Abstract
Several methods of treatment for hepatocellular carcinoma (HCC) are often used in combination for either palliation or cure. We established a multidisciplinary treatment team (MDTT) at the San Francisco Veterans Affairs Medical Center in November 2003 and assessed whether aggressive multimodality treatment strategies may affect survival. A prospective database was established and follow-up information from patients with presumed HCC was collected up to November 2006. Information from the American College of Surgeons (ACS) cancer registry from January 2000 to November 2003 identified patients with HCC that were evaluated at the same institution prior to the establishment of the MDTT. The establishment of a MDTT resulted in the doubling of patient referrals for treatment. Significantly more patients were evaluated at earlier stages of disease and received either palliative or curative therapies. The overall survival (p<0.0001) and length of follow-up (p<0.05) were significantly improved after the establishment of the MDTT. Stage-by-stage comparisons indicate that aggressive multimodality therapy conferred significant survival advantage to patients with American Joint Commission on Cancer (AJCC) stage II HCC (odds ratio 15.50, p<0.001). Multidisciplinary collaboration and multimodality treatment approaches are important in the management of hepatocelluar carcinoma and improves patient survival.
Keywords: Hepatocellular carcinoma, multidisciplinary communication, hepatectomy, palliative therapy
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer in the world. Development of cirrhosis is the most important risk factor. In Asia and Africa, chronic hepatitis B infection is the major etiological factor for cirrhosis and HCC. In the USA, the incidence of HCC is increasing with hepatitis C virus (HCV) infection being responsible for most of that increase 1,2.
The patient population of Veteran Affairs Medical Centers (VAMC) is noteworthy in that the prevalence of chronic HCV infection is much higher than in the general population within the USA. Whereas, the prevalence of HCV is 1.3% in the general public, it is 5–10% in veteran patients who utilize VAMC services 3. Risk factors for HCV infection in the veteran population include history of intravenous drug use, blood transfusions before 1992, combat medical worker, tattoos, incarceration greater than 48 hours, and greater than 15 lifetime sexual partners 4. At the San Francisco VAMC, as one of four resource centers of VA's National Hepatitis C Program, we have an especially broad referral base of veteran patients with HCV-related HCC. This affords us a unique opportunity to investigate the factors that may influence patient care for a growing population of patients who would develop HCV-related HCC in the USA.
Multiple modalities are available to treat HCC. Liver resection and liver transplantation are the two potentially curative treatments. Ablative therapies such as radiofrequency ablation (RFA), transarterial embolization (TAE), transarterial chemoembolization (TACE), and percutaneous ethanol injection (PEI) have been shown to prolong survival 5. Several clinical advances may contribute to improving outcomes in patients with HCC. These factors include early detection of tumors through imaging, improved morbidity and mortality of liver resection and transplantation, and increased use of multimodality treatments 6,7,8. We believe that effective management of HCC requires a multidisciplinary approach with collaboration between hepatologists, oncologists, radiologists, and surgeons. Therefore, we established a multidisciplinary treatment team (MDTT) for HCC at the San Francisco VAMC. Through this team, there was fluid referral of patients between the various disciplines and frequent joint conferences to discuss individual patient cases. The aim of this study was to evaluate the early results of implementing a comprehensive disease-based multidisciplinary management team at a Veterans Affairs Medical Center. Here we describe the results of our MDTT compared to patient outcomes at the same institute before its implementation.
Methods
General
A MDTT for patients with HCC was organized in November 2003 at the San Francisco VAMC and a prospective database was established and maintained. Follow-up information from patients with presumed HCC was collected and analyzed through November 2006 (36 months). Data for analysis included demographics, clinical history, histopathology, imaging studies, laboratory values, operative findings, and clinic follow-up information. For comparison, we examined data from patients treated at our institution during an earlier three-year time interval. The American College of Surgeons (ACS) cancer registry was used to retrospectively identify patients evaluated for HCC between November 2000 and November 2003 (36 months).
Patients
Patients were typically referred after undergoing preliminary investigations at other VAMC affiliated institutions. After referral, additional cross-sectional imaging evaluation was conducted at the San Francisco VAMC in the form of computed tomography (CT) and/or magnetic resonance imaging (MRI). The authors’ approach to treatment and evaluation criteria of tumor resectability, in general, followed the National Comprehensive Cancer Network (NCCN) clinical practice guidelines 9. Decisions regarding treatment modalities offered to patients (palliative or curative) were made based on data from cross-sectional imaging studies, analysis of local tumor-related factors, social issues, and assessment of underlying liver impairment. The vast majority of HCC patients had some degree of underlying liver dysfunction.
