Abstract
Transcription factors belonging to the Per/Arnt/Sim (PAS) domain family are highly conserved and many are involved in circadian rhythm regulation. One member of this family, aryl hydrocarbon receptor (AhR), is an orphan receptor whose physiological role is unknown. Recent findings have led to the hypothesis that AhR has a role in circadian rhythm, which is the focus of the present investigation. First, time-of-day dependent mRNA expression of AhR and its signaling target, cytochrome p4501A1 (Cyp1a1) was determined in C57BL/6J mice by quantitative RT-PCR. Circadian expression of AhR and Cyp1a1 was observed both in the suprachiasmatic nucleus (SCN) and liver. Next, the circadian phenotype of mice lacking AhR (AhRKO) was investigated using behavioral monitoring. Intact AhRKO mice had robust circadian rhythmicity with a similar tau under constant conditions compared to wild-type mice, but a significant difference in tau was observed between genotypes in ovariectomized female mice. Time to re-entrainment following 6-h advances or delays of the light/dark cycle was not significantly different between genotypes. However, mice exposed to the AhR agonist 2,3,7,8-tetracholorodibenzo-p-dioxin (TCDD, 1 μg/kg BW) displayed decreased phase shifts in response to light and had altered expression of Per1 and Bmal1. These results suggest that chronic activation of AhR may affect the ability of the circadian timekeeping system to adjust to alterations in environmental lighting by affecting canonical clock genes. Further studies are necessary to decipher the mechanism of how AhR agonists could disrupt light-induced phase shifts. If AhR does have a role in circadian rhythm, it may share redundant roles with other PAS domain proteins and/or the role of AhR may not be exhibited in the behavioral activity rhythm, but could be important elsewhere in the peripheral circadian system.
Keywords: dioxin toxicity, bHLH-PAS, light entrainment, period, drinking, wheel-running, mice
INTRODUCTION
Many proteins involved in the clockwork mechanism, including CLOCK (circadian locomotor output cycles kaput), BMAL1 (brain and muscle, ARNT-like) and NPAS2 (neuronal PAS protein 2), belong to the basic helix-loop-helix/Per-ARNT-SIM (bHLH/PAS) domain family. Another bHLH/PAS domain protein, aryl hydrocarbon receptor (AhR), has been extensively studied for its role in mediating the toxicity of environmental contaminants, such as the halogenated aromatic hydrocarbons. Among them is the prototypical dioxin, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin).
TCDD is formed as a by-product of combustion processes, during chlorine bleaching of paper-products and in the production of pesticides and herbicides. Human and animal exposure occurs at low levels primarily through the consumption of contaminated food such as fish, meat and dairy products (Travis and Hattemer-Frey 1991). Due to the lipophilicity of TCDD and its slow rate of metabolism, it bioaccumulates in animals and humans and biomagnifies in the environment. It is not readily metabolized in vivo and its half-life can be as long as several to more than 10 yrs in humans (Aylward et al. 2005). Toxic effects of TCDD include teratogenesis, immune impairment, carcinogenesis, and developmental and reproductive dysfunction (Birnbaum 1994; Pohjanvirta and Tuomisto 1994).
AhR activation by TCDD leads to nuclear translocation of the TCDD bound AhR followed by its dimerization with another PAS domain-dependent transcription factor, the aryl hydrocarbon receptor nuclear translocator (ARNT). The AhR:ARNT heterodimer binds to xenobiotic response elements (XREs; also known as dioxin response elements, DREs) to induce a battery of AhR responsive genes including those that are involved in xenobiotic metabolism (Nebert et al. 1993). Endogenous ligands for AhR have been sought for decades (Bjeldanes et al. 1991; McMillan and Bradfield 2007; Rannug et al. 1995; Song et al. 2002). However, they remain elusive and thus, the physiological role of AhR remains unknown.
AhR sequences have been identified in early vertebrates and possible homologs have been suggested even in C. elegans (Hahn et al. 1997). Mice lacking AhR show abnormal liver development, ductus venosus retention, immune system impairment, and abnormal ovarian follicular development (Benedict et al. 2000; Fernandez-Salguero et al. 1995; Lahvis et al. 2005; Schmidt et al. 1996). The evolutionary conservation of AhR and abnormal phenotype of AhR null mice strongly suggests the importance of AhR in normal physiological function. Recently, 55 tryptophan photoproducts have been suggested as potential endogenous AhR ligands, and AhR may have a role in the regulation of biological rhythms (Rannug and Fritsche 2006). The trypotophan photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) blocks glutamate-induced phase shifts in SCN slices (Mukai and Tischkau 2007), lending credence to this hypothesis.
Daily rhythms of AhR protein expression have been reported in liver, lung and thymus (Richardson et al. 1998). In addition, diurnal expression of AhR mRNA, as well as its downstream target gene, cytochrome P4501A1 (Cyp1a1), occurs in liver and pituitary (Huang et al. 2002). Thus, AhR related genes exhibit a circadian rhythm, similar to many other ‘clock’ genes. However, previous studies were done only under light/darkness (LD) conditions. We determined expression patterns of these genes both under LD and constant darkness (DD) conditions to reveal their endogenous rhythms in SCN and liver. We also examined circadian behavior in AhR null (AhRKO) mice. Finally, we tested the effect of TCDD on light-regulation of circadian rhythm. Our results demonstrate that AhR and its signaling targets maintain rhythmicity under DD. AhRKO mice overall have robust rhythmicity under LD, DD and LL conditions and adjust well to jetlag-like light entrainment shifts. However, exposure to TCDD attenuates light-induced phase shifts, suggesting interactions between activation of AhR signaling and the circadian system.
MATERIALS AND METHODS
Animals
Unless noted otherwise, 6-week-old wild-type male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were used. For activity measurements in AhRKO, founding breeders on a C57BL/6J background (Schmidt et al. 1996) were bred at the University of Illinois, Urbana-Champaign. Genotypes of pups resulting from AhR+/− male/female crosses were determined by PCR (Benedict et al. 2000). WT siblings were used as controls in these experiments. Animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Illinois and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Tissue Collection
Male C57BL/6J mice were housed 3–4 per cage in a light-tight chamber, provided with feed and water ad libitum, and entrained for at least 2 weeks under controlled lighting (12L:12D), temperature (22°C), and humidity (39%). Fluorescent light bulbs (13W, company, Sylvania, Danvers, MA) generated 400–500 lux of light to the cages. All other experiments described herein were done under these environmental conditions. Animals were decapitated; liver and SCN were collected at 4 h intervals starting at ZT0 (zeitgeber time, lights on at ZT0, lights off at ZT12). For DD collection, animals were placed in darkness for 48 h prior to collection at 4 h intervals. Circadian time (CT) was reckoned from the previous light/dark cycle. SCN were carefully punched from a 500 μm brain slice using a 1 mm diameter sample corer (Fine Science Tools, Foster City, CA). Tissues were snap-frozen and stored at −80°C until use.
