Abstract
Introduction. Tumor extent (T stage) and lymph node involvement (N stage) have a known combined negative effect on survival in patients with gallbladder adenocarcinoma, but the independent effects of these factors have been less well described. We investigated whether T stage and N stage independently predict survival after surgery for gallbladder adenocarcinoma. Methods. We queried the Surveillance, Epidemiology and End Results database for patients treated with surgical resection for gallbladder adenocarcinoma between 1988 and 2004. Cases were stratified by disease severity based on tumor extent and nodal involvement. Kaplan–Meier and Cox regression methods were used to test the effect of disease severity and to develop multivariate models of the effects of demographic and clinical covariates on survival. Univariate and multivariate models were tested in the entire cohort and in a subsample with pathologically confirmed lymph node status. Results. Four thousand and forty-eight patients who survived the immediate perioperative period comprised the full cohort. The subsample with pathologically confirmed lymph node status included 1298 patients. Age, gender, radiation treatment, tumor grade, tumor extent and lymph node status had statistically significant independent effects on survival in both models (all p<0.03). After accounting for T by N stage interactions, both tumor extent (1.21≤HR≤3.81, all p≤0.005) and lymph node involvement (1.80≤HR≤2.84, p<0.001) had independent effects on survival. Conclusions. Tumor extent and lymph node metastases are independent predictors of survival after surgical resection for gallbladder adenocarcinoma. Tumor penetration of the gallbladder wall and pathologically confirmed lymph node involvement each carry poor prognosis.
Keywords: gallbladder cancer, survival, SEER registry, tumor extent, lymph node metastases
Introduction
Gallbladder adenocarcinoma is an aggressive malignancy with a generally poor prognosis. Primary R0 surgical resection remains the best treatment option. Five-year survival rates in the 1990s, based on American Joint Committee on Cancer (AJCC) 5th edition staging criteria, were 60% for Stage 0, 39% for Stage I, 15% for Stage II, 5% for Stage III and 1% for Stage IV 1. Recent advances in surgical technique and perioperative care have improved survival after extensive local resections. There is an ongoing impetus for an aggressive surgical approach aimed at an R0 resection. Currently most hepatobiliary surgeons advocate radical liver and portal lymph node resections 2. Some surgical centers suggest even more radical resections, including combination hepatectomy with pancreaticoduodenectomy and radical lymph node dissections including peripancreatic and extended portal dissections 3. With implementation of an aggressive surgical resection technique, Dixon et al. have shown an overall five-year survival improvement from 7 to 35% and a significant improvement at every stage 2.
Tumor extent (T stage) and lymph node involvement (N stage) significantly impact survival in patients with gallbladder adenocarcinoma. Some authors suggest that the T stage is the critical prognostic factor in gallbladder adenocarcinoma 4,5. However, others point to nodal metastasis as the crucial indicator of long-term survival 6,7. We investigated the independent effects of tumor extent and lymph node involvement on survival after surgical resection for gallbladder adenocarcinoma. We were also interested in analyzing the effect of radical surgical resection on survival in patients with various T and N stages of gallbladder cancer. To obtain an adequate sample to answer these questions we analyzed data from the Surveillance, Epidemiology and End Results (SEER) registry database for 1988–2004. SEER data previously have demonstrated improved survival in patients with gallbladder cancer who were treated with adjuvant radiation therapy 8. Analysis of the SEER database has also suggested a decreasing incidence and improved survival over the past three decades of treatment of gallbladder cancer 9. Another SEER registry study suggested the importance of lymph node dissection in improving staging and treatment 10. We hypothesized that both increased T stage and N stage would pose independent increased risk of death. Using the SEER registry data, we examined the association between extent of tumor progression and lymph node involvement on survival in patients with gallbladder adenocarcinoma.
Patients and methods
Surveillance, Epidemiology and End Results (SEER) registry and study population
The SEER program is a United States population-based cancer registry supported by the National Cancer Institute and Centers for Disease Control and Prevention spanning 1973–2004. The current registry contains data from 18 sites and samples about 26% of the US population. SEER registrars routinely collect demographic data, primary tumor characteristics including tumor site and spread, primary course of treatment exclusive of chemotherapy, and follow-up for vital status 11.
