Abstract
Using Parkinson's disease as a prototype of neurodegenerative diseases, we propose applications of human stem cells in the development of therapeutics for neurodegenerative diseases. First, in vitro differentiation of human stem cells offers a versatile model for dissecting molecular interactions underlying human dopamine (DA) neuron specification, which may form a foundation for instigating regeneration of DA neurons from progenitors that reside in the brain. Second, stem cells derived from diseased cells or through genetic modification can serve as a platform for unraveling biochemical processes that lead to the cellular pathogenesis of degeneration. This may in turn serve as a template for identifying or developing therapeutics for slowing, stopping, or reversing the disease process. And finally, stem cells, particularly those induced from patients' own cells, provide a reliable source of DA neurons for cell based therapy.
Introduction
Degeneration of dopamine (DA)-producing neurons in the midbrain, especially the substantia nigra, underlies the pathophysiology of Parkinson's disease (PD). How the selective DA neuron degeneration is initiated remains elusive. Pharmacological replacement of the missing DA by its precursor, L-dopa, is generally effective in alleviating symptoms. However, this treatment depends on the availability of DA neurons to synthesize and release DA, and it does not remove the cause of DA neuron degeneration. With fewer DA neurons available to synthesize the transmitter along disease progression, such a chemical replacement therapy loses its efficacy in several years and is accompanied by severe side-effects. As a consequence, alternative therapies are needed for advanced Parkinson's patients.
Alternative therapies that are being practiced or tried include deep brain stimulation, neural protection with trophic factors, and fetal cell transplantation. Stimulation of the subthalamic nucleus or globus pallidus is effective for symptom relief in some advanced PD patients although its effect is variable and it does not address the cause. Protection of the remaining DA neurons by neurotrophic factors such as glial cell line derived neurotrophic factor (GDNF) has been shown to be effective in a small open labeled trial (Gill et al., 2003). Nevertheless, this growth factor has many other target cell types, resulting in side effects. Fetal cell transplantation has shown some efficacy in several open labeled trials since 1987 (Freed et al., 2001;Piccini et al., 1999). In some cases, patients with the fetal tissue graft lived a reasonably healthy life for over a decade (Mendez et al., 2008). However, two NIH-sponsored double-blinded trials showed variable outcomes (Freed et al., 2003). The inconsistent results may be attributed to multiple factors, most notably the donor cells (Redmond, Jr., 2002). Collection of several fetuses and storage of brain tissues for up to a month before transplantation preclude the possibility of standardization or even regular comparison among transplanted patients. Thus, new sources of DA neurons for which the identity, purity, and quantity can be better controlled are now essential before further clinical trials are considered.
Stem cells, capable of renewing themselves as well as differentiating to DA neurons, are potential sources. Several types of stem cells have been reported to give rise to DA neurons or DA-like cells. The most reliable stem cell sources of DA neurons are those from early embryos, embryonic stem cells (ESCs), and those reprogrammed from adult cells, induced pluripotent stem (iPS) cells. While generation of DA neurons from stem cells could serve as a source for potential cell therapy, understanding the molecular underpinnings of human DA neuron specification using the stem cell model may form a foundation for instigating regeneration of DA neurons from progenitors that reside in the brain. Stem cells derived from diseased cells, including those of familial Parkinson's patients, may serve as a platform for unraveling biochemical processes that lead to the cellular pathogenesis of PD. This may in turn serve as a template for identifying or developing therapeutics for slowing, stopping, or reversing the disease process (Krencik et al., 2006).
We will use Parkinson's disease as a prototype to discuss how stem cell biology may contribute to the development of therapeutics for neurodegenerative diseases. This area of research has just begun with little information available. Hence, we will offer our personal perspectives of where the field may go.
Understanding normal human DA neuron development
Specification of midbrain DA neurons has been extensively investigated in vertebrate models. It is generally clear that Wnt1 and fibroblast growth factor (FGF) 8, secreted from the mid-hindbrain boundary (MHB), instruct the midbrain identity of neural progenitors by expressing homeodomain and paired-box transcription factors including Pax2/5 and Engrailed (En) 1 and 2 (Joyner et al., 2000). Together with sonic hedgehog (SHH), a glycoprotein secreted from the floor plate cells, the midbrain progenitors adopt a dopaminergic fate by transcribing Nurr1, ptx3, and genes that are essential for synthesizing and metabolizing DA (Smidt et al., 2007). It remains unclear how these extracellular factors activate the midbrain patterning program and the dopaminergic transcription signaling and how these networks converge on a specific progenitor cell at a particular developmental stage to specify the dopaminergic neuron. In addition, unlike in other parts of the CNS midbrain DA neurons are generated directly from the floor plate cells of the developing midbrain (Ono et al., 2007). The floor plate cells express another set of homeodomain proteins Lmx1a and Msx1, which induce the proneural protein Ngn2 and therefore neuronal differentiation through interaction with Shh signaling (Andersson et al., 2006). Again, how this unique pathway converges on to the dopaminergic pathway remains obscure.
