Abstract
Introduction. Transcatheter arterial embolization (TAE) and chemoembolization (TACE) are increasingly used to treat unresectable primary and metastatic liver tumors. The purpose of this study was to determine the objective response to TAE and TACE in unresectable hepatic malignancies and to identify clinicopathologic predictors of response. Materials and methods. Seventy-nine consecutive patients who underwent 119 TAE/TACE procedures between 1998 and 2006 were reviewed. The change in maximal diameter of 121 evaluable lesions in 56 patients was calculated from pre and post-procedure imaging. Response rates were determined using Response Evaluation Criteria in Solid Tumors (RECIST) guidelines. The Kaplan-Meier method was used to compare survival in responders vs. non-responders and in primary vs. metastatic histologies. Results. TAE and TACE resulted in a mean decrease in lesion size of 10.3%±1.9% (p<0.001). TACE (vs. TAE) and carcinoid tumors were associated with a greater response (p<0.05). Lesion response was not predicted by pre-treatment size, vascularity, or histology. The RECIST partial response (PR) rate was 12.3% and all partial responders were in the TACE group. Neuroendocrine tumors, and specifically carcinoid lesions, had a significantly greater PR rate (p<0.05). Overall survival, however, was not associated with histology or radiologic response. Discussion. TAE and TACE produce a significant objective treatment response by RECIST criteria. Response is greatest in neuroendocrine tumors and is independent of vascularity and lesion size. TACE appears to be superior to TAE. Although an association of response with improved survival was not demonstrated, large cohort studies are necessary to further define this relationship.
Keywords: chemoembolization, regional therapy, RECIST
Introduction
Transcatheter arterial embolization (TAE) and chemoembolization (TACE) are increasingly used as regional therapeutic modalites for the treatment of unresectable hepatic malignancies 1,2,3,4. Despite the increasing application of these embolization techniques, the indications for their use remain ill-defined. In general, TAE and TACE have been used when surgical resection and/or systemic therapy have failed to produce an adequate response or when conventional therapy has been known to be ineffective. Applied in this manner, TAE and TACE appear to have a specific role in the multimodal management of patients with unresectable hepatic malignancies.
TAE and TACE have been used extensively worldwide since their introduction in the early 1980s 5,6,7,8,9,10. Yet, technical issues and questions regarding therapeutic efficacy remain. For example, uniform criteria for patient selection have not been established and therapeutic efficacy for varying histologies is unclear. Furthermore, it has not been determined if the addition of chemotherapy (i.e., TACE) provides a significant benefit over bland embolization (i.e., TAE) alone 1,2,11. Moreover, when TACE is performed, the choice of chemotherapeutic agent(s) is often arbitrary, since no single regimen has a clear therapeutic advantage. Finally, there is no consensus regarding the necessity or timing of repeat embolization. As a result, treatment decisions are largely made according to individual and institutional biases.
Because the procedural details for TAE and TACE have not been firmly established, we conducted a review of our own institutional experience with TAE and TACE in a consecutive series of patients with primary and metastatic hepatic malignancies. The primary aim of our study was to determine the efficacy of TAE and TACE in producing an objective radiographic tumor response in this population of patients. Additional objectives were to identify predictive factors for improved response with respect to histology, tumor vascularity, and pre-treatment lesion size and to examine the potential association of radiographic response with overall survival. Given the lack of methodologic uniformity, we also present an algorithmic approach for the use of TAE and TACE in hepatic malignancies.
Materials and methods
Sample population
Seventy-nine consecutive patients who underwent a total of 119 transcatheter embolization procedures between 1998 and 2006 were identified from the City of Hope National Medical Center, Institutional Review Board (IRB)-approved Liver Tumor Database. All patients were selected for treatment by a multidisciplinary City of Hope Liver Tumor Group. The universal indication for treatment in this patient population was the presence of liver-dominant, unresectable primary hepatocellular carcinoma (HCC) or unresectable metastatic hepatic disease refractory to systemic chemotherapy. TACE was the preferred treatment modality for both primary and metastatic tumors. TAE was used selectively when the administration of chemotherapy was thought to carry prohibitive risk based on the judgment of the individual treating physicians. For HCC, embolization procedures were limited to patients who did not meet criteria for liver transplantation. The primary contraindications to treatment were the presence of severe underlying liver dysfunction (Child's C cirrhosis) or other medical comorbidities with prohibitively high risk for treatment-related complications. Main portal vein thrombosis was also considered a contraindication to treatment. In the setting of peripheral/peritumoral portal vein obstruction, treatment was considered in the absence of severe liver dysfunction.
Embolization technique
Chemoembolization was performed via a percutaneous transarterial approach in an angiography suite. After the administration of conscious sedation and the infiltration of local anesthesia, access to the common femoral artery was established via the Seldinger technique. Initial diagnostic digital subtraction mesenteric and hepatic arteriography was performed to evaluate vascular anatomy and to rule out significant arteriovenous shunting.