Data collection and management
This investigation was reviewed and approved by Vanderbilt University Medical Center Institutional Review Board. We conducted a retrospective, population-based cohort analysis of patients listed in the SEER database who underwent surgical resection for pathologically confirmed diagnosis of gallbladder adenocarcinoma (SEER specific icd-o-3 code 8140 and primary pathology site code C23.9-gallbladder) between 1988 and 2004. Demographic data included age, gender, race, and ethnicity. Clinical staging data included AJCC tumor/node/metastasis (TNM) staging (Table I), available for 2004, and extent of disease (EOD10) classification, available for the years 1988–2003. Data regarding tumor grade, radiation therapy, extent of surgical resection and survival were also recorded. Surgical eras were classified as either 1988–1996 or 1997–2004 based on perceived use of aggressive surgical resection in clinical practice.
Table I. American Joint Committee on Cancer (AJCC) 6th edition staging system 12.
| Stage | TNM classification |
|---|---|
| Stage IA | T1, N0, M0 |
| Stage IB | T2, N0, M0 |
| Stage IIA | T3, N0, M0 |
| Stage IIB | T1-3, N1, M0 |
| Stage III | T4, N any, M0 |
| Stage IV | T any, N any, M1 |
Note: T1, tumor invades lamina propria or muscle layer; T2, tumor invades the perimuscular connective tissue, no extension beyond the serosa or into the liver; T3, tumor perforates the serosa (visceral peritoneum) and/or directly invades the liver and/or one other adjacent organ or structure, such as the stomach, duodenum, colon, or pancreas, omentum or extrahepatic bile ducts; T4, tumor invades main portal vein or hepatic artery or invades multiple extrahepatic organs or structures; N0, no regional lymph node metastasis; N1, regional lymph node metastasis; M0, no distant metastasis; M1, distant metastasis.
The EOD10 data were recoded into the TNM staging paradigm using the SEER EOD10 coding manual and AJCC Staging Manual 6th edition 12,13,14. Patients with missing staging information were excluded from the study. Additionally, those patients who were recorded in the SEER registry as being deceased before one month of follow-up were coded as a perioperative death and were excluded from analysis. Only those patients with pathologically confirmed node positive disease were identified as having lymph node involvement. Patients with documented node negative disease, as well as patients without pathologically examined lymph nodes or without nodes present in the specimen were categorized as node negative. A separate subsample analysis, which was restricted to the patients with pathologically examined lymph nodes, was also performed. SEER registry coding for surgical interventions is based on local interventions, simple/partial surgical resections, total surgical removal, debulking operations, or radical resections. We reclassified these surgery codes as local, total, or radical resections based on SEER manual definitions 14. SEER EOD10 tumor extent code “70”, defined in the manual as either “extension into liver >2 cm or extension into two or more adjacent organs or liver involvement with any other organ”, could be interpreted as either a T3 or T4 lesion. Preliminary analysis confirmed these patients to have survival similar to the T4 lesion patients. Therefore, patients with EOD10 tumor extent code “70” were grouped with Stage III patients. The final classification system for disease severity based on clinical stage, tumor extent, and nodal involvement comprised eight mutually exclusive strata: Stage IA, Stage IB, Stage IIA, Stage IIB – T1N1, Stage IIB – T2N1, Stage IIB – T3N1, Stage III, and Stage IV.
Statistical analysis
Kaplan–Meier survival analysis, with the Log-rank test for between-group comparisons, was used to test the effect of the disease severity classification system on survival 15,16. Cox proportional hazards regression was used to develop a multivariate models of the effects of age, sex, race, ethnicity, radiation therapy, tumor grade, tumor extent, lymph node status, extent of resection, and era of operation on survival in patients without metastatic disease 17. A T stage by N stage interaction effect was included in the Cox multivariate models. Two separate univariate and multivariate analyses were performed. The first analysis tested the effects in the entire cohort. The second analysis tested the effects in the subsample of patients with pathologically confirmed lymph node status.
STATA10 data analysis and statistical software (College Station, TX) was used in data management, statistical analysis and graphics design. A Type I error probability of <0.05 was considered statistically significant. Summary data are reported as mean±SD or percentages.