For investigating clock gene expression in AhRKO and in TCDD-exposed mice, two time-points, CT8 and CT20, were chosen based on the time when clear out of phase-relationship of Per1 and Bmal1 was observed under DD conditions. In this experiment, after 2 weeks of entrainment under LD, the animals were orally dosed with 1 μg/kg BW of TCDD or vehicle (corn oil) during their lights-on period and released into DD 6 days after treatment. Both liver and SCN were collected on the third day in DD.
Real-time Quantitative PCR
Total RNA was isolated using TRIzol (GIBCO BRL, MD) for liver and RNAqueous (Ambion, Austin, TX) for SCN, and qPCR was performed as previously described (Mukai and Tischkau 2007). Primers were designed across two exons to inhibit amplification of genomic DNA using Primer Express 3 (PE Applied Biosystems, UK). Specific primer sequences used were: forward 5′-TTCTTAGGCTCAGCGTCAGCTA-3′ and reverse 3′-GCAAATCCTGCCAGTCTCTGAT-3′ for AhR; forward 5′-CTACAAGCCAACATTTCTATCAGATGA-3′ and reverse 5′-GGTCACATCCTACGACAAACAAAA-3′ for Bmal1; and as previously published (Mukai and Tischkau 2007) for Cyp1a1 and Per1. The Ct (threshold cycle) value was obtained, and relative RNA amount was calculated using the relative standard curve method (Applied Biosystems User Bulletin 2). Serial dilutions of pooled RNA, representative of all times of day, were used to generate standard curves. Linear regression analysis produced an equation for determining doubling efficiency and expression of each transcript. Internal standards were not used because many ‘housekeeping-genes’ exhibit circadian variation.
Activity Recording
WT and AhRKO mice were housed individually in 30 × 19 × 12.5 cm cages (Ancare, Bellmore, NY) equipped with a water bottle attached to a lick sensor (Mini Mitter, Bend, OR). Some females were ovariectomized two weeks before initiation of activity recording to remove the effect of endogenous estrogens on circadian rhythm. The initial intent of ovariectomy was to allow pooling of data from males and females without confounding effects of estrous cyclicity. Other females and all males remained intact. Lick counts were recorded in 1-min bins using VitalView (Mini Mitter, Bend, OR). After entrainment to 12L:12D schedule, animals were 125 moved into DD for 18–21 days and subsequently to LL for 14 days to measure the effect of lighting changes on their circadian period (tau).
For 6-h shift experiments, animals were provided with 4.5-inch diameter activity wheels. Wheel revolutions were recorded upon the circuit closure of hermetically sealed reed switch (Hermetic Switch, Inc., Chickasha, OK), by a neodymium magnet (Magnetic Energies, Inc.; San Antonio, TX) attached to each wheel (Tischkau et al. 2003). After entrainment to a 12L:12D schedule, the light schedule was advanced by 6 h. After 14 days, the cycle was delayed by 6 h. For data analysis, lick and wheel-running counts in 1-min bins were recalculated into 10-min bins by ClockLab (Actimetrics, Evanston, IL). Tau was calculated from activity onset of the last 10 days in DD and LL. Number of days to re-entrainment to a new light schedule was estimated by visual analysis of the actograms. Advance shifts were assessed by activity onset; activity offset was used for delay shifts to account for masking by light.
TCDD and Light Exposure
After entrainment in LD, animals were orally dosed with 1 μg/kg BW of TCDD or vehicle (corn oil) during their lights-on period. They were subsequently placed into DD for 8 days; light pulse experiments were performed to measure phase-resetting after light exposure. Each animal was exposed to light (400 lux) for 30 min starting at CT16, as determined by adding a factor of 24/tau to the predicted onset of activity. Tau was calculated from the slope of a regression line drawn through the onset of activity 5 days prior to the treatment. Light exposure occurred in a separate lit chamber; wheel-running activity data were not collected during the treatment. After 30 min, the cage was returned to DD and collection of activity data was reinitiated. Tau after light treatment was calculated using the activity onset 4–8 days after light exposure.
Statistics
Statistical analysis was performed by one way ANOVA followed by Tukey’s test for pairwise comparisons or by Student’s t-test using SYSTAT (SSI, Richmond, CA). The p values were two-sided and considered statistically significant at p<0.05. All data are shown as mean ± SEM.
RESULTS
Circadian expression of AhR and Cyp1a1 in SCN and in liver
To characterize daily expression patterns of AhR and one of its signaling target genes, we measured mRNA levels for AhR and Cyp1a1 by real-time RT-PCR. Expression of Per1 and Bmal1 served as positive controls. Fig. 1 is double-plotted at CT(ZT)0 and CT(ZT)20 for easier visualization of rhythms. Results show a 24 h oscillation for AhR under both LD and DD, although they were less robust compared to Bmal1 and Per1.
Fig. 1. Circadian mRNA levels of AhR- and clock-related genes in C57BL6/J SCN and liver.