The basic understanding of DA neuron specification has been instrumental to the differentiation of neural progenitors or stem cells to DA neurons in culture. In a rat embryonic explant culture, it was shown that FGF8 and SHH were necessary and sufficient to differentiate the neural progenitors to DA neurons (Ye et al., 1998). However, if the explant comes from the wnt1 null mice, FGF8 and SHH are not sufficient (Prakash et al., 2006). Thus, wnt mediated signaling is also essential for midbrain DA neuron specification.
Translating the information to the differentiation of DA neurons from naïve pluripotent embryonic stem cells (ESCs) becomes less straightforward. A simple reason is that the starting cells are more primitive without pre-defined midbrain information as primary neural tissues. Using mouse ESCs as starting cells, McKay and colleagues first differentiated ESCs to neural progenitors in the absence of morphogens. Following the expansion with FGF2, these neural progenitors were differentiated to DA neurons in the presence of FGF8 and SHH, with an efficiency of 30% (Lee et al., 2000). This simple and efficient method can be readily replicated in other laboratories. However, the majority of the differentiated DA neurons do not appear to carry a midbrain phenotype (Zhao et al., 2004), suggesting that FGF8 and SHH are not sufficient for patterning the midbrain identity. Recently, McKay's group showed that nearly all the DA neurons exhibit a midbrain phenotype including expression of En-1 and ptx3 with minor modification of the protocol (Rodriguez-Gomez et al., 2007). It will be useful to identify the molecular interactions underlying the specification of midbrain DA neurons in this model system.
Dopamine neurons can also be efficiently differentiated from mouse ESCs by co-culturing ESCs with stroma cells, PA6 cells, followed by treatment with FGF8 and SHH (Kawasaki et al., 2000). The effect of PA6 cells is thought to promote neural differentiation from ESCs. It may also influence the midbrain patterning.
Both the co-culture approach and the chemically defined method are employed for differentiating hESCs to DA neurons. In nearly all the reports, FGF8 and SHH are employed to pattern the hESC-derived neuroepithelial cells. The typical yield from co-culture with MS5 cells (Perrier et al., 2004) or in chemically defined cultures (Yan et al., 2005) is about 30% TH-expressing DA neurons among all differentiated progenies, although variable efficiencies are reported (Brederlau et al., 2006;Buytaert-Hoefen et al., 2004;Cho et al., 2008;Iacovitti et al., 2007;Martinat et al., 2006;Park et al., 2005;Schulz et al., 2004;Sonntag et al., 2007;Zeng et al., 2004). Goldman and colleagues reported a much higher yield of DA neurons when co-cultured with immortalized mesencephalic astrocytes (Roy et al., 2006). The important issue is that the proportion of midbrain DA neurons is generally low. Most reports did not assess the expression of midbrain markers such as En-1 and Ptx3 in the DA neurons. Our estimate from the double immunostaining results indicates that around 20% of the DA neurons carry En-1 (Yang et al., 2008). This suggests that the combination of FGF8 and SHH alone, as is the case in mouse ESC differentiation, is not optimal for patterning the hESC-generated neuroepithelial cells to midbrain progenitors. This is a critical issue that requires further investigation.
The less efficient midbrain patterning of the hESC-derived neuroepithelial cells than those from mouse ESCs is at least partly related to the default state of the neuroepithelial cells. Mouse ESCs tend to generate neural progenitors with mostly mid/hind brain phenotypes (Watanabe et al., 2005) and these neural progenitors further generate DA neurons in the presence of SHH. Human ESCs almost exclusively differentiate to neuroepithelia with a forebrain identity(Li et al., 2005;Pankratz et al., 2007). As discussed above, the simple combination of FGF8 and SHH is in fact not effective in patterning the midbrain fate, particularly if the neural progenitors are not somewhat pre-specified. Besides, the commercially available FGF8b, which participates in the patterning of many brain regions including the forebrain and mid/hind brain (Olsen et al., 2006), is unlikely to specify a mesencephalic identity at least based on the amount generally used. The alpha form of FGF8, FGF8a which is now commercially available, is involved mainly in the specification of midbrain identity (Olsen et al., 2006). Still, additional morphogens, such as wnts, may need to be incorporated in order to specify human neuroepithelia to a midbrain fate.
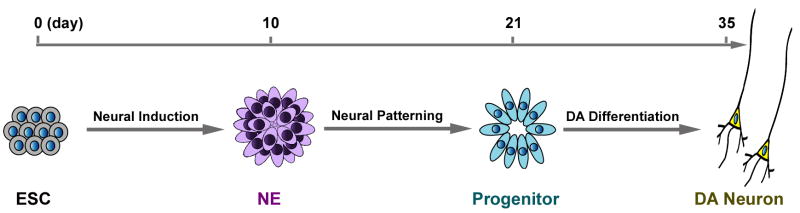
Apparently, specification of midbrain DA neurons requires coordination of multiple factors at multiple dimensions (concentrations, combinations, temporal courses, convergence of intracellular signalings, etc). The defined hESC neural differentiation model will allow dissection of discrete effects of each of the morphogens, such as patterning, specification of transmitter phenotypes, and coupling of regional patterning and transmitter specification (Fig. 1). Revealing the molecular interactions that govern human DA neuron generation will be critical for instigating dopaminergic regeneration from neural stem/progenitor cells that reside in our human brain.