After diagnostic angiography, selective embolization of the distal tumor vasculature was performed. For chemoembolization, doxorubicin (50 mg), mitomycin (10 mg), and cisplatin (150 mg) were emulsified in 15 ml of ethiodol and injected into the distal tumor vasculature. This cocktail of chemotherapeutic agents was chosen based on previous evidence demonstrating significant biologic and morphologic tumor response in HCC, with improved survival compared to previously used regimens 12. The same combination of agents was used for all histologies when performing TACE. Administration of chemotherapy/ethiodol was followed immediately by particulate embolization with polyvinyl alcohol (PVA) microspheres of progressively increasing size (300–700 microns) until stasis of arterial flow to the tumor was achieved. For TAE, ethiodol contrast without chemotherapy was injected initially, followed by the administration of PVA microspheres as above. Treatment was always restricted to a single lobe per session for both TAE and TACE. With large bilobar lesions, additional sessions were scheduled at intervals of approximately one month to embolize the remainder of the untreated tumor vasculature. Retreatment of successfully embolized lesions was not routinely performed.
Assessment of radiologic response
Radiologic response to treatment was assessed by retrospective review of pre and post-procedure imaging. Abdominal computed tomography (CT) imaging with and without intravenous contrast and magnetic resonance imaging (MRI) were selected for response assessment. Pre-procedure studies up to six months prior to the date of treatment were reviewed. Post-procedure studies were examined only if performed ≥30 days after treatment to allow for the development of radiographic changes in the treated lesions. When more than one embolization was performed for the same lesion within a short time interval (<2 months), the combined procedures were considered a single treatment, and radiologic response was assessed after the final embolization.
Response was calculated for both individual lesions and for the overall procedure. Two by two measurements of the three largest treated lesions were recorded. Procedural response was categorized into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) based on the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines 13. We used RECIST size criteria to evaluate treatment response because the methods to evaluate tumor necrosis as a result of embolization have not been established; and the use of RECIST size criteria allowed uniformity in assessing response.
The vascularity of each lesion was categorized as either hypervascular or hypovascular based on the degree of radio-opaque contrast enhancement on arterial phase images. In the case of non-contrast scans, the vascularity of the lesion was coded as missing. All measurements were confirmed by a radiologist experienced in the performance of transcatheter embolization and the assessment of oncologic response.
Of the 119 procedures, six were performed for the same lesion(s) within two months of a previous embolization and were considered part of a single treatment for the purpose of response assessment. Of the remaining procedures, 10 had no available pre-procedure imaging, 26 had no available post-procedure imaging and four had post-procedure imaging performed less than 30 days after treatment. After excluding the above, radiologic response was assessed for a total of 73 procedures in 56 patients, with a total of 121 treated, evaluable lesions.
Data collection
The medical records of the study patients were reviewed for demographic information, details of the embolization procedure, and complications during the post-procedure hospital stay. Renal failure was defined clinically as either post-procedure anuria/oliguria unresponsive to fluid administration, post-procedure dialysis requirement, and/or a rise in post-procedure BUN/creatinine levels. Hepatic failure was defined clinically as increasing ascites and encephalopathy or post-procedure elevation of total bilirubin levels and/or prothrombin time. Procedural mortality was defined as death due to any cause within 30 days of treatment. The design and details of the study were approved by City of Hope IRB.
Statistical analysis
With respect to individual lesion response, the outcome measure of interest was the change in maximal diameter of each lesion. The mean decrease in maximal diameter for all treated lesions was calculated and the significance of the change determined using the paired student's t-test. Lesion response was then stratified according to vascularity (hyper vs. hypo), type of procedure (TAE vs. TACE), histology, and initial lesion size (dichotomized near the median value). Due to the small number of individual histologies in patients with metastatic disease to the liver, metastatic histologies were combined to allow for statistical comparison with HCC. Differences in lesion response by subgroup were assessed using a one-way analysis-of-variance (ANOVA). Differences in RECIST response rates by procedure type and tumor histology were determined using the likelihood-ratio chi-square test. In order to account for possible selection bias caused by the exclusion of early deaths, the procedural analysis was repeated after recategorizing those patients who had no post-procedure imaging and died within six months of treatment as having PD.
Overall patient survival was determined using the Kaplan-Meier method. For the purposes of survival comparison, patients were categorized as “responders” if they had either a CR or PR following any of their embolization procedures, or “non-responders” in the case of stable or progressive radiologic disease. The difference in survival between responders and non-responders was assessed using the log-rank test. A p-value of <0.05 was considered significant. The statistical analysis was performed using Statistical Analysis System (SAS) software version 9.1.3 service pack 3 (SAS Institute Inc., Cary, NC, USA, 2002–2003).
Results
Table I shows the characteristics of the sample population. The mean age at the time of first treatment was 62.7±13.0 years. Fifty-six percent of patients were male. The majority (71%) had a normal or near normal Eastern Cooperative Oncology Group (ECOG) performance status (ECOG status 0 or 1)14. The most common histology was HCC (57%). A significant proportion of patients (43%), however, were treated for liver-dominant metastatic disease. Neuroendocrine tumors (19%), including carcinoids, were the most common metastatic etiology.
