Abstract
Objective:
Damage to the circulatory system resulting from ischemia–reperfusion injury (I/R injury) occurs during heart attacks and hemorrhagic shock. The authors report a method for mitigating microcirculatory injury, using diagnostic frequency continuous-mode ultrasound and how effects are influenced by nitric oxide production impairment.
Methods:
Five groups of hamsters were studied using the dorsal skin fold window chamber: (1) I/R; (2) I/R + ultrasound during ischemia; (3) I/R + ultrasound after ischemia; (4) I/R + Nω-nitro-l-arginine methyl ester (l-NAME); and (5) I/R + l-NAME + ultrasound. Functional capillary density (FCD) and microvascular diameter, flow velocity, and flow were monitored. During the exposures 2.49 MHz continuous ultrasound was used.
Results:
Significant improvements in animals exposed to ultrasound after ischemia were found at 24 h of reperfusion in FCD, arteriolar diameter, and arteriolar and venular flow velocity and flow. Animals exposed to ultrasound during ischemia showed significantly improved FCD. l-NAME treatment reduced the improvement of microvascular function, compared to animals exposed after ischemia.
Conclusions:
The use of continuous-mode diagnostic frequency ultrasound is beneficial in preventing long-term ischemia–reperfusion effects in the microcirculation as shown by the return of microvascular parameters to baseline values, an effect not attained in the absence of ultrasound treatment. The effects may be in part due to the production of nitric oxide consequent to locally induced shear stress effects by ultrasound exposure.
Keywords: diagnostic frequency ultrasound, ischemia–reperfusion, l-NAME, microcirculation
Ischemia–reperfusion injury (I/R injury) is a two-step process that begins with the cessation of blood flow to a tissue or organ, leading to an oxygen deficit and the buildup of toxic metabolites normally removed by the flowing blood. Restoration of blood flow stops and reverses the ischemic damage, but gives rise to cellular injury due to the formation of oxygen radicals [2, 11, 19, 24, 28] resulting from the sudden influx of significant quantities of oxygen. I/R injury occurs in victims of heart attacks, strokes, and hemorrhagic shock. It is a process particularly evident in the microcirculation, where arterioles show impaired vasoactivity [10, 19], blood flow in the capillary network is reduced [4, 11, 19], and leukocyte sticking [2, 10, 11, 29] and migration from the lumen to the surrounding tissue [30] takes place in venules.
There is evidence that the damage consequent to I/R injury may be in part due to the impairment of nitric oxide (NO) production by the microvascular endothelium, a process partly due to shear stress and mechanotransduction [3, 9] resulting from the flowing blood. Mechanotransduction enables NO production [4] and has been proposed to be the underlying cause of the beneficial effects resulting from the exposed tissue (and organisms), recovering from sustained I/R injury, to ultrasound [27]. Ultrasound exposure was shown to stimulate DNA and protein synthesis [22] and NO production [21], and to induce cellular alterations and enhance cellular proliferation [20] in cell cultures. It was also shown to increase capillary perfusion and microvascular diameter and velocity in animals recovering from sustained I/R injury [5].
Currently most experiments using ultrasound to obtain effects in tissues and cell cultures employ low-range frequencies with continuous mode [21, 22, 26] or diagnostic frequencies [1, 5] with pulse mode exposure. Low-frequency, continuous-mode exposures allow for greater tissue penetration without heating. Pulse-mode exposures used in diagnostic procedures have a shorter period of interaction with the system being exposed when compared to the continuous mode. In this context the use of the lower ranges of diagnostic frequencies (MHz) in combination with continuous-mode exposure allows for greater depth in tissue penetration and a longer period of interaction.
The objective of this study was to investigate the efficacy of continuous-mode (i.e., 100% duty cycle) diagnostic frequency ultrasound exposure in mitigating the damage to the microcirculation resulting from I/R injury. Specifically, this study analyzes how functional capillary density (FCD) and microvascular diameter and velocity are influenced by exposure to continuous-mode diagnostic frequency ultrasound after I/R injury. In view of the significant role that NO has in mediating I/R injury and the mechanical nature of the ultrasound intervention we also test the hypothesis that the effects of ultrasound in I/R injury are due to the reversal of NO production impairment in the endothelium.
MATERIALS AND METHODS
Animal Model and Preparation
The dorsal skin fold window chamber model (chamber) was used for investigation in Golden Syrian male hamsters (Charles River, Boston, MA), weight range of 50–75 g. The Guide for the care and use of laboratory animals (National Research Council, 1996) was followed for animal handling and provided care. Experiments are approved by the University of California, San Diego, Animal Subjects Committee. The chamber, a widely used procedure, eliminates anesthetic influence during microcirculation examination. As described previously [8], chamber and carotid artery catheterization surgery was performed under general anesthesia, 50 mg/kg IP injection of pentobarbital sodium [10]. TPX (Westlake Plastics, Placentia, CA) was used in place of the usual glass coverslip. TPX incorporates the protective properties of glass with greater acoustical coupling [7]. Initial microvascular observation carried out at least 3 days after chamber implantation, diminished postsurgical complications.
