Researchers at our AAALAC-accredited animal facility used an inbred strain of mice to generate offspring for bone development studies. Animal care personnel pair one male and one female mouse in individually-ventilated microisolator cages. Each cage housing a breeding unit pair is offered rodent chow (NIH-31; Ziegler Bros., Gardner, PA) and water ad lib.
Animal care personnel requested a veterinarian to examine a mouse with perineal swelling. The mouse was approximately 10 weeks old and was maintained in a breeding unit that had failed to produce any offspring. The absence of offspring was unusual as the mice were murine pathogen free, and husbandry conditions were optimal. The light:dark cycle of the room was set at 12:12 and the temperature maintained at 70° +/− 2°F.
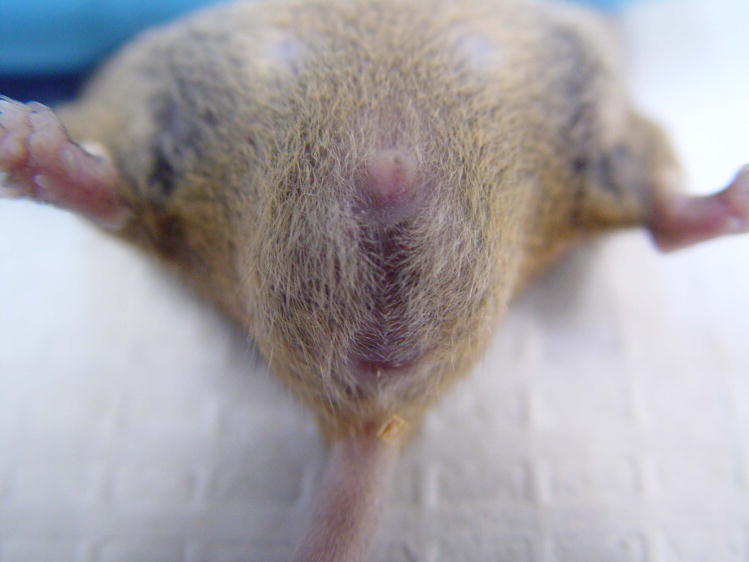
On examination, the mouse was noted to be well-hydrated and in general good physical condition. A soft, reducible swelling was noted in the perineum (Fig. 1). Palpation of the abdomen and the perineum did not appear to be painful. The perineal skin was of normal color and appeared to be of normal temperature.
Figure 1.
A 10-week-old mouse with perineal swelling.
What gender is the mouse? What are your differential diagnoses for the perineal swelling? Could the presenting clinical sign be related to failure to produce any offspring? Is the perineal swelling treatable? How would you treat it?
What’s your diagnosis?
Diagnosis|Imperforate vagina with secondary mucometra
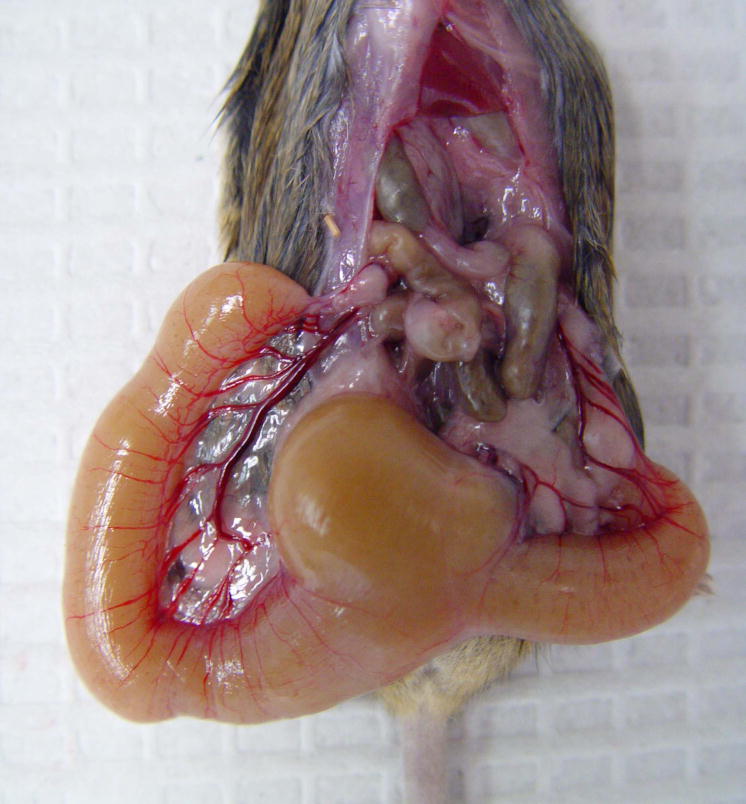
Imperforate vagina is an uncommon condition in mouse breeding colonies, although the incidence can be high within certain strains of inbred and genetically engineered mice1. Because the vaginal opening in not patent in imperforate vagina, the sloughed cells and fluid produced during the estrous cycles is retained, resulting in uterine and vaginal distension (Fig. 2). As the distention progresses, it becomes evident as a “swelling” of the perineum.