Table I. Characteristics of sample population (ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; GIST, gastrointestinal stromal tumor).
| Variable | Total population (n=79) |
|---|---|
| Age (years, mean +/– SD) | 62.7±13.0 |
| Gender | |
| Male | 44 (55.7%) |
| Female | 35 (44.3%) |
| Race | |
| Caucasian/Hispanic | 57 (72.2%) |
| Asian | 16 (20.3) |
| African-American | 6 (7.5%) |
| ECOG status1 | |
| 0 | 38 (48.1%) |
| 1 | 26 (32.9%) |
| 2 | 7 (8.9%) |
| 3 | 6 (7.6%) |
| 4 | 2 (2.5%) |
| Primary diagnosis | |
| HCC | 45 (57%) |
| Metastatic2 | 34 (43%) |
| Neuroendocrine | 15 (19%) |
| Colorectal cancer | 7 (8.9%) |
| GIST | 3 (3.8%) |
| Breast cancer | 2 (2.5%) |
| Cholangiocarcinoma | 2 (2.5%) |
| Other | 5 (6.3%) |
1Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5(6):649–55.
2Includes 2 patients with peripheral/metastatic cholangiocarcinoma.
Procedural details and complications are shown in Tables II and III, respectively. Most procedures were chemoembolizations (84%). The remaining procedures were performed using ethiodol/PVA bland embolization (TAE). One procedure could not be successfully completed and no embolization was performed. This procedure was not included in comparative analysis. With both TAE and TACE, the histologies were almost equally distributed between HCC and metastatic disease. The median length of stay for all procedures was four days (Table II).
Table II. Procedural details (n=119 procedures; TACE, transarterial chemoembolization; TAE, transarterial bland embolization; HCC, hepatocellular carcinoma).
| Results/Frequency (%) | |
|---|---|
| Type of embolization | |
| TACE | 100 (84.0) |
| HCC | 50 |
| Non-HCC | 50 |
| TAE | 18 (15.1) |
| HCC | 10 |
| Non-HCC | 8 |
| Unsuccessful | 1 (0.8) |
| Length of stay, median (range) | Four days (1–15) |
Table III. Procedural complications (n=119 procedures).
| Complication | Frequency (%) |
|---|---|
| Any complication (morbidity)1 | 15 (13%) |
| Bleeding | 2 (2%) |
| Hematoma | 3 (3%) |
| Renal failure | 6 (5%) |
| Hepatic failure | 7 (6%) |
| Infection | 3 (3%) |
| Unable to treat1 | 2 (2%) |
| 30-day all-cause mortality | 7 (5.9%) |
1Unable to treat non-included in overall complication rate
The overall procedural complication rate was 13% (Table III). Organ failure occurred uncommonly, with a 5% incidence of renal failure and a 6% incidence of hepatic failure. Of the seven patients with hepatic failure, two had mild liver dysfunction that resolved spontaneously and four had severe acute liver dysfunction with hepatorenal syndrome. The remaining patient had multi-organ system failure secondary to respiratory insufficiency/respiratory distress which was not directly related to embolization. Of the six cases of renal failure, four were secondary to severe hepatic dysfunction/hepatorenal syndrome and one was secondary to multi-organ system failure. The remaining case had a mild increase in BUN/creatinine which resolved without intervention. Seven 7 deaths occurred within one month of treatment for an overall mortality rate of 5.9%. Five of these deaths occurred during the initial hospital admission, three resulting from severe liver dysfunction after embolization, and two from cardiorespiratory events related to underlying comorbidity. An additional patient was admitted two weeks after discharge with gastric perforation related to non-target embolization of the right gastric artery, and eventually died of septic complications. In all, five of the seven deaths were directly related to the embolization procedure.
Response to TAE/TACE
Table IV shows lesion response collectively for all lesions and stratified by type of embolization, vascularity and initial lesion size. There was a significant overall decrease in lesion size following TAE/TACE with a mean absolute decrease of 6.9 mm±1.4 mm and relative decrease of 10.3%±1.9% (p<0.001). This change was significantly greater with TACE compared to TAE (comparison p<0.04), with no significant decrease in lesion size noted in the TAE subgroup (1.2±2.3 mm, p = 0.62).