Fig. 1. Stem cell differentiation model for dissecting molecular mechanism of human DA neuron specification.
Under a defined condition, human ESCs can be differentiated to primitive neuroepithelial (NE) in 10 days. This uniform population of NE may be used as starting cells to identify molecule(s) that pattern them to midbrain progenitors, assayed by expression of midbrain transcription factors at 3 weeks of differentiation. These progenitors in turn can be used as a template to uncover molecules or pathways that couple the midbrain patterning and specification of DA transmitter for differentiation of DA neurons. This model is applicable for specification of other neuronal subtypes (see Zhang, 2006).
Revealing pathological process of human DA neuron degeneration
Transgenic animals play pivotal roles in revealing disease pathogenesis. Most of these animals are created to model certain aspects of the human diseases. Many neurodegenerative diseases including PD do not naturally occur in the commonly used laboratory animals. Expression of mutant forms of α-synuclein, which causes familial PD, does not result in disease phenotypes including loss of nigra DA neurons in mice(Giasson et al., 2002). Similar phenomena have been observed in the effort of inducing disease phenotypes with mutant DJ-1 and Pink1(Goldberg et al., 2005), two other proteins involved in familial PD. In humans, mutation of the superoxide dismutase (SOD) at a single copy results in amyotrophic lateral sclerosis (ALS). In mice, expression of multiple copies of the mutant SOD is required for generating phenotypes (Bruijn et al., 2004). The most prevalent form of mutant SOD in ALS patients, the A4V, does not generate phenotypes in mice (Furukawa et al., 2006). Hence, model systems with the human background would supplement our current investigations on the pathogenesis of neurodegenerative diseases.
Genetic modification of human stem cells could potentially model cellular and molecular aspects of the disease process but on the human genetic and epigenetic background. Over-expression of dominant disease genes (e.g., α-synuclein) or knock-down of recessive genes (e.g., pink-1, DJ-1) in hESCs could result in the generation of transgenic hESCs. Differentiation of the transgenic hESCs to DA neurons will allow dissecting the effect of disease genes on DA neuron survival and function. We have expressed wild and mutant α-synuclein in hESC-generated neural progenitors and found that the mutant α-synuclein caused death of differentiated human neurons. The death effect of α-synuclein is cell type-specific, i.e., overexpression of α-synuclein results in death of DA neurons whereas the GABA neurons are not affected (Schneider et al., 2007). This preliminary finding is very encouraging as it shows that some of the pathological aspects of α-synuclein toxicity can be reproduced in human neurons as opposed to the lack of effect in mouse cells. Equally importantly, the relatively cell type-specific effect strengthens the value of the in vitro model of human stem cells to dissect the biochemical interactions behind the pathological cellular changes.
It would be technically advantageous to establish transgenic hESC lines than to transfect the differentiated cells in most applications. Insertion of specific sequences including mutations through homologous recombination, a technique that is very well established in animal studies, has been reported for hESCs (Zwaka et al., 2003). However, the recombination efficiency is much lower than in mice and thus it is technically rather demanding for an ordinary laboratory. Gene expression through random insertion is often confounded with inactivation of transgenes following hESC differentiation to functional neuronal and glial cells (Xia et al., 2007). Keller and colleagues have knocked a green fluorescent protein (GFP) reporter gene into the ROSA26 locus of hESCs (Irion et al., 2007). The choice of ROSA26 locus is that this locus is generally resistant to transgene down-regulation. Thus replacement of the GFP with a target disease gene would allow the generation of disease hESC lines. Nevertheless, neurons differentiated from mouse ESCs in which transgenes are knocked into the ROSA26 locus often exhibit a significant down regulation of transgenes (Krencik and Zhang, unpublished observations). Therefore, this locus may not be the best site for inserting transgenes in hESCs, especially for functional analysis in differentiated neurons. We have screened loci in hESCs that are resistant to transgene downregulation following neural differentiation and built in a Cre-mediated cassette into the newly identified sites (Du and Zhang, unpublished). These driver hESC lines, and especially those with transgene expression in a cell type-specific manner and under temporal and quantitative control, will simplify the generation of transgenic hESCs by replacing the cassette with a target gene.