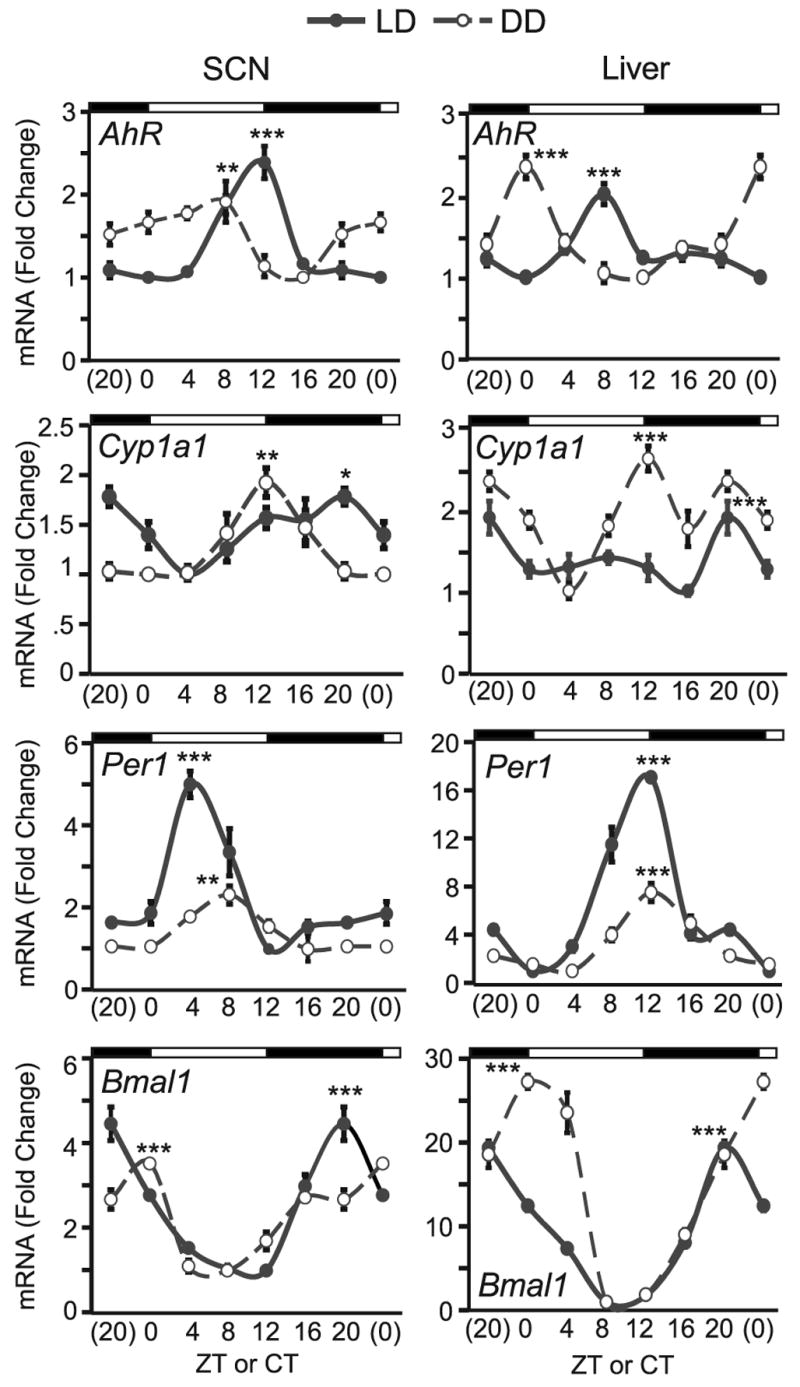
Samples were collected every 4 hours under light/darkness (L12:D12, solid line) and constant darkness (DD, dotted line) schedules. Real time RT-PCR was used to measure AhR, Cyp1a1 and Per1, Bmal1 (top to bottom). Results are shown as mean fold-change to lowest (trough) level ±SEM in SCN (left panel) and liver (right panel). Note that data at CT(ZT)0 and CT(ZT)20 are double plotted simply for easier visualization of the rhythm. The DD data was obtained from mice entrained under same lighting schedule as LD and released into DD for 48 hrs. n=3–4/group, * p<0.05, ** p<0.01, and *** p<0.001 by ANOVA in peak vs. trough expression comparison.
In SCN (Fig. 1, left panel), AhR displayed a peak at ZT12 (2.39 ± 0.2 fold vs. trough expression at ZT0, p<0.0001) under LD. After 48 h in DD, AhR expression peaked 4 h earlier at CT8 (1.91 ± 0.3 fold vs. trough at CT16, p<0.01). Levels of Cyp1a1 were weakly rhythmic in the SCN. Peak expression of Cyp1a1 was delayed compared to AhR; levels were highest at ZT20 (1.78 ± 0.1 fold vs. trough at ZT4, p=0.01) in LD and at CT12 (1.91 ± 0.1 fold vs. trough at CT0, p<0.01) in DD. Per1 levels peaked at ZT4 (5.00 ± 0.3 fold vs. trough at ZT12, p<0.0001) in LD and at CT8 (2.31 ± 0.2 fold vs. trough at CT16, p<0.01) in DD. Bmal1 was characteristically out-of-phase with Per1, peaking at ZT20 (4.46 ± 0.4 fold vs. trough at ZT12, p<0.0001) in LD and at CT0 (3.52 ± 0.1 fold vs. trough at CT8, p<0.0001) in DD.
In liver (Fig. 1, right panel), AhR peaked at ZT8 (2.02 ± 0.1 fold vs. trough at ZT0, p<0.0001) under LD; Cyp1a1 peaked at ZT20 (1.87 ± 0.2 fold vs. trough at ZT16, p<0.001), Per1 peaked at ZT12 (16.95 ± 0.2 fold vs. trough at ZT0, p<0.0001) and Bmal1 peaked at ZT20 (19.32 ± 0.5 fold vs. trough at ZT8, p<0.0001). Expression patterns were different under DD conditions. Under DD, AhR peaked 8 h earlier at CT0 (2.35 ± 0.04 fold vs. trough at CT12, p<0.0001). The Cyp1a1 oscillation had a higher amplitude in DD than in LD, with a peak at CT12 (2.58 ± 0.1 fold vs. trough at CT4, p<0.0001). Per1 had a reduced amplitude compared to LD but still peaked at CT12 (7.46 ± 0.8 fold vs. trough at CT4, p<0.0001) and Bmal1 peaked at CT0 (27.16 ± 0.8 fold vs. trough at CT8, p<0.0001) under DD.
Circadian activity rhythm of AhRKO mice
There were no apparent abnormalities in the circadian rhythm of AhRKO mice (Fig. 2). The circadian period of AhRKO males was not significantly different from that of WT males under both DD and LL conditions (Fig. 2G). Some females were ovariectomized to remove the effect of endogenous estrogens on circadian rhythmicity in order to pool the data with intact males. However, differences between these two groups were observed, therefore the data are shown separately. Data from intact females were also added as a comparison.
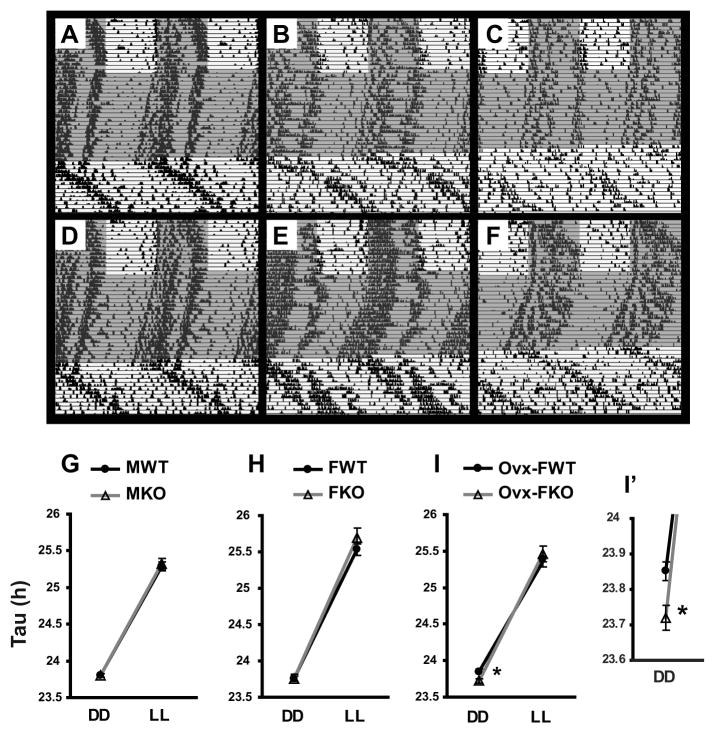
Fig. 2. Double-plotted actogram and circadian periods of drinking activity in wild-type (WT) and AhR knockout (AhRKO) mice under constant light conditions.
