohorts.(26) LVH is a potential mediating factor in the causal pathway between elevated cystatin C and increased cardiovascular disease risk. Whether mild reductions in kidney function are associated with an increased prevalence of LVH has not been studied.
The Multi-Ethnic Study of Atherosclerosis (MESA) was established by the U.S. National Heart, Lung, and Blood Institute (NHLBI) to examine the determinants of sub-clinical cardiovascular disease measures and their associations with cardiovascular disease outcomes. Baseline measures include cystatin C, creatinine, and left ventricular mass determined by magnetic resonance imaging (MRI). Because cystatin C is a sensitive marker for early kidney disease, we hypothesized that lower cystatin C-based eGFR (eGFRcysC) would be associated linearly with a higher prevalence of LVH in subjects with mild and moderate kidney dysfunction.
Methods
Participants and Study Design
A full description of MESA is available elsewhere.(27) MESA recruited 6,814 men and women from four ethnic groups (white, African American, Hispanic, and Chinese) aged 45-84 years. Participants were recruited from six Field Centers: Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; New York, NY; and St. Paul, MN. A probability sample of over 1,000 (ranging from 1,066 to 1,319) participants was selected at each site through population-based approaches [commercial lists of area residents, HCFA lists of area residents (for participants aged 65 and over), area residents enrolled in a union health plan (in New York City), and random digit dialing (New York City and Los Angeles)]. The MESA protocol was approved by the institutional review boards of all participating centers. Informed consent was obtained from all participants, and research ethics guidelines outlined by the Declaration of Helsinki were followed. The baseline visit for the MESA cohort took place between July 2000 and September 2002. For this analysis, we included 4,971 MESA participants with serum creatinine and cystatin C measurements and left ventricular mass obtained by magnetic resonance imaging at the baseline visit.
Predictors
Information on risk factors for cardiovascular disease was obtained at the 2000-2002 MESA examination.(27, 28) Blood collection and processing was conducted using a standardized protocol developed for the Cardiovascular Health Study.(29) Cystatin C was measured from frozen sera at a central laboratory (University of Vermont, Colchester, VT) using a BNII nephelometer (Dade Behring, Inc, Deerfield, Illinois) and a particle-enhanced immunonepholometric assay (N Latex Cystatin C, Dade-Behring).(30) The analytical coefficient of variation for this assay is 2.5%. Estimated GFR was calculated from serum cystatin C concentration using the formula derived and validated by Stevens et al. [eGFRcysC = 76.7 * cystatin C1.19 ].(26) Serum creatinine was measured using a colorimetric method on a Kodak Ektachem 700 Analyzer (Eastman Kodak, Rochester, New York). The analytical coefficient of variation of this assay is 2.2%. Creatinine-based GFR was also estimated using the Modification of Diet in Renal Disease (MDRD) Study equation as creatinine eGFR = 186.3 × (serum creatinine concentration^−1.154) × (age^−0.203) × 1.212 (if African American) × 0.742 (if female).(31) Five participants in the study group lacked serum creatinine measurements, so no creatinine-based GFR was estimated for these participants.
Outcomes
The MESA MRI protocol has been described in detail elsewhere.(27, 32) Briefly, cardiac MRI testing was performed at MESA study sites using a standard protocol and read at a central site (Johns Hopkins University, Baltimore, MD). End-diastolic left ventricular mass was determined using 1.5-T MR scanners: Signa LX and CVi (GE Medical Systems, Waukesha, WI) and Symphony and Sonata (Siemens Medical Systems, Erlangen, Germany). MRI was performed with a four-element, phased array surface coil placed anteriorly and posteriorly, with electrocardiogram gating, and brachial artery pressure monitoring. Imaging consisted of cine images of the left ventricle with a temporal resolution of less than or equal to 50 msec. There were acceptable inter-observer variability in estimating LV mass [technical error of the mean (TEM) 6.0% (95 % CI 4.6-7.4%)] and acceptable intra-observer variability [TEM 6.28 (5.17-7.38)].(32)
Left ventricular mass index (LV mass index) was defined as LV end-diastolic mass divided by body surface area (g/m2). Following the approach of past studies, we defined LVH cutoffs as the upper 95th percentile males and females in the MESA study population without hypertension (no use of antihypertensive medications, systolic blood pressure <140 mm Hg, diastolic blood pressure <90 mm Hg),(33-35) Using this definition, LVH in females was an LV mass index greater than 85.3 g/m2 and LVH in males was an LV mass index greater than 107.8 g/m2. We found that using alternate LVH definitions did not appreciably change the LVH cut points or the results of our analyses: these were a) upper 95th percentile of all MESA participants; b) upper 95th percentile of MESA participants without hypertension or diabetes [fasting glucose ≥ 126 mg/dL (7 mmol/l), or taking insulin or oral diabetes medications]; c) upper 95th percentile of MESA participants without hypertension, diabetes, current smoking, or dyslipidemia [low density lipoprotein cholesterol <160 mg/dL (4.10 mmol/l) and no lipid-lowering medications].