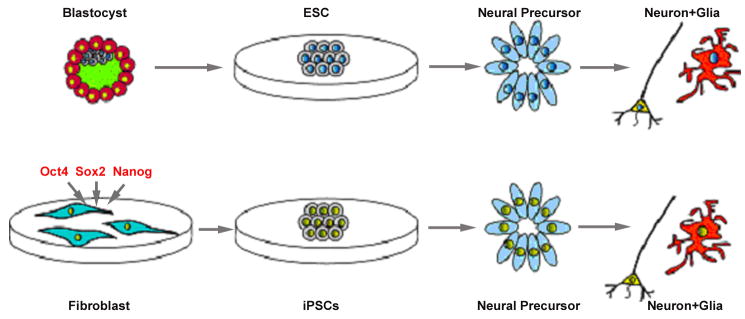
An alternative way to genetic modification of normal hESCs is the establishment of stem cells with natural mutations. Disease-specific ESCs may be generated from PGD (preimplantation genetic diagnosis)-confirmed embryos, as shown for the fragile X syndrome hESCs (Eiges et al., 2007). Disease stem cells may also be generated by reprogramming patients' cells with natural mutations. This can be achieved by transferring mutant DNA into an enucleated oocyte, but this is hampered by ethical and technical issues. A recent development, which is readily reproducible, is to reprogram somatic cells by forced expression of a set of core transcription factors that are crucial for inducing and maintaining the stem cell state, including OCT4, NANOG, and SOX2 alongside chromatin modifying factors c-MYC, KLF4, or LIN28 etc. This technological development has resulted in the creation of induced pluripotent stem (iPS) cells from somatic cells such as fibroblasts, first from mice and lately from humans (Lowry et al., 2008;Park et al., 2008;Takahashi et al., 2006;Takahashi et al., 2007;Yu et al., 2007). The iPS cells generated using different sets of factors in different laboratories show remarkable similarity to hESCs based on morphology, surface marker expression, gene expression profiles, epigenetic status, formation of embryoid bodies in vitro, and teratoma formation in vivo. What is striking is that the viral vectors that are used to induce pluripotency are largely silenced after iPS cell formation, indicating that the iPS cells are reprogrammed and no longer dependent on transgene expression at least at the stem cell stage. IPS cells derived from familiar PD patients could serve as a great model for following the cellular and molecular undertakings of human DA neuron degeneration process when the iPS cells are differentiated to DA neurons (Fig. 2).
Fig. 2. Patient-specific stem cells for pathological/screening studies and for autologous cell therapy.
iPS cells may be established by forced expression of pluripotent transcription factors including Oct4, Sox2, and Nanog. iPS cells produced in this manner exhibit nearly identical phenotypes as ESCs and can be differentiated to neural precursors and mature neurons and glia in the same way as ESCs. iPS cells generated from PD patients may be used for studying cellular and molecular processes underlying DA neuron degeneration, gene therapy (for genetic mutation), and for generating DA neurons for autologous cell therapy.
A prerequisite for the disease iPS cells to serve as a tool for studying the cellular degenerative process is that the disease iPS cells can be directed efficiently to the target cell types such as DA neurons and exhibit pathological features as predicted from in vivo studies or clinical observations. Although human iPS cells can generate teratoma following injection into immune deficient mice, little information is available if human iPS cells can be differentiated to neural cells, including DA neurons, in the same efficiency as hESCs. The good news is that mouse iPS cells have been shown to differentiate to DA neurons efficiently and these neurons appear functional (Wernig et al., 2008). Human iPS cells, generated using retrovirus, can be differentiated to TH-expressing neurons (Takahashi et al., 2007). Our unpublished observation indicates that human iPS cells generated using lentivirus (Yu et al., 2007) can also be differentiated to DA neurons using the identical method for differentiating hESCs. Recently, iPS cells were established from ALS patients and these iPS cells could be differentiated to spinal motor neurons (Dimos et al., 2008), the main target cells of ALS. We predict that disease human iPS cells will provide an unprecedented tool for revealing dynamic cellular and molecular changes along the degenerative process of human neurons, especially those that are not readily studied in transgenic animals.
Identifying molecules that stop the DA neuron degeneration process
Stem cells carrying disease phenotypes, either from genetic modification or from naturally occurring mutations (see above section), can also serve as a template for screening and confirming chemicals/molecules that slow and/or halt the degenerative process. The human stem cell system, which generally requires a long-term culture, may not appear practical for large-scale screening. Technology needs to be developed to adapt the system for easy cell preparation as well as quantifiable and reproducible readout. This will need to be established upon the careful cellular and molecular analysis of the in vitro pathogenesis. Being an in vitro system, the stem cell-differentiated DA neurons may be induced to express a specific phenotype representing a particular pathological stage, e.g., formation of intracellular aggregates, instead of a simple cell live/death by a specific set of insults (Fig. 2). With a built-in quantifiable reporter such as the luciferase gene, one may identify molecules that interfere with the DA neuron degeneration process with a known cellular/molecular target. This will lead to the development of drugs aimed at slowing or halting the DA neuron degeneration rather than the simple symptomatic relief that animal screening is based on. Of course, findings made from the in vitro system will require confirmation from intact animal studies. However, the in vitro human system will speed up the drug discovery at a much lower cost. Molecules screened based on bona fide diseased human dopamine neurons instead of animal cells or immortalized non-neuronal cells would facilitate faster translation to clinical application. It is anticipated that the human stem cell-based screening will become an effective tool for drug discovery.