We quantified the extent of impaired liver function using the Child-Pugh classification system to guide treatment choices. We also used the Model of End-stage Liver Disease (MELD) score since it correlated well with Child-Pugh classification for predicting perioperative mortality. A MELD score of <9 corresponded to Child's A classification, MELD 9–16 corresponded to Child's B, and MELD >16 corresponded to Child's C 10. Individual cases and imaging studies were reviewed at a bimonthly multidisciplinary disease management conference attended by surgeons, radiologists, medical oncologists, and gastroenterologists.
Statistics
The Chi-squared, Fisher's exact, and Mann-Whitney tests were used where indicated to evaluate statistical significance with the InStat 3.0 biostatistical program. Statistical significance was considered when p<0.05. This study was approved by the San Francisco VAMC and University of California, San Francisco Committee on Human Research in accordance with all guidelines.
Results
General
A total of 121 patients with HCC were evaluated by the surgical service between November 2003 and November 2006. As expected from a VAMC population, nearly all of these patients were men. Only one patient was female. Their ages ranged between 48 and 88, with a median age of 58.
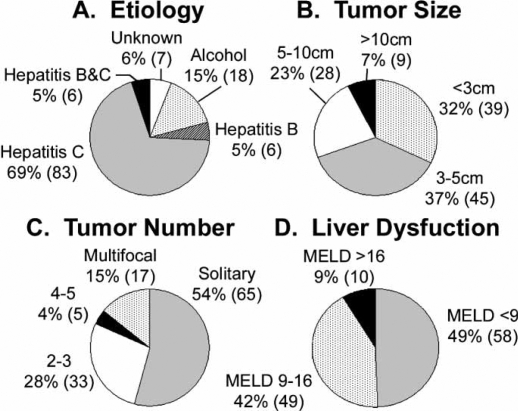
The etiology of HCC in this cohort was heavily weighted toward viral hepatitis infection in nearly 80% of patients. The majority (n=83, 69%), developed HCC from hepatitis C infection. In seven patients (6%) the etiology was unknown and in 18 patients (15%) the cause of HCC was attributed to alcohol consumption alone. Only six patients (5%) had HCC caused by hepatitis B alone while another six patients (5%) developed HCC in the presence of both hepatitis B and hepatitis C (Figure 1A).
Figure 1. .
Hepatocellular carcinoma (HCC) patient characteristics evaluated by the multidisciplinary treatment team (MDTT) at the San Francisco Veterans Affairs Medical Center. A total of 121 patients with HCC were evaluated by the MDTT between November 2003 and November 2006. Graphs show the proportion of patients with the given (A) etiological factor of HCC, (B) tumor size (size of the largest tumor if more than one), (C) tumor number, and (D) degree of liver dysfunction as estimated by MELD score. Percentage of the total patient population is provided, followed by the actual number of patients in parentheses.
The stage of HCC at initial presentation was fairly evenly distributed in the spectrum of early to advanced disease. About one-third of the patients presented with small tumors less than 3 cm, another third had intermediate sized tumors 3–5 cm, and the remaining third had advanced tumors 5 cm and larger (Figure 1B). More than half of the patients had solitary tumors while one-third had two to three tumors. Seventeen patients (15%) had multifocal disease with five or more tumors (Figure 1C).
We used MELD score as a surrogate indicator of Child's classification and degree of liver dysfunction. Nearly half of our patients presented with preserved liver function and low MELD scores (<9). Forty-nine patients (42%) had intermediate MELD scores ranging from 9 to 16, and only 10 patients (9%) had advanced MELD scores (>16) (Figure 1D).