Inclusion Criteria
Animals used were free of edema, preobservation injury to window area, and/or infection to surgical sites.
Assessment of Microcirculatory Parameters
All microscopic observations were preformed using an upright microscope (BX51WI; Olympus, New Hyde Park, NY). Images in the microscopic field of view could also be viewed on a monitor, through the projection of the image onto a charge-coupled device camera (COHU 4815) connected to a video-cassette recorder (AG-7355; JVC, Tokyo, Japan). A 40× (LUMPFL-WIR, numerical aperture 0.8; Olympus) water-immersion objective was used in both transillumination and epiillumination observations. Contrast enhancement between flowing red blood cells (RBC) and tissue was accomplished using a BG12 (420-nm) band pass filter. Epiillumination microscopy used an additional mercury 100-W lamp and appropriate filters. Vessel diameter (D) was obtained online using a video shearing technique [14]. Real-time blood flow velocity (V ) was acquired using the photodiode cross-correlation method (Fiber Optic Photo Diode and Velocity Tracker, model 102B; Vista Electronics, San Diego, CA) [13], using a correction factor, average velocity (Va) was calculated, Va = V /1.6 [17]. Blood flow (Q) was calculated using the measured and calculated parameters, Q = πVa*(D/2)2. FCD was determined in 13–25 stepwise vertically successive microscopic fields, (a region of about 1.7–1.9 mm2). The initial field chosen by an anatomical feature allowed quick recognition in repeated measurements. Functional capillaries had at least one RBC flowing during the observation period.
Only arterioles and venules with baseline diameters less than 60 μm were included in the present study.
Ischemia–Reperfusion Injury
A pressure tourniquet, described previously [10, 18], was used to induce ischemia in the chamber. Flow of the area under ischemia, interrupted by tightening a screw connected to the chamber, was monitored until complete occlusion of all feeding and draining vessels was obtained [18]. Ischemia was ensured through periodic inspection. The release of the tourniquet marked the end of ischemia and start of reperfusion.
Ultrasound Exposure
During ultrasound exposure animals were suspended over the ultrasound transducer using a 3-pronged clamp and support ring stand. The transducer (Valpey Fisher Corporation immersion transducer, part #IL0208HP, nominal frequency 2.25 MHz, element diameter 1 in.) was secured in a water bath. Animal exposure was accomplished using setup 1 or 2. Setup 1: A degassed, water-filled piece of latex glove covered the transducer head. A thin layer of acoustic gel connected the latex and cover slip. Setup 2: An open-ended plastic cone affixed to the face of the transducer, filled with degassed water. In each setup a piece of lightweight rubber absorbed waves continuing through the skin fold. Waves were generated using a function generator (30 MHz Synthesized Function Generator, model DS345; Stanford Research Systems, Sunnyvale, CA) set to produce a continuous sine wave at a frequency of 2.49 MHz with a 10-Vp–p amplitude. Resultant intensity and pressure produced is 8.33 W/cm2 and about 0.5 MPa, respectively. (The transducer was calibrated using a hydrophone in the Ferrara Laboratory, Department of Biomedical Engineering, University of California, Davis).
Experimental Groups
Animals were randomly assigned into one of 5 groups as follows: I/R group (n = 5), I/R + ultrasound during ischemia group (n = 5), I/R + ultrasound after ischemia group (n = 5), I/R +Nω-nitro-l-arginine methyl ester (l-NAME) group (n = 5), and I/R + l-NAME + ultrasound group (n = 5).
Experimental Setup and Protocol
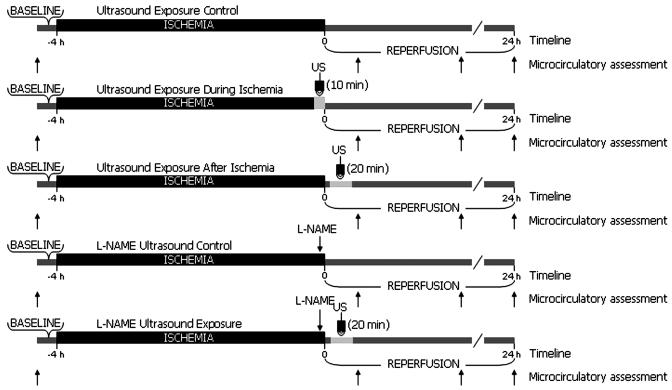
Conscious animals were placed in plexiglass restraining tubes, allowing for protrusion of chamber and minimized movement during observation and ultrasound exposure. Tubes containing hamsters were further secured to custom-made frames and placed on the microscope stage. Similar microcirculatory assessment procedures were preformed in all groups. Transillumination was used for baseline vascular architecture documentation and vessel site selection. Vessels were investigated in terms of diameter, RBC velocity, and blood flow. The same measurement sites were used throughout each experiment to allow direct comparison with baseline values. FCD was also determined during this time. Animals were then subjected to a 4-h period of ischemia and measurements were repeated at 0.5, 2, and 24 h, from the start of reperfusion. Vessel diameter and FCD were assessed using epiillumination during repeated measurements, while transillumination was used for vessel flow velocity. An injection of fluorescein isothiocyanate, bound to dextran (MW 150,000; FITC-Dextran 150 Sigma Chemical, St. Louis, MO; 0.1 mL of a 12.5-mg/mL saline solution) was given 5–10 min before each individual repeated measurement period. Epiillumination and FITC-Dextran normally used for enhanced visualization of vessels was used here to augment damage sustained from I/R [10]. The volume for infusion of l-NAME (Sigma) solution in saline was less than 5% of systemic blood volume, an estimate of 7% of hamster total body weight on day 1 of the experiment. Injections were dosed at 10 mg/kg in each group. The sample solution of 5 mL/g (0.1 to 0.15 ml) was infused through the carotid artery by hand and the catheter was flushed with heparinized saline (Figure 1).