Table IV. Individual lesion response (n=121 assessable lesions in 56 patients; TACE, transarterial chemoembolization; TAE, transarterial bland embolization).
| Decrease in maximaldiameter (mean±SE) | p-Value | |
|---|---|---|
| All lesions (n=121) | ||
| Absolute | 6.93±1.36 mm | p<0.001 |
| Relative | 10.3±1.9% | |
| Type of embolization | ||
| TACE (n=98) | ||
| Absolute | 8.29±1.57 mm | p<0.001 |
| Relative | 12.2±2.2% | |
| TAE (n=23) | ||
| Absolute | 1.17±2.31 mm | P = 0.62 |
| Relative | 2.2±3.3% | |
| p for comparison = 0.04 | ||
| Vascularity1 | ||
| Hypervascular (n=32) | ||
| Absolute | 4.56±3.19 mm | p=0.16 |
| Relative | 6.3±4.3% | |
| Hypovascular (n=57) | ||
| Absolute | 7.07±1.81 mm | p<0.001 |
| Relative | 12.4±2.8% | |
| p for comparison = 0.34 | ||
| Initial Lesion Size2 | ||
| < 6.5 cm (n = 60) Relative | 12.1±3.0% | p<0.001 |
| ≥ 6.5 cm (n = 61) Relative | 8.6±2.6% | p = 0.001 |
| p for comparison = 0.38 | ||
1Includes 89 lesions for which vascularity was assessable. The vascularity of remaining lesions could not be assessed, e.g., due to non-contrast scans.
2Only relative changes compared.
Stratified by vascularity, there was a significant decrease in size after the treatment of hypovascular lesions (7.1±1.8 mm, p<0.001) with a smaller, non-significant decrease in the hypervascular group (4.6±3.2, p = 0.16). On statistical comparison of the two groups, however, the response did not differ significantly by lesion vascularity (p = 0.34). With respect to pre-treatment lesion size, a significant relative decrease was noted for both small and large lesions (12.1±3.0% and 8.6±2.6%, respectively, p<0.01) with no significant difference in response between the two groups (Table IV).
Table V shows lesion response stratified by histology. A significant treatment response was observed in both HCC and metastatic lesions, with no significant difference between the two groups (p=0.67). The response with neuroendocrine tumors, including carcinoids, was slightly better than non-neuroendocrine lesions (9.9 mm + / − 2.1 mm, p<0.001, vs. 5.4 mm±1.8 mm, p = 0.003). However, there is no statistically significant difference between the two groups (comparison p=0.13). Examination of carcinoid lesions alone reveals a significantly better response in this subgroup compared to all other lesions (15.4 mm±3.2 mm vs. 5.5 mm±1.5 mm, comparison p=0.009).
Table V. Individual lesion response stratified by histology (n=121 assessable lesions in 56 patients; HCC, hepatocellular carcinoma).
| Histology | Decrease in maximaldiameter (mean±SE) | p-Value |
|---|---|---|
| HCC (n=45) | ||
| Absolute | 6.28±2.00 mm | p = 0.003 |
| Relative | 9.2±3.1% | |
| Non-HCC (n=76) | ||
| Absolute | 7.32±1.83 mm | p<0.001 |
| Relative | 11.0±2.5% | |
| p for comparison = 0.67 | ||
| Neuroendocrine (n=41) | ||
| Absolute | 9.9±2.1 mm | p<0.001 |
| Relative | 14.6±3.3% | |
| Non-neuroendocrine (n=80) | ||
| Absolute | 5.4±1.8 mm | p=0.003 |
| Relative | 8.1±2.4% | |
| p for comparison = 0.13 | ||
| Carcinoid (n=18) | ||
| Absolute | 15.4±3.22 mm | p<0.001 |
| Relative | 22.7±5.7% | |
| Non-carcinoid (n=103) | ||
| Absolute | 5.46±1.45 mm | p<0.001 |
| Relative | 8.2±2.0% | |
| p for comparison = 0.009 | ||
Table VI shows the procedural response rates by RECIST criteria for the entire population and after stratifying by procedure type and histology. There were no CRs after embolization. The overall radiographic PR rate was 12.3%, with a progression rate of 4.5% (Table VI). Most procedures resulted in SD (82.2%). There was no statistically significant difference in response comparing TAE to TACE (p = 0.16). However, all of the PRs occurred following TACE with no responses in the TAE group. The PD rate was also lower in the TACE group (4.9% vs. 8.3%).
Table VI. Procedural response rates (n=73 procedures with assessable response; TACE, transarterial chemoembolization; TAE, transarterial bland embolization, HCC, hepatocellular carcinoma).
| Partial response | Stable disease | Progressive disease | p-Value | |
|---|---|---|---|---|
| All procedures (n=73) | 9 (12.3%) | 60 (82.2%) | 4 (5.5%) | |
| Procedure type1 | ||||
| TACE (n=61) | 9 (14.8%) | 49 (80.3%) | 3 (4.9%) | 0.17 |
| TAE (n=12) | 0 (0%) | 11 (91.7%) | 1 (8.3%) | |
| Histology | ||||
| HCC (n=33) | 4 (12.1%) | 27 (81.8%) | 2 (6.1%) | 0.98 |
| Metastatic (n=40) | 5 (12.5%) | 33 (82.5%) | 2 (5.0%) | |
| Metastatic neuroendocrine (n=19) | 3 (15.8%) | 16 (84.2%) | 0 (0%) | 0.26 |
| All others (n=54) | 6 (11.1%) | 44 (81.5%) | 4 (7.4%) | |
| Metastatic carcinoid (n=8) | 3 (37.5%) | 5 (62.5%) | 0 (0%) | 0.10 |
| All others (n=65) | 6 (9.2%) | 55 (84.6%) | 4 (6.2%) | |
1Does not include one procedure with failed embolization.