Results
Demographics
There were 5944 patients in the SEER database diagnosed with gallbladder adenocarcinoma between 1988 and 2004. From this group 4365 patients underwent surgical treatment for gallbladder adenocarcinoma and 4048 patients (93%) had sufficient staging information recorded in the SEER database and survived the immediate perioperative period. These 4048 patients comprised the complete cohort for further analysis. Demographic and clinical data are summarized in Table II. Patients averaged 71±13 years. Among the patients 74% were female, 81% were Caucasian and 17% were of Hispanic ethnicity. Radiation therapy was received by 18% of the patients. Specific tumor grade and TNM stage information are shown in Table II.
Table II. Demographic and clinical data: SEER database, cases entered 1988–2004.
| Full study cohort 4048 patients | Pathologically confirmed LN 1298 patients | |
|---|---|---|
| Age | 71±13 | 68±13 |
| Female | 2990 (73.8%) | 952 (73.3%) |
| Caucasian | 3282 (81.1%) | 1,049 (80.8%) |
| Hispanic | 686 (17.0%) | 206 (15.9%) |
| Radiation | 726 (17.9%) | 338 (26.0%) |
| Grade I | 561 (13.9%) | 160 (12.3%) |
| Grade II | 1546 (38.2%) | 496 (38.2%) |
| Grade III | 1508 (37.3%) | 517 (39.8%) |
| Grade IV | 58 (1.4%) | 29 (2.2%) |
| T1 | 907 (22.4%) | 248 (19.1%) |
| T2 | 809 (20.0%) | 297 (22.9%) |
| T3 | 1339 (33.1%) | 477 (36.7%) |
| T4 | 233 (5.8%) | 79 (6.1%) |
| Lymph node involvement | 737 (18.2%) | 737 (56.8%) |
| Metastatic | 845 (20.9%) | 231 (17.8%) |
Only 1298 patients (32%) of the full cohort group had pathologically examined lymph nodes. This subsample was examined separately as a secondary analysis. Demographic and clinical data in this patient subsample were comparable to the overall cohort, with the exception of the proportion of patients with lymph node involvement (Table II). In both samples, 737 patients had positive lymph nodes. In the overall cohort, this constituted 18% of the patients, while in the subsample analysis these patients comprised 57% of the population.
Univariate analyses
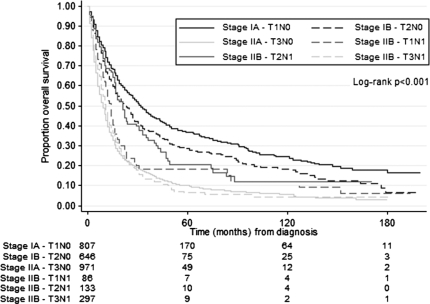
The effect of disease severity on survival is summarized in Table III. In the full study cohort, one- and five-year survival estimates based on Kaplan–Meier analysis were better for Stage IA (72 and 37%), Stage IB (66 and 29%), Stage IIB – T1N1 (53 and 18%) and Stage IIB – T2N1 (69 and 21%) disease; than for Stage IIA (40 and 10%), Stage IIB – T3N1 (43 and 7%), Stage III (21 and 3%) and Stage IV (11 and 3%) disease. Interestingly, in this cohort, patients with Stage IIB – T1N1 and Stage IIB – T2N1 survived significantly longer than patients with Stage IIA disease. Pairwise comparisons revealed improved median survival from Stage IIA: nine month to Stage IIB – T1N1: 14 month (p=0.037) and improved median survival from Stage IIA: nine month to Stage IIB – T2N1: 21 month (p<0.001). Survival did not differ between Stage IIA: nine month and Stage IIB – T3N1: 11 month (p=0.634). One- and five-year survival proportions were similarly improved in patients with Stage IIB – T1N1 and Stage IIB – T2N1 disease compared to patients with Stage IIA disease (Table III). Survival curves for Stage IA through Stage IIB are shown in Figure 1.
