Abstract
Pyoverdin is the hydroxamate siderophore produced by the opportunistic pathogen Pseudomonas aeruginosa under the iron-limiting conditions of the human host. This siderophore includes derivatives of ornithine in the peptide backbone that serve as iron chelators. PvdA is the ornithine hydroxylase, which performs the first enzymatic step in preparation of these derivatives. PvdA requires both FAD and NADPH for activity, and was found to be a soluble monomer most active at pH 8.0. The enzyme demonstrated Michaelis-Menten kinetics using an NADPH oxidation assay, but a hydroxylation assay indicated substrate inhibition at high ornithine concentration. PvdA is highly specific for both substrate and coenzyme, and lysine was shown to be a non-substrate effector and mixed inhibitor of the enzyme with respect to ornithine. Chloride is a mixed inhibitor of PvdA in relation to ornithine but a competitive inhibitor with respect to NADPH, and a bulky mercurial compound (para-chloromercuribenzoate) is a mixed inhibitor with respect to ornithine. Steady state experiments indicate that PvdA:FAD forms a ternary complex with NADPH and ornithine for catalysis. PvdA in the absence of ornithine shows slow substrate-independent flavin reduction by NADPH. Biochemical comparison of PvdA to para-hydroxybenzoate hydroxylase (PHBH from Pseudomonas fluorescens) and flavin-containing monooxygenases (FMOs from Schizosaccharomyces pombe and hog liver microsomes) leads to the hypothesis that PvdA catalysis proceeds by a novel reaction mechanism.
P. aeruginosa is an opportunistic human pathogen that under iron-limiting conditions produces two siderophores, pyochelin and pyoverdin, which contribute to virulence (1, 2). Pyoverdin, a hydroxamate siderophore, chelates iron with high affinity, and has the ability to remove iron from host proteins such as transferrin and lactoferrin (3). Derivatives of ornithine, both hydroxyornithine and formyl-hydroxyornithine, are incorporated into the peptide backbone of pyoverdin and directly coordinate the iron (4).
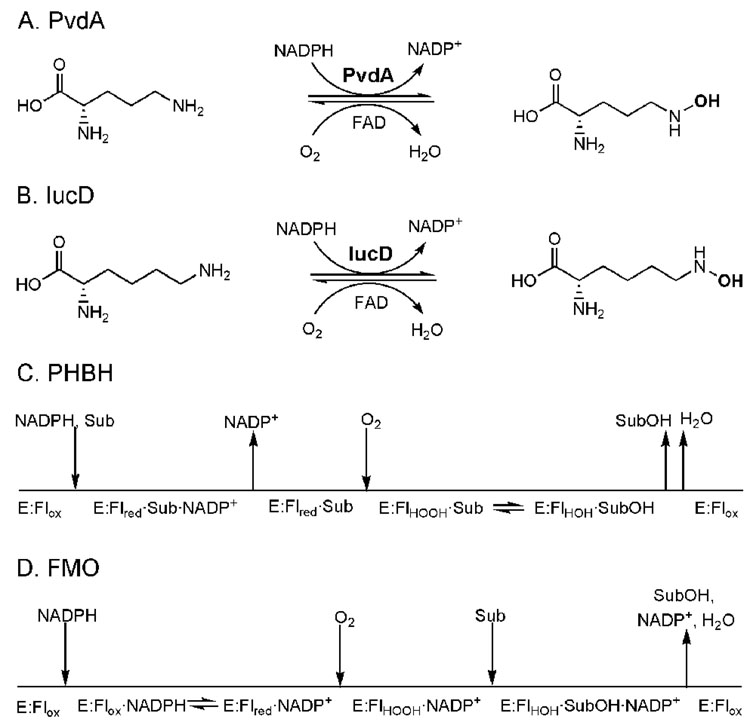
Ornithine hydroxylase (PvdA or L-ornithine N5-oxygenase) is the first enzyme involved in the derivatization of the ornithine, hydroxylating the primary amine of the side chain (Figure 1a). PvdA is part of the pyoverdin locus (5) and PvdA deletion mutants are pyoverdin-deficient (6, 7). PvdA is functionally related to the lysine hydroxylase (IucD) of E. coli, which is required for production of the aerobactin siderophore (Figure 1b), para-hydroxybenzoate hydroxylase (PHBH) of the soil bacterium P. fluorescens, which is important in the biodegradation of lignin from wood (Figure 1c), and flavin-containing monooxygenases (FMOs) from a variety of organisms from bacteria to mammals involved in xenobiotic detoxification (Figure 1d). IucD, PHBH and FMO require two coenzymes: FAD and NADPH (8–10). FAD is reduced by NADPH and can then donate electrons to molecular oxygen (Figure 1). The end result is the production of H2O, and a hydroxyl group is added to the sidechain amine of lysine (IucD), the activated aromatic ring of the hydroxybenzoate (PHBH), or to a variety of nucleophilic substrates (FMO) (9, 11, 12). The mechanism that PHBH and FMO use for hydroxylation is considerably different, and the mechanism for IucD is unknown. PHBH requires substrate to be bound to the enzyme for FAD reduction by NADPH, followed by the dissociation of NADP+ from the enzyme and the binding of molecular oxygen. The reduced flavin reoxidizes, passing through two transient intermediate states before finally forming the oxidized flavin, the product (3,4-dihydroxybenzoate), and water (Figure 1c) (9, 13–15). FMO does not require substrate for FAD reduction or for oxygen binding. Instead, the flavin is reduced by NADPH and then binds molecular oxygen to form a stable flavin hydroperoxide intermediate that reacts with an oxidizable substrate to form the hydroxylated product and water (Figure 1d) (9, 16–18). Recently, PvdA has also been shown to be NADPH and FAD-dependent, but the reaction mechanism has not been elucidated (19).