Figure 2.
A female mouse with severe uterine distension due to an imperforate vagina.
Although there is some disagreement regarding the role of the Müllerian duct and urogenital sinus in the development of the vagina, there is universal agreement on the development of an endodermal membrane (or hymen) that occludes the vaginal opening until puberty1,2,3. At puberty, this barrier degenerates and the vaginal opening becomes patent. In normal weaning age mice, the vaginal orifice can be gently teased open with a cotton-tipped applicator. This is not the case in mice with an imperforate vagina, where opening the vaginal vault requires sharp surgical dissection.
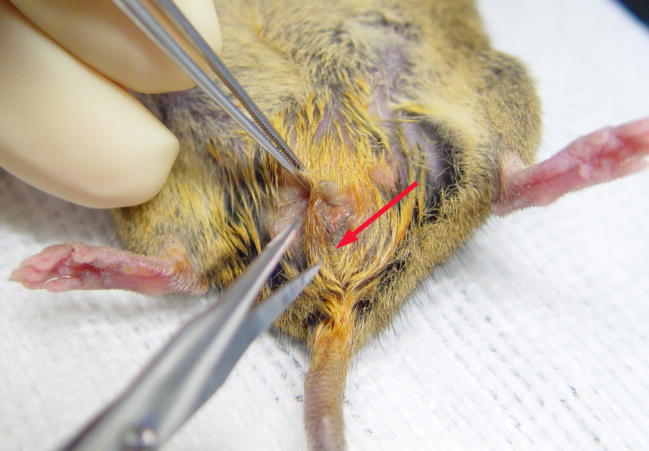
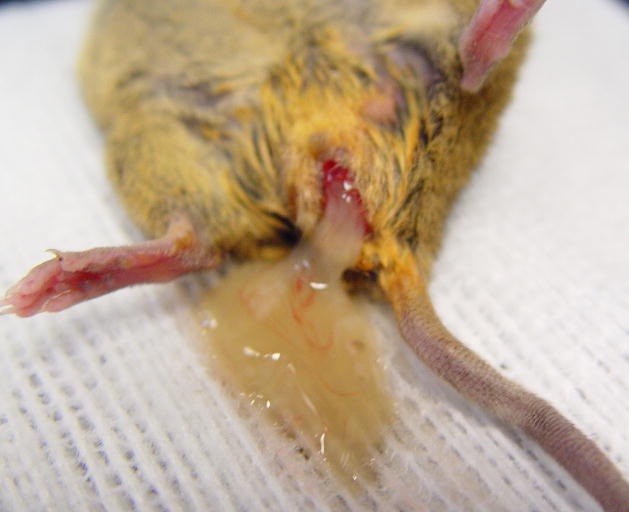
To relieve the pressure of the fluid build up in the uterus and to open the vagina for possible breeding, surgical repair can be attempted4. To improve visualization of the surgical area, a dissecting microscope or magnifying lenses should be used. The mouse of this report was anesthetized with isoflurane and the perineum was cleansed with an iodophor. In the space between the anus and the urinary papilla, we made a transverse incision through the skin along the midline (Fig. 3). The thin layer of subcuticular tissue was then bluntly dissected, exposing an obvious, distended, vaginal membrane (Fig. 4). We sharply transected the vaginal membrane resulting in the release of copious amounts of flocculent, mucoid debris (Fig. 5). Then we dilated the newly formed orifice with blunt scissors to assure an adequate opening and good drainage. Failure to establish adequate dilation will result in stenosis and/or re-closure of the wound during healing. Once an adequate opening was established, we gently massaged the distal abdomen to encourage thorough drainage. Flushing of the vagina and uterus was not done, as we were concerned about damaging the thin, dilated, flaccid uterus and/or causing retrograde expulsion of debris into the abdominal cavity.