Mice were entrained to 12L:12D schedule for 2 weeks, then placed in constant darkness (DD, gray background) for about 3 weeks and then placed constant light (LL, white background) for 2 weeks. Representative actogram of intact male (A,D), intact female (B,E), and ovariectomized female (C, F); WT (A,B,C) and AhRKO (D,E,F). Tau values of intact male (G), intact female (H), and ovariectomized female (I) under constant dark (DD) and constant light (LL). (I′) Higher magnification of (I) at DD. n=5–11/group, * p<0.05 by ANOVA.
Drinking activity of intact females showed estrous cycle-dependent changes (Fig. 2B&E). Changes of activity rhythms caused by estrous cycle have been previously reported in various rodent species (Fitzgerald and Zucker 1976; Gerall et al. 1973; Morin et al. 1977). In this study, some mice showed clear estrous cycle-dependent changes (5 out of 7 and 4 out of 7 in WT and AhRKO, respectively). Of those that showed cyclicity, WT females had a cycle of 3–4 days whereas AhRKO had longer cycles of about 5–6 days. However, the circadian period under DD and LL was not significantly different between intact WT and AhRKO females (Fig. 2H).
The only group that showed significant difference (p<0.05) in circadian period between WT and AhRKO was ovariectomized females (Fig. 2I). AhRKO mice in this group had shorter tau (23.72 ± 0.03h) compared to WTs (23.85 ± 0.03 h). Tau under LL was similar (25.35 ±0.06 and 25.46 ± 0.11 h, WT and AhRKO, respectively). Overall drinking activity appeared to be reduced in ovariectomized (Fig. 2C) compared to intact females (Fig. 2B), or males (Fig. 2A) as indicated on the actogram.
Light entrainment shifts of AhRKO mice
To determine whether mice lacking AhR can re-entrain to new light conditions, animals were subjected to 6-h advance and delay shifts (Fig. 3). Wheel-running activity was used to measure days until re-entrainment after 6-h shifts. This change was made because significant drinking during the light phase in the previous study made analysis of activity onset difficult and forced the removal of some samples. We also wanted to determine whether activity differences could be observed using a different behavior. Clear differences between drinking (Fig. 2A&D) and wheel-running activity (Fig. 3A) are present in the actograms; however, no significant differences in wheel-running activity were observed between genotypes.
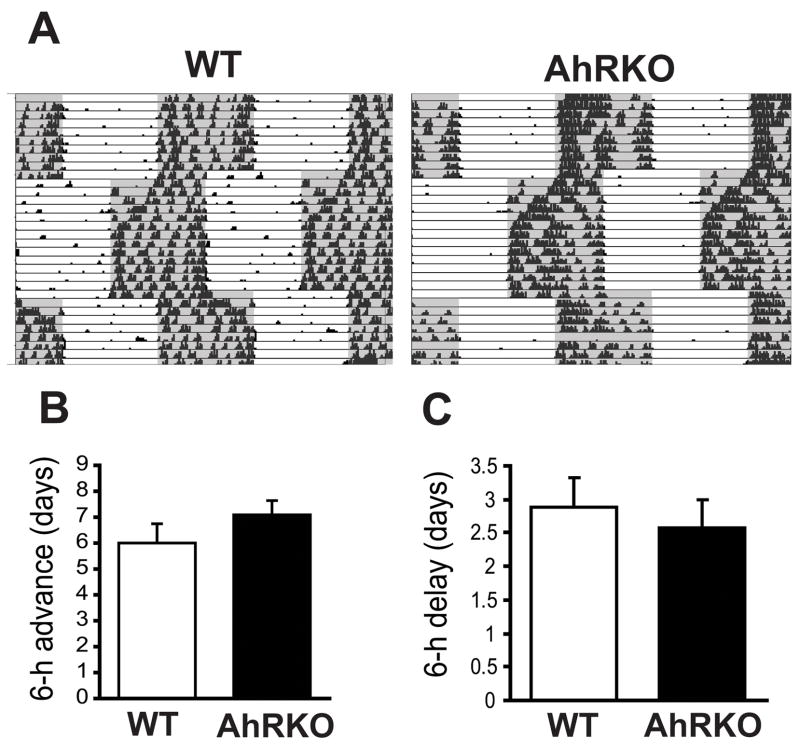
Fig. 3. Re-entrainment of wild-type and AhR knockout (AhRKO) mice to 6-h jetlag shifts.
(A) Double plotted actogram measuring wheel-running activity during 6-h shifts. Days it took to re-entrain to new light schedule after 6-h advance shift (B) and delay shift (C). n=10–11/group
The time needed for mice to entrain to a 6-h advance shift varied from 2–10 days. There were no significant differences between WT (6.00 ± 0.75 days) and AhRKO mice (7.09 ± 0.55 days; Fig. 3B), although 3 out of 10 WT re-entrained as fast as 2–4 days, whereas no AhRKOs (n=11) re-entrained that quickly. Activity offset was used to measure days to re-entrainment to delay shifts to avoid the effect of masking by light. There was no significant difference between the genotypes (2.87 ± 0.44 and 2.58 ± 0.42 days; WT and AhRKO, respectively; Fig. 3C).
Effect of TCDD exposure on light-pulse induced phase shifts
To study whether exposure to dioxins affects the response to nocturnal light exposure, circadian period and phase shifting was assessed in TCDD- and vehicle-treated mice. Rhythms in TCDD-treated mice under DD were as robust as in vehicle-treated mice (Fig. 4A). Tau was not altered by TCDD treatment before light exposure (23.70 ± 0.04 and 23.71 ±0.05 h, vehicle-and TCDD-treated) or after light exposure (23.68 ± 0.04 and 23.72 ± 0.04 h, vehicle- and TCDD-treated) (Fig. 4B). However, the amplitude of phase delay after light exposure was significantly reduced in TCDD-treated (−1.69 ± 0.09 h) compared to vehicle-treated mice (−2.20 ±0.16 h, p<0.01; Fig. 4C).
Fig. 4. Effect of TCDD on light-induced phase shifts.