Figure 1.
Reaction schemes for the ornithine hydroxylase (PvdA) from P. aeruginosa (a), the lysine hydroxylase (IucD) from E. coli (b), p-hydroxybenzoate hydroxylase (PHBH) from P. fluorescens (c), and flavin-containing monooxygenase (FMO) from hog liver microsomes (d). The substrate for PHBH (p-hydroxybenzoate) and FMO (nucleophilic and some electrophilic compounds) is labeled as Sub and the flavin states are labeled as oxidized (Flox), reduced (Flred), hydroperoxyflavin intermediate (FlHOOH), and hydroxyflavin intermediate (FlHOH).
Here we report a comprehensive biochemical characterization of PvdA, including the kinetics of product formation and NADPH oxidation, demonstration of substrate inhibition, inhibition by lysine, chloride, and mercurial compounds, the oligomerization state in solution, and steady state kinetics. We put these new data into the framework of what is known for IucD, PHBH and FMO and begin to determine the mechanism of the reaction, and its relation to the mechanisms of the functional homologues. This work is a first step toward using PvdA as an antimicrobial drug target to reduce the virulence of P. aeruginosa (and possibly related bacterial species that require an amine hydroxylase for siderophore production), which infect immunocompromised patients and patients with cystic fibrosis.
EXPERIMENTAL PROCEDURES
Cloning of PvdA
The pvdA gene was amplified from PAO1 P. aeruginosa genomic DNA by polymerase chain reaction using Herculase (Stratagene) with 10 % DMSO as an adjuvant. The forward primer (5’-GAA TTC CAT ATG ACT CAG GCA ACT GCA ACC-3’) includes an NdeI site (underlined), whereas the reverse primer (5’-CCC AAG CTT TCA GCT GGC CAG GGC GTG-3’) contains a HindIII site (underlined). The amplified 1329 base pair fragment was digested with NdeI and HindIII and ligated into the pET28b plasmid (Novagen) digested with the same enzymes. The resultant plasmid encodes the pvdA gene with an N-terminal histidine tag. This plasmid was transformed into the BL21(DE3) E. coli strain (Stratagene) for overproduction of the PvdA protein.
PvdA protein overexpression and purification
BL21(DE3) E. coli containing the PvdA expression plasmid were grown in LB medium containing 50 µg/ml kanamycin at 37 °C with shaking (225 rpm) until an OD600 of ~0.8 was reached. Protein expression was induced with 0.2 mM isopropyl-β-D-thiogalactopyranoside and the cells were harvested by centrifugation (6,000 × g, 10 min, 4 °C) after 3 – 4 hours. The cell pellet was resuspended in 15 ml 20 mM potassium phosphate pH 8.0, 500 mM NaCl, 50 mM sodium citrate, 5 mM imidazole (buffer A) per liter of culture. Cells were disrupted using a French pressure cell (35,000 psi) and cellular debris was removed by centrifugation (12,000 × g, 30 min, 4 °C). The supernatant was applied to a Chelating Sepharose Fast Flow column (Amersham Biosciences) charged with nickel chloride and pre-equilibrated in buffer A. PvdA protein eluted at approximately 300 mM imidazole using a linear gradient of 5 – 500 mM imidazole in buffer A. The pooled fractions were applied to a Superdex 200 size exclusion column (Amersham Biosciences) equilibrated with 100 mM potassium phosphate pH 8.0, 100 mM sodium citrate. The fractions containing PvdA were pooled and concentrated using an Amicon Stirred Cell with a YM-30 membrane to 9 – 13 mg/ml as determined by the Bradford assay and stored at −80 °C for use in the activity assays.
Oligomerization Studies
To determine the hydrodynamic radii of the PvdA species in solution, a PD2000DLSPlus Dynamic Light Scattering detector (Precision Detectors) was used with a PvdA concentration of 4.7 mg/ml. The hydrodynamic radii were also determined in the elution buffer in the presence of ten fold excess FAD or in the presence of ten fold excess FAD and ornithine.
NADPH oxidation assay
The standard assay buffer contained 100 mM potassium phosphate pH 8.0, 0.03 mM FAD, and 0.15 mM NADPH. PvdA (0.25 mg, 5 µM) was incubated in 1 ml assay buffer for 2 min at 24°C before the reaction was initiated by the addition of 5 mM L-ornithine. The NADPH oxidation was monitored at 366 nm (ε= 2850 M−1 cm−1) using a BioMate 3 spectrometer (Thermo Spectronics) at 24 °C for 40 s with 5 s time points according to the protocol described for IucD (10).