Spectrum of treatments for hepatocellular carcinoma (HCC)
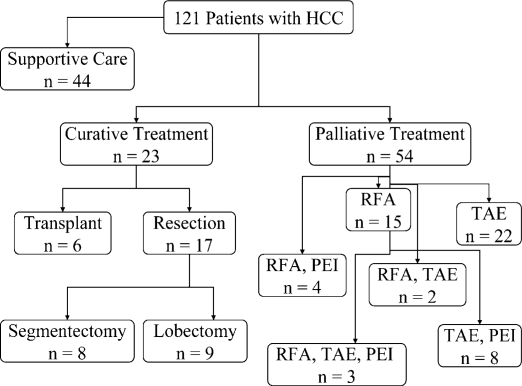
Between November 2003 and November 2006, 121 patients with presumed HCC who fulfilled the study criteria as outlined above were identified. Forty-four patients (36%) had disease that was too advanced to treat and were offered supportive care only. The remaining 77 patients (64%) were offered either palliative (n=54) or curative (n=23) treatments. Patients who were not candidates for resection or liver transplantation were treated with ablative therapies such as RFA, TAE and PEI for palliation (Figure 2). A substantial proportion of patients in both the curative arm, nine of 23 (40%), and palliative arm, 17 of 56 (30%), received treatment from two modalities or more.
Figure 2. .
Treatments received by hepatocellular carcinoma (HCC) patients after establishment of the multidisciplinary treatment team (MDTT). A total of 121 patients with HCC were evaluated by the MDTT between November 2003 and November 2006. Forty-four patients had disease that was too advanced for treatment and received supportive care only. Treatments with curative intent included liver resection and transplantation. Segmentectomies were resections of three or fewer segments. Lobectomies included formal right and left lobectomies as well as extended lobectomies. Ablative therapies such as radiofrequency ablation (RFA), transarterial embolization (TAE), and percutaneous ethanol injection (PEI) were considered palliative. These treatments were either given alone as a single modality (i.e. RFA or TAE) or in combination with other modalities (i.e., RFA and PEI, RFA and TAE, TAE and PEI, or RFA, TAE, and PEI).
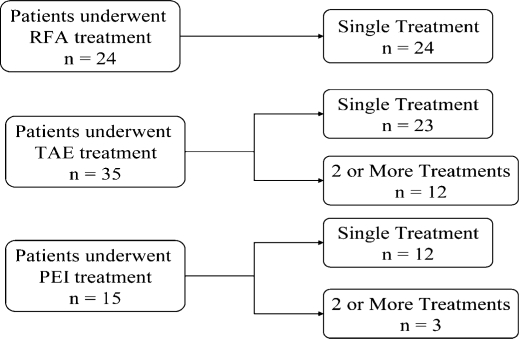
Fifty-four patients (45%) had treatments aimed toward palliation. Twenty-four patients underwent RFA, of which, 15 had a single RFA treatment session (Figure 3). In two patients, percutaneous RFA of the liver tumor(s) was done under CT guidance. The majority (n=22) were treated in the operating room using either a laparoscopic or open approach. Treatment by TAE (bland particle) of the hepatic artery was done in 35 patients in the radiology suite. Of these, 12 patients (34%) had two or more arterial embolizations in separate sessions. Twenty-two patients had TAE alone. Finally, 15 patients received PEI in conjunction with other treatment modalities (RFA, TAE, or both). The range of treatments given to these patients illustrates our multidisciplinary multimodality treatment philosophy.
Figure 3. .
Palliative treatments given by the multidisciplinary treatment team (MDTT). The total number of patients who underwent radiofrequency ablation (RFA), transarterial embolization (TAE), and percutaneous ethanol injection (PEI) as palliative therapies is listed. Whether the patient received a single treatment of that modality or repeated treatments of the same modality is shown. Patients treated with RFA all had only a single treatment with RFA. In contrast, 12 of 35 (34%) of the patients treated with TAE had two or more repeated treatments with TAE.
Twenty-three patients (19%) were treated with curative intent. Six patients were transplanted and 17 underwent liver resections. The six liver transplant patients were treated by RFA, TAE, or both to control disease prior to definitive treatment. Among the 17 patients submitted to partial hepatectomy, eight underwent anatomically based segmentectomies, five had standard hemi-lobectomies, and four had extended lobectomies (Table I). The median follow-up for resection patients was 19.3 months. At last follow-up, nine patients were alive without disease (53%). There were seven recurrences (41%) and four patients (23%) died of recurrent disease. The median time to recurrence was 15.8 months. There was one death (post-operative day 50) from liver failure (patient #17).