Stratified by histology, there was no difference in response comparing HCC to metastatic disease (p = 0.98). Of the five responses in the metastatic subgroup, three occurred in patients with neuroendocrine tumors, one in a patient with colorectal metastases, and one in metastatic breast cancer with PR rates of 15.8% (one of 19), 8.3% (one of 12) and 33.3% (one of three) in these groups, respectively. The radiographic response rates were slightly but non-significantly better in metastatic neuroendocrine tumors compared to all other histologies combined (PR 15.8% vs 11.1%, respectively, p = 0.26). Similarly, there was a trend toward improved radiologic response in carcinoid tumors compared to non-carcinoid disease (PR 37.5% vs 9.2%, p=0.10). The comparison fails to reach significance likely due to a lack of power given the small sample size in the carcinoid group (n=8).
In the case of procedures with no post-procedure scans where a death occurred less than six months from the time of treatment, categorization of this group as PD did not change the relationships observed above (data not shown). This indicates that, although there is the potential for the introduction of selection bias by patients who died prior to post-procedure scanning, the bias does not appear to have affected the above results.
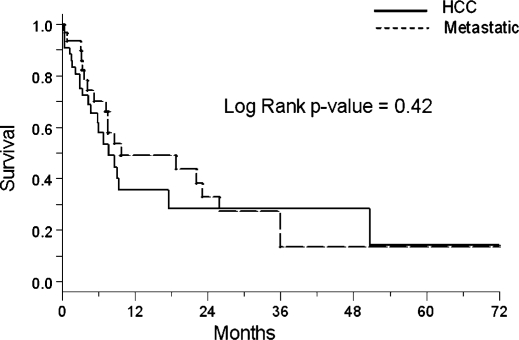
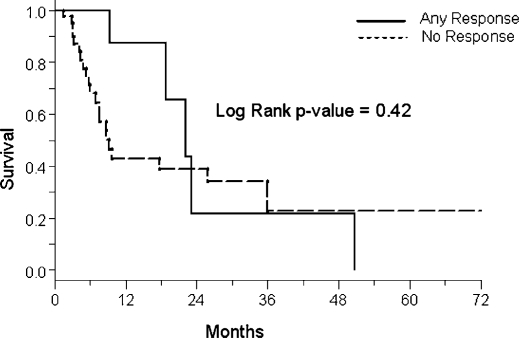
The median survival (MS) for the entire sample population was 8.7 months. The median survival for patients who had an assessable radiologic response was 17.6 months. Figure 1 shows the Kaplan-Meier survival curves comparing patients with HCC and metastatic disease. There is no significant difference in survival between the two groups (MS 7.7 vs. 9.7 months, respectively, p = 0.42). The Kaplan-Meier curves stratified by procedural response are shown in Figure 2. As evident from the overlapping curves, there is no significant survival advantage in patients with a PR to an embolization procedure compared to patients with SD or progression (MS 22.5 vs. 26.3 months, respectively, p = 0.93).
Figure 1. .
Survival estimates stratified by histology.
Figure 2. .
Survival estimates stratified by response.
Discussion
TAE and TACE have been among the most commonly used regional modalities for the treatment of unresectable hepatic malignancies 1,2,3. However, multi-institutional phase III studies demonstrating the benefit of TAE and TACE are lacking and uniform therapeutic indications and patient selection criteria have yet to be established. As a result, it is unclear which patients truly benefit from embolization therapy. In addition, there is no consensus whether TACE is better than TAE alone. In light of these uncertainties, we performed an institutional review of TAE and TACE in a consecutive series of patients with primary and metastatic hepatic malignancies. The purpose of the study was to provide an objective evaluation of the results of TAE and TACE using consistent and uniform criteria for response and to identify tumor, patient, and procedure based predictive factors for improved response. Using our findings in the context of existing evidence, we also provide an algorithmic approach for the use of TAE and TACE in unresectable hepatic malignancies.
In the current study, treatment response was assessed in three ways: (1) change in size of individual lesions; (2) procedural response based on RECIST criteria; and (3) overall survival. Our results demonstrated that both TAE and TACE were well tolerated and performed safely. Our complication rate was 13% with a procedural mortality of 5.9%. Both figures are at the lower range of reported rates 15,16,17,18. Of note, we did not include post-embolization syndrome as a complication because an accurate estimate of its incidence was not possible from retrospective chart review.
With respect to treatment response, TAE and TACE resulted in a measurable and statistically significant decrease in the size of treated lesions. The procedural response rate was 12% by RECIST criteria and was independent of pre-treatment lesion size and vascularity. Primary HCC and metastatic disease responded similarly to treatment. Carcinoid tumors had the largest relative reduction in lesion size (23%) and the highest RECIST response rate (37%). Radiologic response rates in the literature have ranged between 16 and 61% 1,11,19. The lower response rate observed in our series may be secondary to a number of factors. Although decreases in lesion size occurred commonly after embolization, the change in maximal diameter was often not large enough to be captured by RECIST criteria. A clinically significant decrease in tumor activity may still have been present in such cases. It is now known that response to therapy is often not reflected by changes in lesion size and other lesion characteristics, such as necrosis, may be equally critical in assessing treatment response 20,21,22,23.