Figure 1.
Schematic outline of the experimental protocol for each group. US, ultrasound.
Ischemia–Reperfusion and Ultrasound Exposure Groups
Experimental procedure of the I/R and I/R + ultrasound groups followed the general protocol as mentioned above. In addition, the I/R + ultrasound during ischemia group was exposed to ultrasound for a 10-min period, during the last 10 min of ischemia. The I/R + ultrasound after ischemia group was exposed to ultrasound for a 20-min period, 5 min following the onset of reperfusion.
Ischemia–Reperfusion l-NAME and Ultrasound Exposure Groups
Experimental procedure for the I/R + l-NAME and I/R + l-NAME + ultrasound groups followed the general protocol for baseline and repeated microcirculation assessments. l-NAME was administered to both groups as a bolus injection 5 min before the release of the tourniquet. Animals in the I/R + l-NAME + ultrasound group were exposed to ultrasound for a period of 20 min, 5 min following the onset of reperfusion.
Statistical Analysis
Results are presented as means ± standard deviation. Data graphs are presented normalized to baseline values. Data comparisons made within groups and between group controls and treatments were analyzed using the Kruskal-Wallis nonparametric test with Dunn's post test or the Mann-Whitney non-parametric test. Differences were considered significant for p < .05. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software; San Diego, CA).
RESULTS
Ischemia–Reperfusion and Ultrasound Exposure Groups
In this study we focus on the changes due to ultrasound exposure not those due to ischemia–reperfusion. Ultrasound treatment significantly increased FCD 24 h after release of the tourniquet in both I/R + ultrasound during ischemia and after ischemia groups relative to unexposed animals; FCD was below baseline for all groups at all time points with the exception of one animal exposed to ultrasound after ischemia (p < .01, Figure 2).
Figure 2.
Changes in functional capillary density (FCD) due to ischemia–reperfusion injury and exposure to ultrasound. FCD of groups receiving ultrasound exposure, for 10 min during ischemia and for 20 min after ischemia showed a significant increase from I/R at 24 h. Data are presented as means ± standard deviations. I/R, I/R group; D, I/R + ultrasound during ischemia group; A, I/R + ultrasound after ischemia group. * – p < .05 vs. I/R.
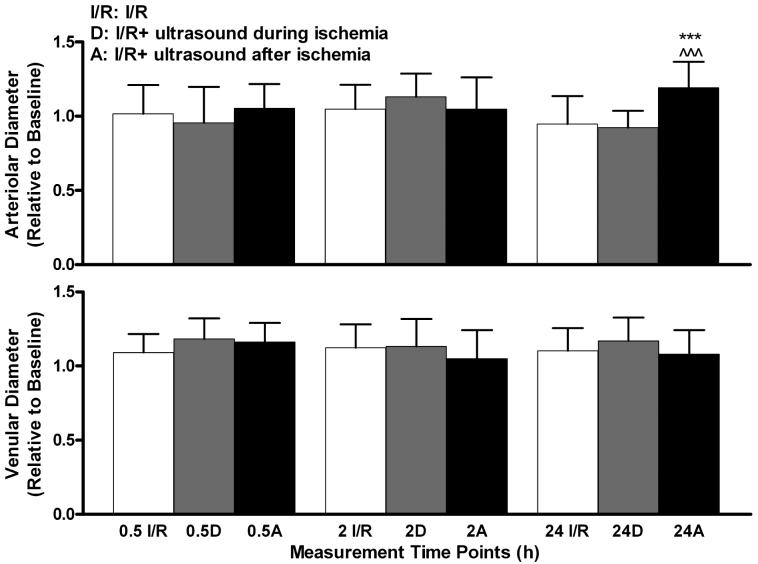
Vessel diameters increased for most time points during reperfusion in all animal groups. At 24 h, arteriolar diameters of animals exposed to ultrasound after ischemia were significantly increased above those of both the I/R and I/R + ultrasound during ischemia groups (p < .001, Figure 3).
