Abstract
Background. Radical re-resection is offered to patients with non-metastatic, invasive, incidental gallbladder cancer. Data evaluating 18F-fluorodeoxyglucose positron emission tomography–computed tomography (18F-FDG PET–CT) in patients with incidental gallbladder cancer is sparse. Aim. To evaluate the efficacy of integrated 18F-FDG PET–CT in determining occult metastatic or residual local–regional disease in patients with incidental gallbladder cancer. Methods. Patients referred with incidental gallbladder cancer for radical re-resection were evaluated using multidetector computed tomography (MDCT) and PET–CT. Based on preoperative imaging, 24 out of 92 patients were found suitable for surgery. The two imaging modalities were evaluated with respect to residual and resectable disease. Results. In determining residual disease, MDCT had a sensitivity and positive predictive value (PPV) of 42.8%, each, while PET–CT had a sensitivity and PPV of 28.5 and 20%, respectively. In determining resectability, MDCT had a sensitivity, PPV, and accuracy of 100, 87.5, and 87.5%, respectively, as compared to PET–CT (sensitivity=100%, PPV=91.3%, accuracy=91.6%). Conclusions. From our study, it appears that in patients with incidental gall bladder cancer without metastatic disease, PET–CT and MDCT seem to have roles complementing each other. PET–CT was able to detect occult metastatic or residual local–regional disease in some of these patients, and seems to be useful in the preoperative diagnostic algorithm of patients whose MDCT is normal or indicates locally advanced disease.
Keywords: gallbladder, incidental, imaging, PET–CT
Introduction
Radical re-resection remains the most effective tool in the management of patients with incidental gallbladder cancer 1,2. It is indicated in all patients with lesions >T1b and T2 (determined on the histopathological examination of the excised specimen of the simple cholecystectomy) 3,4,5,6,7,8,9, in the absence of metastatic disease. Surgery does not offer any survival benefit in gallbladder cancer in patients with distant metastasis. Further, incomplete resection is associated with an equally dismal prognosis 10,11. It is therefore, important to detect patients who have advanced disease and are unlikely to benefit from an exploratory surgery 12. This would facilitate offering these patients palliative treatment options or enrolling them into research protocols.
The currently used imaging modalities for preoperative staging of the disease include multidetector computed tomography (MDCT) scanning and magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP). MDCT is a useful tool in staging of the disease by identifying the presence of residual disease in the gallbladder fossa and also regional metastasis to the lymph nodes draining the gall bladder, liver, and ascites. However, there does exist the possibility of understaging the disease since these imaging modalities may not detect distant spread as well as peritoneal and/or omental deposits.
PET is a functional imaging modality that avails the high utilization of glucose in tumor cells. This is utilized in imaging for cancer where the higher rates of phosphorylation and the low rates of dephosphorylation result in the accumulation of FDG-6-phosphate (generated by the introduction of 18F-FDG into tumor cells). The poor anatomic localization of the positive PET lesions is overcome by combining the PET images with contrast-enhanced CT images (PET–CT).
Data evaluating 18F-fluorodeoxyglucose positron emission tomography–computed tomography (18F-FDG PET–CT) in patients with incidental gallbladder cancer is sparse. However, there is no study comparing, or exploring the complementary role on the use of PET–CT and MDCT in the preoperative evaluation of patients with incidental gallbladder cancer who are being considered for radical re-resection.
We designed this study with intent to evaluate the efficacy of integrated 18F-FDG PET–CT in determining the presence of occult metastatic or residual local–regional disease after the gallbladder has been removed and also to determine the benefit of adding a PET–CT to the already existing information from MDCT in determining resectability. We hoped to determine the role of PET–CT in the diagnostic algorithm prior to radical re-resection for incidental gallbladder cancer.