Table I. Liver resections for curative intent of the multidisciplinary treatment team (MDTT).
| Patient | Operation | Size (cm) | Grade | Margins | Nodes | Vascular invasion | Recurrence | Follow-up (months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Segmentx | 3.1 | mod | − | − | − | − | 7.9 | AWOD |
| 2 | Segmentx | 7.8 | mod | − | − | − | − | 4.8 | AWOD |
| 3 | Segmentx | 1.6* | n/a | n/a | n/a | n/a | − | 13.0 | AWOD |
| 4 | Hemihepx | 8.0 | well to mod | − | − | − | − | 8.3 | AWOD |
| 5 | Hemihepx | 8.5 | mod | − | − | − | − | 0.3 | AWOD |
| 6 | Hemihepx | 11.5 | mod | − | − | − | − | 7.4 | AWOD |
| 7 | Hemihepx | 11.5 | poor | − | − | + | − | 3.1 | AWOD |
| 8 | Ext. Hemihepx | 3; mf | mod to poor | − | − | − | − | 26.6 | AWOD |
| 9 | Ext. Hemihepx | 8.0 | mod to well | − | − | − | − | 14.4 | AWOD |
| 10 | Segmentx | 3.5 | mod | − | − | − | + | 19.4 | AWD |
| 11 | Segmentx | 4.0 | mod | − | − | − | + | 4.9 | AWD |
| 12 | Ext. Hemihepx | 8.8 | mod | + | − | − | + | 24.5 | AWD |
| 13 | Segmentx | 2.0 | mod | − | − | − | + | 3.1 | DOD |
| 14 | Segmentx | 2.0 | mod | − | − | − | + | 3.7 | DOD |
| 15 | Segmentx | 4.1 | mod to well | − | − | − | + | 15.8 | DOD |
| 16 | Hemihepx | 1.5 | well to mod | − | − | − | + | 18.2 | DOD |
| 17 | Ext. Hemihepx | 6; mf | mod to poor | − | − | − | − | 2.8 | DWOD** |
Note: A total of 17 patients underwent curative liver resection. Operations performed were segmentectomy (segmentx), hemihepatectomy (hemihepx), or extended hemihepatecomy (ext. hemihepx). Pathological findings included tumor size (mf denoted multifocal disease), grade (differentiation classified as well, well to moderate, moderate, moderate to poor, and poor), resection margins (+, positive resection margins; −, negative resection margins), nodes (−, negative regional nodes), and vascular invasion (+, present; −, absent). Tumor recurrence either occurred (+) or not (−) during the follow-up period calculated from the time of surgery. Survival outcomes at the time of last follow-up were alive without disease (AWOD), alive with disease (AWD), death of disease (DOD), or death without disease (DWOD).
*Patient three had a 1.6 cm tumor on pre-operative tri-phasic liver computed tomography imaging but no tumor was found on the pathological specimen.
**Patient 17 died of liver failure on post-operative day 50 without development of recurrent hepatocellular carcinoma.
Pre- and post-multidisciplinary treatment team (MDTT) outcomes
To determine whether establishment of the MDTT improved HCC patient outcomes at the San Francisco VAMC, we compared our three-year prospective database to information obtained from the ACS cancer registry collected at our institution in the previous three years. Comparing the two time periods showed that the number of patients referred to the surgical service for HCC treatment doubled from 62 to 112 after the implementation of the MDTT (Table II). Through the improved referral system of the MDTT, significantly more patients were evaluated with earlier stages of HCC disease (p<0.0001). Prior to the establishment of the MDTT, very few patients evaluated had American Joint Committee on Cancer (AJCC) stage 1 and 2 disease. In fact, 40% of the patients presented to medical attention with stage four metastatic HCC. However, in the three years after the MDTT, this ratio reversed in that nearly 30% of the patients evaluated were with stage 1 disease and another 33% with stage 2 disease. As a result, a greater proportion of patients received either curative or palliative treatments for HCC (37% pre-MDTT vs. 64% post-MDTT, p<0.0001).
Table II. Treatment and survival of patients before and after establishment of the multidisciplinary treatment team (MDTT).
| Pre-MDTT November 2000–November 2003 | Post-MDTT November 2003–November 2006 | |
|---|---|---|
| Total # of patients | 62 | 121 |
| AJCC stage* | ||
| I | 3 (5%) | 35 (29%) |
| II | 11 (18%) | 40 (33%) |
| IIIA | 16 (26%) | 34 (28%) |
| IIIB | 3 (5%) | 2 (2%) |
| IIIC | 4 (6%) | 4 (3%) |
| IV | 25 (40%) | 5 (4%) |
| Treatment | ||
| Not treated | 39 (63%) | 44 (36%) |
| Treated** | 23 (37%) | 77 (64%) |
| Palliative | 19 (31%) | 54 (45%) |
| Curative | 4 (6%) | 23 (19%) |
| Outcomes | ||
| Survival** | 13 (21%) | 79 (65%) |
| Median Follow-up*** (months) | 4.5 | 9.5 |
Statistical significance by the *Chi-squared test, p<0.0001, **Fisher's exact test, p<0.0001, or ***Mann-Whitney test, p<0.05.