Figure 3.
Changes in arteriolar and venular diameters due to ischemia–reperfusion injury and exposure to ultrasound. Top: I/R + ultrasound after ischemia animals showed significant increase of arteriolar diameters at 24 h, compared to both I/R and I/R + ultrasound during ischemia animals. Bottom: No significant venular diameter difference from I/R was found for either of the groups exposed to ultrasound. Values are presented as means ± standard deviations. I/R, I/R group; D, I/R + ultrasound during ischemia; A, I/R + ultrasound after ischemia group. * * * – p < .001 vs. I/R. ∧∧∧ – p < .001 vs. IR + ultrasound during ischemia.
A common trend was seen in the arteriolar flow velocity of all groups. Flow velocity values recorded at 0.5 h into reperfusion were observed to decrease at 2 h and then to increase at 24 h. All groups showed significance in the decrease at 2 h, with the greatest significance seen in the I/R group (p < .001). A significant increase at 24 h was only seen in the I/R + ultrasound after ischemia group. At 24 h both arteriolar and venular flow velocity of the I/R + ultrasound after ischemia group increased significantly over that of the I/R group (p < .05). Arteriolar flow velocity of the I/R + ultrasound after ischemia group was also significantly increased at 24 h compared with the group exposed to ultrasound during ischemia (p < .001, Figure 4).
Figure 4.
Changes in arteriolar and venular flow velocity due to ischemia–reperfusion injury and ultrasound exposure. Top: Arteriolar flow velocity of I/R + ultrasound after ischemia animals significantly increased at 24 h, compared to both I/R and I/R + ultrasound during ischemia animals. Bottom: I/R + ultrasound after ischemia animals showed significant increases in venular flow velocity at 24 h, compared to the I/R group. Values are presented as means ± standard deviations. I/R, I/R group; D, I/R + ultrasound during ischemia group; A, I/R + ultrasound after ischemia group. +++ – p < .001 vs. I/R 0.5 h assessment. ββ – p < .01 vs. I/R + ultrasound during ischemia 0.5 h assessment. Φ – p < .05 vs. I/R + ultrasound after ischemia 0.5 h assessment. * * * – p < .001 vs. I/R. ∧∧∧ – p < .001 vs. I/R + ultrasound during ischemia. * – p < .05 vs. I/R.
Blood flow was maintained in all vessels of all groups throughout the reperfusion period. Calculated flows showed substantial increases in the arteriolar and venular flow of the group exposed to ultrasound after ischemia compared to the I/R group only at 24 h. A similar trend seen in the arteriolar flow velocity of each group was evident in the calculated blood flow. Blood flow values 0.5 h into reperfusion were observed to decrease at 2 h and then to increase at 24 h (Figure 5). All groups were significantly decreased at 2 h, with a statistically significant increase at 24 h, above both 0.5 and 2 h flows, seen in the I/R + ultrasound after ischemia group (Figure 5).
Figure 5.
Calculated changes in arteriolar and venular flow due to ischemia–reperfusion injury and ultrasound exposure. Top: In the I/R + ultrasound after ischemia group arteriolar flow significantly increased at 24 h, compared to both the I/R and the I/R + ultrasound during ischemia groups. Bottom: Venular flow significantly increased at 24 h, for the I/R + ultrasound after ischemia group compared to the I/R group. Values are presented as means ± standard deviations. I/R, I/R group; D, I/R + ultrasound during ischemia group; A, I/R + ultrasound after ischemia group. +++ – p < .001 vs. I/R 0.5 h assessment. β – p < .05 vs. I/R + ultrasound during ischemia 0.5 h assessment. Φ – p < .05 vs. I/R + ultrasound after ischemia 0.5 h assessment. * * * – p < .001 vs. I/R. ∧∧∧ – p < .001 vs. I/R + ultrasound during ischemia. ∇∇ – p < .01 vs. I/R + ultrasound after ischemia 0.5 h assessment. ΨΨΨ – p < .001 vs. I/R + ultrasound after ischemia 2 h assessment. * – p < .05 vs. I/R.
Ischemia–Reperfusion l-NAME and Ultrasound Exposure Groups
The degree of I/R injury impacted by NO production inhibition was determined by changes in FCD and vessel diameter, flow velocity, and flow, relative to baseline at each measurement. The effect of the l-NAME bolus injection was seen in arteriolar and venular flow velocity and flow, at 0.5 h of reperfusion, being statistically different from I/R animal arteriolar flow velocity (Figure 8). Figure 6 shows how NO inhibition affects FCD at each repeated measurement. FCD was decreased in all animals, compared to baseline values. It was higher in I/R + l-NAME group animals at the 0.5- and 24-h measurements; however, this observation did not reach statistical significance.