Developing stem cell based therapy for PD
Cell therapy, either cell replacement or cell-mediated delivery of active molecules, remains hopeful for PD patients given the foundation of fetal cell based clinical trials. There are a number of issues that need to be addressed before such a therapy becomes viable, ranging from inconsistent cell source to poor survival, integration, and functional regulation of grafted DA neurons, as well as potential risks of immune rejection, overgrowth or tumor formation. At present, the fundamental issue, which is also relevant to this review, is the generation of consistent, defined, and functional population of transplantable human DA neurons.
Methods for obtaining enriched population of midbrain DA neurons
There are diverse stem cell sources including some non-neural stem cells that are reported to generate DA neurons or DA-like cells. Among them ESCs, including those from humans, can consistently and efficiently differentiate to functional and transplantable DA neurons. Human ESCs are thus likely the ideal sources at present. Although no data are yet available, human iPS cells will likely become the ultimate source given the possibility of generating patient's iPS cells, especially when non-genetic and/or safe approaches are developed to induce stem cells.
Differentiation to DA neurons, as discussed in the previous section, has been achieved mainly through co-culture with stromal cells or midbrain astrocytes, or using chemically defined cultures employing recombinant growth factors. Both methods generate a similar proportion of DA neurons among the general differentiated cell population. Several issues still need to be solved or improved in order to make use of stem cell-derived DA neurons toward therapeutic application. The first issue is the identity of the in vitro generated DA neurons. DA neurons with the midbrain phenotype will likely be critical for a successful transplant therapy (Chung et al., 2005) in terms of survival, phenotypic stability, and synapse formation with target cells. From the reports to date, DA neurons carrying the midbrain phenotypes such as expression of En-1, ptx3, and Girk2, are still a minority. Despite extensive publications on DA neuron differentiation from hESCs, this critical issue still awaits careful investigations.
The second issue is the purity of the DA neuron cultures. As in nearly all differentiation systems, culture methods to date result in a mixture that contains DA neurons. It is largely unknown what the non-target (DA neurons) populations are in the mixture. When we dissect a small region of the fetal brain, we can predict what is in the tissue, at least in terms of regional identity. In the ESC-differentiated mixture, there could be a very diverse population of cells, particularly if the restriction to midbrain is not complete, which is the case at the present. In hESC differentiation cultures for spinal motor neurons, we found that RA and SHH (or a small molecule purmorphamine) almost completely restrict the neural cells to the ventral spinal cord fate. Therefore, besides the target motor neuronal cell population that comprises about 80% of the population the remaining 20% cells are interneurons of the ventral spinal cord (Li et al., 2008). Knowing what the non-target cells are in the differentiated progenies is as important as the target cells themselves.
One may argue that the best way to solve the purity issues is to sort the target DA neurons or to remove non-DA neuronal cells. Indeed, it would be a critical step from the safety standpoint. Nevertheless, chemical and physical separation strategies will impose a detrimental effect on the viability of neurons, including DA neurons. Enrichment of mouse DA neurons by FACS severely affects the survival of DA neurons, and thus the consequence of transplants (Hedlund et al., 2008). One option is to enrich the DA neuron progenitors, which are generally more resistant to cell separation. However, such a specific cell surface marker for DA neuron progenitors is not available. An alternative at this moment is to use a molecule covering a broader lineage, e.g., ventral neural progenitors. A recent cell lineage tracing study indicates that corin, a cell surface molecule expressed by floor plate progenitors, is expressed by the midbrain progenitors that are destined to DA neurons (Ono et al., 2007). Cells sorted with corin will likely suffice the need for enriching midbrain DA neurons and removing the proliferative anterior neural progenitors, if the neuroepithelia are restricted to the midbrain region. Even if the progenitors are patterned to a broader region than just midbrain, progenitors sorted by floor plate markers like corin may differentiate to additional neuronal types such as noradrenergic and serotonergic neurons from the mid/hind brain region. Although the cardinal symptoms of PD result from loss of DA neurons in the midbrain, there are also other neurons, including noradrenergic neurons of the locus coeruleus and serotonergic neurons of the raphe, both of which are involved in the progression of PD and symptoms of depression and dementia (Ahlskog, 2007). The presence of non-DA neurons may not necessarily be detrimental and isolation of the ventral neural progenitors from the differentiated progenies is feasible.