Our data suggest that the addition of chemotherapy may provide an advantage over bland embolization alone. TACE was associated with a significantly greater decrease in individual lesion size compared to TAE. PRs were observed only after TACE, with no partial responders following TAE, although there was no significant difference on statistical comparison. Interestingly, TACE has never been shown to be more effective than TAE in a trial directly comparing these treatments. However, only TACE has been shown to provide a survival benefit over best supportive care in prospective randomized studies 11,24.
The overall survival observed in our population was less than 10 months and is indicative of the relatively advanced stage of disease in our treatment population (mean pre-treatment lesion size of 9 cm, >50% of patients multiple lesions). None of the patients underwent surgical resection or transplantation after treatment. There was no significant difference in survival comparing patients who had a radiologic response after TAE/TACE to those with either stable or PD. This finding is likely influenced by the small sample size – specifically the small number of responders – which does not allow detection of a small potential survival benefit. Additionally, it is possible that the non-responders in this group benefited similarly to responders, but the treatment benefit was not captured by RECIST criteria.
Survival rates following TAE/TACE have varied widely in the literature, with one and two-year figures as high as 82 and 63%, respectively, in HCC 1,11. Of the several single institution prospective trials, only two have identified a survival advantage with TACE in HCC, both using highly selected patient populations and multiple, planned retreatments 11,24. In the setting of metastatic disease, significant response to second-line TACE has been demonstrated in patients with colorectal hepatic metastases 25,26,27,28,29. Additionally, several retrospective series have demonstrated the potential for prolonged survival in metastatic melanoma 30,31,32, peripheral cholangiocarcinoma 33, and metastatic breast cancer treated with TACE 34. TAE and TACE have been particularly applicable in metastatic neuroendocrine tumors, with response rates ranging from 33 to 80% independent of specific tumor type 35.
Our study has a number of limitations. First, because response was assessed only in patients who had both pre-procedure as well as post-procedure imaging, our findings are subject to some degree of selection bias. We attempted to correct this selection bias by recategorizing response as “progressive disease” in patients who had no post-procedure scan and died within six months of treatment. Recategorization in this manner did not significantly change the reported response rates or comparisons. Additionally, while we were able to identify the histology of the malignancy being treated from the medical record, information about the underlying primary disease and the extent of liver dysfunction could not be consistently assessed from the medical record.
Given the results of our series and evidence from the existing literature, we recommend the following algorithmic approach to the selection and treatment of patients via transcatheter embolization. For HCC patients who are not eligible for surgery or transplant, the extent of the tumor burden should be assessed with triple-phase contrast CT or gadolinium-enhanced MRI. Additional studies should be used to confirm liver-only or liver-dominant disease. Symptomatic patients with pain referable to the mass may benefit from a reduction in tumor volume. Asymptomatic patients with liver-dominant disease, even in the presence of extra-hepatic disease, also warrant consideration for treatment. In such cases, treatment should be undertaken only if the extent of extrahepatic disease is small. Ablative therapies, such as radiofrequency ablation can be considered for lesions <5 cm. For larger lesions, TAE or TACE is the next best option. Chemoembolization appears preferable to bland embolization, although the choice of chemotherapeutic agent is debatable. Cisplatin, doxorubicin, and mitomycin with emulsification in lipiodol is our cocktail of choice 12.
In the case of metastatic hepatic disease, the primary therapy should be surgery and/or systemic chemotherapy. When conventional strategies have failed or are known to be ineffective, such as with neuroendocrine tumors, embolization therapy may be considered. With neuroendocrine tumors, TACE is generally effective and should be employed. The preference of TACE over TAE in this setting is based on our observations as well as other series demonstrating response to chemoembolization 36,37,38. In addition, a recent study demonstrated a trend toward improved survival with TACE vs. TAE with neuroendocrine metastases 39. With other histologies, in the absence of clinical trials, TACE should be considered with the same regimen listed above. In the setting of colorectal hepatic metastases, current first-line chemotherapeutic agents are very effective. Second and third-line regimens incorporating biologic targeted therapies have also been shown to produce response. TACE remains an alternative when these measures hav e failed. TAE should also be considered in patients who are unfit for chemotherapy secondary to medical comorbidities. In addition, with multiple lesions of varying size, combined RFA and TACE can be used to treat the same or opposite lobes.
With respect to patient selection, treatment should be avoided in patients with decompensated liver disease (Child's C cirrhosis). Main portal vein thrombosis is a contraindication to arterial embolization. Based on our findings, large lesion size should not preclude treatment, as response was independent of size in our study. Additionally, hypovascularity should not preclude treatment, as a significant response to TACE was demonstrated in patients with both hypervascular and hypovascular lesions.