Statistical methods
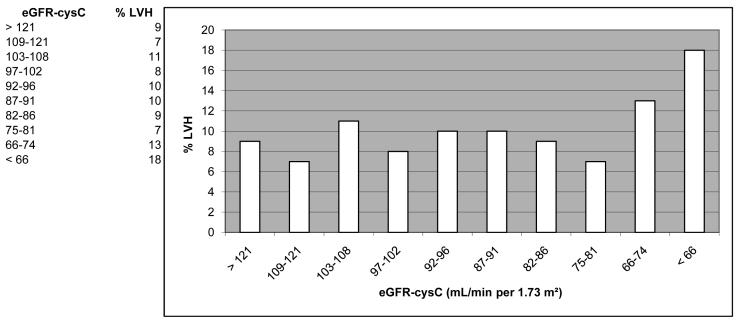
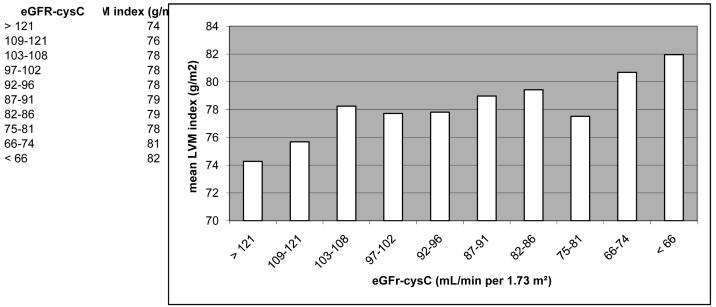
First the baseline characteristics of participants included in or excluded from the study were compared. Participants were then categorized into ascending quintiles of cystatin C and the distributions of demographic characteristics, and cardiovascular risk factors were compared using ANOVA for continuous variables and χ2 test for categorical variables. The percent prevalence of LVH and mean LV mass index were graphed by deciles of cystatin C eGFR (Figures 1a and 1b). Analysis of these histogram plots suggested a non-linear association across eGFRcysC deciles. We tested the non-linearity assumption by regressing continuous LV mass index (g/m2) on continuous eGFRcysC (mL/min per 1.73 m2). The slope for the association between a eGFRcysC ≤75 mL/min per 1.73 m2 was −0.112 (i.e., suggesting higher LV mass with lower eGFR), while the slope for an eGFRcysC >75 mL/min per 1.73 m2 approximated unity (beta coefficient = 0.008, suggesting no association). In subsequent analyses, eGFRcysC was therefore modeled only in the following categories: >75, 60-75, and <60 mL/min per 1.73 m2, or >75 and ≤75 mL/min per 1.73 m2. Because the study hypothesis focused on subjects with normal and mild-to-moderately impaired kidney function, we excluded subjects with a creatinine-based eGFR <60 mL/min per 1.73 m2 for the multivariate analyses. Logistic regression models were then used to determine if eGFRcysC ≤75 mL/min per 1.73 m2 was independently associated with a higher prevalence of LVH after adjusting for potential confounders, i.e., age, sex, race, MESA study site, current smoking, and diabetes mellitus.
Figure 1.
Figure 1a. Percent left ventricular hypertrophy (LVH) by decile of cystatin C-based estimated glomerular filtration rate (eGFRcysC, ml/min/1.73 m2) in 4,971 MESA participants. (LVH cut points for MESA were 107.8 g/m2 in males, and 85.3 g/m2 in females).
Figure 1b. Mean left ventricular (LV) mass index (g/m2 body surface area) by deciles of cystatin C-based estimated glomerular filtration rate (eGFRcysC, ml/min/1.73 m2) in 4,971 MESA participants.
Because hypertension is a potential mediator between elevated cystatin C and LVH, hypertension was added to the multivariate model in a separate step. We did not adjust for body mass index (BMI, kg/ m2) because of its close association with the body surface area (m2) component of the LV mass index. We tested whether the strength of the association of eGFRcysC ≤75 mL/min per 1.73 m2 with LVH differed by race/ethnic category by entering an interaction term (cystatin C * race/ethnic category) into the model. We also adjusted for use of angiotensin converting enzyme inhibitors or angiotensin II receptor blockers, and for use of any antihypertensive medications; these models included systolic and diastolic blood pressures in place of hypertension. The results from models including antihypertensive medications were similar to the fully adjusted models without antihypertensive medication variables, so use of medications was not included in the final fully adjusted model
We estimated the association of an eGFRcysC ≤75 mL/min per 1.73 m2 with a higher mean LV mass index in linear regression models before and after adjusting for the covariates listed above. For the linear regression models, a Sidak test was used for pairwise comparisons using the highest eGFRcysC category as the reference group (i.e. highest with middle, highest with lowest). A P-value of <0.05 was considered statistically significant. Statistical analyses were performed using S-Plus (release 6.1, Insightful Inc, Seattle, WA) and SPSS statistical software (release 14.0.2, SPSS Inc, Chicago, IL).