Approaches for enhancing survival and integration of grafted DA neurons
A number of factors can affect the survival of grafted DA neurons and subsequent functional integration, ranging from cell preparation to the host environment. Developmentally, the survival of a neuron depends on the synaptic engagement with its target cells. Differentiation of ESCs is essentially the recapitulation of development. The DA neurons that form synaptic connections with striatal neurons are mostly originated from the midbrain, especially the nigra. Hence, it is reasonable to speculate that the extremely poor survival of grafted hESC-derived DA neurons, seen in recent reports (Ben Hur et al., 2004;Brederlau et al., 2006;Yang et al., 2008;Zeng et al., 2004) is at least partly attributed to the identity of the DA neurons, i.e., most of the DA neurons do not possess the midbrain traits. Indeed, we found that the majority of DA neurons that survived in the graft for up to 5 months and contributed to functional improvement of the transplanted animals exhibited a mesencephalic phenotype by expressing Girk2 (Yang et al., 2008). More complete restriction to midbrain DA neuronal lineage will likely improve the survival and subsequent functional integration.
Generally speaking, neural progenitors survive better than more mature neurons during cell preparation and transplantation. We compared graft survival and the number of DA neurons in the graft among neural progenitors (3 weeks following ESC differentiation), putative DA neuronal progenitors (4-5 weeks) and more mature DA neurons (7 weeks). Transplantation of the above cell types all resulted in survival of grafts. Somewhat counter intuitively, more mature DA neurons with a longer term culture survived more and contribute to locomotor functional recovery (Yang et al., 2008), whereas the DA neuronal progenitors with a shorter culture period resulted in fewer DA neurons with variable functional consequence (Yang and Zhang, unpublished observation). It is not clear why the progenitors generate fewer DA neurons in the graft. It is possible that human (primate) DA neuron progenitors are particularly vulnerable to environmental changes or may change cell fate in the adult striatal environment. In clinical practice of fetal brain tissue transplant, a strict developmental stage of 6-8 week-old human embryos is essential for successful cell transplantation (Freed et al., 2001). Our observation closely matches clinical finding given that in vitro differentiation is essentially the recapitulation of in vivo development.
The better survival of DA neurons in the graft with older cultures may also be attributed to the presence of mesencephalic glial cells. We have shown that astrocytes, marked by S-100β, generally appear after 6-8 weeks of culture in our chemically defined condition and their presence is essential for neuronal synaptogenesis and synaptic function (Johnson et al., 2007). As discussed above, DA neuron differentiation is significantly enhanced by the presence of mesencephalic astrocytes (Roy et al., 2006). The presence of astrocytes could significantly influence the survival of transplanted neurons.
Strategies for avoiding over-growth and immune rejection
Safety is perhaps the number one concern for cell-based therapy. The nature of ESCs is their ability to differentiate to cell and tissue types that would normally be seen in an embryo, such as formation of a teratoma when placed in an excessive numbers in an immune deficient animal. Differentiated progenies are likely cells beyond the germ layer stem cell stage. Therefore, generation of teratomas from hESC-differentiated progenitor or neuronal cultures, which requires at least 3 weeks of differentiation, is extremely unlikely. If it happens, it usually points to the unsuccessful differentiation system and/or abnormal karyotyping of the inappropriately and excessively expanded ESCs. Hence, the perception that ESC-derived progenies are more prone to generating tumors than other types of cultured stem/progenitors is somewhat misleading.
Prolonged expansion of hESCs, like any other somatic cells, will result in karyotypic instability (Draper et al., 2004). Karyotypically altered hESCs often do not differentiate to the target cell types as predicated. In a double blinded assessment of the neural differentiation potential of the NIH approved hESC lines through the National Stem Cell Bank, poor differentiation in our defined system almost always suggests the abnormality of the ESCs, which later turned out to be karyotypically abnormal in each of the cases (unpublished). Hence, karyotypic instability is a critical concern of safety. This translates into the appropriate culture system for maintaining the stem cells and certain limit of expansion.
Of more concern regarding safety is the overgrowth of ESC-derived progenitors. To date, transplantation of hESC-differentiated dopamnergic cultures can result in locomotor functional improvement. However, some of these transplants are accompanied by large grafts in the recipient rat brain (Roy et al., 2006), which raised skepticism over whether the symptom improvement is attributed by the grafted dopamine neurons. Examination of dividing cells in the graft revealed that nearly all the proliferating cells carry an anterior phenotype by expressing Otx2 or Foxg1(Yang et al., 2008). As discussed in the previous section, hESC-differentiated neural progenitors are predominantly of a forebrain phenotype and a simple treatment with FGF8 and SHH is not sufficient to restrict them to the midbrain fate. We have previously shown that transplantation of hESC-derived neural progenitors into the neonatal immune deficient mouse brain will continue to divide for about 3 months before exiting cell cycle (Guillaume et al., 2006). We therefore predict that more complete restriction of the progenitors to the midbrain fate and/or removal of these anterior progenitors will allow us a better control of cell proliferation following transplantation.