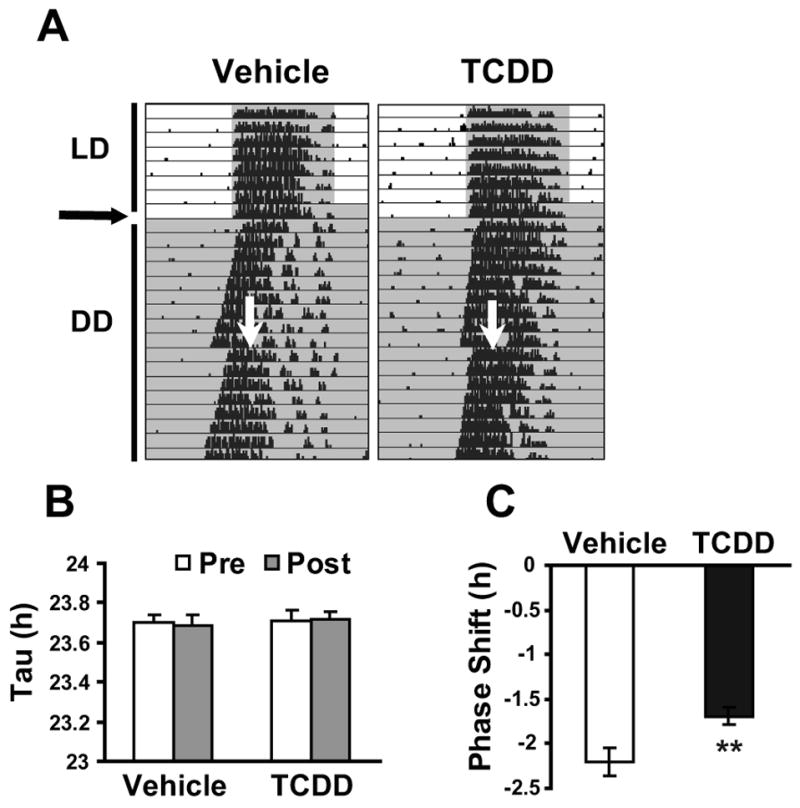
(A) Representative actogram of wheel-running activity. Mice were entrained in 12L:12D schedule and then 1μg/kg BW of TCDD or vehicle was orally exposed at horizontal arrow. Subsequently animals were placed under DD and given a 30-min light pulse at vertical arrow. (B) Circadian period pre- and post-light exposure at CT16–16.5 in vehicle and TCDD-treated mice. (C) Phase shift after light exposure at CT16–16.5 of vehicle and TCDD treated mice. n=9–10/group, **p<0.01 by t-test.
Per1 and Bmal1 expression in AhRKO and TCDD-treated mice
To determine whether deletion of AhR or TCDD exposure affect clock gene expression, we examined Per1 and Bmal1 levels in SCN and liver of AhRKO and TCDD-treated mice at two timepoints, selected based on relatively high and low levels of expression of these genes under DD as in Fig. 1. Per1 and Bmal1 levels were not changed in the SCN of AhRKO mice, compared to controls at both CT8 and CT20 (Fig. 5A&C). However, Per1 levels at CT8 were decreased 27% (p<0.05) in the SCN of TCDD- vs. vehicle-treated mice (Fig. 5A); TCDD had no effect on Per1 in the SCN at CT20. Bmal1 levels in the SCN were not changed at CT8 with TCDD treatment (Fig. 5C); at CT20 SCN levels of Bmal1 were increased 21% (p<0.05) in TCDD-treated mice.
Fig. 5. Expression of Per1 and Bmal1 mRNA in SCN and liver of AhRKO and TCDD-treated mice.
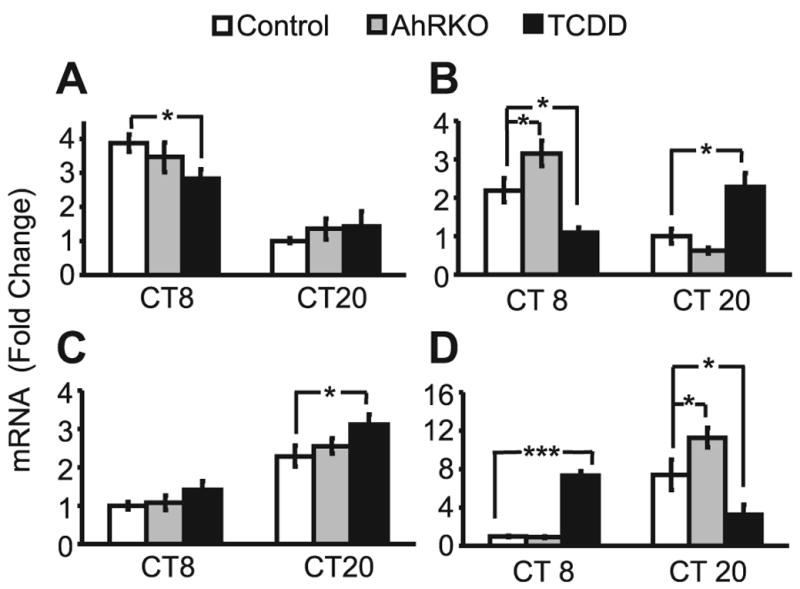
SCN and liver were collected at CT8 and CT20 during the third day in DD, from control (WT, vehicle), AhRKO (vehicle), and TCDD-treated mice. Real time RT-PCR was used to measure mRNA expression of Per1 (A,B) and Bmal1 (C, D) in SCN (A, C) and liver (B, D). Results are shown in mean fold-change compared to lowest control level ±SEM. n=4 per group. *p<0.05, **p<0.01 by ANOVA.
Effects of AhR deletion and TCDD treatment were more prominent in the liver (Fig. 5B, D). At CT8, levels of Per1 were increased 44% (p<0.05) in AhRKO vs. WT control, but substantially decreased (51% vs. control, p<0.01) in TCDD treated mice (Fig. 5B). At CT20, Per1 was not changed in AhRKO, but was increased 129% by TCDD exposure. Bmal1 levels were unchanged at CT8, but were increased 52% (p<0.05) in AhRKO at CT20, compared to control at respective timepoints (Fig. 5D). With TCDD treatment, Bmal1 levels were increased 633% (p<0.001) at CT8, but decreased by 56% (p<0.05) at CT20.
DISCUSSION
Previous studies indicate that AhR protein expression exhibits a similar oscillatory pattern in the liver, lungs, and thymus of rats (Richardson et al. 1998), with a peak approximately at ZT5 under LD. AhR mRNA (Huang et al. 2002) expression was also clearly rhythmic in rat liver, although the time of peak relative to the light schedule (14L:10D) was not reported. However, whether expression of AhR or its signaling targets are under control of the endogenous circadian oscillator has remained an open question. Whereas AhR and its dimerization partner ARNT are present in the SCN and other brain regions (Petersen et al. 2000), circadian variation in the SCN has not, to our knowledge, been reported. Our results demonstrate circadian variation of AhR transcripts in both SCN and liver under LD and DD, although the overall amplitude of oscillation was smaller than the clock genes, Per1 and Bmal1.