Materials and methods
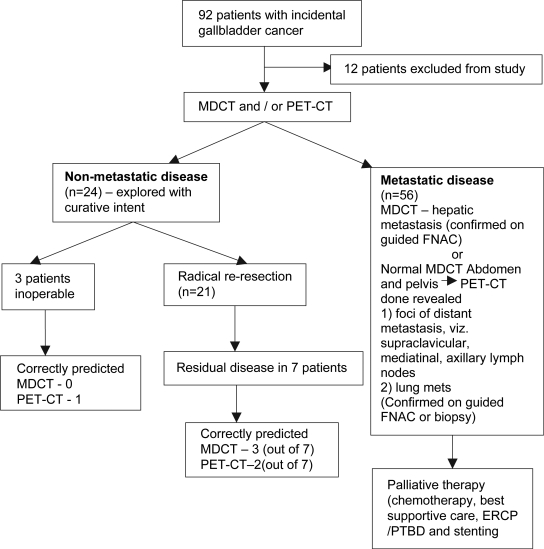
Patients referred to the Department of Gastrointestinal Surgery, Tata Memorial Hospital between 1st December 2006 and 31st October 2007, with incidental gallbladder cancer and planned for radical re-resection (based on the histopathological review of the resected specimen indicating the tumor to be ≥pT1b) were evaluated using MDCT (see Figure 1). In patients with no evidence of metastasis on MDCT and with no evidence of disease in the gallbladder fossa or with locally advanced disease, a PET–CT was performed to rule out distant metastasis. Of a total of 92 patients, 56 patients had advanced metastatic disease based on MDCT and PET–CT imaging and were thus advised palliative chemotherapy while 12 patients were excluded from the study for logistical reasons. Thus 24 patients in whom the MDCT and PET–CT scan findings were suggestive of localized disease or no disease were included in the study. These patients underwent radical re-resection which included the clearance of the following nodes: cystic, pericholedochal, hepatic hilar, hepatic, retroportal, posterior pancreatoduodenal, and celiac. In addition, a non-anatomical 3 cm-wedge resection of the gall bladder bed (segments IV B and V) was performed. In all patients, the cystic duct stump was identified and revised (with negative margins confirmed by frozen section). In case of a positive revised margin (on frozen section) of the cystic duct that was flush with the common bile duct, the patient was subjected to a radical extrahepatic bile duct excision with a hepaticodochojejunostomy.
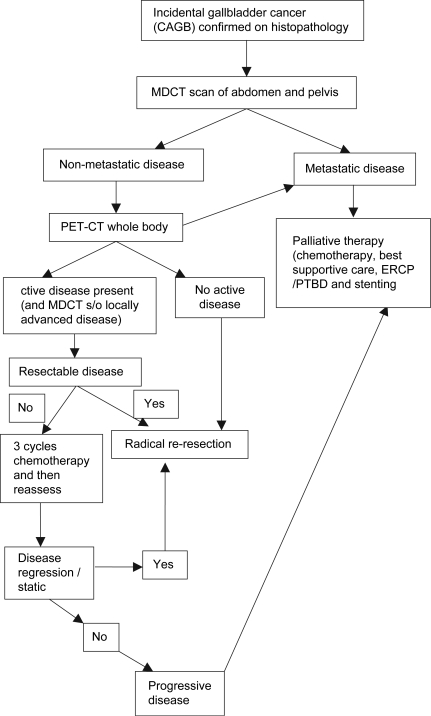
Figure 1. .
Algorithm for evaluation and management of incidental gallbladder cancers.
Preoperatively, all patients were investigated with routine blood investigations, including blood counts, liver and renal functions, and serum tumor marker CA 19-9.
MDCT
Preoperative evaluation included a contrast-enhanced CT study of the abdomen and pelvis on a 16-slice MDCT scanner. Sections were acquired from the domes of diaphragm to the ischial tuberosities. Hundred milliliters of nonionic iodinated contrast was injected @ 3 ml/second and images acquired after a 65-second delay. Images were acquired with 5 mm collimation and reconstructed at 1.25 mm. Coronal and Sagittal reformats were also studied along with bone and soft tissue settings. CT parameters used were 120 kvp, 200 mA, pitch of 1.375 and FOV was 35 cm. The criteria used for unresectability were: distant metastasis (liver or peritoneal lesions), extensive contiguous organ invasion (liver, duodenum), invasion of biliary confluence, invasion of main portal vein, or proper hepatic artery. Lymph nodes were considered positive if they were greater than 10 mm in maximum axial diameter or if there were features of necrosis (heterogeneous contrast enhancement).
A prepared proforma was used to evaluate every MDCT so as to accurately and uniformly interpret the scan.
PET–CT
The PET–CT scan was performed at a median duration of 51 days (range 17–152) after the first surgery.
Patient preparation and PET–CT imaging protocol
All patients were asked to fast for 4–6 hours prior to the study and blood glucose levels were checked and confirmed to be less than 150 mg/dl. The studies were performed one hour following intravenous administration of 370 MBq (10 mCi) of 18F-FDG during which period patients were asked to rest. Patients were asked to drink 750 ml of water soluble iodinated oral contrast to opacify the bowel for the CT component of the study. No intravenous iodinated contrast was administered. Patients were positioned supine with their arms to their sides and were asked to breathe normally during image acquisition.