Due to the xenotransplant nature (human cells to rodent brain) for which daily immuno-suppression is necessary, the test for long-term safety of the grafted cells is difficult. Additionally, the cellular interactions between the grafted human cells and the host rodent cells may also be different from those in allo-transplant. Thus, a better model is needed to assess the efficacy and safety of stem cell progenies in a long term. Recipient animals with an immune deficient background, such as SCID mice, will generally serve the purpose.
An even better model to assess the long-term efficacy and safety is autologous primate transplant model. Non-human primates can be reproducibly induced to develop PD by 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine. Given the success in establishing rodent and human iPS cells, it is realistic to produce monkey iPS cells from biopsied tissues like skin. Differentiation of DA neurons from monkey ES cells can be achieved like human ES cells. Hence, DA neurons differentiated from monkey iPS cells can then be transplanted back to the PD monkey. This will avoid immune rejection, the ultimate frontier stem cell therapy has to face. This will in turn allow long-term observation of the efficacy and safety. This experimental system mimics future application of human iPS cell-derived DA neurons for PD patients (Fig. 2), a model for personalized regenerative medicine.
Acknowledgments
Studies from our laboratory described in the article have been supported by the NIH-NINDS (NS045926, NS046587, NS061243), the Michael J. Fox Foundation, the ALS Association, the National Multiple Sclerosis Society, and partly from the NICHD (P30 HD03352). We thank Ben Thiede and Hong Zhang for preparing the cartoons.
References
- Ahlskog JE. Beating a dead horse: dopamine and Parkinson disease. Neurology. 2007;69:1701–1711. doi: 10.1212/01.wnl.0000296942.14309.4a. [DOI] [PubMed] [Google Scholar]
- Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Ben Hur T, Idelson M, Khaner H, Pera M, Reinhartz E, Itzik A, Reubinoff BE. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22:1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Bergquist F, Riebe I, Nannmark U, Carta M, Hanse E, Takahashi J, Sasai Y, Funa K, Brundin P, Eriksson PS, Li JY. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the Mechanisms Involved in Motor Neuron Degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- Buytaert-Hoefen KA, Alvarez E, Freed CR. Generation of Tyrosine Hydroxylase Positive Neurons from Human Embryonic Stem Cells after Coculture with Cellular Substrates and Exposure to GDNF. Stem Cells. 2004;22:669–674. doi: 10.1634/stemcells.22-5-669. [DOI] [PubMed] [Google Scholar]
- Cho MS, Lee YE, Kim JY, Chung S, Cho YH, Kim DS, Kang SM, Lee H, Kim MH, Kim JH, Leem JW, Oh SK, Choi YM, Hwang DY, Chang JW, Kim DW. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005;14:1709–1725. doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced Pluripotent Stem Cells Generated from Patients with ALS Can Be Differentiated into Motor Neurons. Science. 2008 doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Draper JS, Smith K, Gokhale P, Moore HD, Maltby E, Johnson J, Meisner L, Zwaka TP, Thomson JA, Andrews PW. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22:53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- Eiges R, Urbach A, Malcov M, Frumkin T, Schwartz T, Amit A, Yaron Y, Eden A, Yanuka O, Benvenisty N, Ben-Yosef D. Developmental Study of Fragile X Syndrome Using Human Embryonic Stem Cells Derived from Preimplantation Genetically Diagnosed Embryos. Cell Stem Cell. 2007;1:568–577. doi: 10.1016/j.stem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Fu R, Deng HX, Siddique T, O'Halloran TV. Disulfide cross-linked protein represents a significant fraction of ALS-associated Cu, Zn-superoxide dismutase aggregates in spinal cords of model mice. Proc Natl Acad Sci U S A. 2006;103:7148–7153. doi: 10.1073/pnas.0602048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, Shen J. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Guillaume DJ, Johnson MA, Li XJ, Zhang SC. Human embryonic stem cell-derived neural precursors develop into neurons and integrate into the host brain. J Neurosci Res. 2006;84:1165–1176. doi: 10.1002/jnr.21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund E, Pruszak J, Lardaro T, Ludwig W, Vinuela A, Kim KS, Isacson O. Embryonic stem cell-derived Pitx3-enhanced green fluorescent protein midbrain dopamine neurons survive enrichment by fluorescence-activated cell sorting and function in an animal model of Parkinson's disease. Stem Cells. 2008;26:1526–1536. doi: 10.1634/stemcells.2007-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovitti L, Donaldson AE, Marshall CE, Suon S, Yang M. A protocol for the differentiation of human embryonic stem cells into dopaminergic neurons using only chemically defined human additives: Studies in vitro and in vivo. Brain Res. 2007;1127:19–25. doi: 10.1016/j.brainres.2006.