Abstract
Background. This study aimed to assess the impact of wait times on patient survival following liver transplantation for hepatocellular carcinoma (HCC) in a single donor service area. Patients and methods. Patients listed in the New England Organ Bank (NEOB) from 1996 to 2005 for liver transplantation with a diagnosis of HCC were identified from the United Network for Organ Sharing database. The following data were extracted: date of listing, date removed from the wait list, indication for wait list removal, patient death and date of last known follow-up. Kaplan-Meier survival estimates were calculated from the time of listing for transplant (intention to treat liver transplant survival, ITT OLT) and compared to those calculated from the date of transplant (liver transplant, OLT). Results. There were 63 new registrations to the transplant list during the study period. Sixty-one patients were removed from the waiting list: transplanted 41 (65%), death seven (11%), candidate condition deteriorated/too sick to transplant eight (13%), medically unsuitable one (2%), other one (2%), transferred to another center two (3%), and transplanted at another center one (2%). Three-year survival following liver transplantation for primary liver cancer was 85%. When the results were analyzed using an intention to treat analysis there was a 10–20% decrease in survival rate at every time point due to wait list drop-out. Conclusion. Wait list drop-out adversely affects liver transplant survival in transplant centers served by the NEOB. These data should be considered when recommending transplant versus resection as first line therapy for stage I or II HCC in our region.
Keywords: liver, transplant, hepatocelullar carcinoma
Introduction
The optimal therapy for patients with hepatocellular carcinoma (HCC), American Liver Tumor Study Group Stage I or II remains controversial. While the five-year survival rates appear equivalent in selected patients undergoing either surgical resection or transplantation, liver transplantation appears to offer the best disease free survival 1. Time to transplantation is influenced by organ availability, which varies by region; therefore, the decision for transplant versus surgical resection as first line therapy should be in part based on local/regional intention to treat survival rates for both therapies. This study aimed to assess the impact of wait times on patient survival following liver transplantation for HCC in a donor specific area (DSA).
Methods
Patients listed in the New England Organ Bank (NEOB) and in the USA from 1996 to 2005 for liver transplantation with a diagnosis of hepatoma/HCC, hepatoma and cirrhosis, and fibrolamellar HCC (primary liver cancer) were identified from the United Network for Organ Sharing (UNOS) database. The following data were extracted: date of listing for liver transplant, date removed from the wait list, indication for removal from the wait list, patient death and date of last known follow-up.
Wait list drop-out was defined as removal from the liver transplant wait list for any of the following indications: medically unsuitable, candidate condition deteriorated/too sick to transplant, died and other.
The Kaplan-Meier survival estimates for all-cause mortality was calculated For NEOB patients from the time of listing for transplant (intention to treat liver transplant survival, ITT OLT) and compared to that calculated from the date of transplant (liver transplant, OLT). Observations were right-censored at 20 April, 2007, based on the assumption that all deaths occurring up to this date would have been included in the database. The log-rank statistic was used to cautiously assess the leftward shift associated with an ITT OLT protocol.
Results
Registrations for liver transplantation for primary liver cancer in Region I and in the USA are shown in Table I. The data is shown for the 10-year study period. Data for years 2004–2005 are reported separately to reflect the most up-to-date MELD (Model for End-stage Liver Disease) allocation variance for HCC. The transplant rate for listed patients ranges from 64 to 72% with an upward trend in Region I following the implementation of MELD.
Table I. Number of patients registered with UNOS in the New England Organ Bank (MAOB) and the USA with a diagnosis of primary liver cancer.
| Existing registrations | New registrations | At risk population* | Transplanted (%) | Dropout (%) | |
|---|---|---|---|---|---|
| MAOB | |||||
| 2004–2005 | 11 | 32 | 43 | 31 (72%) | 9 (21%) |
| 1996–2005 | 1 | 63 | 64 | 42 (66%) | 17 (27%) |
| USA | |||||
| 2004–2005 | 159 | 680 | 830 | 530 (64%) | 108 (13%) |
| 1996–2005 | 32 | 1812 | 1818 | 1200 (65%) | 410 (23%) |
*At risk population=[(existing registrations at start of time period + new registrations during time period)–(number of patients removed from wait list for the indication: candidate improved/transplant not warranted)].
Average wait time for transplanted patients was 143 days (range 2–501 days).
Wait list drop-out was defined as removal from the wait list for any of the following indications: death, medically unsuitable, candidate condition deteriorated/too sick to transplant and other. Wait list drop-out trended down following the implementation of MELD in both NEOB and in the USA (27–21%, p=0.48 and 23–13%, p=0.00001, respectively). Indications for wait list removal are shown in Table II.