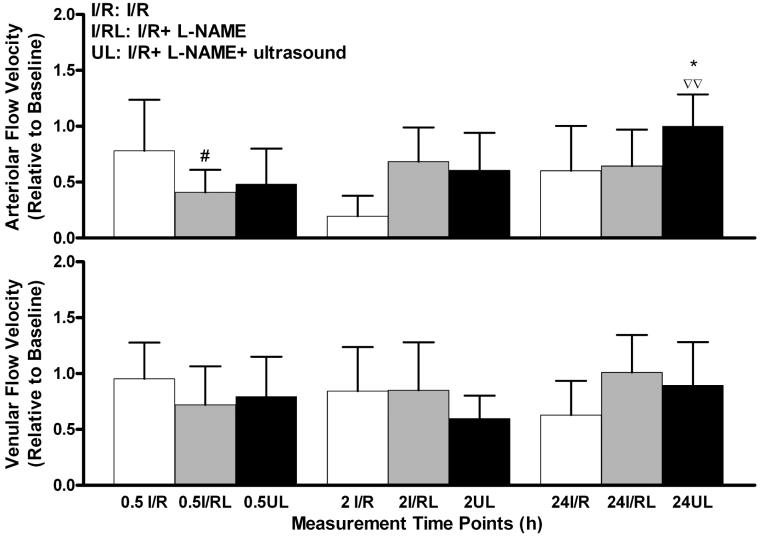
Figure 8.
Changes in arteriolar and venular flow velocity due to ischemia–reperfusion injury, exposure to ultrasound, and NO production inhibition using l-NAME. Top: The influence of l-NAME is seen in the 0.5-h measurement, with a significant decrease compared to I/R. Arteriolar velocity exhibited an increase from 0.5 h through the 24-h time point. A significant increase was seen at 24 h in I/R + l-NAME + ultrasound animals, compared to I/R + l-NAME. Bottom: Venular velocity of both groups was highest at the 24-h time point but not significantly different. Values are presented as means ± standard deviation. I/R, I/R group; I/RL, I/R + l-NAME group; UL, I/R + l-NAME + ultrasound group. #p < .05 vs. I/R. * – p < .05 vs. I/R + l-NAME. ∇∇ – p < .01 vs. I/R + l-NAME + ultrasound 0.5-h assessment.
Figure 6.
Changes in functional capillary density (FCD) due to ischemia–reperfusion injury, exposure to ultrasound, and NO production inhibition using l-NAME. FCD of the I/R + l-NAME group is greater than that of the I/R + l-NAME + ultrasound group, at 0.5 and 24 h, without statistical significance. Data are presented as means ± standard deviation. I/R, I/R group; I/RL, I/R + l-NAME group; UL, I/R + l-NAME + ultrasound group.
Figure 7 shows the changes in arteriolar and venular diameter during reperfusion, comparing the I/R + l-NAME and I/R + l-NAME + ultrasound groups. The majority of arteriolar and venular values were increased compared to baseline. Changes in arteriolar and venular diameter between l-NAME groups were not significant.
Figure 7.
Changes in arteriolar and venular diameters due to ischemia–reperfusion injury, exposure to ultrasound, and NO production inhibition using l-NAME. Top: Most arteriolar diameters dilated compared to baseline values. Bottom: Venular diameters also show mostly dilation at all observation time points compared to baseline values, without statistical significance. Values are presented as means ± standard deviation. I/R, I/R group; I/RL, I/R + l-NAME group; UL, I/R + l-NAME + ultrasound group.
Vessel flow velocity decreased compared to baseline, for both arterioles and venules up to 24 h after termination of ischemia (Figure 8).Values increased at 24 h in each l-NAME group for both arterioles and venules, although significant increases compared to the I/R + l-NAME were seen only in arterioles, exposed to ultrasound (p < .05). A significant increase was seen from the 0.5-h to the 24-h measurement in the animals treated with l-NAME and exposed to ultrasound (Figure 8).
Blood flow was maintained in all animals after receiving l-NAME treatment and exposure to ultrasound. A statistically significant difference in arteriolar flow was seen at 24 h compared to the I/R + l-NAME group (Figure 9). The arteriolar flow of the I/R + l-NAME + ultrasound group showed a significant increase from the 0.5-h to the 24-h measurement. Both arteriolar and venular flow also showed statistical significance in the increased flow from the 2-h to the 24-h measurement (Figure 9). Flow of one arteriole and one venule did not return in the I/R + l-NAME group after the ischemic period. Arteriolar flow did not return through 24 h of reperfusion; while venular flow returned at 24 h. Vessels lacking flow at any time point were excluded from all graphs.
Figure 9.
Changes in arteriolar and venular flow due to ischemia–reperfusion injury, exposure to ultrasound, and NO production inhibition using l-NAME. Top: Arteriolar flow increased in I/R + l-NAME + ultrasound animals, while the increase at 2 h was not maintained through 24 h in I/R + l-NAME animals. Statistical significance was seen at 24 h for I/R + l-NAME + ultrasound compared to I/R + l-NAME animals. Bottom: Venular flow exhibited a stepwise increase for I/R + l-NAME animals, while the decrease at 2 h increased at 24 h for ultrasound exposed animals. No significant differences were seen between the groups. Values are presented as means ± standard deviation. I/R, I/R group; I/RL, I/R + l-NAME group; UL, I/R + l-NAME + ultrasound group. ** – p < .01 vs. I/R + l-NAME ∇∇ – p < .01 vs. I/R + L-NAME + ultrasound 2-h assessment. +++ – p < .001 vs. I/R + l-NAME + ultrasound 0.5-h assessment.