Results
Of the 6,814 MESA participants, 1,810 did not have MRI measurement of LV mass, and 33 lacked serum cystatin C measurement, leaving 4,971 with both MRI assessment of LV mass and serum cystatin C measurement who were included in this analysis. Compared with excluded participants, participants included in the study had a lower mean BMI and systolic blood pressure, a lower prevalence of hypertension and diabetes, and were more likely to be Chinese, and less likely to be African American (Table 1). While creatinine eGFR was similar between included and excluded participants, eGFRcysC was slightly higher on average in included participants. The mean age for the participants included in this analysis was 62 years old, 39% of participants were white, 26% African American, 22% Hispanic, and 13% Chinese.
Table 1.
Characteristics of participants in the Multi-Ethnic Study of Atherosclerosis (MESA) included in or excluded from the study.
| Excluded | Selected | p-value* | |
|---|---|---|---|
| N | 1843 | 4971 | |
| Age (years) | 64 (10) | 62 (10) | <0.001 |
| Male | 842 (46) | 2371 (48) | 0.14 |
| Race/ethnicity | |||
| White | 679 (37) | 1945 (39) | 0.09 |
| Chinese | 151 (8) | 652 (13) | <0.001 |
| African American | 619 (34) | 1276 (26) | <0.001 |
| Hispanic | 394 (21) | 1098 (22) | 0.53 |
| BMI† (kg/m2) | 30.0 (6) | 27.7 (5) | <0.001 |
| Ever Smoked | 970 (53) | 2404 (49) | 0.001 |
| Current Smoking | 256 (14) | 631 (13) | 0.2 |
| Systolic blood pressure (mmHg) | 130 (22) | 125 (21) | <0.001 |
| Diastolic blood pressure (mmHg) | 72 (10) | 72 (10) | 0.3 |
| Taking antihypertensive medications | 781 (42) | 1755 (35) | <0.001 |
| Hypertension‡ | 949 (52) | 2109 (42) | <0.001 |
| Diabetes§ | 331 (18) | 640 (13) | <0.001 |
| Creatinine – based eGFR (ml/min/1.73m2)∥ | 81 (21) | 81 (17) | 0.4 |
| Cystatin C (mg/L) | 0.92 (0.25) | 0.88 (0.24) | <0.001 |
| eGFRcysC(ml/min/1.73m2)** | 89 (22) | 94 (32) | <0.001 |
Data are means (standard deviations) or number (%).
P values are from a t-test for trend or chi squared test.
BMI = body mass index
Hypertension was defined as either systolic blood pressure of 140 mm Hg or greater, diastolic blood pressure of 90 mm Hg or greater, or treatment with antihypertensive medications for blood pressure control.
Diabetes was defined as fasting glucose >= 126 mg/dL(7 mmol/l), taking insulin, or oral diabetes medication.
creatinine-based eGFR = estimated GFR using the creatinine-based Modification of Diet in Renal Disease (MDRD) equation.(31)
cystatin C-based eGFR(26)
Participants with cystatin C concentration in the highest quintiles were older, and more likely to be male (Table 2). The highest two quintiles of cystatin C had a higher proportion of Whites and fewer Chinese compared with lower quintiles. The number of African American and Hispanic participants appeared evenly distributed across cystatin C categories. Mean systolic blood pressure, mean body mass index, and the prevalence of diabetes, hypertension, and microalbuminuria were all greater in the highest quintile of cystatin C compared with lower quintiles. Within the highest quintile of cystatin C, 65% of participants had a creatinine eGFR ≥ 60 ml/min/1.73 m2 and 75% had an eGFRcysC ≥ 60 ml/min/1.73 m2. The prevalence of LVH and the LV mass index were distinctly higher in the lowest two eGFRcysC deciles compared with higher deciles (Figures 1a and 1b).
Table 2.