Table II. Indications for removal from the liver transplant wait list for patients with a listing diagnosis of primary liver cancer.
| Deceased donor liver transplant | Death | Medically unsuitable | Candidate condition deteriorated, too sick to transplant | Other | |
|---|---|---|---|---|---|
| MAOB* | |||||
| 2004–2005 | 31 | 3 | 1 | 5 | 0 |
| 1996–2005 | 42 | 7 | 1 | 8 | 1 |
| USA | |||||
| 2004 | 530 | 37 | 8 | 40 | 23 |
| 1996–2005 | 1200 | 182 | 17 | 145 | 66 |
*MAOB, New England Organ Bank.
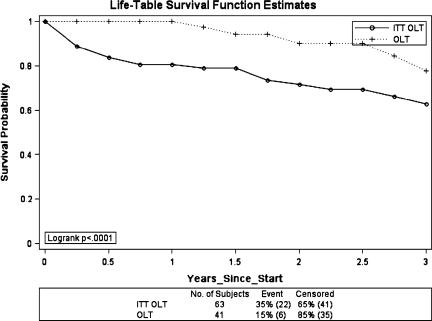
Kaplan-Meier survival curves were calculated using the methodology described previously. Survival was calculated from the OLT survival and from the date of listing for transplant (ITT OLT) (Figure 1). The intention to treat survival is 10–20% lower at every time point when compared to OLT survival. This difference is a result of wait list drop-out.
Figure 1. .
Kaplan-Meier Survival estimates following liver transplantation for hepatocellular carcinoma. OLT, liver transplant survival; ITT OLT, intention to treat liver transplant survival.
Discussion
Liver transplantation and surgical resection currently represent the only curative therapies for HCC. While ablation using radiofrequency, cryotherapy or microwave technology may lead to complete necrosis in up to 65% of small tumors (<3 cm), the results are poorer for larger lesions (>3 cm) and recurrence rates remain high 10,11. Transarterial chemoembolization is an effective treatment for local control in selected patients, but should not be considered a stand alone therapy with curative intent.
Optimal therapy for patients with HCC, American Liver Tumor Study Group Stage I or II remains controversial. While five-year patient survival appears equivalent in selected patients undergoing either surgical resection or transplantation, liver transplantation appears to offer the best disease free five-year survival for those patients who actually receive a transplant 1 (Table III).
Table III. Survival following liver transplantation for primary liver cancer.
| N | One-year survival | Three-year survival | Five-year survival | |
|---|---|---|---|---|
| Cillo 2* | 40 | 89% | 71% | 63% |
| Margarit 1 | 36 | 78% | 65% | |
| Marui 3* | 59 | 56% | ||
| Broelsch 4 | 46 | 61% | ||
| Yao 5 | 46 | 91% | 73% | |
| Llovet 6* | 87 | 84% | 69% | 69% |
| Mazzaferro 7 | 48 | 75%¶ | ||
| UNOS/OPTN | 1201 | 86.3%[euro] | 70%£ | 57.3%¥ |
*Intention to treat survival data.
¶Four-year survival.
[euro]Based on 2002–2004 transplants.
£Based on 1999–2002 transplants.
¥Based on 1997–2000 transplants.
Llovet et al. reported an 87 and 74% three- and five-year survival, respectively with surgical resection for HCC in selected patients without portal hypertension. In their experience, when mean wait times for liver transplantation increased from 62 to 242 days, wait list drop-out increased from 0 to 23% and ITT survival for transplant decreased to 54% in two years 6.
Maraui et al. reported the New Zealand experience for patients listed for liver transplantation with a diagnosis of HCC within the Milan criteria. With a median wait time of 63 days (range 0–832 days) they report a drop-out rate of 17% (10/59) and an ITT five-year survival of 56% 3.
Yao et al. reported the cumulative probability of wait list drop-out to be 7.3%, 25.3% and 43.6% at six, 12 and 24 months, respectively in their experience with mean wait times for blood group O and A patients, listed as status 2B, exceeding 600 days. They reported 11 drop outs in 46 patients listed for liver transplantation with a diagnosis of HCC, with a two-year intention to treat survival of 73% 5.
Cillo et al. reported an impressive 85% ITT three-year survival in patients transplanted for HCC exceeding the Milan criteria with a 69% ITT three-year survival for patients within the Milan Criteria with a median wait time of 11.8 months. The difference in their results may be attributable to bridging therapy for patients awaiting transplantation. In their experience, 83/100 patients received some form of treatment while awaiting transplantation including surgical resection, radiofrequency ablation, transarterial chemoembolization, alcohol injection or some combination of these modalities 12.
Despite the excellent results reported by Cillo et al. the impact of neoadjuvant treatment in patients awaiting liver transplantation remains unclear with conflicting reports in the literature and a lack of large randomized, prospective studies 12,13.
In the present study, patients undergoing liver transplantation for HCC in the NEOB DSA had an 85% three-year survival. When the survival data was adjusted for wait list drop-out, a 10–20% reduction in patient survival was noted at every time point out to three years. This trend continues out to at least five years; however, the present study was inadequately powered to report the data beyond three years.