Hydroxylation assay
The amount of hydroxylated product formed by PvdA was assayed using a variation of the Csaky iodine oxidation reaction (20–23). The standard assay buffer is the same as described for the NADPH oxidation assay. The reaction was initiated by the addition of 5 mM L-ornithine to 0.25 mg (5 µM) enzyme in 1 ml assay buffer at 24 °C. At ten second intervals up to 40 s, 83 µl of the assay mixture was withdrawn and added to 42̣N µl 0.2 N perchloric acid to terminate the reaction. For each time point, 50 µl of the terminated reaction mixture was transferred into a glass 96 well plate and the reaction mixture was neutralized by the addition of 50 µl 5% (w/v) sodium acetate solution. To each well, 50 µl 1% (w/v) sulfanilic acid in 25% (v/v) acetic acid and 20 µl 1.3% (w/v) potassium iodide in glacial acetic acid were added and the reaction was allowed to incubate at room temperature for 5–7 min. Excess iodine was removed with 20 µl 0.1 N sodium thiosulfate and the color was developed by adding 20 µl 0.6% (w/v) α-naphthylamine in 30% (v/v) acetic acid. The absorbance at 562 nm was measured after 15 min using an Elx800 plate reader (Bio-Tek). A standard curve of hydroxylamine hydrochloride was used to calculate the amount of hydroxylated product produced.
Determination of enzyme specificity and kinetic parameters
A variety of permutations of the standard assay conditions were tested. Using the NADPH oxidation assay, pH optimization was conducted with 100 mM potassium phosphate at pH 6.0 – 8.0; 100 mM Tris-HCl at pH 7.0 – 9.0; 100 mM glycine at pH 9.0 – 10.0; or 33 mM potassium phosphate, 33 mM Tris-SO4, 33 mM glycine at pH 6.0 – 10.0; varying in steps of 0.5 pH units in the presence and absence of substrate. An ionic strength of 100 mM promoted protein solubility and stability, and was used for all experiments. Both the NADPH oxidation and hydroxylation assays were used to determine enzyme specificity. For the coenzyme substitution reactions, FAD was replaced by FMN or NADPH by NADH at comparable concentrations. For the substrate substitution reactions, 5 mM L-ornithine was substituted with 5 mM DL-2,3-diaminopropionic acid, DL-2,4-diaminobutyric acid, L-lysine, 5-aminopentanoic acid, 1,4-diaminobutane, D-ornithine, or Lnorleucine, all purchased from Sigma. The Michaelis-Menten kinetics of PvdA were assayed similarly, varying the L-ornithine concentrations from 0 – 15 mM.
Determination of FAD dissociation constant
PvdA was diluted into 100 mM potassium phosphate pH 8.0 to a final concentration of 2.5 µM. The FAD concentration was varied from 0 to 60 uM. Protein fluorescence was excited at 280 nm and the emission was detected at 330 nm.
Steady state kinetics
Using the NADPH oxidation assay and 1 ml standard assay buffer with 5 µM PvdA and 0.03 mM FAD, NADPH concentration (0.05 – 0.125 mM) was varied relative to ornithine concentration (0.5 – 10 mM) and the initial velocities were measured.
Inhibition assays
The inhibition of PvdA by L-lysine was investigated using the hydroxylation assay. The standard assay conditions were as described above with a few modifications. To increase the signal-to-noise ratio, the substrate, coenzymes and enzyme were increased in concentration as follows: 25 µM PvdA, 0.15 mM FAD, and 0.75 mM NADPH and the L-ornithine concentrations ranged from 0.15 – 2 mM in 1 ml assay buffer. L-lysine was added in concentrations ranging from 0 – 10 mM. Inhibition by chloride was characterized using the NADPH oxidation assay and standard assay conditions. Sodium chloride concentrations ranged from 0 to 250 mM over a L-ornithine concentration range of 0.5 – 10 mM. Inhibition by p-chloromercuribenzoate (PCMB) was characterized using the NADPH oxidation assay and standard assay conditions. The PCMB concentration ranged from 0 to 10 µM over a L-ornithine concentration range of 0.075 – 5 mM.
PvdA Flavin Reoxidation
To determine the rate constants for flavin reduction and reoxidation, 40 µM PvdA was incubated in 100 mM potassium phosphate pH 8.0 with 20 µM FAD. Upon the addition of 40 µM NADPH, the absorbance from 300 – 800 nm was measured at 20 s time points using a Cary 50 Bio Spectrophotometer (Varian) at 24 °C. The change in absorbance at 451 nm was used to determine the rate constants for reduction and reoxidation.
Data anaylsis
All reactions were carried out in triplicate with standard deviations as indicated. Data analysis was done using SigmaPlot 8.0 ®. All curves were fit to the data points using least squares linear regression analysis and standard algorithms, except the substrate inhibition which was fit using the equation: Vo = Vmax[Substrate] / (Km + [Substrate] + ([Substrate]2/KI)) (23).
RESULTS
PvdA protein production and purification
The pvdA gene was cloned from PAO1 P. aeruginosa genomic DNA and an overexpression plasmid was generated such that expression in BL21(DE3) E. coli yielded the PvdA protein of 51.6 kDa including an N-terminal his6 tag (a construct with a C-terminal his6 tag was insoluble and was produced in inclusion bodies; J. Martin Bollinger, personal communication). Protein purification was carried out using nickel affinity and size exclusion chromatographies yielding 20 – 45 mg of PvdA at ~95% purity per liter of culture. The purified protein did not contain FAD as determined by the lack of an absorbance peak at 450 nm.