Table III. One- and five-year survival by disease severity stratification.
| Full study cohort 4048 patients |
Pathologically confirmed LN 1298 patients |
|||||
|---|---|---|---|---|---|---|
| Disease severity | No. of patients | One-year (%) | Five-year (%) | No. of patients | One-year (%) | Five-year (%) |
| Stage IA | 814 | 72.1 | 36.6 | 160 | 84.2 | 47.4 |
| Stage IB | 659 | 65.3 | 28.5 | 155 | 88.0 | 53.8 |
| Stage IIA | 982 | 39.6 | 10.3 | 155 | 65.5 | 24.9 |
| Stage IIB – T1N1 | 86 | 52.7 | 18.2 | 86 | 52.7 | 18.2 |
| Stage IIB – T2N1 | 135 | 68.6 | 20.7 | 135 | 68.6 | 20.7 |
| Stage IIB – T3N1 | 298 | 43.0 | 6.5 | 298 | 43.0 | 6.5 |
| Stage III | 229 | 21.2 | 2.9 | 78 | 28.9 | 7.9 |
| Stage IV | 845 | 11.3 | 2.6 | 231 | 21.1 | 4.5 |
Figure 1. .
Survival by disease severity in the full study cohort. Patients with Stage IA through Stage IIB disease are included in the Kaplan–Meier plot. Survival estimates were significantly better for patients with Stage IA, Stage IB, Stage IIB – T1N1, and Stage IIB – T2N1 disease; than for patients with Stage IIA, and Stage IIB – T3N1 disease.
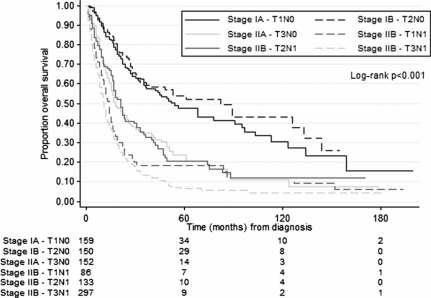
Univariate analysis for the subsample of patients with pathologically confirmed lymph node status differed in specific respects from the full cohort (Table III). Kaplan–Meier one- and five-year survival estimates in patients with pathologically confirmed lymph node status were better for patients with Stage IA (84 and 47%) and Stage IB (88 and 54%) disease, than for those with Stage IIA (65 and 23%), Stage IIB – T1N1 (53 and 18%), Stage IIB – T2N1 (68 and 21%), Stage IIB – T3N1 (43 and 6%), Stage III (29 and 8%), and Stage IV (21 and 4%) disease. Pairwise comparisons did not reveal statistically significant differences between patients with Stage IA and Stage IB disease (p=0.423). Survival in this subsample was better in Stage IIA patients compared to patients with Stage IIB – T1N1 disease (p=0.041) and patients with Stage IIB – T3N1 disease (p<0.001). It did not differ between patients with Stage IIA and Stage IIB – T2N1 disease (p=0.960). Survival curves for the subsample analysis between Stage IA and Stage IIB are shown in Figure 2.
Figure 2. .
Survival by disease severity in the patient subsample with pathologically confirmed lymph node status. Survival estimates were significantly better for patients with Stage IA and Stage IB disease, than for patients with Stage IIA, Stage IIB – T1N1, Stage IIB – T2N1, and Stage IIB – T3N1 disease.
Multivariate analysis
The multivariate Cox model testing of the independent effects of tumor extent and lymph node involvement on survival in 2930 patients without metastatic disease is summarized in Table IV. Of the 3203 patients with non-metastatic disease, 273 patients were excluded from multivariate analysis due to either missing tumor grade or surgical procedure. This model demonstrates statistically significant independent risk of death in patients with progressive tumor extent and node positive disease. Additionally, increasing age, male gender, African American race, increasing tumor grade, increasing tumor extent, and presence of lymph node involvement all conferred independent risk of death in patients with gallbladder adenocarcinoma (all p<0.04). Radiation treatment conferred 22% risk reduction in this model (p<0.001). There was a greater independent risk associated with progressive tumor extent (HR=1.21, CI=1.05–1.39, p=0.008 for T2 vs. T1; HR=2.29, CI=2.02–2.59, p<0.001 for T3 vs. T1 and HR=3.81, CI=3.14–4.62, p<0.001 for T4 vs. T1) than associated with lymph node involvement (HR=1.80, CI=1.37–2.37, p<0.001).
