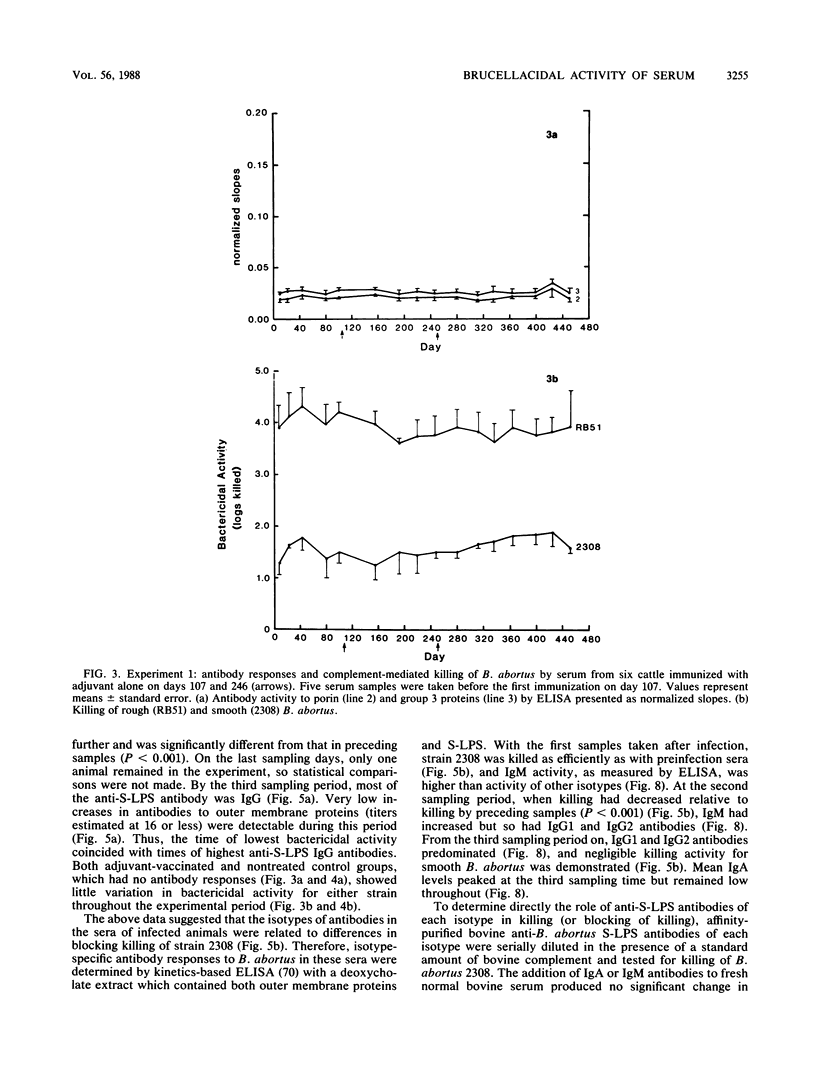
Abstract
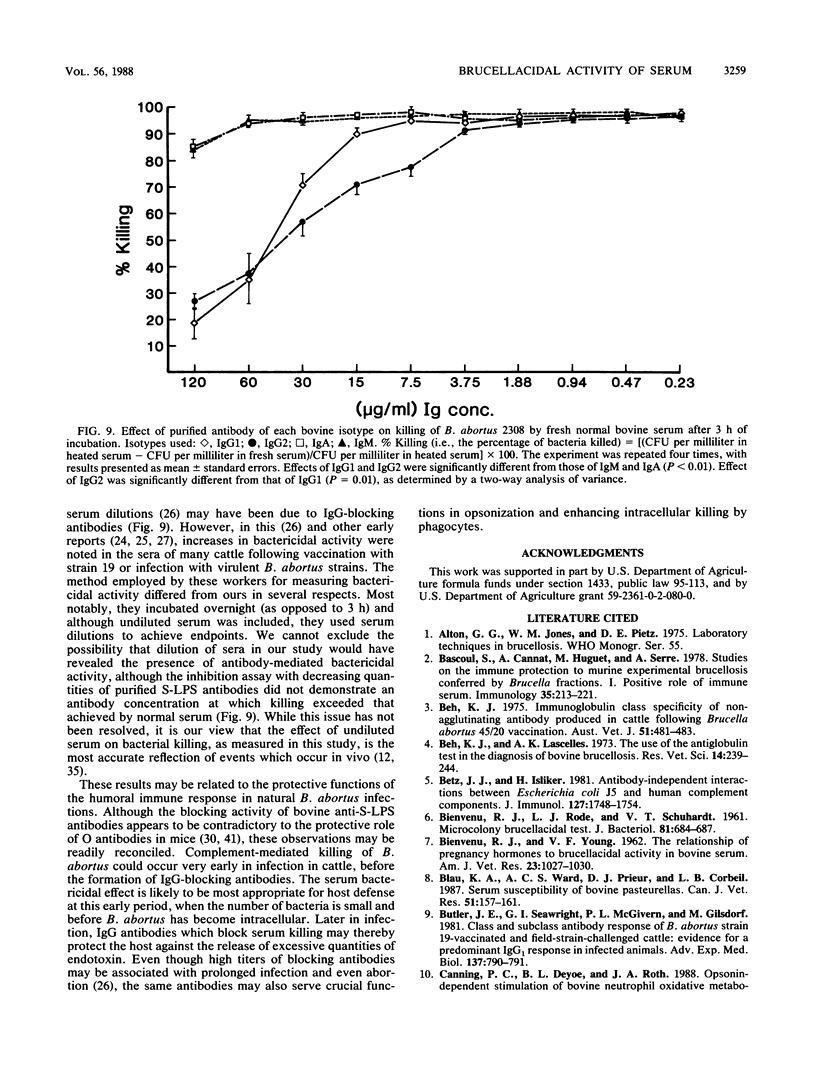
Studies of the serum bactericidal system in bovine brucellosis were undertaken to investigate the role of the humoral immune response in protection of cattle against the facultative intracellular parasite Brucella abortus. Fresh sera from normal control cattle, infected cattle, and cattle immunized with B. abortus cell envelopes were collected before treatment and during the course of immunization or infection. Normal fresh bovine serum or fresh agammaglobulinemic serum from colostrum-deprived calves was effective in killing smooth virulent B. abortus 2308, but rough strains RB51 (a rough mutant of strain 2308) and 45/20 were much more sensitive to serum. The difference in susceptibility to serum was shown to be correlated with differences in lipopolysaccharide chemotype, with the more resistant strain 2308 having O polysaccharide and the more susceptible strains 45/20 and RB51 lacking O side chains. By treatment of fresh serum with MgCl2 and EGTA [ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid] killing was shown to occur via the classical pathway of complement activation. When antibody to B. abortus was present, killing of strain RB51 increased but killing of smooth strain 2308 decreased. The earliest antibody response in serum from infected animals did not interfere with killing. When affinity-purified bovine immunoglobulins specific for B. abortus smooth lipopolysaccharide were added to fresh normal bovine serum, immunoglobulin G1 (IgG1) and IgG2 isotypes blocked killing but IgM and IgA isotypes did not. Thus, it appears that serum from previously unexposed animals or animals early during infection can kill smooth B. abortus, an appropriate defense mechanism before the organism becomes intracellular. At later stages of infection, blocking antibodies predominate.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIENVENU R. J., Jr, YOUNG V. F. The relationships of pregnancy hormones to brucellacidal activity in bovine serum. Am J Vet Res. 1962 Sep;23:1027–1030. [PubMed] [Google Scholar]
- Bascoul S., Cannat A., Huguet M. F., Serre A. Studies on the immune protection to murine experimental brucellosis conferred by Brucella fractions. I. Positive role of immune serum. Immunology. 1978 Aug;35(2):213–221. [PMC free article] [PubMed] [Google Scholar]
- Beh K. J. Immunoglobulin class specificity of non-agglutinating antibody produced in cattle following Brucella abortus 45/20 vaccination. Aust Vet J. 1975 Oct;51(10):481–483. doi: 10.1111/j.1751-0813.1975.tb02385.x. [DOI] [PubMed] [Google Scholar]
- Beh K. J., Lascelles A. K. The use of the antiglobulin test in the diagnosis of bovine brucellosis. Res Vet Sci. 1973 Mar;14(2):239–244. [PubMed] [Google Scholar]
- Betz S. J., Isliker H. Antibody-independent interactions between Escherichia coli J5 and human complement components. J Immunol. 1981 Nov;127(5):1748–1754. [PubMed] [Google Scholar]
- Bienvenu R. J., Rode L. J., Schuhardt V. T. MICROCOLONY BRUCELLACIDAL TEST. J Bacteriol. 1961 May;81(5):684–687. doi: 10.1128/jb.81.5.684-687.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau K. A., Ward A. C., Prieur D. J., Corbeil L. B. Serum susceptibility of bovine pasteurellas. Can J Vet Res. 1987 Apr;51(2):157–161. [PMC free article] [PubMed] [Google Scholar]
- Cho H. J., Ingram D. G. Mechanisms of prozone formation in agglutination reaction. Can J Microbiol. 1972 Apr;18(4):449–456. doi: 10.1139/m72-070. [DOI] [PubMed] [Google Scholar]
- Corbeil L. B., Blau K., Prieur D. J., Ward A. C. Serum susceptibility of Haemophilus somnus from bovine clinical cases and carriers. J Clin Microbiol. 1985 Aug;22(2):192–198. doi: 10.1128/jcm.22.2.192-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Limet J. Evidence of heterogeneity of lipopolysaccharides among Brucella biovars in relation to A and M specificities. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):27–37. doi: 10.1016/0769-2609(87)90051-2. [DOI] [PubMed] [Google Scholar]
- Forget A., Borduas A. G. An immunological enhancement phenomenon in experimental brucella infection of the chicks. Int Arch Allergy Appl Immunol. 1977;53(2):190–194. doi: 10.1159/000231751. [DOI] [PubMed] [Google Scholar]
- Forget A., Riendeau C. Enhancement activity of anti-brucella sera in experimental Brucella abortus infection in mice. Rev Can Biol. 1977 Sep;36(3):239–243. [PubMed] [Google Scholar]
- Griffiss J. M. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J Immunol. 1975 Jun;114(6):1779–1784. [PubMed] [Google Scholar]
- Hall W. H., Manion R. E., Zinneman H. H. Blocking serum lysis of Brucella abortus by hyperimmune rabbit immunoglubulin A. J Immunol. 1971 Jul;107(1):41–46. [PubMed] [Google Scholar]
- Harmon B. G., Adams L. G. Assessment of bovine mammary gland macrophage oxidative burst activity in a chemiluminescence assay. Am J Vet Res. 1987 Jan;48(1):119–125. [PubMed] [Google Scholar]
- Hoffmann E. M., Houle J. J. Failure of Brucella abortus lipopolysaccharide (LPS) to activate the alternative pathway of complement. Vet Immunol Immunopathol. 1983 Nov;5(1):65–76. doi: 10.1016/0165-2427(83)90032-6. [DOI] [PubMed] [Google Scholar]
- Huddleson I. F., Wood E. E., Cressman A. R., Bennett G. R. The Bactericidal Action of Bovine Blood for Brucella and Its Possible Significance. J Bacteriol. 1945 Sep;50(3):261–277. doi: 10.1128/jb.50.3.261-277.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- LIVE I., GUILIANI L. A. Protective effect of sera from animals treated with different Brucella antigens upon Brucella infection in mice. J Immunol. 1953 Oct;71(4):187–191. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Limet J., Plommet A. M., Dubray G., Plommet M. Immunity conferred upon mice by anti-LPS monoclonal antibodies in murine brucellosis. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):417–424. doi: 10.1016/s0769-2625(87)80052-1. [DOI] [PubMed] [Google Scholar]
- MacDonald J. T., Maheswaran S. K., Opuda-Asibo J., Townsend E. L., Thies E. S. Susceptibility of Pasteurella haemolytica to the bactericidal effects of serum, nasal secretions and bronchoalveolar washings from cattle. Vet Microbiol. 1983 Nov;8(6):585–599. doi: 10.1016/0378-1135(83)90007-x. [DOI] [PubMed] [Google Scholar]
- Madraso E. D., Cheers C. Polyadenylic acid-polyuridylic acid (poly A : U) and experimental murine brucellosis. II. Macrophages as target cells of poly A : U in experimental brucellosis. Immunology. 1978 Jul;35(1):77–84. [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. A., Katzenstein D., Norquist D., Chikami G., Wunderlich A., Braude A. I. Role of blocking antibody in disseminated gonococcal infection. J Immunol. 1978 Nov;121(5):1884–1888. [PubMed] [Google Scholar]
- McEwan A. D., Fisher E. W., Selman I. E., Penhale W. J. A turbidity test for the estimation of immune globulin levels in neonatal calf serum. Clin Chim Acta. 1970 Jan;27(1):155–163. doi: 10.1016/0009-8981(70)90390-6. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Musoke A. J. Biologic activities of bovine IgG subclasses. Adv Exp Med Biol. 1981;137:359–366. [PubMed] [Google Scholar]
- McGuire T. C., Pfeiffer N. E., Weikel J. M., Bartsch R. C. Failure of colostral immunoglobulin transfer in calves dying from infectious disease. J Am Vet Med Assoc. 1976 Oct 1;169(7):713–718. [PubMed] [Google Scholar]
- McNaught D. J., Chappel R. J., Allan G. S., Bourke J. A., Rogerson B. A. The effects of IgG2 and of antigen concentration on prozoning in the complement fixation test for bovine brucellosis. Res Vet Sci. 1977 Mar;22(2):194–197. [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect Immun. 1986 Aug;53(2):245–251. doi: 10.1128/iai.53.2.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J., Hunter D. M., Sowa B. A., Wu A. M., Adams L. G. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986 Mar;51(3):961–963. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Berman D. T., Boettcher L. A. Biological activities of Brucella abortus lipopolysaccharides. Infect Immun. 1981 Jan;31(1):362–370. doi: 10.1128/iai.31.1.362-370.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti P. The epidemiology of bovine brucellosis. Adv Vet Sci Comp Med. 1980;24:69–98. [PubMed] [Google Scholar]
- Nielsen K., Rosenbaum B., Ballinger R., Stiller J. Effects of various treatments of bovine complement on its lytic efficacy measured by two different tests. Vet Immunol Immunopathol. 1984 Jul;6(3-4):273–283. doi: 10.1016/0165-2427(84)90053-9. [DOI] [PubMed] [Google Scholar]
- Nielsen K., Rosenbaum B., Harper S., Adams L. G., Williams J. D. Failure of bovine complement levels measured by radial hemolysis to correlate with tube titration. Am J Vet Res. 1983 Oct;44(10):1935–1937. [PubMed] [Google Scholar]
- Nielsen K., Stilwell K., Stemshorn B., Duncan R. Ethylenediaminetetraacetic acid (disodium salt)-labile bovine immunoglobulin M Fc binding to Brucella abortus: a cause of nonspecific agglutination. J Clin Microbiol. 1981 Jul;14(1):32–38. doi: 10.1128/jcm.14.1.32-38.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon P. Resistance against a subcutaneous Brucella challenge of mice immunized with living or dead Brucella or by transfer of immune serum. Ann Immunol (Paris) 1977 Nov-Dec;128(6):1025–1037. [PubMed] [Google Scholar]
- Patterson J. M., Deyoe B. L., Stone S. S. Identification of immunoglobulins associated with complement fixation, agglutination, and low pH buffered antigen tests for brucellosis. Am J Vet Res. 1976 Mar;37(3):319–324. [PubMed] [Google Scholar]
- Perez Perez G. I., Blaser M. J. Lipopolysaccharide characteristics of pathogenic campylobacters. Infect Immun. 1985 Feb;47(2):353–359. doi: 10.1128/iai.47.2.353-359.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B. H., Lee T. J., Snyderman R., Brooks G. F. Neisseria meningitidis and Neisseria gonorrhoeae bacteremia associated with C6, C7, or C8 deficiency. Ann Intern Med. 1979 Jun;90(6):917–920. doi: 10.7326/0003-4819-90-6-917. [DOI] [PubMed] [Google Scholar]
- Plommet M., Plommet A. M. Immune serum-mediated effects on brucellosis evolution in mice. Infect Immun. 1983 Jul;41(1):97–105. doi: 10.1128/iai.41.1.97-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Vayo H. E., Tam M. R., Blake M. S. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986 Nov 1;164(5):1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J. M., Verstreate D. R., Perera V. Y., Winter A. J. Outer membrane proteins from rough strains of four Brucella species. Infect Immun. 1984 Oct;46(1):188–194. doi: 10.1128/iai.46.1.188-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurig G. G., Jones L. M., Speth S. L., Berman D. T. Antibody response to antigens distinct from smooth lipopolysaccharide complex in Brucella infection. Infect Immun. 1978 Sep;21(3):994–1002. doi: 10.1128/iai.21.3.994-1002.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurig G. G., Pringle A. T., Breese S. S., Jr Localization of brucella antigens that elicit a humoral immune response in Brucella abortus-infected cells. Infect Immun. 1981 Dec;34(3):1000–1007. doi: 10.1128/iai.34.3.1000-1007.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele N. P., Munson R. S., Jr, Granoff D. M., Cummins J. E., Levine R. P. Antibody-dependent alternative pathway killing of Haemophilus influenzae type b. Infect Immun. 1984 May;44(2):452–458. doi: 10.1128/iai.44.2.452-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller J. M., Nielsen K. H. Affinity purification of bovine antibodies to Brucella abortus Lipopolysaccharide. J Clin Microbiol. 1983 Feb;17(2):323–326. doi: 10.1128/jcm.17.2.323-326.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulitzeanu D. Mechanism of immunity against brucella. Nature. 1965 Mar 13;205(976):1086–1088. doi: 10.1038/2051086a0. [DOI] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W., Kroll H. P. Killing of an encapsulated strain of Escherichia coli by human serum. Infect Immun. 1983 Jan;39(1):122–131. doi: 10.1128/iai.39.1.122-131.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C. Immunoglobulin patterns of antibodies against Brucella in man and animals. J Immunol. 1966 Mar;96(3):457–463. [PubMed] [Google Scholar]
- Winter A. J., Hall C. E., Jacobson R. H., Verstreate D. R., Meredith M. P., Castleman W. L. Effect of pregnancy on the immune response of cattle to a Brucella vaccine. J Reprod Immunol. 1986 Dec;9(4):313–325. doi: 10.1016/0165-0378(86)90032-x. [DOI] [PubMed] [Google Scholar]
- Winter A. J., Verstreate D. R., Hall C. E., Jacobson R. H., Castleman W. L., Meredith M. P., McLaughlin C. A. Immune response to porin in cattle immunized with whole cell, outer membrane, and outer membrane protein antigens of Brucella abortus combined with trehalose dimycolate and muramyl dipeptide adjuvants. Infect Immun. 1983 Dec;42(3):1159–1167. doi: 10.1128/iai.42.3.1159-1167.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Braude A. I. Treatment of gram-negative bacteremia with antiserum to core glycolipid. I. The experimental basis of immunity to endotoxin. Eur J Cancer. 1979;15 (Suppl):71–76. [PubMed] [Google Scholar]