Oligomerization Studies
The average hydrodynamic radius for apo-PvdA in the activity assay buffer was 4.77 nm with a small percentage as larger species suggesting aggregation, as determined by dynamic light scattering (Figure 2). The addition of excess FAD or excess FAD and excess ornithine yielded a hydrodynamic radius comparable to apo-protein.
Figure 2.
Hydrodynamic radii as determined by dynamic light scattering for PvdA in elution buffer. The fraction of species with a particular hydrodynamic radius is plotted for apo-PvdA (filled circles), for PvdA with excess FAD (open circles), and for PvdA with excess FAD and ornithine (filled triangles).
pH optimum for catalytic activity
The initial velocities for NADPH oxidation by PvdA were measured using a pH range of 6.0 to 10.0 (Figure 3). The pH for maximal turnover is 8.0 – 8.5. Therefore, all further assays were performed using potassium phosphate pH 8.0 as the buffer system. The substrate-independent NADPH oxidation was monitored using a pH range of 6.0 – 10.0 and no change was observed over the entire pH range, indicating that the uncoupling of the enzyme is not pH dependent.
Figure 3.
Effect of pH on PvdA activity. The rate of NADPH oxidation was measured in the presence of ornithine as a decrease in absorbance at 366 nm using 100 mM potassium phosphate at pH 6.0 – 8.0 (filled circles); 33 mM potassium phosphate, 33 mM Tris-SO4, 33 mM glycine at pH 6.0 – 10.0 (open circles); 100 mM Tris-HCl at pH 7.0-9.0 (filled triangles); and 100 mM glycine at pH 9.0 – 10.0 (open triangles). The rate of NADPH oxidation was measured in the absence of ornithine using 100 mM potassium phosphate at pH 6.0 – 8.0 (filled squares), 100 mM Tris-HCl at pH 7.0 – 9.0 (open squares), and 100 mM glycine at pH 9.0 – 10.0 (filled diamonds).
Coenzyme specificity
Using the NADPH oxidation and hydroxylation assays, the coenzymes required for PvdA activity were determined (Table 1). PvdA is dependent on FAD for activity and FMN is not an effective coenzyme. NADPH is the electron donor for PvdA catalysis, and no coenzyme oxidation was detected when substituted with NADH; however, hydroxylated product was detected at 14% of full activity, suggesting the possibility of non-specific product formation in the presence of NADH. Substrate-independent NADPH oxidation was detected at 2 % of full activity.
Table 1.
Summary of the PvdA Coenzyme and Substrate Specificities
| Test conditions | Hydroxylated product formation (nmol/min/mg) | NADPH oxidation (nmol/min/mg) |
|---|---|---|
| Omission Test | ||
| No omission | 321 +/− 3 | 534 +/− 14 |
| − FAD | 22 +/− 12 | * |
| − NADPH | * | * |
| − L-ornithine | * | 11 +/− 1 |
| Specificity | ||
| −FAD | ||
| + FMN | * | * |
| − NADPH | ||
| + NADH | 46 +/− 1 | * |
| − L-ornithine | ||
| + DL-2,3-diaminopropionic acid | * | 8 +/− 4# |
| + DL-2,4-diaminobutyric acid | * | * |
| + L-lysine | * | 428 +/− 4 |
| + 5-aminopentanoic acid | * | * |
| + 1,4-diaminobutane | * | 21 +/− 2 |
| + D-ornithine | * | 5 +/− 3# |
| + L-norleucine | * | * |
below the limits of detection (5 nmol for the hydroxylation assay, and <1 nmol for the NADPH oxidation assay)
less than substrate-independent NADPH oxidation.
Substrate specificity
Shortening of the side chain length by one or two methylene groups (DL-2,4-diaminobutyric acid or DL-2,3-diaminopropionic acid, respectively) resulted in no significant activity in either assay (Table 1). By extending the side chain by one methylene group (L-lysine), NADPH oxidation occurred at 80 % of the rate of L-ornithine; however, no hydroxylated product was formed. Changing the chirality of the substrate to D-ornithine resulted in no enzymatic activity using either assay (Table 1 and (19)). The α-amino and α-carboxyl groups are required for activity and may be involved in substrate binding since no NADPH oxidation or hydroxylated product was detected with 5-aminopentanoic acid and very little NADPH oxidation (4 %) and no hydroxylated product was detected for 1,4-diaminobutane (Table 1). Exchanging the side chain amine for a methyl group (L-norleucine) resulted in no NADPH oxidation or production formation above background, which was anticipated as this substitution eliminates the amine to be hydroxylated.
Determination of kinetic constants
Both the NADPH oxidation assay and the hydroxylation assay were used to determine the kinetic parameters of PvdA at L-ornithine concentrations ranging from 0 to 15 mM (Figure 4). Increasing the ratio of FAD to protein did not affect the kinetic parameters (for example, increase the Vmax or increase the kcat), nor did it increase the uncoupling of the reaction. Substrate inhibition is observed for the hydroxylation assay at ornithine concentrations over 5 mM, but not for the NADPH oxidation assay. Over 5 mM ornithine, the initial velocity for the hydroxylation assay continued to decrease with increasing ornithine concentration at all concentrations tested up to 50 mM ornithine (data not shown). The Vmax, Km, and kcat values are consistent for the two assays (Table 2).