Table IV. Cox multivariate model of the effects of clinical and demographic covariates on survival.
| Full study cohort 2930 patients |
Pathologically confirmed LN 999 patients |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age at diagnosis | 1.02 | 1.01–1.03 | <0.001 | 1.02 | 1.01–1.03 | <0.001 |
| Female sex | 0.88 | 0.80–0.96 | 0.006 | 0.79 | 0.67–0.94 | 0.007 |
| African American race | 1.20 | 1.01–1.41 | 0.033 | 0.99 | 0.71–1.37 | 0.946 |
| Hispanic ethnicity | 0.99 | 0.88–1.11 | 0.827 | 1.04 | 0.84–1.29 | 0.710 |
| Radiation | 0.78 | 0.70–0.87 | <0.001 | 0.65 | 0.54–0.79 | <0.001 |
| Surgery | ||||||
| Total vs. Local | 0.99 | 0.84–1.17 | 0.898 | 0.87 | 0.64–1.18 | 0.369 |
| Radical vs. Local | 0.91 | 0.75–1.11 | 0.352 | 0.85 | 0.61–1.20 | 0.354 |
| Era (1997–2004 vs. 1988–96) | 0.93 | 0.85–1.02 | 0.115 | 0.82 | 0.70–0.97 | 0.022 |
| Tumor grade | ||||||
| II vs. I | 1.27 | 1.11–1.44 | <0.001 | 1.28 | 0.99–1.66 | 0.064 |
| III vs. I | 1.84 | 1.61–2.10 | <0.001 | 1.71 | 1.32–2.22 | <0.001 |
| IV vs. I | 2.00 | 1.42–2.82 | <0.001 | 2.13 | 1.27–3.57 | 0.004 |
| Tumor extent (T stage) | ||||||
| T2 vs. T1 | 1.21 | 1.05–1.39 | 0.008 | 0.92 | 0.64–1.31 | 0.626 |
| T3 vs. T1 | 2.29 | 2.02–2.59 | <0.001 | 2.13 | 1.55–2.91 | <0.001 |
| T4 vs. T1 | 3.81 | 3.14–4.62 | <0.001 | 3.23 | 1.97–5.31 | <0.001 |
| LN involvement (N stage) | 1.80 | 1.37–2.37 | <0.001 | 2.84 | 1.99–4.04 | <0.001 |
A separate multivariate model was developed for the subsample of patients with pathologically confirmed lymph node status without distant metastases. Of the 1067 patients without distant metastases, 999 had complete data and were included in the multivariate analysis. Age, male gender, increasing tumor grade, increasing tumor extent and lymph node involvement all conferred statistically significant independent risk of death (all p<0.01). Radiation therapy conferred a 35% risk reduction (p<0.001), and patients undergoing operation after 1997 had an 18% risk reduction compared to patients treated before 1997 (p=0.022). In this model, the independent effect of lymph node involvement (HR = 2.84, CI = 1.99–4.04, p<0.001) was more pronounced than in the full cohort. Tumor extent at the T2 level did not convey additional risk (p=0.626); however both T3 (HR = 2.13, CI = 1.55–2.91, p<0.001) and T4 (HR = 3.23, CI 1.97–5.31) lesions conveyed significant independent risks (Table IV).
Both models included significant T stage by N stage interaction effects (p<0.05) which reflected increased risk due to lymph node involvement across progression of tumor extent. These interaction effects produced a non-multiplicative combination of T stage and N stage risk that is summarized in Table V. In the full cohort, T stage contributes significant risk at every level, while the effect of N stage provided considerably less additional risk. The additional risk due to nodal involvement was greatest in patients with T1 stage disease. However, the negative effect of N stage was more pronounced in patients with the pathologically confirmed lymph node status. In this subsample, the incremental risk of nodal metastases was significant at every T stage, and was most pronounced for T1 and T2 lesions.
Table V. Effect of T stage and N stage on survival in patients with gallbladder adenocarcinoma without distant metastases – interaction model adjusted for age, sex, race, ethnicity, radiation therapy, extent of surgery, era of operation and tumor grade. Data entries are hazard ratios (95% CI).
| Full study cohort |
Pathologically confirmed LN |
|||
|---|---|---|---|---|
| N0 | N1 | N0 | N1 | |
| T1 | 1.0* | 1.80 (1.37–2.37) | 1.0* | 2.84 (1.99–4.04) |
| T2 | 1.21 (1.05–1.39) | 1.44 (1.14–1.83) | 0.92 (0.64–1.31) | 2.26 (1.63–3.13) |
| T3 | 2.29 (2.02–2.59) | 2.35 (1.97–2.77) | 2.13 (1.55–2.91) | 3.75 (2.82–4.97) |
| T4 | 3.81 (3.14–4.62) | 3.85 (2.76–5.37) | 3.23 (1.97–5.31) | 6.77 (4.48–10.23) |
*HR 1.0 for T1N0 used as reference.