Importantly, implementation of the MDTT improved overall HCC patient survival. Only 13 of 62 patients (21%) survived during the pre-MDTT follow-up period. In contrast, a significantly higher percentage, 79 of 121 patients (65%), survived during the post-MDTT follow-up period (p<0.0001, Table II). This observation is especially remarkable considering that the median length of follow-up for HCC patients was also significantly increased after the establishment of the MDTT (4.5 months pre-MDTT vs. 9.5 months post-MDTT, p<0.05). The overall survival odds ratio post-MDTT was 7.10 compared to pre-MDTT (95% confidence interval, 3.46–14.52; p<0.0001), indicating a substantial survival advantage for HCC patients at our institution after the implementation of the MDTT (Table III).
Table III. AJCC stage-by-stage and overall survival odds ratios after establishment of the multidisciplinary treatment team (MDTT).
| Survival odds ratio | 95% confidence interval | p-Value | |
|---|---|---|---|
| Stage I | 1.44 | 0.12–17.92 | NS |
| Stage II | 15.50 | 2.82–85.09 | <0.001 |
| Stage III | 2.19 | 0.66–7.23 | NS |
| Stage IV | 21.00 | 1.83–240.66 | 0.01 |
| Overall | 7.10 | 3.46–14.52 | <0.0001 |
Note: Survival odds ratios were calculated in comparison to before the establishment of the MDTT by the Fisher's exact test. NS indicates not statistically significant.
The comparison between the pre- and post-MDTT databases showed that significantly more patients presented with early stage HCC after the establishment of the MDTT (Table II). In order to demonstrate that the improvement in overall survival post-MDTT was not only the increased number of early disease stage patients, we performed a stage-by-stage comparison for survival (Table III). There was no statistically significant difference in survival for patients with stage I disease likely because there were too few patients pre-MDTT (only three ) for statistical analysis. However, comparing patients with stage II disease, there was a remarkable survival advantage after the implementation of the MDTT. The odds ratio for survival post-MDTT for stage II patients was 15.50 (95% confidence interval 2.82–85.09, p<0.001) compared to pre-MDTT. This suggested that the aggressive multimodality treatment strategy adopted by the MDTT extended survival for stage II patients. For stage III patients, a there was a trend toward a survival advantage post-MDTT (odd ratio 2.19), but it was not statistically significant. Of note, there was a statistically significant improvement in survival for stage IV patients even though the number of stage IV patients evaluated post-MDTT were very few.
Discussion
HCC is an aggressive tumor that has a poor prognosis if left untreated. The natural history of untreated HCC has a median survival between one and eight months and a five-year survival of 3% 11,12. It's a disease that is increasing in incidence in the USA likely associated with the increasing prevalence of HCV infection. From 1993 to 1999, the incidence of HCC among patient 65 years of age or older increased from 14.2 per 100,000 to 18.1 per 100,000. In the same period, HCV-related HCC increased from 11 to 21% 2. Because the prevalence of chronic HCV infection is much higher in veterans than in the general population 3, these trends predict that HCC incidence within the veteran patient population will continue to increase significantly in the coming years. Our medical center is one of only four national HCV treatment referral centers within the VAMC system. Accordingly, we have implemented a MDTT to address the growing demands and special challenges of treating patients with HCC.
Since establishment of the MDTT, we have adopted an aggressive, yet flexible, multidisciplinary treatment strategy. Patients are offered transplantation or surgical resection whenever possible since these remain the only curative modalities. We use local regional ablative therapies as a bridge to transplantation since pre-operative therapy with TACE or RFA has been shown to provide good five-year disease-free survival rates after liver transplant 13,14. There may also be a potential benefit to tumor downstaging with locoregional therapies 15. However, pre-transplant management remains controversial because other studies did not document improved survival with these pre-operative therapies 16,17. In our study, more than half of our patients presented with solitary tumors <5 cm and within Milan criteria for transplantation, however, many patients were not transplant candidates due to ongoing psychosocial comorbidities.