Peak mRNA expression of AhR in both SCN and liver under LD occurred at ZT12 and ZT8, respectively. Although apparently contradictory to a previous report (Richardson et al. 1998), because the protein expression peak in the liver in that report (ZT6) precedes the mRNA expression peak in the current study, differences may simply be related to species, rat versus mouse. Expression of the AhR target gene, Cyp1a1, was out-of-phase with AhR (4–8 h delay). Under DD, peak expression levels of both AhR and Cyp1a1 were advanced. Whether this reflects shortening of the circadian period under DD conditions, or direct effects of light on expression patterns of these genes, remains to be explored.
Interestingly, the amplitude of Cyp1a1 increased in DD compared to in LD, especially in liver. Although absolute values could not be compared between LD and DD because the PCR was performed at different times, it is possible that Cyp1a1 expression is increased during the day, resulting in higher basal expression (CT4 under DD). Increased trough expression could result in attenuated amplitude of oscillation. UV light exposure upregulates down-stream target genes of AhR not only in skin but also in liver (Goerz et al. 1996), providing a potential mechanism for increased baseline Cyp1a1 expression during the day. Additional experiments that examine AhR signaling in response to light exposure are required to test this hypothesis. Expression patterns of clock genes Per1 and Bmal1 are similar to previous reports (Karman and Tischkau 2006; Oishi et al. 1998).
AhRKO mice had no apparent abnormalities in behavioral circadian rhythmicity. Day/night differences in Per1 and Bmal1 transcripts followed the same pattern in AhRKO mice compared to controls. AhRKO mice re-entrained to a new light schedule similar to wild-type controls. However, in 6-h advance shift experiments, there was tendency for AhRKO mice to take longer to adjust to the new light schedule. An increased sample size may produce significant differences between genotypes. The only difference observed was the circadian period between AhRKOs and WTs of ovariectomized females. Although the explanation for this remains obscure, potential interactions between AhR and estrogen receptor (Klinge et al. 2000; Ohtake et al. 2003) may prove to be important. Estrogen replacement in ovariectomized females would help to clarify whether the changes observed are estrogen-dependent.
Robust behavioral rhythmicity in AhRKO mice, nevertheless, does not substantiate immediate rejection of the hypothesis that AhR has a role in circadian rhythm. Robust rhythmicity is observed in mice bearing single mutations of known clock genes (Dudley et al. 2003; van der Horst et al. 1999; Zheng et al. 2001). In fact, deletion of a single clock gene rarely causes complete disruption of rhythmicity (Dudley et al. 2003; van der Horst et al. 1999; Zheng et al. 2001), with the exception of severe disruption observed in Bmal1 knockouts (Bunger et al. 2000). Conversely, deletion of two clock genes often results in clear behavioral arrythmicity (Bae et al. 2001; Oster et al. 2002; van der Horst et al. 1999). Robust rhythmicity in Clock null mice (Debruyne et al. 2006) occurs because NPAS2 can functionally substitute for CLOCK (Debruyne et al. 2007). Similarly, a protein that shares redundancy with AhR may compensate for the complete absence of AhR. Therefore, it would be interesting to study the circadian phenotype of AhR mutant or AhRKO mice that have deletion of another clock gene such as Clock or Npas2.
TCDD affects circadian hormone rhythms (Jones et al. 1987; Pohjanvirta et al. 1989; Yellon et al. 2000) and feeding behaviors (Kelling et al. 1985; Seefeld et al. 1984). US Vietnam war veterans have sleep disorders that are dependent on TCDD exposure from the defoliant, Agent Orange (Liu et al. 2004). TCDD alters circadian expression of clock genes in murine hematopoietic stem and progenitor cells (Garrett and Gasiewicz 2006), in SCN of deer mice (Miller et al. 1999) and C57BL6 mice (Filipski et al. 2004). Oral TCDD exposure (10 μg/kg BW) has reportedly caused phase advances of behavior rhythm in deer mice (Miller et al. 1999), as well as behavioral splitting and arrythmicity in mice with a high dose of TCDD (Frame et al. 2004).
However, we did not observe these phase advancing or arrhythmic effects in our behavioral studies. C57BL/6J mice exposed to TCDD showed robust rhythmicity in DD for at least 3 weeks following exposure. Transient shifts of wheel-running activity immediately after TCDD exposure were not apparent. Because many previous reports are available only in abstract form (Frame et al. 2004; Li et al. 2004; Miller et al. 1999), it is difficult to identify the precise cause of these discrepancies, but differences in TCDD dose and species/strain of animal used may contribute.
TCDD treated mice showed a small but significant reduction in light-induced phase resetting at CT16. This result is consistent with the inhibition of glutamate-induced phase shifts in brain slices preincubated with the AhR agonist, 6-formylindolo[3,2-b]carbazole (FICZ) (Mukai and Tischkau 2007). Several factors may contribute to the difference between complete inhibition of the phase shift in vitro and partial inhibition of the phase shift in vivo by AhR agonists. First, it may be due to a reduced ability of TCDD to reach the SCN when administered systemically in vivo (Pohjanvirta et al. 1990). Although Cyp1a1 induction was observed after incubation of SCN 2.2 with FICZ, this was never observed in SCN in vivo with FICZ administered i.p. (Mukai and Tischkau 2007) or with TCDD administered p.o. (unpublished data; M. Mukai and S. Tischkau). Direct application of AhR agonists onto the SCN or into the third ventricle in vivo could be used to circumvent this problem. Second, the inhibitory effect of an AhR agonist on the phase shift at the level of the SCN firing rate rhythm may not translate efficiently to other mechanisms that could be involved in driving the wheel-running activity rhythm. Altered Per1 and Bmal1 mRNA expression at CT8 and CT20 with TCDD exposure suggest some changes in the clockworks. A phase-response-curve for light-induced phase shifts in TCDD treated animals will be necessary to determine whether these animals actually respond less to light overall, or whether their phase response curve is shifted, thus resulting in decreased response at CT16, the time-point selected in this study. The use of AhRKO mice is planned for future studies.
The mechanism that underlies the attenuation of the light response in TCDD-treated animals requires further investigation. However, immediate metabolism of endogenous ligands by the highly induced xenobiotic metabolic enzymes in certain peripheral tissues may also be important. Moreover, because AhR will be constantly occupied by TCDD, the cyclic pattern of AhR signaling, which is generated physiologically by binding of putative endogenous ligands, such as tryptophan photoproducts, will be disrupted. This may lead to an aberrant light signal.
It is also possible that activation of AhR by TCDD blocks light-induced phase shifts by interfering with induction of Per1 or Per2. Interrelationships between AhR signaling and the Per genes have been demonstrated; TCDD-induced AhR signaling is altered in the mammary glands of Per1ldc and Per1ldc/Per2ldc (Qu et al. 2007). The physiological significance of these interactions requires further investigation.