I/R + Ultrasound After Ischemia and I/R + l-NAME + Ultrasound Exposure Groups
We compared the changes in flow in the two groups (Figures 5 and 9). Flow was decreased at 0.5 and 24 h in both groups treated with l-NAME compared to the group I/R + ultrasound after ischemia. Decreased flow in the I/R + l-NAME + ultrasound group at the 0.5 h assessment was statistically significantly different ( p < .001) from the I/R + ultrasound after ischemia group at the 0.5 h point, while there was no significant difference at 24 h (p = .1).
DISCUSSION
The principal finding of this study is that exposure to diagnostic frequency continuous ultrasound after 4 h of ischemia improves FCD and microvascular flow 24 h after reperfusion. This result was obtained by exposing the tissue to continuous wave ultrasound for 20 min, 5 min from the onset of reperfusion. Exposure of the tissue to the same ultrasound settings for 10 min, during the last 10 min of the ischemic period, resulted in significant improvement of FCD seen 24 h into reperfusion. These improvements were negated by the inhibition of NO production by treatment with l-NAME prior to reperfusion. Although ultrasound did improve l-NAME-treated animals at 24 h after reperfusion, the degree of recovery was significantly reduced by comparison to the untreated group.
Twenty minutes of ultrasound exposure resulted in microcirculatory improvements in all microvessels, while 10 min exposure only improved FCD. The difference in effects may be due to several factors. It is hypothesized that vessel blood flow may be necessary to produce beneficial effects through the production of mediators. This hypothesis is contradicted in part by the observations of Suchkova et al. [26] who found that tissue perfusion increased and acidosis decreased during thrombosis throughout the application of ultrasound (40 kHz, continuous-wave mode). However, the increased perfusion was thought to result from collateral vessel flow, which is not a possibility in our experimental setup using complete ischemic isolation of blood flow to the area under investigation. A second possibility for explaining the differences in ultrasound exposure effects is based on the existence of a threshold time limit. The 10-min exposure did not reach the time needed to successfully influence the damaged microcirculation. This result could be related to the existence of a threshold of total acoustic energy exposure that determines positive effects in endothelial cell cultures proposed by Raz et al. [20] that is dependent on the amount of energy transferred.
Experimental studies examining the influence of preventative treatments or treatment administered after sustained injury, followed over extended observation periods, report attainment of statistical significance when assessed after an extended period with little or no significance in short-term assessments. Significant differences between control and endothelial cell cultures exposed to different parameters of ultrasound irradiation became evident days after the first 24-h observation [20]. Hangai-Hoger et al. [12] observed a significant improvement of FCD in animals resuscitated from septic shock with polyethylene glycol conjugated bovine albumin (PEG-BSA-24). This result was only apparent from systemic and microvascular measurements 24 h after initial treatment.
The efficacy of the dosage of l-NAME in inhibiting NO bioavailability was previously established in other investigations in the same preparation and tissue. In these studies of vasoconstriction and arterial flow, reduction was found with the dosage of 10 mg/kg [23].
Observations of l-NAME-treated animals suggest the existence of both fast- and slow-acting responses to ultrasound exposure. l-NAME treatment may influence only the earlier stimulation effect, leaving the slower response to continue its action, resulting in the decrease of significant microvascular improvements. l-NAME being a nitric oxide synthase (NOS) inhibitor directly affects the arterioles because of the NO's effects on smooth muscle. The majority of microcirculatory improvements observed in animals not treated with l-NAME were in the arterioles, suggesting that improvements previously seen in venules and capillaries were indirect effects of ultrasound stimulation. The moderate improvement of arteriolar flow velocity and flow may have led to the elimination of improvements in the downstream vessels. It may also suggest that decreased improvements of venules may be due to other factors not investigated in this study, such as the accumulation of leukocytes in venules, which may be reduced with the presence of NO [15, 16]. This hypothesis may also partially explain the reduction in FCD.
Decreased initial reperfusion flow would lessen vessel exposure to oxygen radicals, while still rapidly flushing away built-up metabolites. Animals exposed to ultrasound after ischemia showed decreased initial arteriolar flow compared to control animals 0.5 h into reperfusion. Two hours into reperfusion the decrease in arteriolar flow is hypothesized to be evidence of the amount of damage caused by ischemia and initial reperfusion. This effect may be in part responsible for lowering reperfusion damage, since it inherently limits the availability of oxygen, directly implicating the formation of ROS.