As previously demonstrated and in our series, only a fraction (19% in our series, 15–30% in other series) of patients with HCC are surgical candidates due to advanced disease stage or inadequate liver reserve 5. Unfortunately, the risk of disease recurrence following resection remains high due to de novo tumor development in the remnant cirrhotic liver. Recurrence rates are estimated to be about 50% at three years post-resection 18,19.
When patients were not candidates for resection or liver transplantation because of performance status, severity of chronic liver disease, tumor-related factors, or social reasons, they received a variety of ablative therapies and close follow-up to control progression of disease. Ablative therapies (RFA, TAE, or PEI) are generally not curative and patients who receive them as the initial primary treatment are at similar or increased risk for tumor progression and recurrence compared to resection patients 20. In the absence of effective medical therapies, it is our philosophy that an aggressive multimodality treatment approach should be pursued along with frequent surveillance imaging to offer the best chance for extended survival.
In the present study, 30–40% of patients treated with curative or palliative intent were given two or more treatment modalities. There is evidence that combined modality treatment of HCC is efficacious and improves survival. Treatment with TACE prior to RFA is effective for tumors greater than 3 cm, whereas RFA alone is recommended only for tumors less than 2 cm 21. While, in general, RFA is a superior ablative therapy compared to PEI 22, there are a few situations in which PEI is preferred. We use PEI for small lesions (<3 cm) in the periphery of the liver or those adjacent to portal structures. In these anatomic locations, PEI offers effective tumor kill with less radial tissue damage to important neighboring structures, such as the biliary tree, stomach, duodenum, and colon which can be easily damaged by other heat energy modalities, such as RFA. We also use PEI in combination with TAE or TACE since the two modalities together have demonstrated better response rates compared to TACE alone 23,24.
The establishment of the MDTT accomplished several important objectives that improved patient outcomes. First of all, within a three- year period, we were able to double the number of HCC referrals at our institution. Second, we significantly increased the number of patients evaluated for early stage HCC (AJCC stage 1 and 2) who were amenable to either curative or palliative treatments. This may be due to improved screening, heightened awareness of clinicians to at risk individuals, and better communication between the various disciplines involved in caring for HCC patients. Identifying patients at an early disease stage is important since more curative treatment options are available according to the Barcelona Clinic Liver Cancer System recommendations endorsed by the American Association for the Study of Liver Diseases (AASLD) 20. Third, after implementation of the MDTT, with effective collaboration between surgical, medical, and radiological specialties, more patients received either curative or palliative treatments and many received combined modality treatments. Finally, the efforts of the MDTT resulted in significantly improved overall patient survival and follow-up. The improvement in survival post-MDTT was not only due to more patients evaluated at earlier disease stages, since stage-by-stage comparisons demonstrated a remarkable survival advantage especially for stage II patients after the establishment of the MDTT. These results indicated that aggressive multimodality therapy increased survival for AJCC stage II patients. Whether aggressive therapy may benefit advanced stage HCC (stage III and IV) will likely require further investigation.
In conclusion, HCC continues to be a challenging clinical problem. This is especially true in the veteran population in which the increased HCV prevalence predicts that a higher number of veteran patients will eventually develop HCC. We have shown that multidisciplinary collaboration and multimodality treatment approaches are important in the treatment of patients with HCC and lead to improved outcomes.
Acknowledgements
We thank Ryan Garrett for maintaining our patient database. We also thank Linda M. Fuhrman, N.P. and Michael Byrd for their outstanding clinical assistance. The authors would like to graciously thank Pamela Derish for her critical review of the manuscript. This work was supported by a grant from the Department of Veterans Affairs, Biomedical Laboratory Research and Development and a grant from the Northern California Institute for Research and Education.