Survival following surgical resection for HCC in selected candidates may be as high as 57–75% at five years (Table IV). These survival rates compare very favorably to intention to treat survival rates for liver transplantation in the NEOB DSA; making a case for surgical resection as first line therapy.
Table IV. Patient survival following surgical resection for primary liver cancer.
| N | One-year survival | Three-year survival | Five-year survival | |
|---|---|---|---|---|
| Cillo 2* | 131 | 75% | 52% | 31% (58%)§ |
| Tanaka 8 | 86 | 89.6% | ||
| Margarit 1 | 37 | 92% | 70% | |
| Broelsch 4 | 139 | 65% | ||
| Fong 9 | 154 | 81% | 54% | 37% (57%)§ |
| Llovet 6* | 77 | 85% | 62% | 51% (75%)§ |
*Intention to treat survival data.
§Five-year survival in “best candidates” for surgical resection shown in parenthesis.
There is no single, correct answer to the debate: transplant versus resection for stage I and II HCC. Liver transplantation offers the best disease-free survival for patients who actually get transplanted; however, transplant patients are subjected to wait list drop-out. Surgical resection may offer a much shorter time to definitive treatment, but has a higher rate of recurrence.
Wait times for liver transplantation vary by region and DSA. In addition, ITT survival data for transplantation and resection vary by center. The ideal treatment for patients with stage I and II HCC should be based on local experience, regional wait times and individual program's intention to treat survival for both treatment modalities.
Limitations of our study include the following. First, we are comparing liver transplant data from the NEOB DSA to surgical resection data from the published literature. Second, because wait times and outcomes vary by region and center, we suggest caution in applying the NEOB data to centers outside of our DSA. Finally, our data are only relevant for selected patients who are deemed potential candidates for both surgical resection and liver transplantation based on tumor size, location and degree of liver dysfunction.
In conclusion, there is no universally appropriate answer to the debate of transplantation versus resection for treatment of stage I and II HCC. The decision needs to be made based on local ITT survival for both therapies, which will vary based on local wait times for transplantation and experience.
Long wait times for liver transplantation result in wait list drop-out which adversely affects ITT survival in transplant centers served by the NEOB. These data should be deliberately and actively used in the patient counseling and decision-making process when recommending transplant versus resection as first line therapy for stage I or II HCC in our region.
References
- 1.Margarit C, Escartin A, Castells L, Vargas V, Allende E, Bilbao I. Resection for hepatocellular carcinoma is a good option in Child-Turcotte-Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transpl. 2005;11:1242–51. doi: 10.1002/lt.20398. [DOI] [PubMed] [Google Scholar]
- 2.Cillo U, Vitale A, Brolese A, Zanus G, Neri D, Valmasoni M, et al. Partial hepatectomy as first-line treatment for patients with hepatocellular carcinoma. J Surg Onc. 2007;95:213–20. doi: 10.1002/jso.20641. [DOI] [PubMed] [Google Scholar]
- 3.Marui Y, McCall J, Gane E, Holden A, Duncan D, Yeong ML, et al. Liver transplantation for hepatocellular carcinoma in New Zealand: a prospective intent to treat analysis. N Z Med J. 2005;118:1532–45. [PubMed] [Google Scholar]
- 4.Broelsch CE, Frilling A, Malago M. Hepatoma-resection or transplantation. Surg Clin N Am. 2004;84:495–511. doi: 10.1016/j.suc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873–83. doi: 10.1053/jlts.2002.34923. [DOI] [PubMed] [Google Scholar]
- 6.LLovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–40. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. NEJM. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka S, Noguchi N, Ochiai T, Kudo A, Nakamura N, Ito K, et al. Outcomes and recurrence of initially respectable hepatocellular carcinoma meeting Milan criteria: rationale for partial hepatectomy as first strategy. J Am Coll Surg. 2007;204:1–6. doi: 10.1016/j.jamcollsurg.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a western center. Ann Surg. 1999;229:790–800. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900–9. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pompili M, Mirante VG, Rondinara G, Fassati LR, Piscaglia F, Agnes S, et al. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11:1117–26. doi: 10.1002/lt.20469. [DOI] [PubMed] [Google Scholar]
- 12.Cillo U, Vitale A, Grigoletto, Gringeri E, D'Amico F, Valmsaoni M, et al. Intention-to-treat analysis of liver transplantation in selected, aggressively treated HCC patients exceeding the Milan criteria. Am J Transpl 2007:7:972–81. [DOI] [PubMed] [Google Scholar]
- 13.Lopez P, Villanueva A, Roayaie S, Llovet J. Neoadjuvent therapies for hepatocellular carcinoma before liver transplantation: a critical appraisal. Liver Transpl. 2006;12:1747–54. doi: 10.1002/lt.21018. [DOI] [PubMed] [Google Scholar]