Figure 3.
Surgical repair of an imperforate vagina. The initial incision is made along the midline (arrow) between the urinary papilla and anus.
Figure 4.
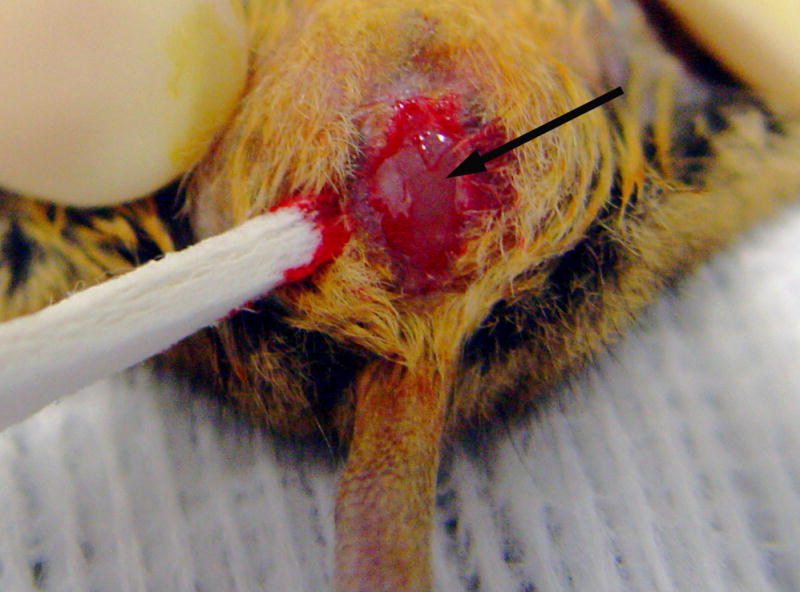
Surgical repair of an imperforate vagina. Once the skin between the urinary papilla and anus is incised, the distended vaginal membrane can be visualized (arrow).
Figure 5.
Surgical repair of an imperforate vagina. After incision of the vaginal membrane, fluid should readily drain from the uterus.
Postoperatively, we instilled a triple antibiotic ophthalmic ointment with hydrocortisone (Foguera, Melville, NY) into the vaginal orifice followed by 1–2 drops of the local anesthetic agent bupivacaine (Marcaine® 0.5%, Hospira, Lake Forest, IL) along the incision site. The antibiotic trimethoprim-sulfa (30 mg/kg, Medical Arts Pharmacy, Baltimore, MD) was administered subcutaneously twice daily in 1 ml of 0.9% NaCl. This treatment was continued for 5 to 7 days with the exception of the bupivacaine, which we discontinued after three days. The tube tip of the ophthalmic ointment helps to maintain the dilation of the vagina when the ointment is instilled intravaginally. The hydrocortisone is used to minimize contracture and closure of the newly formed vaginal os.
Bacterial swabs for culture that we obtained at surgical repair failed to yield bacterial growth. However, we felt it was prudent to treat the mouse with post-procedural antibiotics to prevent infection due to abnormal uterine and vaginal tone.
In our hands, we have found the procedure described is an effective treatment for the relief of perineal and abdominal distention in female mice due to imperforate vagina. However, the percent of post-surgical mice that return to breeding soundness has been disappointing. Several of the mice have “plugged” but do not continue to successful pregnancies. Although not confirmed by histological analysis, we suspect that this might be due to the presence of other reproductive abnormalities and/or permanent damage due to the extreme distension of the uterus. This problem may be partially circumvented by early detection and prompt surgical repair.
Because of the disappointing ability of the surgically repaired mice to return to successful reproduction, our institution offers this treatment primarily as a salvage procedure so that valuable females can be used for other experimental manipulations. We perform the surgical repair in order to relieve the discomfort that may result from the uterine and vaginal distension. Post-surgical complications are rare but include utero-vaginal prolapse and rectal vaginal fistula. Contracture and re-closure of the vaginal os has been a more frequent complication. In vitro fertilization (IVF) can be used to rescue valuable mice that do not return to breeding soundness after attempting surgical rescue5.