Impact of surgery
The type of surgical interventions was recoded from the SEER registry as three groups (local, total, and radical) and included in the multivariate model. In the full study cohort, after excluding patients with metastatic disease, the number of patients in the three groups was: local n=295 (9.2%), total n=2,474 (77.2%), or radical n=411 (12.8%). Twenty-three patients (0.7%) did not have a specific operative code recorded. We did not demonstrate a significant difference in survival due to either total vs. local resection (HR = 0.99, CI = 0.84–1.17, p=0.898) or radical vs. local resection (HR = 0.91, CI = 0.75–1.11, p=0.352). Distribution of operative procedures was similar between the full cohort and in the subsample of patients with pathologically confirmed lymph node status. Consistent with the findings in the full cohort, neither total vs. local resection (HR = 0.87, CI = 0.64–1.18, p=0.369) nor radical vs. local resection (HR = 0.82, CI = 0.70–0.97, p=0.354) provided a statistically significant benefit in this subsample.
Discussion
Gallbladder adenocarcinoma continues to present challenges in diagnosis and treatment. Most patients with symptomatic gallbladder malignancy present with incurable, advanced stage, disease 18. A number of single-center studies suggest improved outcomes with aggressive surgical management 2,3. The goal of surgical treatment of gallbladder carcinoma is complete R0 tumor resection with negative histologic margins. While the rationale for extended cholecystectomy as well as hemi- or extended hepatectomy and portal lymph node dissection has been universally accepted among hepatobiliary surgeons, the efficacy of more radical procedures including extrahepatic biliary resection, radical lymph node dissection, and pancreaticoduodenectomy has remained controversial 19. A number of studies document improved survival with aggressive resections for patients with T2, T3 and even T4 lesions 7,20,21. Other studies suggest success of lymphadenectomy in improving survival in patients with N1 disease 22,23.
Our aim was to evaluate independent effects of tumor extent and lymph node metastases on survival in gallbladder adenocarcinoma. The SEER registry provides a rich source of data for a population-based cohort study. Unfortunately only 32% of all patients undergoing surgical resection for gallbladder adenocarcinoma between 1988 and 2004 had pathologically examined lymph nodes as part of the specimen. Excluding these patients could markedly reduce the representative distribution of the study sample and would lead to a substantial loss of statistical power. For these reasons we developed two separate analyses. In the first analysis, we investigated the univariate and multivariate effects in our full patient cohort. In the second analysis we focused on those patients with pathologically confirmed lymph node status. Tumor extent and lymph node involvement exerted statistically significant independent negative effects on patient survival in both analyses. Univariate effects differed between the full cohort, in which patients without pathologically documented lymph node status were classified as N0, and the subsample of patients with pathologically confirmed lymph nodes. In the full cohort, univariate analysis suggested that patients with Stage IIB – T1N1 and Stage IIB – T2N1 had better survival than patients with Stage IIA – T3N0. However, this finding did not persist in the subsample of patients with confirmed N stage. In fact, patients with T1N0 and T2N0 lesions had comparatively better survival to patients with either T3 lesions or N1 lesions. This finding implies that a number of patients with undocumented lymph node status included in the T3N0 group in the full cohort actually had N1 lesions. Subsample analysis confirmed that 155 patients in T3N0 group had pathologically confirmed negative lymph nodes, which leaves 827 patients from the full cohort with undocumented lymph node status. Unfortunately it is impossible to determine which of these patients from the full cohort have N0 or N1 lesions.
However, the multivariate analyses were more consistent between the overall cohort and the subsample of patients with pathologically confirmed lymph node status. Both multivariate models included significant T stage by N stage interaction effects and demonstrated significant independent effects of tumor extent and lymph node involvement on survival in gallbladder adenocarcinoma. In patients without distant metastatic disease, both models demonstrated that lymph node involvement conferred a significantly increased risk of death, while the presence of a T3 lesion more than doubled and a T4 lesion more than tripled this risk. The effect of T2 lesions was statistically significant in the full model, but not in the subsample analysis. The effect of N1 stage in the full model increased the risk by 80%, but almost tripled the risk in the patients with pathologically confirmed lymph nodes.