Characteristics of the 4,971 MESA participants included in the analysis by quintile of cystatin C.
| Characteristic | Cystatin C Quintile (mg/L) | p-value | ||||
|---|---|---|---|---|---|---|
| ≤ 0.74 | 0.75-0.82 | 0.83-0.90 | 0.91-1.02 | ≥1.03 | ||
| N | 1125 | 1075 | 963 | 948 | 860 | |
| Age (years) | 56 (9) | 59 (9) | 62 (9) | 64 (9) | 69 (9) | <0.001 |
| Male | 404 (36) | 512 (48) | 491 (51) | 514 (54) | 450 (52) | <0.001 |
| Race/ethnicity | <0.001 | |||||
| White | 376 (33) | 368 (34) | 407 (42) | 410 (43) | 384 (45) | |
| Chinese | 207 (18) | 144 (13) | 103 (11) | 112 (12) | 86 (10) | |
| African American | 318 (28) | 306 (29) | 229 (24) | 204 (22) | 219 (26) | |
| Hispanic | 224 (20) | 257 (24) | 224 (23) | 222 (23) | 171 (20) | |
| BMI† (kg/m2) | 26.6 (4.8) | 27.4 (4.6) | 27.9 (4.9) | 28.2 (5.0) | 29.0 (5.2) | <0.001 |
| Ever Smoked | 491 (44) | 517 (48) | 468 (49) | 483 (51) | 445 (52%) | 0.002 |
| Current Smoking | 118 (11) | 137 (13) | 128 (13) | 125 (13) | 123 (14) | 0.02 |
| Systolic blood pressure (mm Hg) | 121 (20) | 123 (20) | 126 (20) | 127 (21) | 132 (24) | <0.001 |
| Diastolic blood pressure (mm Hg) | 71 (11) | 72 (10) | 72 (10) | 72 (10) | 71 (11) | 0.8 |
| Taking antihypertensive medications | 268 (24) | 291 (27) | 342 (36) | 371 (39) | 483 (56) | <0.001 |
| Hypertension‡ | 350 (31) | 365 (34) | 408 (42) | 447 (47) | 539 (63) | <0.001 |
| Diabetes§ | 147 (13) | 124 (12) | 99 (10) | 99 (10) | 171 (20) | 0.001 |
| Microalbuminuria ∥ | 70 (6) | 54 (5) | 53 (6) | 64 (7) | 126 (15) | <0.001 |
| Taking ACE inhibitor | 89 (8) | 83 (8) | 93 (10) | 112 (12) | 164 (19) | <0.001 |
| Taking angiotensin II receptor blocker | 19 (2) | 28 (3) | 26 (3) | 32 (3) | 56 (7) | <0.001 |
| Creatinine (mg/dL)** | 0.83 (0.15) | 0.90 (0.15) | 0.94 (0.17) | 0.98 (0.17) | 1.17 (0.56) | <0.001 |
| eGFR (creatinine; ml/min/1.73m2)†† | 92 (16) | 86 (15) | 80 (14) | 77 (13) | 65 (16) | <0.001 |
| 15-29 ml/min/1.73m2 [n, (%)] | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 14 (2) | |
| 30-59 | 8 (1) | 15 (1) | 33 (3) | 68 (7) | 292 (34) | |
| ≥60 | 1117 (99) | 1160 (99) | 927 (97) | 880 (93) | 552 (64) | |
| eGFRcysC (cystatin C;ml/min/1.73m2)‡‡ | 124 (51) | 102 (3) | 91 (3) | 81 (3) | 64 (10) | <0.001 |
| 15-29 ml/min/1.73m2 [n, (%)] | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 12 (1) | <0.001 |
| 30-59 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 192 (22) | |
| ≥60 | 1125 (100) | 1075 (100) | 963 (100) | 948 (100) | 656 (76) | |
Note: Data are means (standard deviations) or number (%).
P values are from an ANOVA test for trend or chi squared test for trend.
BMI = body mass index
Hypertension was defined as either systolic blood pressure of 140 mm Hg or greater, diastolic blood pressure of 90 mm Hg or greater, or treatment with antihypertensive medications for blood pressure control.
Diabetes was defined as fasting glucose >= 126 mg/dL (7 mmol/l), taking insulin, or oral diabetes medication.