Imaging was performed on a Discovery ST PET–CT system (GE medical systems).
It combines a 16 slice CT scanner with a dedicated PET (BGO plus crystal, dimensions 3.8 mm×3.8 mm×3.8 cm).
A CT was performed over 5–7 bed positions from the skull base to the mid-thigh level using multislice (16 slice) CT component of the system. CT parameters included 140 kV, 110 mA, 0.8 s/rotation, pitch of 1.75:1, FOV 50 cm, length of scan 1.0–1.6 m, 0.625 spatial resolution and slice thickness of 3.75 mm.
This was followed immediately by acquisition of PET data in the same anatomic locations with 15.4 cm axial FOV acquired in 2D mode with 2–3 min/bed position.
The total acquisition time accumulating between 100 and 150 million useful events varied between 15 and 20 minutes.
Image reconstruction and interpretation
CT data obtained was used for attenuation correction of PET images, and images were reconstructed using a standard vendor provided reconstruction algorithm which incorporated ordered subset expectation maximization (OSEM). Image fusion was performed using co-ordinate-based fusion software and subsequently reviewed at a workstation (Xeleris) that provided multiplanar reformatted images and displayed PET images, CT images and PET–CT fusion images.
Studies were interpreted independently by a Nuclear Medicine specialist and a Radiologist. The CT data was used for anatomical localization and corroboration of the PET findings.
Before surgery, the MDCT images were interpreted by a radiologist who was blinded to the results of the PET–CT scan.
The algorithm used for management has been shown in Figure 1.
Perioperative mortality was defined as death during the hospitalization following surgery or within 30 days of surgery.
The total hospital course was defined from the date of surgery until the patient was discharged.
All statistical analyses were performed using SPSS Version 14.0 for Windows. The continuous data were expressed as mean±standard deviation. The sensitivity (number of true positives, i.e. patients with occult metastatic or residual local–regional disease correctly detected by the test), specificity (number of true negatives, i.e. patients who do not have occult metastatic or residual local–regional disease and correctly detected by the test), and the positive (patients in whom the test is positive and actually have occult metastatic or residual local–regional disease) and negative (patients in whom the test is negative and in whom there is actually no occult metastatic or residual local–regional disease) predictive values (positive predictive value, PPV and NPV) and accuracy were calculated individually for PET–CT and MDCT using histopathology as the gold standard.
Results
Patient demography and tumor characteristics
Of the 24 patients, there were eight male and 16 female patients. The mean age was 45.3±11.4 years. In 12 patients, the first surgery (simple cholecystectomy) had been performed laparoscopically, while 12 patients underwent prior open cholecystectomy.
Review of the histopathology of the first surgery indicated the histology as adenocarcinoma (well differentiated =2, moderately differentiated =16, and poorly differentiated =6). By pT-stage, seven patients had T1b disease while, 12 and five patients had T2 and T3 disease, respectively.
The median CA19-9 level was 6.8 U/ml (range 0–2021).
Resectability
Although 24 patients were explored with an intent to perform a radical re-resection based on the preoperative imaging suggestive of localized or no active disease, three patients were found to have metastatic disease (peritoneal/omental) or locally advanced disease (fixed portal mass). Thus, the intended procedure could be completed in 21 patients (87.5%)
Although preoperative MDCT had predicted resectability in 24 patients, three patients had unresectable disease. MDCT had indicated the presence of a mass in the gallbladder fossa in two of the three patients. However, it neither predicted the presence of disseminated disease, nor were there findings suggestive of unresectability in these three patients (sensitivity = 100%, PPV = 87.5%, accuracy = 87.5%). PET–CT predicted resectability in 23 patients (sensitivity = 100%, PPV = 91.3%, accuracy = 91.6%). In the three patients who were unresectable, PET–CT had indicated the presence of regional disease in one of the three patients.
There was no statistical difference between MDCT and PET–CT in predicting resectability once distant disease was excluded.
Residual disease
On the histopathological examination of the excised scar, gallbladder wedge, dissected lymph nodal tissue (hepatic, portal, pericholedochal, retroduodenal, paraaortic/interaortocaval), residual disease was found in seven patients.
Of the seven patients with histopathologically proven residual disease, MDCT had predicted the likelihood of residual disease in three patients (sensitivity = 42.8%, PPV = 42.8%) while PET–CT had predicted the likelihood of residual disease in two patients (sensitivity = 28.5%, PPV = 20%).