In the full cohort, hazard ratios increased substantially with increase in T stage, but increased only slightly with lymph node involvement. However, in patients with documented lymph node status, lymph node involvement conveyed a substantially increased risk across the T stages. Once more, this finding implies that a number of patients with undocumented lymph node status may have had N1 disease. Given that these patients did not have documented metastatic disease, we can only speculate as to why they did not undergo a more aggressive lymph node dissection.
Unfortunately, the SEER registry for years 1988–2004 does not have sufficient information to test the effect of an aggressive surgical approach in this patient population with stringency. The coding of surgical procedure is based on “completeness” of resection and does not provide the specifics of the surgical intervention. As such, the precise surgical intervention, and whether the planned intervention was an R0 resection with an intent to cure, is unknown. We characterized operative interventions as local, total, and radical resections based on SEER coding and did not show a significant impact on survival in the multivariate models. Interestingly, only 12.8% of the patients without distant metastatic disease underwent a radical resection. It is not known how many patients were diagnosed after a routine cholecystectomy and required re-resections as part of treatment.
As we have documented, in the SEER registry, only 32% of the patients surgically treated for gallbladder adenocarcinoma had pathologically documented presence of lymph nodes within the resection specimen. Moreover, only about 13% of the patients underwent aggressive surgical treatment. Yet, our analysis suggests that tumor penetration through the gallbladder wall and pathologically documented lymph node involvement each carry a particularly poor prognosis in this patient population. While the evidence is indirect, we believe that these data suggests that lymph node status must be better documented at the time of resection with a more thorough regional lymphadenectomy. In addition, in an attempt to improve survival, patients with T3 lesions must be treated with a radical resection.
The SEER registrars are continually striving to improve the quantity and quality of data within the database. While this cancer database is the standard for quality among cancer registries in USA, some of the patient and treatment information is limited. The registry includes data from both community and academic hospitals; however, information is not stratified by hospital type or volume of complex biliary procedures. As with other complex hepato-pancreato-biliary procedures, it is likely that the multimodality treatment and high surgical volume would lead to improved outcomes 24,25. Some of the tumor staging and grading data is missing. As previously discussed, information regarding operative procedure lacks specificity and cannot be used to identify an operative procedure. Information about patients’ comorbidities, performance status, tumor resection margin, and chemotherapy is not available. Despite these limitations, well-designed SEER registry studies have provided clinicians with a wealth of information. Continuous improvements in SEER data collection and management will increase utility of this registry in study of quality and outcomes in cancer treatment.
Our study, based on population data captured by the SEER registry between 1988 and 2004, demonstrates that both tumor extent and lymph node metastases are independent predictors of survival in gallbladder adenocarcinoma. Patients with tumor extent limited to the perimuscular connective tissue without positive lymph nodes had best prognosis. Tumor penetration of gallbladder wall and pathologically confirmed lymph node involvement carried particularly poor prognosis.
Acknowledgements
Supported in part by the Institutional National Research Service Award T32 HS 013833 from the Agency of Healthcare Research and Quality, US Department of Health and Human Services. The authors like to thank Dr Marie Griffin (Department of Preventive Medicine) and Dr William Dupont (Department of Biostatistics) for helpful discussions.
Footnotes
Presented at the 8th American Hepato-Pancreato-Biliary Association Meeting, Ft. Lauderdale, Florida, March 2008.