Another, more common, reproductive anomaly of female mice is the presence of transverse vaginal septa (Fig. 6). These mice are detected during estrous determination, “plug” checking, or during physical examination for failure to breed. This anomaly is easily repaired by briefly anesthetizing the mouse with an inhalant anesthetic and sharply dissecting the membrane after surgical cleansing of the perineal area with an iodophor. A single application of a long-acting local anesthetic is applied topically. In contrast to the procedure for treatment of imperforate vagina, the percentage of mice that return to breeding soundness after the procedure to treat transverse vaginal septa is high.
Figure 6.
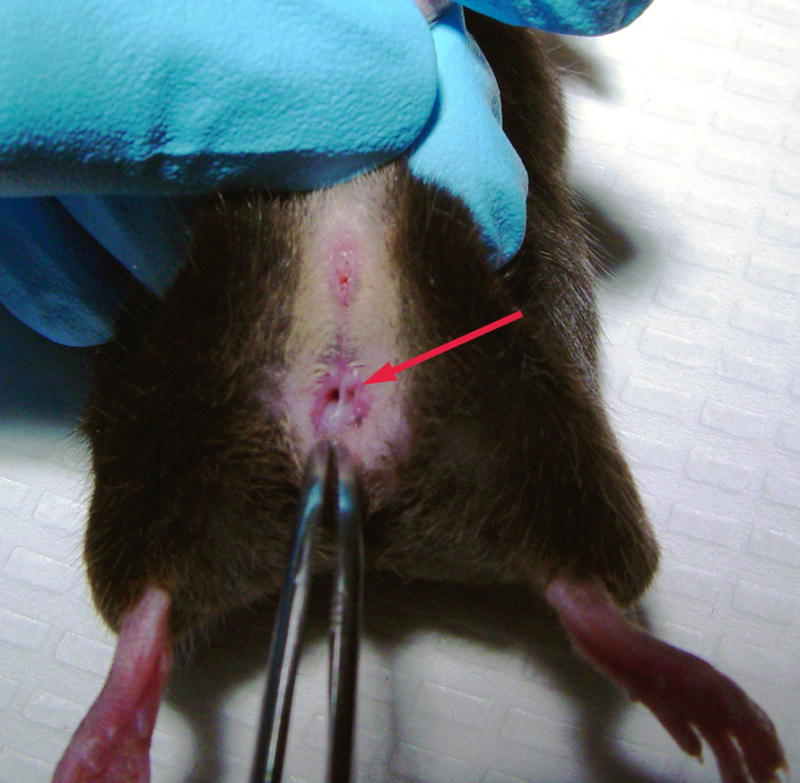
Vaginal septa (arrow) are often a frequent occurrence in some inbred lines of mice.
We have found it is important to educate animal care and investigative staff in recognizing these, and other, reproductive anomalies in breeding colony mice. Because of the swollen perineum, mice with imperforate vaginas are often mistaken as males. The presenting complaint may be failure of a breeding unit to produce pups when the problem is a normal appearing female “mated” with a second female with an imperforate vagina. Mice with vaginal septa also commonly present with the complaint of failure to breed. In these animals early detection and repair is paramount. The mode of inheritance for both imperforate vagina and transverse vaginal septa is unclear. However, if a particular pair appears to be regularly producing these anomalies, it may be wise to eliminate these individuals from the breeding stock.
Acknowledgments
This research was supported [in part] by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
References
- 1.Sundberg JP, Brown KS. Imperforate vagina and mucometra in inbred laboratory mice. Lab An Sci. 1994;44(4):380–382. [PubMed] [Google Scholar]
- 2.Kaufman MH, Bard JBL. The Anatomical Basis of Mouse Development. Academic Press; San Diego, CA: 1999. pp. 121–123. [Google Scholar]
- 3.Kaufman MH. The atlas of mouse development. Academic Press; San Diego, CA: 1992. p. 255. [Google Scholar]
- 4.Masangkay JS, Kondo K. Imperforate vagina in mice: percent incidence and surgical repair. Exp Anim. 1983;32(3):139–144. doi: 10.1538/expanim1978.32.3_139. [DOI] [PubMed] [Google Scholar]
- 5.Didion BA, Hauser ME, Eisen EJ. Use of in vitro fertilization and embryo transfer to circumvent infertility caused by an inherited imperforate vagina in mice. J In Vitro Fert Embryo Transf. 1991;8(3):167–172. doi: 10.1007/BF01131709. [DOI] [PubMed] [Google Scholar]