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irion S, Luche H, Gadue P, Fehling HJ, Kennedy M, Keller G. Identification and targeting of the ROSA26 locus in human embryonic stem cells. Nat Biotechnol. 2007;25:1477–1482. doi: 10.1038/nbt1362. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AL, Liu A, Millet S. Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr Opin Cell Biol. 2000;12:736–741. doi: 10.1016/s0955-0674(00)00161-7. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Krencik R, Zhang SC. Stem cell neural differentiation: a model for chemical biology. Curr Opin Chem Biol. 2006;10:592–597. doi: 10.1016/j.cbpa.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, Zhang SC. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinat C, Bacci JJ, Leete T, Kim J, Vanti WB, Newman AH, Cha JH, Gether U, Wang H, Abeliovich A. Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc Natl Acad Sci U S A. 2006;103:2874–2879. doi: 10.1073/pnas.0511153103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Olsen SK, Li JY, Bromleigh C, Eliseenkova AV, Ibrahimi OA, Lao Z, Zhang F, Linhardt RJ, Joyner AL, Mohammadi M. Structural basis by which alternative splicing modulates the organizer activity of FGF8 in the brain. Genes Dev. 2006;20:185–198. doi: 10.1101/gad.1365406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Nakatani T, Sakamoto Y, Mizuhara E, Minaki Y, Kumai M, Hamaguchi A, Nishimura M, Inoue Y, Hayashi H, Takahashi J, Imai T. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007;134:3213–3225. doi: 10.1242/dev.02879. [DOI] [PubMed] [Google Scholar]
- Pankratz MT, Li XJ, Lavaute TM, Lyons EA, Chen X, Zhang SC. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Minn YK, Lee JY, Choi DH, Chang MY, Shim JW, Ko JY, Koh HC, Kang MJ, Kang JS, Rhie DJ, Lee YS, Son H, Moon SY, Kim KS, Lee SH. In vitro and in vivo analyses of human embryonic stem cell-derived dopamine neurons. J Neurochem. 2005;92:1265–1276. doi: 10.1111/j.1471-4159.2004.03006.x. [DOI] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. From the Cover: Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini P, Brooks DJ, Bjorklund A, Gunn RN, Grasby PM, Rimoldi O, Brundin P, Hagell P, Rehncrona S, Widner H, Lindvall O. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat Neurosci. 1999;2:1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- Prakash N, Brodski C, Naserke T, Puelles E, Gogoi R, Hall A, Panhuysen M, Echevarria D, Sussel L, Weisenhorn DM, Martinez S, Arenas E, Simeone A, Wurst W. A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo. Development. 2006;133:89–98. doi: 10.1242/dev.02181. [DOI] [PubMed] [Google Scholar]
- Redmond DE., Jr Cellular replacement therapy for Parkinson's disease--where we are today? Neuroscientist. 2002;8:457–488. doi: 10.1177/107385802237703. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gomez JA, Lu JQ, Velasco I, Rivera S, Zoghbi SS, Liow JS, Musachio JL, Chin FT, Toyama H, Seidel J, Green MV, Thanos PK, Ichise M, Pike VW, Innis RB, McKay RD. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells. 2007;25:918–928. doi: 10.1634/stemcells.2006-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Schneider BL, Seehus CR, Capowski EE, Aebischer P, Zhang SC, Svendsen CN. Over-expression of alpha-synuclein in human neural progenitors leads to specific changes in fate and differentiation. Hum Mol Genet. 2007;16:651–666. doi: 10.1093/hmg/ddm008. [DOI] [PubMed] [Google Scholar]
- Schulz TC, Noggle SA, Palmarini GM, Weiler DA, Lyons IG, Pensa KA, Meedeniya AC, Davidson BP, Lambert NA, Condie BG. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004;22:1218–1238. doi: 10.1634/stemcells.2004-0114. [DOI] [PubMed] [Google Scholar]
- Smidt MP, Burbach JP. How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci. 2007;8:21–32. doi: 10.1038/nrn2039. [DOI] [PubMed] [Google Scholar]
- Sonntag KC, Pruszak J, Yoshizaki T, van Arensbergen J, Sanchez-Pernaute R, Isacson O. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells. 2007;25:411–418. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Zhang SC. Genetic modification of human embryonic stem cells. Biotechnol Genet Eng Rev. 2007;24:297–309. doi: 10.1080/02648725.2007.10648105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang ZJ, Oldenburg M, Ayala M, Zhang SC. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells. 2008;26:55–63. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zeng X, Cai J, Chen J, Luo Y, You ZB, Fotter E, Wang Y, Harvey B, Miura T, Backman C, Chen GJ, Rao MS, Freed WJ. Dopaminergic differentiation of human embryonic stem cells. Stem Cells. 2004;22:925–940. doi: 10.1634/stemcells.22-6-925. [DOI] [PubMed] [Google Scholar]
- Zhao S, Nichols J, Smith AG, Li M. SoxB transcription factors specify neuroectodermal lineage choice in ES cells. Mol Cell Neurosci. 2004;27:332–342. doi: 10.1016/j.mcn.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells. Nat Biotechnol. 2003;21:319–321. doi: 10.1038/nbt788. [DOI] [PubMed] [Google Scholar]