References
- 1.Donohue JH, Stewart AK, Menck HR. The national cancer data base report on carcinoma of the gallbladder, 1989–1995. Cancer. 1998;83(12):2618–28. doi: 10.1002/(sici)1097-0142(19981215)83:12<2618::aid-cncr29>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 2.Dixon E, Vollmer CM, Jr, Sahajpal A, Cattral M, Grant D, Doig C, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg. 2005;241(3):385–94. doi: 10.1097/01.sla.0000154118.07704.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirai Y, Wakai T, Hatakeyama K. Radical lymph node dissection for gallbladder cancer: indications and limitations. Surg Oncol Clin N Am. 2007;16(1):221–32. doi: 10.1016/j.soc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg. 2007;11(5):671–81. doi: 10.1007/s11605-006-0075-x. [DOI] [PubMed] [Google Scholar]
- 5.Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232(4):557–69. doi: 10.1097/00000658-200010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sikora SS, Singh RK. Surgical strategies in patients with gallbladder cancer: nihilism to optimism. J Surg Oncol. 2006;93(8):670–81. doi: 10.1002/jso.20535. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett DL, Fong Y, Fortner JG, Brennan MF, Blumgart LH. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224(5):639–46. doi: 10.1097/00000658-199611000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mojica P, Smith D, Ellenhorn J. Adjuvant radiation therapy is associated with improved survival for gallbladder carcinoma with regional metastatic disease. J Surg Oncol. 2007;96(1):8–13. doi: 10.1002/jso.20831. [DOI] [PubMed] [Google Scholar]
- 9.Kiran RP, Pokala N, Dudrick SJ. Incidence pattern and survival for gallbladder cancer over three decades – an analysis of 10301 patients. Ann Surg Oncol. 2007;14(2):827–32. doi: 10.1245/s10434-006-9224-4. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz RE, Smith DD. Lymph node dissection impact on staging and survival of extrahepatic cholangiocarcinomas, based on US population data. J Gastrointest Surg. 2007;11(2):158–65. doi: 10.1007/s11605-006-0018-6. [DOI] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology and End Results (SEER) program: Public use data (1973–2004). National Cancer Institute. http://seer.cancer.gov/data/. Last accessed July 2007. [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M. Gallbladder. In: AJCC cancer staging manual. 6th ed. New York: Springer; 2002. [Google Scholar]
- 13.SEER Extent of Disease – 1988. Codes and coding instructions. 3rd ed. http://seer.cancer.gov/data/documentation.html. Last accessed July 1998. [Google Scholar]
- 14.SEER program coding and staging manual 2007. Appendix C. http://seer.cancer.gov/tools/codingmanuals/Last accessed July 2007. [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 16.Peto R, Pike MC. Conservatism in the approximation E(0-E)2/E in the log rank test for survival data or tumor incidence data. Biometrics. 1973;29:579–84. [PubMed] [Google Scholar]
- 17.Cox DR. Regression models and life tables. J R Stat Assoc. 1972;29:187–220. [Google Scholar]
- 18.Miller G, Jarnagin WR. Gallbladder carcinoma. Eur J Surg Oncol. 2008;34(3):306–12. doi: 10.1016/j.ejso.2007.07.206. [DOI] [PubMed] [Google Scholar]
- 19.Pitt HA. Gallbladder cancer: what is an aggressive approach? Ann Surg. 2005;241(3):395–6. doi: 10.1097/01.sla.0000154119.55201.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wise PE, Shi YY, Washington MK, Chapman WC, Wright JK, Sharp KW, et al. Radical resection improves survival for patients with pT2 gallbladder carcinoma. Am Surg. 2001;67(11):1041–7. [PubMed] [Google Scholar]
- 21.Foster JM, Hoshi H, Gibbs JF, Iyer R, Javle M, Chu Q, et al. Gallbladder cancer: defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol. 2007;14(2):833–40. doi: 10.1245/s10434-006-9097-6. [DOI] [PubMed] [Google Scholar]
- 22.Shimada H, Endo I, Togo S, Nakano A, Izumi T, Nakagawara G. The role of lymph node dissection in the treatment of gallbladder carcinoma. Cancer. 1997;79(5):892–9. doi: 10.1002/(sici)1097-0142(19970301)79:5<892::aid-cncr4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87(4):418–22. doi: 10.1046/j.1365-2168.2000.01384.x. [DOI] [PubMed] [Google Scholar]
- 24.Birkmeyer JD, Finlayson SR, Tosteson AN, Sharp SM, Warshaw AL, Fisher ES. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125(3):250–6. [PubMed] [Google Scholar]
- 25.Ho V, Heslin MJ. Effect of hospital volume and experience on in-hospital mortality for pancreaticoduodenectomy. Ann Surg. 2003;237(4):509–14. doi: 10.1097/01.SLA.0000059981.13160.97. [DOI] [PMC free article] [PubMed] [Google Scholar]