Microalbuminuria was defined as spot urine measurement, albumin(mg) / creatinine (g) ≥ 30 mg/g
ACE inhibitor = Angiotensin Converting Enzyme Inhibitor
to convert to micromoles per liter, multiply by 88.4
eGFR = estimated GFR using the creatinine-based Modification of Diet in Renal Disease (MDRD) equation.(31) Five participants lack serum creatinine measurements, and so no eGFR was available for these participants.
eGFR = estimated GFR using the cystatin C-based equation eGFR = 76.7 × cystatin C−1.18 [reference: Stevens et al. (26)]
Participants with a creatinine eGFR <60 mL/min per 1.73 m2 were then excluded and logistic regression models were conducted. After adjusting for age, sex, race/ethnicity, MESA site, current smoking, and diabetes, each lower category of eGFRcysC below 75 mL/min per 1.73 m2 was associated with a higher odds of LVH. Participants with a eGFRcys 60-75 mL/min per 1.73 m2 had 1.6-fold the odds of LVH compared with participants with an eGFR >75 (Table 3, P < 0.001). Participants in the lowest eGFRcysC category had 2.0 the odds of LVH compared with the >75 mL/min per 1.73 m2 category (P = 0.041). These associations were attenuated after additional adjustment for hypertension. The association between the lowest categories of eGFRcysC and LVH was similar across race/ethnic categories (P for interaction = 0.50). Similar results were estimated in models with eGFRcysC categorized as >75 or ≤75 mL/min per 1.73 m2 (Table 3).
Table 3.
Association of categories of cystatin C-based estimated glomerular filtration rate (eGFRcysC) with LVH (cut point of 107.8 g/m2in males, 85.3 g/m2 in females) in 4,536 MESA participants with a creatinine-based eGFR > 60 ml/min/1.73 m2
| Demographic Adjusted* | Adjusted for demographic variables, smoking, and diabetes† |
Adjusted for demographic variables, smoking, diabetes, and hypertension‡ |
|||||
|---|---|---|---|---|---|---|---|
| Number | OR (95% CI) |
P value |
OR (95% CI) |
P value | OR (95% CI) |
P value | |
| Cystatin C eGFR∥ | |||||||
| > 75 | 3874 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| 60-75 | 587 | 1.60 (1.22, 2.11) | 0.001 | 1.57 (1.20, 2.07) | 0.001 | 1.45 (1.10, 1.92) | 0.008 |
| <60 | 75 | 2.09 (1.10, 3.97) | 0.03 | 1.97 (1.03, 3.75) | 0.04 | 1.76 (0.98, 3.41) | 0.09 |
| Cystatin C eGFR | |||||||
| > 75 | 3874 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| <=75 | 662 | 1.65 (1.27, 2.14) | <0.001 | 1.61 (1.24, 2.09) | <0.001 | 1.49 (1.14, 1.94) | 0.003 |
adjusted for age, sex, race/ethnicity, and MESA site
diabetes defined as fasting serum glucose ≥ 126 mg/dL (7 mmol/l) or taking insulin or oral diabetes medications
hypertension defined as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg, or taking antihypertensive medications
eGFR = glomerular filtration rate estimated using cystatin C(26)
In linear regression models with continuous LV mass index as the dependent variable, the eGFRcysC ≤75 mL/min per 1.73 m2 was associated with a 2.3 g/m2 higher mean LV mass index compared with an eGFRcysC >75 mL/min per 1.73 m2 after adjustment for age, sex, ethnicity, MESA site, current smoking, and diabetes (Table 4). This association was not eliminated after additional adjustment for hypertension. A similar association between a lower eGFRcysC and higher mean LV mass index was observed for an eGFRcysC 60-75 compared with participants with an eGFRcysC >75 mL/min per 1.73 m2, but evidence for a higher mean LV mass at a eGFRcysC <60 was absent (Table 4).
Table 4.
Adjusted LV mass index (g/m2 body surface area) by categories of cystatin C-based estimated glomerular filtration rate (eGFRcysC) in 4,536 MESA participants with a creatinine-based eGFR > 60 ml/min/1.73 m2
| Demographic Adjusted* | Adjusted for demographic variables, smoking, and diabetes† |
Adjusted for demographic variables, smoking, diabetes, and hypertension‡ |
|||||
|---|---|---|---|---|---|---|---|
| Number | Mean LV mass index (g/m2) (95% CI) |
P value | Mean LV mass index (g/m2) (95% CI) |
P value | Mean LV mass index (g/m2) (95% CI) |
P value | |
| Cystatin C eGFR∥ | |||||||
| > 75 | 3874 | 77.6 (77.2, 78.1) | Ref | 77.6 (77.2, 78.1) | Ref | 77.7 (77.3, 78.1) | Ref |
| 60-75 | 587 | 80.1 (78.9, 81.3) | <0.001 | 80.0 (78.8, 81.1) | 0.001 | 79.6 (78.4, 80.7) | 0.009 |
| <60 | 75 | 78.8 (75.6, 82.0) | 0.8 | 78.5 (75.2, 81.7) | 0.9 | 77.8 (74.7, 80.9) | 0.9 |
| Cystatin C eGFR | |||||||
| > 75 | 3874 | 77.6 (77.2, 78.1) | Ref | 77.6 (77.2, 78.1) | Ref | 77.7 (77.3, 78.1) | Ref |
| <=75 | 662 | 79.9 (78.8, 81.1) | <0.001 | 79.8 (78.7, 80.9) | <0.001 | 79.4 (78.3, 80.5) | 0.005 |
adjusted for age, sex, race/ethnicity, and MESA site
diabetes defined as fasting serum glucose ≥ 126 mg/dL (7 mmol/l) or taking insulin or oral diabetes medications
hypertension defined as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg, or taking antihypertensive medications
pairwise comparisons using the highest eGFR category as the reference group (i.e. highest with middle, highest with lowest). Sidak test used.