Tables I and II show a complete site-wise break-up of the specificity, NPV and accuracy of MDCT and PET–CT in detecting residual disease in patients with incidental gallbladder cancer. Figure 2 summarises the final outcomes of all the 92 patients with incidental gallbladder cancer.
Table I. Analysis of MDCT based on the detection of residual disease in the various regions on the final histopathological assessment (n = 24).
| Specificity | NPV | Accuracy | |
|---|---|---|---|
| Scar | 95 | 82.6 | 79.1 |
| GB fossa | 89.4 | 94.4 | 80.9 |
| Liver metastasis | 100 | 100 | 100 |
| Hepatic node | 90 | 94.7 | 85.7 |
| Portal node | 100 | 90.4 | 90.4 |
| Retroduodenal node | 95.2 | 100 | 95.2 |
| Pericholedochal node | 100 | 90.4 | 90.4 |
| Paraaortic node | 90.4 | 100 | 90.4 |
| Regional disease | 100 | 91.3 | 91.6 |
Table II. Analysis of PET–CT based on the detection of residual disease in the various regions on the final histopathological assessment (n = 24).
| Specificity | NPV | Accuracy | |
|---|---|---|---|
| Scar | 80 | 80 | 66.6 |
| GB fossa | 89.4 | 94.4 | 85.7 |
| Liver metastasis | 100 | 100 | 100 |
| Hepatic node | 100 | 95.2 | 95.2 |
| Portal node | 100 | 90.4 | 90.4 |
| Retroduodenal node | 95.2 | 100 | 95.2 |
| Pericholedochal node | 100 | 90.4 | 90.4 |
| Paraaortic node | 100 | 100 | 100 |
| Regional disease | 80.9 | 85 | 70.8 |
Figure 2. .
Flow chart of the 92 patients with incidental gallbladder cancer who were considered candidates for potentially curative surgery and their final outcomes.
Discussion
Owing to the biological aggressiveness of the disease, there appears to be no long-term survival in patients with gallbladder cancers with distant metastasis – macroscopic or occult. The detection of distant disease helps in avoiding an unnecessary exploration.
At present, the most commonly employed and effective method for the preoperative staging of gallbladder cancer is MDCT which allows fast scanning with thin sections and high resolution volumetric reconstructions. This permits a more accurate detection of liver infiltration by the tumor in the gallbladder infiltration while minimizing partial volume artifacts, thereby improving T staging of the tumor 13,14,15. However, as yet there are no studies evaluating the role of MDCT in detecting residual disease or determining resectability (for radical re-resection) in patients with incidental gallbladder cancer.
While it does constitute an important investigation in the preoperative setting, the inability to pick up peritoneal seedlings and small hepatic metastasis coupled with the fact that there remains the possibility of missing regional lymphadenopathy, there exists a chance of understaging of the disease as pointed out by Donohue et al. 16.
The use of 18F-FDG in the diagnostic work-up of oncological patients is well established 17,18. Studies exploring the benefit of PET specifically for gallbladder cancer are few 19,20,21,22,23,24. These studies, which have essentially focused on the use of PET imaging for the preoperative evaluation of primary gallbladder cancers, have reported sensitivities of 75–100%. Till date there is only one study comparing PET–CT versus contrast-enhanced CT in primary/occult metastatic or residual local–regional gallbladder cancers 25.
Determination of resectability
MDCT remains a useful investigation for detecting gross metastatic disease in the abdomen and pelvis in patients with incidental gallbladder cancer who are being considered for radical re-resection. The addition of PET–CT to the diagnostic algorithm further helps in narrowing down those patients with metastatic disease outside the fields of study of the MDCT. In our study there was only one patient in whom the PET–CT had indicated the likelihood of unresectability by virtue of increased regional FDG uptake. Based on the MDCT we, however, did explore this patient only to find the disease not amenable to a resection. While this is only a single case, it does highlight that there may be a need to further clarify the features on PET–CT that may indicate the likelihood of unresectability. This may further improve the clinical application of PET–CT in patients with incidental gallbladder cancer.
On comparing our resectability rates between two time periods, i.e. from January 2003 to November 2006 and the current series, our resectability rates appear to have improved. Out of 72 patients explored, resectable disease was found in 53 patients (73.6%) as opposed to 21 out of 24 patients in the current series (87.5%). It is important to note that the (R0) resectability rate in the entire group is 87.5% which is indicative of the benefit of combining the two investigative modalities.