In conclusion, our experience demonstrates that arterial embolization procedures can be performed safely with low procedural morbidity and mortality. Using uniform criteria, we show a modest but significant PR to embolization, with significant reductions in lesion size. Response was consistent regardless of size or vascularity of lesions. Our data suggest a greater benefit with TACE compared to TAE. This finding requires confirmation in further trials. The objective tumor response achieved with TACE and TAE merits their continued use in patients with advanced primary and metastatic liver malignancies.
References
- 1.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40(3):225–35. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 2.Liapi E, Geschwind JF. Transcatheter and ablative therapeutic approaches for solid malignancies. J Clin Oncol. 2007;25(8):978–86. doi: 10.1200/JCO.2006.09.8657. [DOI] [PubMed] [Google Scholar]
- 3.Stuart K. Chemoembolization in the management of liver tumors. Oncologist. 2003;8(5):425–37. doi: 10.1634/theoncologist.8-5-425. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan KL. Hepatic artery chemoembolization. Semin Oncol. 2002;29(2):145–51. doi: 10.1053/sonc.2002.31671. [DOI] [PubMed] [Google Scholar]
- 5.Patt YZ, Chuang VP, Wallace S, Benjamin RS, Fuqua R, Mavligit GM. Hepatic arterial chemotherapy and occlusion for palliation of primary hepatocellular and unknown primary neoplasms in the liver. Cancer. 1983;51(8):1359–63. doi: 10.1002/1097-0142(19830415)51:8<1359::aid-cncr2820510807>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Takayasu K, Shima Y, Muramatsu Y, Moriyama N, Yamada T, Makuuchi M, et al. Hepatocellular carcinoma: treatment with intraarterial iodized oil with and without chemotherapeutic agents. Radiology. 1987;163(2):345–51. doi: 10.1148/radiology.163.2.3031724. [DOI] [PubMed] [Google Scholar]
- 7.Takayasu K, Suzuki M, Uesaka K, Muramatsu Y, Moriyama N, Yoshida T, et al. Hepatic artery embolization for inoperable hepatocellular carcinoma; prognosis and risk factors. Cancer Chemother Pharmacol. 1989;23(Suppl):S123–5. doi: 10.1007/BF00647257. [DOI] [PubMed] [Google Scholar]
- 8.Shimamura Y, Gunven P, Takenaka Y, Shimizu H, Shima Y, Akimoto H, et al. Combined peripheral and central chemoembolization of liver tumors. Experience with lipiodol-doxorubicin and gelatin sponge (L-TAE) Cancer. 1988;61(2):238–42. doi: 10.1002/1097-0142(19880115)61:2<238::aid-cncr2820610206>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Kanematsu T, Inokuchi K, Sugimachi K, Furuta T, Sonoda T, Tamura S, et al. Selective effects of Lipiodolized antitumor agents. J Surg Oncol. 1984;25(3):218–26. doi: 10.1002/jso.2930250317. [DOI] [PubMed] [Google Scholar]
- 10.Okamura J, Horikawa S, Fujiyama T, Monden M, Kambayashi J, Sikujara O, et al. An appraisal of transcatheter arterial embolization combined with transcatheter arterial infusion of chemotherapeutic agent for hepatic malignancies. World J Surg. 1982;6(3):352–7. doi: 10.1007/BF01653556. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized controlled trial. Lancet. 2002;359(9319):1734–9. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 12.Solomon B, Soulen MC, Baum RA, Haskal ZJ, Shlansky-Goldberg RD, Cope C. Chemoembolization of hepatocellular carcinoma with cisplatin, doxorubicin, mitomycin-C, ethiodol, and polyvinyl alcohol: prospective evaluation of response and survival in a US population. J Vasc Interv Radiol. 1999;10(6):793–8. doi: 10.1016/s1051-0443(99)70117-x. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–55. [PubMed] [Google Scholar]
- 15.Groupe d'Etude et de Traitement du Carcinome HepatocellulaireA comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med 1995;332(19):1256–61. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda K, Saitoh S, Koida I, Tsubota A, Arase Y, Chayama K, et al. A prospective randomized evaluation of a compound of tegafur and uracil as an adjuvant chemotherapy for hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Am J Clin Oncol. 1995;18(3):204–10. doi: 10.1097/00000421-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P'eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82(1):122–6. doi: 10.1002/bjs.1800820141. [DOI] [PubMed] [Google Scholar]
- 18.Izumi R, Shimizu K, Iyobe T, Ii T, Yagi M, Matsui O, et al. Postoperative adjuvant hepatic arterial infusion of lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology. 1994;20(2):295–301. [PubMed] [Google Scholar]