eGFR = glomerular filtration rate estimated using cystatin C(26)
Discussion
In this study of men and women from a population-based multi-ethnic cohort with predominantly normal or mildly impaired kidney function, we found a non-linear association of cystatin C with LV mass. The association between eGFRcysC concentration and prevalence of LVH was apparent only for participants with an eGFRcysC ≤ 75 mL/min per 1.73 m2, even after excluding participants with more advanced kidney dysfunction (creatinine-based eGFR <60 mL/min per 1.73 m2).
LVH is a predictor of increased risk for cardiovascular events in both patients with end stage renal disease and chronic kidney disease.(7-9, 11, 12) Past studies have established that increased LV mass and LVH are highly prevalent in end-stage renal disease patients at the initiation of dialysis,(6) and that untreated LVH progresses in patients undergoing dialysis.(8) A number of studies of patients with chronic kidney disease have demonstrated a progressive increase in LV mass and in the prevalence of LVH with declining estimated GFR,(14, 16) but few participants in these studies had a creatinine-based eGFR ≥ 60 ml/min/1.73 m2.
Cystatin C is superior to creatinine-based measures in detecting risk of death and cardiovascular disease events in elderly subjects with mild kidney dysfunction.(22) Only 9% of the middle-aged and elderly participants from the MESA cohort included in this study had an creatinine-based eGFR < 60 ml/min/1.73 m2, but a substantial proportion (36%) had a cystatin C concentration ≥ 1.0 mg/L (eGFRcysC ≤ 76.7 mL/min per 1.73 m2), a level associated in past studies as the threshold for independent risk for cardiovascular events.(22, 24, 25) In this analysis of participants with no prior diagnosis of cardiovascular disease, eGFRcysC was only associated with LVH at the lowest two deciles of eGFRcysC (<75 ml/min/1.73 m2). Even after excluding patients with a creatinine-based eGFR <60 ml/min/1.73 m2, there was a dose-response relationship between each lower category of eGFRcysC and odds of LVH, but only at an eGFRcysC ≤ 75 mL/min per 1.73 m2. Past studies linking a cystatin C concentration ≥ 1.0 mg/L (eGFRcysC ≤ 76.7 mL/min per 1.73 m2) with risk for cardiovascular events found that cystatin C had a stronger association with risk for cardiovascular death and heart failure than it did with myocardial infarction or stroke, events more primarily linked with atherosclerosis.(22, 25) Indeed, past cross-sectional studies have found only modest associations between mild-to-moderate kidney dysfunction, measured with cystatin C, and subclinical markers of atherosclerosis such as valvular calcification(36) and carotid intima-medial thickness.(37)
Our results are also cross-sectional, but suggest that LVH could be one of the mediators of the association between cystatin C and risk for heart failure and fatal cardiovascular events. If so, increased LV mass may only partially mediate the association between cystatin C and increased cardiovascular risk. Adjusted difference in mean LV mass index between the >75 and ≤ 75 mL/min per 1.73 m2 eGFRcysC was only 2.2 g/m2, only a portion of the 13 g/m2 standard deviation in LV mass index in the MESA population without LVH,(38) and past studies have linked increased cardiovascular risk to full standard deviation higher differences in LV mass index.(3)
Two main determinants of an increased prevalence of LVH in patients with chronic kidney disease are hypertension and anemia.(15, 39) The prevalence of hypertension was high in participants with the highest quintile of cystatin C (63%). After adjusting for hypertension, the association between eGFRcysC ≤ 75 mL/min per 1.73 m2 and LVH was attenuated, indicating that hypertension may at least partially determine the association between cystatin C and LVH. It is possible that we did not measure the full impact of hypertension burden as a mediating factor because we assigned the diagnosis of hypertension based on the use of antihypertensive medications or an elevated blood pressure at a single office visit, leading to a potential underestimation of the burden of hypertension in the lowest deciles of eGFRcysC. Additionally, antihypertensive medications are known to reduce LV mass, possibly diminishing the association of hypertension with LVH. However, we found similar results from models which directly adjusted for use of antihypertensive medications or mean blood pressure in mm Hg. Anemia is a potent determinant of LVH in patients with chronic kidney disease,(15) though the prevalence of anemia only noticeably increases at a creatinine-based eGFR < 60ml/min/1.73 m2.(40, 41) Anemia may at least partly explain the association between cystatin C and LVH at the lowest two deciles of eGFRcysC, in which most participants had mild or moderate kidney dysfunction, and 40% had an creatinine eGFR < 60ml/min/1.73 m2. MESA did not measure hemoglobin levels, however, so we were unable to evaluate the impact of anemia on the association between cystatin C and LVH.