The false negative results of PET–CT in our series corroborate with the findings of Anderson et al. 23 who studied the role of PET in 14 patients with gallbladder cancer. They felt that these results were due to the small size of the lesions which escaped detection by the equipment. In fact, low sensitivity for peritoneal disease has also been reported for PET in the case of gastric cancer 26. This low sensitivity is also seen with MDCT.
Determination of residual disease
As seen in Tables I and II, the specificity, accuracy, and NPV are high. This indicates the ability of the two diagnostic imaging modalities to correctly diagnose the absence of disease. The low sensitivity of PET in detecting microscopic disease has been reported earlier in relation to ovarian cancer 27. There were seven patients with false positive FDG uptake in the scar. The PET–CT in these four patients was performed at mean interval of 53±23.1 days after the first surgery. This may be argued in favor of FDG avidity to the inflammatory process within the scar 28. In fact the only previous report of a false positive FDG uptake in post-surgical gallbladder cancer patients was noted by Anderson et al. 23 who felt that this may occur if the PET–CT is performed within one month of the first surgery.
Our study proves that PET–CT is capable of detecting occult metastatic or residual local–regional gallbladder cancer and thus provides the basis for further exploring the role of PET–CT in patients with incidental gallbladder cancer.
Our study is unable to provide sufficient evidence to state that PET–CT improves resectability rates. However, it does provide the impetus for further exploring whether there exist PET–CT criteria that can clearly determine resectability. With further improvements in the ability of PET–CT imaging to detect smaller lesions, the role of nuclear imaging in preoperative staging of malignant disease will only increase.
In conclusion, it appears that the prime advantage of PET–CT over MDCT is its ability to detect occult metastatic disease in the rest of the body as opposed to MDCT of the abdomen and pelvis which is useful in the loco-regional staging of the disease. This study thus provides an important indication for the use of PET–CT, that is, in patients with incidental gallbladder cancer since the detection of clinically occult metastasis using PET–CT scan will help identify those patients who will not benefit from a radical resection and who would be better served by palliative care strategies.
References
- 1.Foster JM, Hoshi H, Gibbs JF, Iyer R, Javle M, Chu O, et al. Gallbladder cancer: defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol. 2007;14:833–40. doi: 10.1245/s10434-006-9097-6. [DOI] [PubMed] [Google Scholar]
- 2.Shirai Y, Yoshida K, Tsukada K, Muto T. Inapparent carcinoma of the gall bladder: an appraisal of a radical second operation after simple cholecystectomy. Ann Surg. 1992;215:326–31. doi: 10.1097/00000658-199204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yildrim E, Celen O, Gulben K, Berberoglu U. The surgical management of incidental gallbladder carcinoma. Eur J Surg Oncol. 2005;31:45–52. doi: 10.1016/j.ejso.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167–76. doi: 10.1016/s1470-2045(03)01021-0. [DOI] [PubMed] [Google Scholar]
- 5.Muratore A, Amisano M, Vigano L, Massucco P, Capussotti L. Gallbladder cancer invading the perimuscular connective tissue: results of reresection after prior non-curative operation. J Surg Oncol. 2003;83:212–5. doi: 10.1002/jso.10258. [DOI] [PubMed] [Google Scholar]
- 6.Ogura Y, Mizumoto R, Isaji S, Kusuda T, Matsuda S, Tabata M. Radical operations for carcinoma of the gallbladder: present status in Japan. World J Surg. 1991;15:337–43. doi: 10.1007/BF01658725. [DOI] [PubMed] [Google Scholar]
- 7.Ouchi K, Suzuki M, Tominaga T, Saijo S, Matsuno S. Survival after surgery for cancer of the gallbladder. Br J Surg. 1994;81:1655–7. doi: 10.1002/bjs.1800811131. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto Y, Fujii H, Aoyama H, Yamamoto M, Sugahara K, Suda K. Surgical treatment of primary carcinoma of the gallbladder based on the histologic analysis of 48 surgical specimens. Am J Surg. 1992;163:239–45. doi: 10.1016/0002-9610(92)90109-5. [DOI] [PubMed] [Google Scholar]