- 19.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37(2):429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 20.Michaelis LC, Ratain MJ. Measuring response in a post-RECIST world: from black and white to shades of grey. Nat Rev Cancer. 2006;6(5):409–14. doi: 10.1038/nrc1883. [DOI] [PubMed] [Google Scholar]
- 21.Rosner GL, Stadler W, Ratain MJ. Randomized discontinuation design: application to cytostatic antineoplastic agents. J Clin Oncol. 2002;20(22):4478–84. doi: 10.1200/JCO.2002.11.126. [DOI] [PubMed] [Google Scholar]
- 22.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 23.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349(5):427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–71. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 25.Abramson RG, Rosen MP, Perry LJ, Brophy DP, Raeburn SL, Stuart KE. Cost-effectiveness of hepatic arterial chemoembolization for colorectal liver metastases refractory to systemic chemotherapy. Radiology. 2000;216(2):485–91. doi: 10.1148/radiology.216.2.r00au26485. [DOI] [PubMed] [Google Scholar]
- 26.Popov I, Lavrnic S, Jelic S, Jezdic S, Jasovic A. Chemoembolization for liver metastases from colorectal carcinoma: risk or a benefit. Neoplasma. 2002;49(1):43–8. [PubMed] [Google Scholar]
- 27.Vogl TJ, Zangos S, Eichler K, Yakoub D, Nabil M. Colorectal liver metastases: regional chemotherapy via transarterial chemoembolization (TACE) and hepatic chemoperfusion: an update. Eur Radiol. 2007;17(4):1025–34. doi: 10.1007/s00330-006-0372-5. [DOI] [PubMed] [Google Scholar]
- 28.Tellez C, Benson AB, III, Lyster MT, Talamonti M, Shaw J, Braun MA, et al. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer. 1998;82(7):1250–9. doi: 10.1002/(sici)1097-0142(19980401)82:7<1250::aid-cncr7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 29.Lang EK, Brown CL., Jr Colorectal metastases to the liver: selective chemoembolization. Radiology. 1993;189(2):417–22. doi: 10.1148/radiology.189.2.8210369. [DOI] [PubMed] [Google Scholar]
- 30.Bedikian AY, Legha SS, Mavligit G, Carrasco CH, Khorana S, Plager C, et al. Treatment of uveal melanoma metastatic to the liver: a review of the M.D. Anderson Cancer Center experience and prognostic factors. Cancer. 1995;76(9):1665–70. doi: 10.1002/1097-0142(19951101)76:9<1665::aid-cncr2820760925>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 31.Vogl T, Eichler K, Zangos S, Herzog C, Hammerstingl R, Balzer J, et al. Preliminary experience with transarterial chemoembolization (TACE) in liver metastases of uveal malignant melanoma: local tumor control and survival. J Cancer Res Clin Oncol. 2007;133(3):177–84. doi: 10.1007/s00432-006-0155-z. [DOI] [PubMed] [Google Scholar]
- 32.Mavligit GM, Charnsangavej C, Carrasco CH, Patt YZ, Benjamin RS, Wallace S. Regression of ocular melanoma metastatic to the liver after hepatic arterial chemoembolization with cisplatin and polyvinyl sponge. JAMA. 1988;260(7):974–6. [PubMed] [Google Scholar]
- 33.Burger I, Hong K, Schulick R, Georgiades C, Thuluvath P, Choti M, et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol. 2005;16(3):353–61. doi: 10.1097/01.RVI.0000143768.60751.78. [DOI] [PubMed] [Google Scholar]
- 34.Giroux MF, Baum RA, Soulen MC. Chemoembolization of liver metastasis from breast carcinoma. J Vasc Interv Radiol. 2004;15(3):289–91. doi: 10.1097/01.rvi.0000116190.44877.04. [DOI] [PubMed] [Google Scholar]
- 35.Ruszniewski P, O'Toole D. Ablative therapies for liver metastases of gastroenteropancreatic endocrine tumors. Neuroendocrinology. 2004;80(Suppl 1):74–8. doi: 10.1159/000080746. [DOI] [PubMed] [Google Scholar]
- 36.Therasse E, Breittmayer F, Roche A, De BT, Indushekar S, Ducreux M, et al. Transcatheter chemoembolization of progressive carcinoid liver metastasis. Radiology. 1993;189(2):541–7. doi: 10.1148/radiology.189.2.7692465. [DOI] [PubMed] [Google Scholar]
- 37.Ruszniewski P, Rougier P, Roche A, Legmann P, Sibert A, Hochlaf S, et al. Hepatic arterial chemoembolization in patients with liver metastases of endocrine tumors. A prospective phase II study in 24 patients. Cancer. 1993;71(8):2624–30. doi: 10.1002/1097-0142(19930415)71:8<2624::aid-cncr2820710830>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 38.Clouse ME, Perry L, Stuart K, Stokes KR. Hepatic arterial chemoembolization for metastatic neuroendocrine tumors. Digestion. 1994;55(Suppl 3):92–7. doi: 10.1159/000201208. [DOI] [PubMed] [Google Scholar]
- 39.Ruutiainen AT, Soulen MC, Tuite CM, Clark TW, Mondschein JI, Stavropoulos SW, et al. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Interv Radiol. 2007;18(7):847–55. doi: 10.1016/j.jvir.2007.04.018. [DOI] [PubMed] [Google Scholar]