The strength of this study is that it is the first to evaluate the association of cystatin C with LV mass determined by MRI in a multi-ethnic cohort of relatively healthy participants without diagnosed clinical cardiovascular disease. Our study has several limitations. Few participants with advanced chronic kidney disease or end-stage renal disease were included MESA, and the participants included in this analysis differed from excluded participants regarding age, race/ethnicity, and several cardiovascular disease risk factors. We were therefore not able to describe the full spectrum of the association between kidney dysfunction and LV mass. However, the primary objective of this study was to characterize the association of cystatin C with LV mass in persons with normal or mildly impaired kidney function. With this in mind, we restricted the study population to participants with a creatinine-based eGFR ≥ 60 mL/min per 1.73 m2 in our main analysis. Though we adjusted for age, demographic characteristics, and cardiovascular disease risk factors, we cannot rule out the possibility that unmeasured confounders account for the associations we observed. Conversely, kidney disease may lead to elevated blood pressure, so adjustment for hypertension may lead to over adjustment and an underestimation of the association of cystatin C with LVH. Because of this possibility, we reported models with and without adjustment for hypertension. This analysis was cross-sectional, so we cannot assume a causal association between the highest quintile of cystatin C and increased LVH until follow up left ventricular mass measurements are available from MESA. MESA did not directly measure GFR, so we cannot be certain that the association of elevated cystatin C with LVH is solely due to its approximation of impaired GFR. The formula used to estimate GFR from cystatin C concentration was derived from and validated in chronic kidney disease cohorts with predominantly advance kidney disease, and may be imprecise in estimating GFR ≥ 60 ml/min/1.73 m2. However, the cystatin C estimating formula has been shown to be accurate in the range of a measured GFR ≤ 60 ml/min/1.73 m2.(26) The generalizability of GFR estimating equations based on cystatin C or creatinine will be improved with validation in cohorts representing the full range of GFR ≥ 60 ml/min/1.73 m2.
In summary, we found that cystatin C concentration was not linearly associated with increased LV mass, but only mild-to-moderate kidney dysfunction (eGFRcysC ≤ 75 mL/min per 1.73 m2) was independently associated with LVH. Because a cystatin C above a similar threshold (≥ 1.0 mg/L, or eGFRcysC ≤ 76.7 mL/min per 1.73 m2) predicts an increased and independent risk for cardiovascular events(22), our results favor the hypothesis that LVH is a mediator between cystatin C and cardiovascular disease risk.
Acknowledgement and funding source
This research was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95166 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. Dade Behring Inc. donated to MESA and the NHLBI the reagents used to measure cystatin C.
Abbreviations
- LV
left ventricle
- LVH
left ventricular hypertrophy
- MRI
magnetic resonance imaging
- eGFR
estimated glomerular filtration rate
- eGFRcysC
glomerular filtration rate estimated using serum cystatin C concentration
- MESA
Multi-Ethnic Study of Atherosclerosis
- HCFA
Health Care Finance Administration
- NHLBI
National Heart, Lung, and Blood Institute
Footnotes
Conflicts of interest: The authors have no conflicts of interest to report.
Clinical Trials Registration: MESA is registered with the U.S. National Library of Medicine (www.clinicaltrials.gov), registration number NCT00005487.