References
- 1.Marrero JA. Hepatocellular carcinoma. Curr Opin Gastroenterol. 2006;22:248–53. doi: 10.1097/01.mog.0000218961.86182.8c. [DOI] [PubMed] [Google Scholar]
- 2.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127:1372–80. doi: 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Dominitz JA, Boyko EJ, Koepsell TD, Heagerty PJ, Maynard C, Sporleder JL, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41:88–96. doi: 10.1002/hep.20502. [DOI] [PubMed] [Google Scholar]
- 4.Briggs ME, Baker C, Hall R, Gaziano JM, Gagnon D, Bzowej N, Wright TL. Prevalence and risk factors for hepatitis C virus infection at an urban Veterans Administration Medical Center. Hepatolog. 2001;34:1200–5. doi: 10.1053/jhep.2001.29303. [DOI] [PubMed] [Google Scholar]
- 5.Cormier JN, Thomas KT, Chari RS, Pinson CW. Management of hepatocellular carcinoma. J Gastrointest Surg. 2006;10:761–80. doi: 10.1016/j.gassur.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int. 2008;7:237–57. [PubMed] [Google Scholar]
- 7.Volk ML, Marrero JA. Early detection of liver cancer: diagnosis and management. Curr Gastroenterol Rep. 2008;10:60–6. doi: 10.1007/s11894-008-0010-2. [DOI] [PubMed] [Google Scholar]
- 8.Chan AC, Poon RT, Ng KK, Lo CM, Fan ST, Wong J. Changing paradigm in the management of hepatocellular carcinoma improves the survival benefit of early detection by screening. Ann Surg. 2008;247:666–73. doi: 10.1097/SLA.0b013e31816a747a. [DOI] [PubMed] [Google Scholar]
- 9.Benson AB, Bekaii-Saab T, Ben-Josef E, Blumgart L, Clary BM, Curley SA, et al. Hepatobiliary cancers. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4:728–50. doi: 10.6004/jnccn.2006.0064. [DOI] [PubMed] [Google Scholar]
- 10.Farnsworth N, Fagan SP, Berger DH, Awad SS. Child-Turcotte-Pugh versus MELD score as a predictor of outcome after elective and emergent surgery in cirrhotic patients. Am J Surg. 2004;188:580–3. doi: 10.1016/j.amjsurg.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 11.Akashi Y, Koreeda C, Enomoto S, Uchiyama S, Mizuno T, Shiozaki Y, et al. Prognosis of unresectable hepatocellular carcinoma: an evaluation based on multivariate analysis of 90 cases. Hepatology. 1991;14:262–8. [PubMed] [Google Scholar]
- 12.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–28. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Maluf DG, Stravitz RT, Williams B, Cotterell AH, Mas VR, Heuman D, et al. Multimodality therapy and liver transplantation in patients with cirrhosis and hepatocellular carcinoma: 6 years, single-center experience. Transplant Proc. 2007;39:153–9. doi: 10.1016/j.transproceed.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557–63. doi: 10.1053/jlts.2003.50106. [DOI] [PubMed] [Google Scholar]
- 15.Yao FY, Hirose R, LaBerge JM, Davern TJ, Bass NM, Kerlan RK, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505–14. doi: 10.1002/lt.20526. [DOI] [PubMed] [Google Scholar]
- 16.Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, et al. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005;11:767–75. doi: 10.1002/lt.20418. [DOI] [PubMed] [Google Scholar]
- 17.Porrett PM, Peterman H, Rosen M, Sonnad S, Soulen M, Markmann JF, et al. Lack of benefit of pre-transplant locoregional hepatic therapy for hepatocellular cancer in the current MELD era. Liver Transpl. 2006;12:665–73. doi: 10.1002/lt.20636. [DOI] [PubMed] [Google Scholar]
- 18.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–82. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grazi GL, Ercolani G, Pierangeli F, Del Gaudio M, Cescon M, Cavallari A, Mazziotti A. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234:71–8. doi: 10.1097/00000658-200107000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 21.Yamakado K, Nakatsuka A, Ohmori S, Shiraki K, Nakano T, Ikoma J, et al. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol. 2002;13:1225–32. doi: 10.1016/s1051-0443(07)61969-1. [DOI] [PubMed] [Google Scholar]
- 22.Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237:171–9. doi: 10.1097/01.SLA.0000048443.71734.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartolozzi C, Lencioni R, Caramella D, Vignali C, Cioni R, Mazzeo S, et al. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology. 1995;197:812–8. doi: 10.1148/radiology.197.3.7480761. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Nakamura S, Numata K, Okazaki H, Endo O, Inoue S, et al. Hepatocellular carcinoma: treatment with percutaneous ethanol injection and transcatheter arterial embolization. Radiology. 1992;185:457–60. doi: 10.1148/radiology.185.2.1329143. [DOI] [PubMed] [Google Scholar]