Recent discoveries of new PAS domain proteins and their roles in circadian rhythm raise questions regarding whether this ancient family of proteins has evolved as a central mediator of circadian timing among different organisms and tissues. For example, NPAS2, a peripheral replacement for CLOCK in some tissues, is important in the food-entrainable oscillator. Npas2 mouse mutants cannot adapt well to restricted feeding during daylight, leading ultimately to death (Dudley et al. 2003). AhR is expressed abundantly in various tissues, including the SCN (Petersen et al. 2000), other parts of the brain and the rest of the body (Abbott et al. 1995; Carver et al. 1994; Roman and Peterson 1998). It is possible that, like Npas2, AhR has evolved to have a specific role in the circadian control of peripheral clocks in a whole organismic system. In this study, both AhR deletion and TCDD exposure had greater effect on Per1 and Bmal1 mRNA expression in the peripheral clock, liver, vs. the master clock, SCN. In contrast, to the minimal effect observed in the SCN, the day/night pattern of both Per1 and Bmal1 were strikingly reversed after TCDD treatment in the liver. Determining whether the differences between tissues are due to distribution of TCDD or different responsiveness to TCDD will require further investigation. Although more complete investigation is necessary, it is possible that TCDD exposure caused a phase shift in the molecular clockworks in the liver.
In today’s society where people live under schedules that ignore the natural photoperiod provided by sunlight, such as in night-shift workers and international travelers who experience jetlag, the ability to adjust the circadian rhythm is important. Chronic disruption of circadian rhythm manifests as various physiological problems that may lead to more serious diseases, such as sleep and eating disorders and cancer (Haus and Smolensky 2006). Xenobiotics that activate AhR are ubiquitous in the environment. Although the AhR-mediated toxicity of TCDD has been studied for decades, mechanisms remain ambiguous. Symptoms associated with dioxin exposure, such as sleep disruption, suggest a relationship with the circadian timing system. Therefore, further investigation on the inhibitory effect of dioxins on light regulation of circadian rhythm is necessary to understand potential novel mechanisms of action of TCDD.
Acknowledgments
The authors thank Dr. Jennifer Mitchell, Bethany Karman and Stacey Krager for technical advice and support. Certain primer sets for qPCR were designed by Jason Hickok. This research was supported by NIEHS grant ES012948 (to SAT), an Eli Lilly Pre-doctoral Fellowship (to MM), and a grant from the Illinois State Governor’s Venture Technology Fund.
References
- Abbott BD, Birnbaum LS, Perdew GH. Developmental expression of two members of a new class of transcription factors: I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Dev Dyn. 1995;204:133–143. doi: 10.1002/aja.1002040204. [DOI] [PubMed] [Google Scholar]
- Aylward LL, Brunet RC, Carrier G, Hays SM, Cushing CA, Needham LL, Patterson DG, Jr, Gerthoux PM, Brambilla P, Mocarelli P. Concentration-dependent TCDD elimination kinetics in humans: toxicokinetic modeling for moderately to highly exposed adults from Seveso, Italy, and Vienna, Austria, and impact on dose estimates for the NIOSH cohort. J Expo Anal Environ Epidemiol. 2005;15:51–65. doi: 10.1038/sj.jea.7500370. [DOI] [PubMed] [Google Scholar]
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Benedict JC, Lin TM, Loeffler IK, Peterson RE, Flaws JA. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci. 2000;56:382–388. doi: 10.1093/toxsci/56.2.382. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS. The mechanism of dioxin toxicity: relationship to risk assessment. Environ Health Perspect. 1994;102(Suppl 9):157–167. doi: 10.1289/ehp.94102s9157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci U S A. 1991;88:9543–9547. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver LA, Hogenesch JB, Bradfield CA. Tissue specific expression of the rat Ah-receptor and ARNT mRNAs. Nucleic Acids Res. 1994;22:3038–3044. doi: 10.1093/nar/22.15.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron. 2006;50:465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Debruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Grechez-Cassiau A, Guettier C, Hastings MH, Francis L. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K, Zucker I. Circadian organization of the estrous cycle of the golden hamster. Proc Natl Acad Sci U S A. 1976;73:2923–2927. doi: 10.1073/pnas.73.8.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame LT, Li W, Miller JD, Dickerson RL. A proposed role for the arylhydrocarbon receptor (AhR) in non-photic feedback to the master circadian clock. Toxocologist. 2004:954. [Google Scholar]
- Garrett RW, Gasiewicz TA. The aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin alters the circadian rhythms, quiescence, and expression of clock genes in murine hematopoietic stem and progenitor cells. Mol Pharmacol. 2006;69:2076–2083. doi: 10.1124/mol.105.021006. [DOI] [PubMed] [Google Scholar]
- Gerall AA, Napoli AM, Cooper UC. Daily and hourly estrous running in intact, spayed and estrone implanted rats. Physiol Behav. 1973;10:225–229. doi: 10.1016/0031-9384(73)90302-8. [DOI] [PubMed] [Google Scholar]
- Goerz G, Barnstorf W, Winnekendonk G, Bolsen K, Fritsch C, Kalka K, Tsambaos D. Influence of UVA and UVB irradiation on hepatic and cutaneous P450 isoenzymes. Arch Dermatol Res. 1996;289:46–51. doi: 10.1007/s004030050151. [DOI] [PubMed] [Google Scholar]
- Hahn ME, Karchner SI, Shapiro MA, Perera SA. Molecular evolution of two vertebrate aryl hydrocarbon (dioxin) receptors (AHR1 and AHR2) and the PAS family. Proc Natl Acad Sci U S A. 1997;94:13743–13748. doi: 10.1073/pnas.94.25.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control. 2006;17:489–500. doi: 10.1007/s10552-005-9015-4. [DOI] [PubMed] [Google Scholar]
- Huang P, Ceccatelli S, Rannug A. A study on diurnal mRNA expression of CYP1A1, AHR, ARNT, and PER2 in rat pituitary and liver. Environmental Toxicology and Pharmacology. 2002;11:119–126. doi: 10.1016/s1382-6689(01)00111-9. [DOI] [PubMed] [Google Scholar]
- Jones MK, Weisenburger WP, Sipes IG, Russell DH. Circadian alterations in prolactin, corticosterone, and thyroid hormone levels and down-regulation of prolactin receptor activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1987;87:337–350. doi: 10.1016/0041-008x(87)90295-x. [DOI] [PubMed] [Google Scholar]
- Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: Effects of luteinizing hormone. Biol Reprod. 2006;75:624–632. doi: 10.1095/biolreprod.106.050732. [DOI] [PubMed] [Google Scholar]
- Kelling CK, Christian BJ, Inhorn SL, Peterson RE. Hypophagia-induced weight loss in mice, rats, and guinea pigs treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam Appl Toxicol. 1985;5:700–712. doi: 10.1016/0272-0590(85)90194-0. [DOI] [PubMed] [Google Scholar]
- Klinge CM, Kaur K, Swanson HI. The aryl hydrocarbon receptor interacts with estrogen receptor alpha and orphan receptors COUP-TFI and ERRalpha1. Arch Biochem Biophys. 2000;373:163–174. doi: 10.1006/abbi.1999.1552. [DOI] [PubMed] [Google Scholar]
- Lahvis GP, Pyzalski RW, Glover E, Pitot HC, McElwee MK, Bradfield CA. The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol Pharmacol. 2005;67:714–720. doi: 10.1124/mol.104.008888. [DOI] [PubMed] [Google Scholar]
- Li W, Dickerson RL, Frame LT. Low dose in vivo exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD or dioxin) alters expression of the clock-associated protein, period, in the suprachiasmatic nucleus (SCN) and liver of C57B6 mice. Toxicologist. 2004:91. [Google Scholar]
- Liu Y, Michalek JE, Frame LT. Serum 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) levels and sleep disorders in US air force veterans of the Vietnam War. Toxicologist. 2004:90. [Google Scholar]
- McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor is activated by modified low-density lipoprotein. Proc Natl Acad Sci U S A. 2007;104:1412–1417. doi: 10.1073/pnas.0607296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Settachan D, Frame LT, Dickerson R. 2,3,7,8-Tetracholorodibenzo-p-dioxin phase advances the deer mouse (Peromyscus maniculatus) circadian rhythm by altering expression of clock proteins. Organohalogan Compounds. 1999;42:23–28. [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196:305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- Mukai M, Tischkau SA. Effects of tryptophan photoproducts in the circadian timing system: searching for a physiological role for aryl hydrocarbon receptor. Toxicol Sci. 2007;95:172–181. doi: 10.1093/toxsci/kfl126. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Puga A, Vasiliou V. Role of the Ah receptor and the dioxin-inducible [Ah] gene battery in toxicity, cancer, and signal transduction. Ann N Y Acad Sci. 1993;685:624–640. doi: 10.1111/j.1749-6632.1993.tb35928.x. [DOI] [PubMed] [Google Scholar]
- Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J, Chambon P, Yanagisawa J, Fujii-Kuriyama Y, Kato S. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Antiphase circadian expression between BMAL1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochem Biophys Res Commun. 1998;253:199–203. doi: 10.1006/bbrc.1998.9779. [DOI] [PubMed] [Google Scholar]
- Oster H, Yasui A, van der Horst GT, Albrecht U. Disruption of mCry2 restores circadian rhythmicity in mPer2 mutant mice. Genes Dev. 2002;16:2633–2638. doi: 10.1101/gad.233702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Curran MA, Marconi SA, Carpenter CD, Lubbers LS, McAbee MD. Distribution of mRNAs encoding the arylhydrocarbon receptor, arylhydrocarbon receptor nuclear translocator, and arylhydrocarbon receptor nuclear translocator-2 in the rat brain and brainstem. J Comp Neurol. 2000;427:428–439. doi: 10.1002/1096-9861(20001120)427:3<428::aid-cne9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: effects, mechanisms, and animal models. Pharmacol Rev. 1994;46:483–549. [PubMed] [Google Scholar]
- Pohjanvirta R, Tuomisto J, Linden J, Laitinen J. TCDD reduces serum melatonin levels in Long-Evans rats. Pharmacol Toxicol. 1989;65:239–240. doi: 10.1111/j.1600-0773.1989.tb01164.x. [DOI] [PubMed] [Google Scholar]
- Pohjanvirta R, Vartiainen T, Uusi-Rauva A, Monkkonen J, Tuomisto J. Tissue distribution, metabolism, and excretion of 14C-TCDD in a TCDD-susceptible and a TCDD-resistant rat strain. Pharmacol Toxicol. 1990;66:93–100. doi: 10.1111/j.1600-0773.1990.tb00712.x. [DOI] [PubMed] [Google Scholar]
- Qu X, Metz RP, Porter WW, Cassone VM, Earnest DJ. Disruption of clock gene expression alters responses of the aryl hydrocarbon receptor signaling pathway in the mouse mammary gland. Mol Pharmacol. 2007;72:1349–1358. doi: 10.1124/mol.107.039305. [DOI] [PubMed] [Google Scholar]
- Rannug A, Fritsche E. The aryl hydrocarbon receptor and light. Biol Chem. 2006;387:1149–1157. doi: 10.1515/BC.2006.143. [DOI] [PubMed] [Google Scholar]
- Rannug U, Rannug A, Sjoberg U, Li H, Westerholm R, Bergman J. Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands. Chem Biol. 1995;2:841–845. doi: 10.1016/1074-5521(95)90090-x. [DOI] [PubMed] [Google Scholar]
- Richardson VM, Santostefano MJ, Birnbaum LS. Daily Cycle of bHLH-PAS Proteins, Ah Receptor and Arnt, in Multiple Tissues of Female Sprague-Dawley Rats. Biochemical and Biophysical Research Communications. 1998;252:225–231. doi: 10.1006/bbrc.1998.9634. [DOI] [PubMed] [Google Scholar]
- Roman BL, Peterson RE. In utero and lactational exposure of the male rat to 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs prostate development. 1. Effects on gene expression. Toxicol Appl Pharmacol. 1998;150:240–253. doi: 10.1006/taap.1997.8362. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seefeld MD, Corbett SW, Keesey RE, Peterson RE. Characterization of the wasting syndrome in rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1984;73:311–322. doi: 10.1016/0041-008x(84)90337-5. [DOI] [PubMed] [Google Scholar]
- Song J, Clagett-Dame M, Peterson RE, Hahn ME, Westler WM, Sicinski RR, DeLuca HF. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci U S A. 2002;99:14694–14699. doi: 10.1073/pnas.232562899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischkau SA, Weber ET, Abbott SM, Mitchell JW, Gillette MU. Circadian clock-controlled regulation of cGMP-protein kinase G in the nocturnal domain. J Neurosci. 2003;23:7543–7550. doi: 10.1523/JNEUROSCI.23-20-07543.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis CC, Hattemer-Frey HA. Human exposure to dioxin. Sci Total Environ. 1991;104:97–127. doi: 10.1016/0048-9697(91)90010-c. [DOI] [PubMed] [Google Scholar]
- van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, Buijs R, Bootsma D, Hoeijmakers JH, Yasui A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Singh D, Garrett TM, Fagoaga OR, Nehlsen-Cannarella SL. Reproductive, neuroendocrine, and immune consequences of acute exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in the Siberian hamster. Biol Reprod. 2000;63:538–543. doi: 10.1095/biolreprod63.2.538. [DOI] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]