References
- 1.Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study) Am J Cardiol. 2001;87:1051–1057. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- 2.Verdecchia P, Porcellati C, Reboldi G, Gattobigio R, Borgioni C, Pearson TA, Ambrosio G. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation. 2001;104:2039–2044. doi: 10.1161/hc4201.097944. [DOI] [PubMed] [Google Scholar]
- 3.Verdecchia P, Carini G, Circo A, Dovellini E, Giovannini E, Lombardo M, Solinas P, Gorini M, Maggioni AP. Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol. 2001;38:1829–1835. doi: 10.1016/s0735-1097(01)01663-1. [DOI] [PubMed] [Google Scholar]
- 4.Liao Y, Cooper RS, Durazo-Arvizu R, Mensah GA, Ghali JK. Prediction of mortality risk by different methods of indexation for left ventricular mass. J Am Coll Cardiol. 1997;29:641–647. doi: 10.1016/s0735-1097(96)00552-9. [DOI] [PubMed] [Google Scholar]
- 5.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 6.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 7.Stack AG, Saran R. Clinical correlates and mortality impact of left ventricular hypertrophy among new ESRD patients in the United States. Am J Kidney Dis. 2002;40:1202–1210. doi: 10.1053/ajkd.2002.36881. [DOI] [PubMed] [Google Scholar]
- 8.Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE. Long-term evolution of cardiomyopathy in dialysis patients. Kidney Int. 1998;54:1720–1725. doi: 10.1046/j.1523-1755.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- 9.Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36:286–290. doi: 10.1038/ki.1989.192. [DOI] [PubMed] [Google Scholar]
- 10.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 11.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. Jama. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 12.Weiner DE, Tighiouart H, Vlagopoulos PT, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16:1803–1810. doi: 10.1681/ASN.2004070597. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 14.Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis. 1996;27:347–354. doi: 10.1016/s0272-6386(96)90357-1. [DOI] [PubMed] [Google Scholar]
- 15.Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125–134. doi: 10.1016/s0272-6386(99)70118-6. [DOI] [PubMed] [Google Scholar]
- 16.Stewart GA, Gansevoort RT, Mark PB, Rooney E, McDonagh TA, Dargie HJ, Stuart R, Rodger C, Jardine AG. Electrocardiographic abnormalities and uremic cardiomyopathy. Kidney Int. 2005;67:217–226. doi: 10.1111/j.1523-1755.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 17.Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis. 2005;46:320–327. doi: 10.1053/j.ajkd.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 18.Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995;8:221–228. doi: 10.1016/0895-7061(94)00178-E. [DOI] [PubMed] [Google Scholar]
- 19.Cranney GB, Lotan CS, Dean L, Baxley W, Bouchard A, Pohost GM. Left ventricular volume measurement using cardiac axis nuclear magnetic resonance imaging. Validation by calibrated ventricular angiography. Circulation. 1990;82:154–163. doi: 10.1161/01.cir.82.1.154. [DOI] [PubMed] [Google Scholar]
- 20.Coll E, Botey A, Alvarez L, Poch E, Quinto L, Saurina A, Vera M, Piera C, Darnell A. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 21.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37:79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 22.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 23.Shlipak MG, Katz R, Fried LF, Jenny NS, Stehman-Breen CO, Newman AB, Siscovick D, Psaty BM, Sarnak MJ. Cystatin-C and mortality in elderly persons with heart failure. J Am Coll Cardiol. 2005;45:268–271. doi: 10.1016/j.jacc.2004.09.061. [DOI] [PubMed] [Google Scholar]
- 24.Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 25.Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 26.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr., Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 28.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 29.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 30.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 32.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 33.Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol. 1987;59:956–960. doi: 10.1016/0002-9149(87)91133-7. [DOI] [PubMed] [Google Scholar]
- 34.Salton CJ, Chuang ML, O'Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, Edelman RR, Levy D, Manning WJ. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol. 2002;39:1055–1060. doi: 10.1016/s0735-1097(02)01712-6. [DOI] [PubMed] [Google Scholar]
- 35.Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch-Herold M, Sinha S, Kronmal R, Arnett D, Crouse JR, 3rd, Heckbert SR, Bluemke DA, Lima JA. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2005;112:984–991. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 36.Ix JH, Shlipak MG, Katz R, Budoff MJ, Shavelle DM, Probstfield JL, Takasu J, Detrano R, O'Brien KD. Kidney function and aortic valve and mitral annular calcification in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2007;50:412–420. doi: 10.1053/j.ajkd.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Rodondi N, Yerly P, Gabriel A, Riesen WF, Burnier M, Paccaud F, Bovet P. Microalbuminuria, but not cystatin C, is associated with carotid atherosclerosis in middle-aged adults. Nephrol Dial Transplant. 2007;22:1107–1114. doi: 10.1093/ndt/gfl733. [DOI] [PubMed] [Google Scholar]
- 38.Edvardsen T, Rosen BD, Pan L, Jerosch-Herold M, Lai S, Hundley WG, Sinha S, Kronmal RA, Bluemke DA, Lima JA. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging--the Multi-Ethnic Study of Atherosclerosis (MESA) American heart journal. 2006;151:109–114. doi: 10.1016/j.ahj.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 39.Sarnak MJ. Cardiovascular complications in chronic kidney disease. Am J Kidney Dis. 2003;41:11–17. doi: 10.1016/s0272-6386(03)00372-x. [DOI] [PubMed] [Google Scholar]
- 40.Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988-1994) Arch Intern Med. 2002;162:1401–1408. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 41.Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504–510. doi: 10.1681/ASN.V132504. [DOI] [PubMed] [Google Scholar]