Figure 4.
Kinetic analysis of PvdA as determined by NADPH oxidation and hydroxyornithine production. Initial velocities were measured as a function of NADPH oxidation versus L-ornithine concentration (filled circles) and as the amount of hydroxyornithine production versus L-ornithine concentration (open circles). The curve for the NADPH oxidation assay was fit to the standard equation for Michaelis-Menten reactions and the curve for the hydroxylation assay was fit to Vo = Vmax [Substrate] / (Km + [Substrate] + ([Substrate]2/KI)) for substrate inhibition due to the decrease in activity at substrate concentrations above 5 mM.
Table 2.
Summary of the PvdA Kinetic Parameters
| Hydroxylated product formation assay | NADPH oxidation assay | |
|---|---|---|
| Vmax | 479 +/− 54 nmol/min/mg | 528 +/− 8 nmol/min/mg |
| Km | 600 +/− 70 µM | 593 +/− 12 µM |
| kcat | 24 +/− 3 min−1 | 26.4 +/− 0.4 min−1 |
| kcat/Km | 670 M−1s−1 | 742 M−1s−1 |
| KI* | 31 +/− 5 mM | n.a. |
inhibition constant determined for substrate inhibition
n.a. = not applicable
FAD dissociation constant
The dissociation constant for FAD was determined to be 26 +/− 5 µM. The difference of 2.5 fold from the previously reported Kd (19) may be due to the elimination of the chloride (an inhibitor) from our final protein storage buffer, and thus from the buffer system for determination of the dissociation constant.
Ternary complex formation
Using steady state kinetics and the NADPH oxidation assay, initial velocities were measured for PvdA with excess FAD varying both NADPH and ornithine concentrations. The resulting velocities, plotted using double reciprocal plots, indicate the formation of a ternary complex since lines are not parallel but intersect in the upper left quadrant (Figure 5) (24).
Figure 5.
Ternary complex formation of FAD loaded PvdA with NADPH and ornithine. Double reciprocal plot of NADPH oxidation as a function of L-ornithine concentration. NADPH concentrations used were 0.05 mM (filled circles), 0.075 mM (open circles), and 0.125 mM (filled triangles).
Inhibition assays
Using the hydroxylation assay, addition of L-lysine resulted in mixed inhibition (Table 3). Addition of sodium chloride into the reaction mixture resulted in inhibition of NADPH oxidation. Mixed inhibition was observed with respect to L-ornithine, whereas the inhibition is competitive with respect to NADPH. Using the NADPH oxidation assay, mixed inhibition by p-chloromercuribenzoate (PCMB), a mercury compound that binds cysteines not involved in disulfide bonding, was observed.
Table 3.
Summary of PvdA Inhibition
| Inhibitor | Varied Component | Type of Inhibition | KI* | KI'# |
|---|---|---|---|---|
| L-lysine | L-ornithine | Mixed | 5.4 +/− 1.4 mM | 4.3 +/− 1.5 mM |
| Chloride | L-ornithine | Mixed | 134 +/− 31 µM | 213 +/− 36 µM |
| NADPH | Competitive | 67 +/− 6 µM | n.a. | |
| PCMB | L-ornithine | Mixed | 4.1 +/− 0.6 µM | 2.9 +/− 0.4 µM |
inhibition constant for competitive inhibition; KI = [E][I]/[EI]
inhibition constant for uncompetitive inhibition; KI’ = [ES][I]/[ESI]
n.a. = not applicable
Flavin re-oxidation
Using sub-stoichiometric amounts of FAD and stoichiometric amounts of NADPH, the aerobic reduction of flavin by NADPH in PvdA in the absence of ornithine was monitored as a decrease in absorbance at 451 nm. The rate constant was determined as 0.036 +/− 0.007 s−1 and no semiquinone intermediates were detected (no absorbance peaks between 600 and 800 nm). The aerobic oxidation of the reduced flavin was observed over time as an increase in absorbance with a rate constant determined as 0.019 +/− 0.002 s−1 (Figure 6).
Figure 6.
Flavin reoxidation as a function of time. Absorbance spectra of PvdA:FAD complex were measured at 300–800 nm. Representative absorbance spectra of the oxidized flavin (——), the reduced flavin (— —), and at 60 seconds (– – –) and 120 seconds (......) after reduction. The rate constant for flavin reduction and reoxidation was determined by the change in absorbance at 451 nm to be 0.036 +/− 0.007 s−1 and 0.019 +/− 0.002 s−1 respectively. PvdA concentration used was 40 µM and the FAD concentration was 20 µM.