- 9.Wagholikar GD, Behari A, Krishnani N, Kumar A, Sikora SS, Saxena R, et al. Early gallbladder cancer. J Am Coll Surg. 2002;194:137–41. doi: 10.1016/s1072-7515(01)01136-x. [DOI] [PubMed] [Google Scholar]
- 10.Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557–69. doi: 10.1097/00000658-200010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behari A, Sikora SS, Wagholikar GD, Kumar A, Saxena R, Kapoor VK. Longterm survival after extended resections in patients with gallbladder cancer. J Am Coll Surg. 2003;196:82–8. doi: 10.1016/s1072-7515(02)01611-3. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins WG, DeMatteo RP, Jarnagin WR, Ben-Porat L, Blumgart LH, Fong Y. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 2004;11:310–5. doi: 10.1245/aso.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimitsu K, Honda H, Shinozaki K, Aibe H, Kuroiwa T, Irie H, et al. Helical CT of the local spread of carcinoma of the gall bladder: evaluation according to the TNM system in patients who underwent surgical resection. Am J Roentgenol. 2002;179:423–8. doi: 10.2214/ajr.179.2.1790423. [DOI] [PubMed] [Google Scholar]
- 14.Kim BS, Ha HK, Lee IJ, Kim JH, Eun HW, Bae IY, et al. Accuracy of CT in local staging of gall bladder carcinoma. Acta Radiol. 2002;43:71–6. doi: 10.1080/028418502127347475. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Fernandez A, Gomez-Rio M, Medina-Benitez A, Moral JV, Ramos-Font C, Ramia-Angel JM, et al. Application of modern imaging methods in diagnosis of gall bladder cancer. J Surg Oncol. 2006;93:650–64. doi: 10.1002/jso.20533. [DOI] [PubMed] [Google Scholar]
- 16.Donohue JH. Present status of the diagnosis and treatment of gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2001;8:530–4. doi: 10.1007/s005340100021. [DOI] [PubMed] [Google Scholar]
- 17.Coleman RE. Clinical PET in oncology. Clin Positron Imaging. 1998;1:15–30. doi: 10.1016/s1095-0397(97)00004-6. [DOI] [PubMed] [Google Scholar]
- 18.Czernin J, Allen-Auerbach M, Schelbert HR. Improvements in cancer staging with PET/CT: literature-based evidence as of September. J Nucl Med ;48:s. 2006;2007:78s–88. [PubMed] [Google Scholar]
- 19.Koh T, Taniguchi H, Yamaguchi A, Kunishima S, Yamagishi H. Differential diagnosis of gallbladder cancer using positron emission tomography with fluorine-18-labeled fluorodeoxyglucose (FDG-PET) J Surg Oncol. 2003;84:74–81. doi: 10.1002/jso.10295. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Fernandez A, Gomez-Rio M, Llamas-Elvira JM, Ortega-Lozano S, Ferron-Orihuela JA, Ramia-Angel JM, et al. Positron emission tomography with fluorine-18-fluoro-2-deoxy-D-glucose for gallbladder cancer diagnosis. Am J Surg. 2004;188:171–5. doi: 10.1016/j.amjsurg.2003.12.070. [DOI] [PubMed] [Google Scholar]
- 21.Koh T, Taniguchi H, Kunishima S, Yamagashi H. Possibility of differential diagnosis of small polypoid lesions in the gallbladder using FDG-PET (case report) Clin Positron Imaging. 2000;3:213–8. doi: 10.1016/s1095-0397(00)00100-x. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama Y, Yamamoto Y, Fukunaga K, Kimura N, Miki A, Sasakawa Y, et al. Dual-time-point 18F-FDG PET for the evaluation of gallbladder carcinoma. J Nucl Med. 2006;47:633–8. [PubMed] [Google Scholar]
- 23.Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluordeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90–7. doi: 10.1016/j.gassur.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Corvera CU, Blumgart LH, Akhurst T, DeMatteo RP, D'Angelica M, Fong Y, Jarnagin WR. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206:57–65. doi: 10.1016/j.jamcollsurg.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum S, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43–50. doi: 10.1016/j.jhep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Jadvar H, Tatlidil R, Garcia AA, Conti PS. Evaluation of recurrent gastric malignancy with [F-18]-FDG positron emission tomography. Clin Radiol. 2003;58:215–21. doi: 10.1016/s0009-9260(02)00477-4. [DOI] [PubMed] [Google Scholar]
- 27.Nanni C, Rubello D, Farsad M, De Iaco P, Sansovini M, Erba P, et al. 18F-FDG PET/CT in the evaluation of recurrent ovarian cancer: a prospective study on forty-one patients. Eur J Surg Oncol. 2005;31:792–7. doi: 10.1016/j.ejso.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Abouzied MM, Crawford ES, Nabi HA. 18F-FDG Imaging: pitfalls and artifacts. J Nucl Med Technol. 2005;33:145–55. [PubMed] [Google Scholar]