The animal groups treated with l-NAME also show evidence of the benefit of reduced initial arteriolar flow. The 0.5- and 24-h flows of the I/R + l-NAME + ultrasound group are decreased compared to the I/R + ultrasound after ischemia group, further supporting the hypothesis that an intermediate initial flow is best for long-term recovery. The increase in arteriolar flow at 2 h for each l-NAME group from 0.5 h, as compared to the decrease seen in the groups not treated with l-NAME, is thought to be an artifact of l-NAME treatment. The use of l-NAME as the only NOS inhibitor in the study does not allow for a definitive distinction between the three forms of NOS, endothelial, inducible, and neuronal, although it does have a greater capacity to prevent eNOS and nNOS production over that of iNOS [6, 25].
In summary this study provides evidence of a new approach in the therapeutic use of ultrasound. It also suggests that further improvements may be obtained without increased ultrasound exposure by including delivery of naturally occurring beneficial mediators of vascular health, such as l-arginine. Evidence for the benefits of diagnostic cardiac [5], high-frequency [1, 21, 22], and low-frequency [21, 22, 26] ultrasound have been previously demonstrated. Our recent observations expand the range of beneficial ultrasound applications, showing its applicability in conditions of microvascular I/R injury.
Acknowledgments
The authors thank Froilan P. Barra and Cynthia Walser for the surgical preparation of the animals. The authors also gratefully acknowledge Dustin E. Kruse (University of California, Davis) for his discussion on and assistance with ultrasonics and the calibration of our ultrasound transducer. This work has been supported by the NIH Grant HL 40696.
REFERENCES
- 1.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moye LA, Hill MD, Wojner AW. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 2.Becker BF, Kupatt C, Massoudy P, Zahler S. Reactive oxygen species and nitric oxide in myocardial ischemia and reperfusion. Z Kardiol. 2000;89(Suppl 9):IX/88–91. doi: 10.1007/s003920070037. [DOI] [PubMed] [Google Scholar]
- 3.Bertuglia S, Giusti A. Microvascular oxygenation, oxidative stress, NO suppression and superoxide dismutase during postischemic reperfusion. Am J Physiol Heart Circ Physiol. 2003;285:H1064–H1071. doi: 10.1152/ajpheart.00124.2003. [DOI] [PubMed] [Google Scholar]
- 4.Bertuglia S, Giusti A. Influence of ACTH-(1-24) and plasma hyperviscosity on free radical production and capillary perfusion after hemorrhagic shock. Microcirculation. 2004;11:227–238. doi: 10.1080/10739680490425930. [DOI] [PubMed] [Google Scholar]
- 5.Bertuglia S, Giusti A, Picano E. Effects of diagnostic cardiac ultrasound on oxygen free radical production and microvascular perfusion during ischemia reperfusion. Ultrasound Med Biol. 2004;30:549–557. doi: 10.1016/j.ultrasmedbio.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Boer R, Ulrich WR, Klein T, Mirau B, Haas S, Baur I. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol. 2000;58:1026–1034. [PubMed] [Google Scholar]
- 7.Booi RC, Krucker JF, Goodsitt MM, O'Donnell MO, LeCarpentier GL, Roubidoux MA, Fowlkes JB, Carson PL. Evaluation of thin compression paddles for mammographically compatible ultrasound. Ultrasonics Symposium, 2004. IEEE. 2004;3:2129–2132. doi: 10.1016/j.ultrasmedbio.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endrich B, Asaishi K, Gotz A, Messmer K. Technical report—a new chamber technique for microvascular studies in unanesthetized hamsters. Res Exp Med (Berl) 1980;177:125–134. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- 9.Frangos JA, Huang TY, Clark CB. Steady shear and step changes in shear stimulate endothelium via independent mechanisms: superposition of transient and sustained nitric oxide production. Biochem Biophys Res Commun. 1996;224:660–665. doi: 10.1006/bbrc.1996.1081. [DOI] [PubMed] [Google Scholar]
- 10.Friesenecker B, Tsai AG, Instaglietta M. Capillary perfusion during ischemia–reperfusion in subcutaneous connective tissue and skin muscle. Am J Physiol. 1994;267:H2204–H2212. doi: 10.1152/ajpheart.1994.267.6.H2204. [DOI] [PubMed] [Google Scholar]
- 11.Grace PA. Ischaemia–reperfusion injury. Br J Surg. 1994;81:637–647. doi: 10.1002/bjs.1800810504. [DOI] [PubMed] [Google Scholar]