DISCUSSION
PvdA is most closely related to a lysine hydroxylase from E. coli (IucD). Both enzymes hydroxylate the primary amines of amino acid sidechains (Figure 1), and share 46 % sequence similarity. The structure of IucD has not been determined and there has been debate about the solubility and cellular localization (membrane or cytoplasm) of this enzyme (25–28). Initial attempts to overexpress IucD led to insoluble protein, and hydrophobic stretches of the enzyme were identified that could serve as transmembrane helices (27). These sequences were later suggested to be involved in coenzyme binding, FAD at the N-terminus and NADPH in the center of the protein (25). In support of this latter hypothesis, IucD can be produced in an active, soluble form by the addition of an N-terminal his tag (28). This purified protein is tetrameric in solution (10, 29). PvdA is also soluble when produced with an N-terminal histidine tag, and is monomeric in solution (Figure 2). Neither IucD nor PvdA co-purify with the required flavin coenzyme, and both bind FAD in the micromolar range (19, 30).
PvdA is also functionally related to two previously well-characterized enzymes, flavin-containing monooxygenase (FMO) and para-hydroxybenzoate hydroxylase (PHBH). FMOs are individually substrate promiscuous and hydroxylate a wide variety of nucleophilic substrates including primary, secondary, and tertiary amines, as well as sulfur, phosphorous, selenium, and iodine containing groups (9). FMOs in mammalian systems (in particular, the hog liver microsomal FMO (mFMO), which has been biochemically characterized) are membrane bound tetramers/octamers, but in bacterial and unicellular eukaryotes FMOs are cytoplasmic dimers (9, 31, 32). The structure of FMO from Schizosaccharomyces pombe (yFMO) is composed of two domains: a large domain containing the FAD binding sequence and the FMO identifying sequence (residues 1 – 175, 292 – 457), and a smaller insertion domain and is involved in stabilizing the NADPH (residues 176 – 291) (33). Sequence similarity between PvdA and yFMO is 37% (determined using the ALIGN algorithm, Genestream Search, CRBM Montepellier, France). PHBH and PvdA are relatively as similar in terms of primary structure (34 %). Sequence similarity between PvdA and either yFMO or PHBH is not concentrated in areas of ligand binding, but is found throughout the proteins. PHBH hydroxylates the activated aromatic ring of p-hydroxybenzoate, and is very substrate specific (8). PHBH is a dimer in solution and the x-ray crystal structure shows three domains: an N-terminal Rossmann-fold for FAD binding (residues 1 – 174), a middle domain composed primarily of β-structure for substrate binding (residues 175 – 295), and a helical C-terminal domain involved in dimerization (residues 296 – 394) (34). PHBH binds FAD stably in the nanomolar range and FAD is copurified with both mFMO and yFMO (33, 35).
PvdA is FAD and NADPH dependent (Table 1 and (19)). For PHBH and mFMO, NADPH reduces FAD for the hydroxylation of substrate, with the hydroxyl group derived from molecular oxygen (Figure 1) (15, 35). Similar to other flavin-containing enzymes (9), the oxidized flavin in PvdA shows two peaks at 380 nm and 451 nm (Figure 6), whereas fully reduced flavin shows only one peak at 380 nm (data not shown). Flavin reduction in PHBH in the presence of substrate occurs at a rate of 256 s−1, but in the absence of substrate, reduction occurs slowly at 0.41 min−1 (13). Reoxidation of the reduced flavin in the absence of substrate has a millisecond half-life (36). In mFMO, the flavin reduction is independent of substrate and the reduced enzyme reacts with molecular oxygen to form a stable flavin-peroxide intermediate (half-life of 2 hrs at pH 7.2) until substrate binds (9). In this initial characterization of flavin reduction and oxidation, PvdA does not require substrate for flavin reduction (Figure 6) similar to mFMO. However, the reoxidation of the flavin in PvdA is quicker than in mFMO by two orders of magnitude but slower than in PHBH by four orders of magnitude.
PHBH has been reported to have a kcat of 1370 min−1 at 25 °C (13, 16, 17). In contrast, the kinetic analysis of PvdA yields a slow turnover number, 26.4 min−1 by NADPH oxidation and 24 min−1 by the hydroxylation assay at 25 °C (Table 2), which is comparable to IucD (23). Kinetic parameters have not been reported for yFMO, and those reported for mFMO are an order of magnitude slower than PvdA, albeit at 4 °C (16, 17). PHBH and IucD exhibit substrate inhibition at high substrate concentrations (8, 30). Similarly, the hydroxylation assay plot shows an inhibition of PvdA activity at high L-ornithine concentrations with a KI of 31 +/− 5 mM (Figure 4, Table 2). However, this substrate inhibition is not seen with the NADPH oxidation assay (Figure 4 and (19)). Therefore, the excess substrate does not inhibit the oxidation of NADPH but only the formation of hydroxylated product, promoting uncoupling of the reaction.
The PvdA active site is very specific for substrate. The peptide groups and the sidechain amine are required for catalysis and presumably important for substrate positioning and binding (Table 1). Shortening of the side chain length resulted in no significant activity. Extending the side chain by one methylene group (L-lysine) resulted in significant NADPH oxidation without the formation of the hydroxylated product, indicating that the reaction was uncoupled. As with PHBH (37), uncoupling of the NADPH oxidation from product formation in PvdA leads to the production of hydrogen peroxide (19). Therefore, L-lysine acts as a non-substrate effector for PvdA. We hypothesize that L-lysine leads to closing of the active site by an induced fit mechanism allowing for electron transfer from NADPH to FAD. However, L-lysine is sufficiently different in size such that it cannot be hydroxylated efficiently, resulting in uncoupling the enzyme similar to what is seen for non-substrate effectors of PHBH and IucD (13, 30, 37, 38). Lysine is a mixed inhibitor (Table 3), also indicating that lysine is sufficiently similar to ornithine to compete for binding at the active site. Ornithine and lysine bind both in the active site (as substrate and competitive inhibitor) and at a second site leading to substrate inhibition (ornithine) or uncompetitive inhibition (lysine). Most probably, this effect is due to two molecules of substrate (substrate inhibition), or one substrate molecule and one inhibitor molecule (mixed inhibition) binding to the active site simultaneously, as described canonically for invertase (39). In contrast, competitive inhibition was observed for non-substrate effectors of PHBH (37) and 5-aminopentanoic acid, L-2,4-diaminobutyric acid, and L-homoserine for PvdA (19).
IucD has been reported to have increased activity with increased chloride concentration (29). In contrast, inhibition by halides, including chloride, has been described for PHBH (40). The crystal structure of PHBH demonstrates three chloride binding sites, one of which interferes with flavin intermediate formation and substrate hydroxylation (9, 41). Whereas chloride could merely disrupt ionic interactions for decreased enzyme efficiency, competitive chloride inhibition in PvdA with respect to NADPH suggests specific binding of the monovalent anion in the coenzyme-binding site. Both IucD and PHBH are inhibited by bulky mercurial compounds (~60%), and the activity was completely regained by the addition of reducing agents (8, 10, 42, 43). p-Chloromercuribenzoate is a mixed inhibitor of PvdA with respect to ornithine (Table 3). While these mercurial compounds are not site specific, these data suggest that binding of a bulky mercurial compound to a cysteine possibly in the active or coenzyme binding sites disrupts activity for all three of the enzymes.
As demonstrated using steady state kinetics, FAD loaded PHBH forms a ternary complex with NADPH and a nonsubstrate effector, 6-hydroxynicotinate (13). From this work, it was deduced that PHBH uses a rapid equilibrium random order mechanism (as opposed to a compulsory order mechanism) for catalysis. Yeast FMO:FAD forms a ternary complex with NADPH and substrate; however, the reaction was determined as a compulsory order mechanism (9, 17). PvdA:FAD also forms a ternary complex with substrate and NADPH (Figure 5); however, it cannot be determined from this primary plot if the mechanism will be a random order or compulsory order (24).
Conclusions
PvdA shares many biochemical similarities to PHBH: substrate specificity, substrate inhibition, non-substrate effectors, and inhibition by halides and bulky mercurials. However, some of the characteristics seen in PvdA are shared with mFMO: primary amine hydroxylation and the reduction of the flavin in the absence of substrate. Some characteristics of PvdA are different from either PHBH or the FMOs, including the oligomeric state in solution (monomer), and the rate of reoxidation of the flavin in the absence of substrate. The sequence similarity between PvdA and PHBH (18 % identity and 34 % similarity) and between PvdA and yFMO (19 % identity and 37 % similarity) are comparable to that of PHBH and yFMO (18 % identity, 38 % similarity). However, the structural similarity between PHBH and yFMO is predominantly in the FAD binding domains, with a DALI Z-score of 10.5 for less than half of the protein (~180 amino acids), and a root mean squared deviation of 4.0 Å (44). Taken together, our data suggest that catalysis by PvdA will likely proceed by a novel reaction mechanism, and PvdA will have a distinct three-dimensional structure to those previously determined for PHBH and the FMOs.
Acknowledgements
We would like to thank T.C. Gamblin for the use of the Bio-Tek Elx800 plate reader and the Varian Cary 50 Bio Spectrophotometer. We thank B. Palfey for helpful discussions.
ABBREVIATIONS AND TEXTUAL FOOTNOTES
- PvdA
ornithine hydroxylase from Pseudomonas aeruginosa
- IucD
lysine hydroxylase from Escherichia coli
- PHBH
para-hydroxybenzoate hydroxylase from Pseudomonas fluorescens
- FMO
flavin-containing monooxygenase
- mFMO
mammalian or microsomal (hog liver) FMO
- yFMO
yeast (Schizosaccharomyces pombe) FMO
- FAD
flavin adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- FMN
flavin-mononucleotide
- NADH
nicotinamide adenine dinucleotide
- DTT
dithiothreitol
- PCMB
para-chloromercuribenzoate
- Vo
initial velocity
- Vmax
velocity at maximal substrate concentration
- Km
Michaelis constant
- kcat
turnover number
- KI
inhibition constant
Footnotes
This publication was made possible by the National Center for Research Resources of the National Institute of Health (NIH Grant Number P20 RR-17708).
REFERENCES
- 1.Cox CD. Effect of pyochelin on the virulence of Pseudomonas aeruginosa. Infect Immun. 1982;36:17–23. doi: 10.1128/iai.36.1.17-23.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer JM, Neely A, Stintzi A, Georges C, Holder IA. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crosa JH. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989;53:517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer JM. Pyoverdines: pigments, siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch Microbiol. 2000;174:135–142. doi: 10.1007/s002030000188. [DOI] [PubMed] [Google Scholar]
- 5.Lamont IL, Martin LW. Identification and characterization of novel pyoverdine synthesis genes in Pseudomonas aeruginosa. Microbiology. 2003;149:833–842. doi: 10.1099/mic.0.26085-0. [DOI] [PubMed] [Google Scholar]
- 6.Visca P, Serino L, Orsi N. Isolation and characterization of Pseudomonas aeruginosa mutants blocked in the synthesis of pyoverdin. J Bacteriol. 1992;174:5727–5731. doi: 10.1128/jb.174.17.5727-5731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visca P, Ciervo A, Orsi N. Cloning and nucleotide sequence of the pvdA gene encoding the pyoverdin biosynthetic enzyme L-ornithine N5-oxygenase in Pseudomonas aeruginosa. J Bacteriol. 1994;176:1128–1140. doi: 10.1128/jb.176.4.1128-1140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosokawa K, Stanier RY. Crystallization and properties of p-hydroxybenzoate hydroxylase from Pseudomonas putida. J Biol Chem. 1966;241:2453–2460. [PubMed] [Google Scholar]
- 9.Palfey BA, Massey V. Flavin-Dependent Enzymes. In: Sinnott M, editor. Comprehensive Biological Catalysis. San Diego: Academic Press; 1998. pp. 83–154. [Google Scholar]
- 10.Plattner HJ, Pfefferle P, Romaguera A, Waschutza S, Diekmann H. Isolation and some properties of lysine N6-hydroxylase from Escherichia coli strain EN222. Biol Met. 1989;2:1–5. doi: 10.1007/BF01116193. [DOI] [PubMed] [Google Scholar]
- 11.Ballou DP, Entsch B, Cole LJ. Dynamics involved in catalysis by single-component and two-component flavin-dependent aromatic hydroxylases. Biochem Biophys Res Commun. 2005;338:590–598. doi: 10.1016/j.bbrc.2005.09.081. [DOI] [PubMed] [Google Scholar]
- 12.Cashman JR. Human flavin-containing monooxygenase: substrate specificity and role in drug metabolism. Curr Drug Metab. 2000;1:181–191. doi: 10.2174/1389200003339135. [DOI] [PubMed] [Google Scholar]
- 13.Howell LG, Spector T, Massey V. Purification and Properties of p-Hydroxybenzoate Hydroxylase from Pseudomonas fluorescens. J Biol Chem. 1972;247:4340–4350. [PubMed] [Google Scholar]
- 14.Spector T, Massey V. p-Hydroxybenzoate Hydroxylase from Pseudomonas fluorescens, Evidence for an Oxygenated Flavin Intermediate. J Biol Chem. 1972;247:5632–5636. [PubMed] [Google Scholar]
- 15.Husain M, Massey V. Kinetic studies on the reaction of p-hydroxybenzoate hydroxylase. Agreement of steady state and rapid reaction data. J Biol Chem. 1979;254:6657–6666. [PubMed] [Google Scholar]
- 16.Beaty NB, Ballou DP. The reductive half-reaction of liver microsomal FAD-containing monooxygenase. J Biol Chem. 1981;256:4611–4618. [PubMed] [Google Scholar]
- 17.Beaty NB, Ballou DP. The oxidative half-reaction of liver microsomal FAD-containing monooxygenase. J Biol Chem. 1981;256:4619–4625. [PubMed] [Google Scholar]
- 18.Jones KC, Ballou DP. Reactions of the 4a-hydroperoxide of liver microsomal flavin-containing monooxygenase with nucleophilic and electrophilic substrates. J Biol Chem. 1986;261:2553–2559. [PubMed] [Google Scholar]
- 19.Ge L, Seah SY. Heterologous expression, purification, and characterization of an L-ornithine N(5)-hydroxylase involved in pyoverdine siderophore biosynthesis in Pseudomonas aeruginosa. J Bacteriol. 2006;188:7205–7210. doi: 10.1128/JB.00949-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Csaky TZ. On the Estimation of Bound Hydroxylamine in Biological Materials. Acta Chem Scand. 1948;2:450–454. [Google Scholar]
- 21.Tomlinson G, Cruickshank WH, Viswanatha T. Sensitivity of substituted hydroxylamines to determination by iodine oxidation. Anal Biochem. 1971;44:670–679. doi: 10.1016/0003-2697(71)90258-2. [DOI] [PubMed] [Google Scholar]
- 22.Gillam AH, Lewis AG, Andersen RJ. Quantitative Determination of Hydroxamic Acids. Anal Chem. 1981;53:841–844. [Google Scholar]
- 23.Stehr M, Smau L, Singh M, Seth O, Macheroux P, Ghisla S, Diekmann H. Studies with lysine N6-hydroxylase. Effect of a mutation in the assumed FAD binding site on coenzyme affinities and on lysine hydroxylating activity. Biol Chem. 1999;380:47–54. doi: 10.1515/BC.1999.006. [DOI] [PubMed] [Google Scholar]
- 24.Cornish-Bowden A. Fundamentals of Enzyme Kinetics. London: Portland Press Ltd; 1995. [Google Scholar]
- 25.Stehr M, Diekmann H, