Abstract
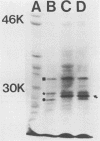
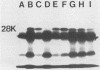
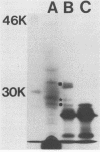
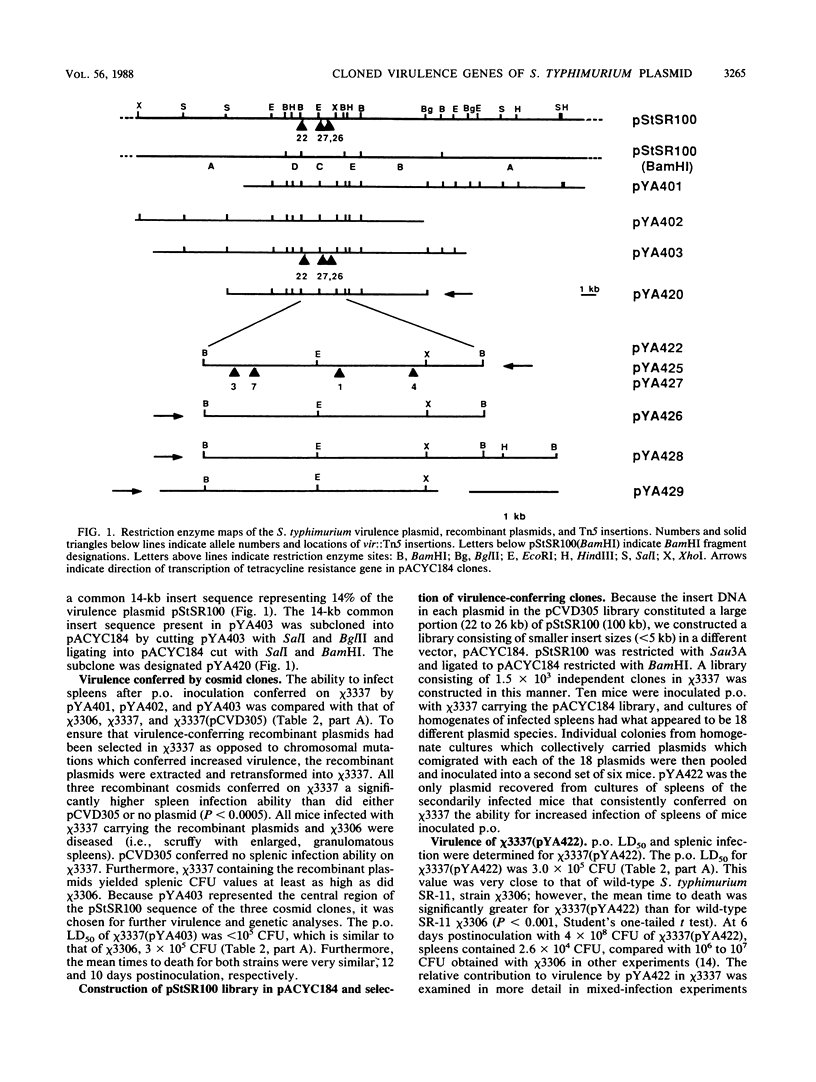
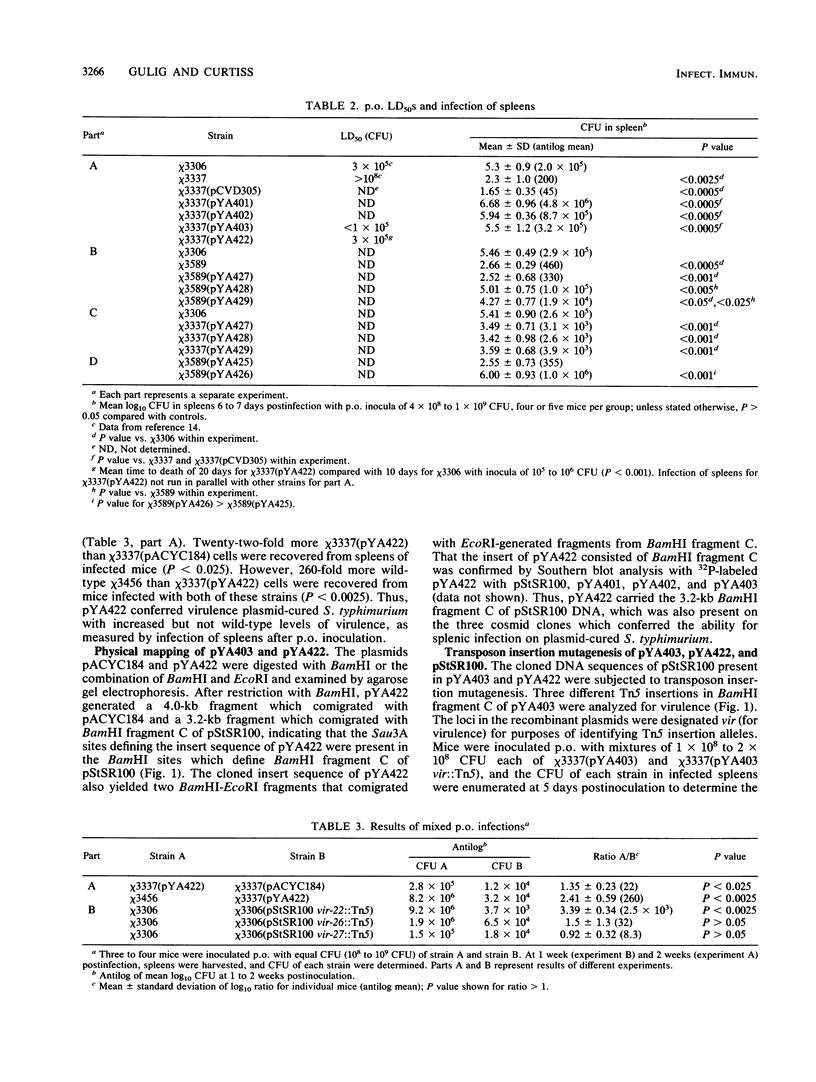
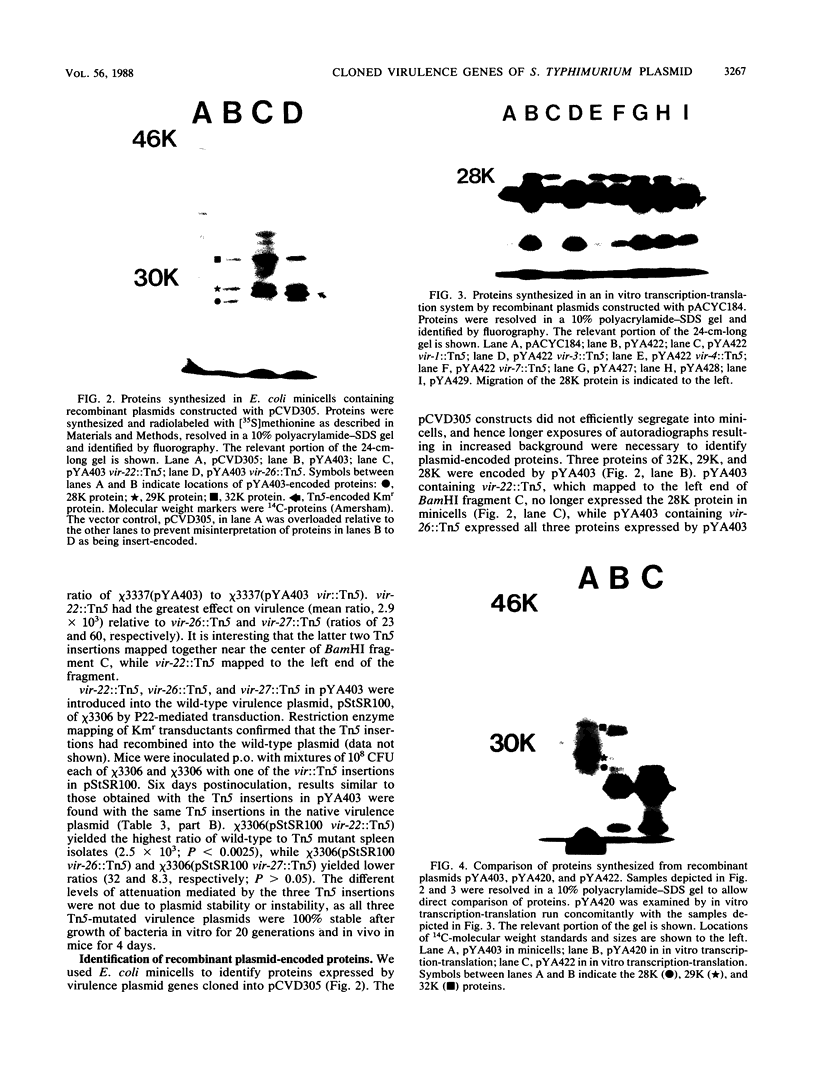
We have cloned regions of the 100-kilobase (kb) plasmid, pStSR100, of Salmonella typhimurium SR-11 that confer virulence to plasmid-cured S. typhimurium. Cells carrying recombinant plasmids that conferred virulence were selected by inoculating mice orally with recombinant libraries in virulence plasmid-cured S. typhimurium and harvesting isolates that infected spleens. Three plasmids, pYA401, pYA402, and pYA403, constructed with the cosmid vector pCVD305 conferred wild-type levels of virulence to plasmid-cured S. typhimurium and had a common 14-kb DNA insert sequence. Another recombinant plasmid, pYA422, constructed with the vector pACYC184, conferred to plasmid-cured S. typhimurium a wild-type 50% lethal dose (LD50) level, but mice died more slowly than when infected with wild-type S. typhimurium. Furthermore, pYA422 conferred the ability to cause a higher, but not a wild-type, level of splenic infection on plasmid-cured S. typhimurium. pYA422 had a 3.2-kb insert sequence which mapped to the center of the 14-kb common sequence of the cosmid clones. Transposon Tn5 insertion mutations in pYA403 inhibited virulence to various degrees, and when transduced into the native virulence plasmid of S. typhimurium, these Tn5 insertions decreased virulence to degrees similar to those observed when the Tn5 insertions were present in pYA403. vir-22::Tn5 in pStSR100 greatly lowered infection of spleens relative to unmutagenized virulence plasmid, while vir-26::Tn5 and vir-27::Tn5 lowered splenic infection to lesser degrees. At least three proteins were encoded by pYA403 containing 23 kb of insert sequence and subclone pYA420, containing the 14-kb common insert sequence present in all of the cosmid clones. One of these proteins, with an apparent molecular weight of 28,000, was also encoded by pYA422. The Tn5 insertion that most attenuated virulence, vir-22::Tn5, inhibited synthesis of the 28,000-molecular-weight protein. The vir-22::Tn5 insertion was complemented by recombinant plasmids encoding only the 28,000-molecular-weight protein, suggesting a role of this protein in virulence. However, recombinant plasmids, exemplified by pYA422, that encoded only the 28,000-molecular-weight protein did not confer full virulence.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird G. D., Manning E. J., Jones P. W. Evidence for related virulence sequences in plasmids of Salmonella dublin and Salmonella typhimurium. J Gen Microbiol. 1985 Jul;131(7):1815–1823. doi: 10.1099/00221287-131-7-1815. [DOI] [PubMed] [Google Scholar]
- Baxter-Gabbard K. L. A simple method for the large-scale preparation of sucrose gradients. FEBS Lett. 1972 Jan 15;20(1):117–119. doi: 10.1016/0014-5793(72)80031-0. [DOI] [PubMed] [Google Scholar]
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Beninger P. R., Chikami G., Tanabe K., Roudier C., Fierer J., Guiney D. G. Physical and genetic mapping of the Salmonella dublin virulence plasmid pSDL2. Relationship to plasmids from other Salmonella strains. J Clin Invest. 1988 May;81(5):1341–1347. doi: 10.1172/JCI113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Curtiss R., 3rd Analysis of recombinant DNA using Escherichia coli minicells. Methods Enzymol. 1983;101:347–362. doi: 10.1016/0076-6879(83)01026-5. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Production, properties and utility of bacterial minicells. Curr Top Microbiol Immunol. 1975;69:1–84. doi: 10.1007/978-3-642-50112-8_1. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Curtiss R., 3rd Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987 Dec;55(12):2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S., Reed R. R., Steitz J. A., Low K. B. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):135–140. doi: 10.1101/sqb.1981.045.01.022. [DOI] [PubMed] [Google Scholar]
- Hackett J., Kotlarski I., Mathan V., Francki K., Rowley D. The colonization of Peyer's patches by a strain of Salmonella typhimurium cured of the cryptic plasmid. J Infect Dis. 1986 Jun;153(6):1119–1125. doi: 10.1093/infdis/153.6.1119. [DOI] [PubMed] [Google Scholar]
- Hackett J., Wyk P., Reeves P., Mathan V. Mediation of serum resistance in Salmonella typhimurium by an 11-kilodalton polypeptide encoded by the cryptic plasmid. J Infect Dis. 1987 Mar;155(3):540–549. doi: 10.1093/infdis/155.3.540. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmuth R., Stephan R., Bunge C., Hoog B., Steinbeck A., Bulling E. Epidemiology of virulence-associated plasmids and outer membrane protein patterns within seven common Salmonella serotypes. Infect Immun. 1985 Apr;48(1):175–182. doi: 10.1128/iai.48.1.175-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Jacobs W. R., Barrett J. F., Clark-Curtiss J. E., Curtiss R., 3rd In vivo repackaging of recombinant cosmid molecules for analyses of Salmonella typhimurium, Streptococcus mutans, and mycobacterial genomic libraries. Infect Immun. 1986 Apr;52(1):101–109. doi: 10.1128/iai.52.1.101-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rabert D. K., Svinarich D. M., Whitfield H. J. Association of adhesive, invasive, and virulent phenotypes of Salmonella typhimurium with autonomous 60-megadalton plasmids. Infect Immun. 1982 Nov;38(2):476–486. doi: 10.1128/iai.38.2.476-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Michiels T., Popoff M. Y., Durviaux S., Coynault C., Cornelis G. A new method for the physical and genetic mapping of large plasmids: application to the localisation of the virulence determinants on the 90 kb plasmid of Salmonella typhimurium. Microb Pathog. 1987 Aug;3(2):109–116. doi: 10.1016/0882-4010(87)90069-6. [DOI] [PubMed] [Google Scholar]
- Pardon P., Popoff M. Y., Coynault C., Marly J., Miras I. Virulence-associated plasmids of Salmonella serotype Typhimurium in experimental murine infection. Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):47–60. doi: 10.1016/s0769-2609(86)80093-x. [DOI] [PubMed] [Google Scholar]
- Popoff M. Y., Miras I., Coynault C., Lasselin C., Pardon P. Molecular relationships between virulence plasmids of Salmonella serotypes typhimurium and dublin and large plasmids of other Salmonella serotypes. Ann Microbiol (Paris) 1984 May-Jun;135A(3):389–398. doi: 10.1016/s0769-2609(84)80080-0. [DOI] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Vandenbosch J. L., Rabert D. K., Jones G. W. Plasmid-associated resistance of Salmonella typhimurium to complement activated by the classical pathway. Infect Immun. 1987 Nov;55(11):2645–2652. doi: 10.1128/iai.55.11.2645-2652.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]