- 12.Hangai-Hoger N, Nacharaju P, Manjula BN, Cabrales P, Tsai AG, Acharya SA, Intaglietta M. Microvascular effects following treatment with polyethylene glycol-albumin in lipopolysaccharide-induced endotoxemia. Crit Care Med. 2006;34:108–117. doi: 10.1097/01.ccm.0000190623.97200.82. [DOI] [PubMed] [Google Scholar]
- 13.Intaglietta M, Tompkins WR. System for the measurement of velocity of microscopic particles in liquids. IEEE Trans Biomed Eng. 1971;18:376–379. doi: 10.1109/tbme.1971.4502869. [DOI] [PubMed] [Google Scholar]
- 14.Intaglietta M, Tompkins WR. On-line measurement of microvascular dimensions by television microscopy. J Appl Physiol. 1972;32:546–551. doi: 10.1152/jappl.1972.32.4.546. [DOI] [PubMed] [Google Scholar]
- 15.Koeppel TA, Thies JC, Schemmer P, Trauner M, Gebhard MM, Otto G, Post S. Inhibition of nitric oxide synthesis in ischemia/reperfusion of the rat liver is followed by impairment of hepatic microvascular blood flow. J Hepatol. 1997;27:163–169. doi: 10.1016/s0168-8278(97)80297-8. [DOI] [PubMed] [Google Scholar]
- 16.Kurose I, Wolf R, Grisham MB, Aw TY, Specian RD, Granger DN. Microvascular responses to inhibition of nitric oxide production: role of active oxidants. Circ Res. 1995;76:30–39. doi: 10.1161/01.res.76.1.30. [DOI] [PubMed] [Google Scholar]
- 17.Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 18.Menger MD, Steiner D, Messmer K. Microvascular ischemia–reperfusion injury in striated muscle: significance of “no reflow.”. Am J Physiol. 1992;263:H1892–H1900. doi: 10.1152/ajpheart.1992.263.6.H1892. [DOI] [PubMed] [Google Scholar]
- 19.Menger MD, Pelikan S, Steiner D, Messmer K. Microvascular ischemia–reperfusion injury in striated muscle: significance of “reflow paradox.”. Am J Physiol. 1992;263:H1901–H1906. doi: 10.1152/ajpheart.1992.263.6.H1901. [DOI] [PubMed] [Google Scholar]
- 20.Raz D, Zaretsky U, Einav S, Elad D. Cellular alterations in cultured endothelial cells exposed to therapeutic ultrasound irradiation. Endothelium. 2005;12:201–213. doi: 10.1080/10623320500227317. [DOI] [PubMed] [Google Scholar]
- 21.Reher P, Doan N, Bradnock B, Meghji S, Harris M. Therapeutic ultrasound for osteoradionecrosis: an in vitro comparison between 1 MHz and 45 kHz machines. Eur J Cancer. 1998;34:1962–1968. doi: 10.1016/s0959-8049(98)00238-x. [DOI] [PubMed] [Google Scholar]
- 22.Reher P, Harris M, Whiteman M, Hai HK, Meghji S. Ultrasound stimulates nitric oxide and prostaglandin E2 production by human osteoblasts. Bone. 2002;31:236–241. doi: 10.1016/s8756-3282(02)00789-5. [DOI] [PubMed] [Google Scholar]
- 23.Sakai H, Hara H, Tsai AG, Tsuchida E, Intaglietta M. Constriction of resistance arteries determines l-NAME-induced hypertension in a conscious hamster model. Microvasc Res. 2000;60:21–27. doi: 10.1006/mvre.2000.2240. [DOI] [PubMed] [Google Scholar]
- 24.Seal JB, Gewertz BL. Vascular dysfunction in ischemia–reperfusion injury. Ann Vasc Surg. 2005;19:572–584. doi: 10.1007/s10016-005-4616-7. [DOI] [PubMed] [Google Scholar]
- 25.Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 26.Suchkova VN, Baggs RB, Francis CW. Effect of 40-kHz ultrasound on acute thrombotic ischemia in a rabbit femoral artery thrombosis model: enhancement of thrombolysis and improvement in capillary muscle perfusion. Circulation. 2000;101:2296–2301. doi: 10.1161/01.cir.101.19.2296. [DOI] [PubMed] [Google Scholar]
- 27.Suchkova VN, Baggs RB, Sahni SK, Francis CW. Ultrasound improves tissue perfusion in ischemic tissue through a nitric oxide dependent mechanism. Thromb Haemost. 2002;88:865–870. [PubMed] [Google Scholar]
- 28.Tauber S, Menger MD, Lehr HA. Microvascular in vivo assessment of reperfusion injury: significance of prostaglandin E(1) and I(2) in postischemic “no-reflow” and “reflow-paradox.”. J Surg Res. 2004;120:1–11. doi: 10.1016/S0022-4804(03)00332-9. [DOI] [PubMed] [Google Scholar]
- 29.Wood JG, Johnson JS, Mattioli LF, Gonzalez NC. Systemic hypoxia promotes leukocyte–endothelial adherence via reactive oxidant generation. J Appl Physiol. 1999;87:1734–1740. doi: 10.1152/jappl.1999.87.5.1734. [DOI] [PubMed] [Google Scholar]
- 30.Wood JG, Johnson JS, Mattioli LF, Gonzalez NC. Systemic hypoxia increases leukocyte emigration and vascular permeability in conscious rats. J Appl Physiol. 2000;89:1561–1568. doi: 10.1152/jappl.2000.89.4.1561. [DOI] [PubMed] [Google Scholar]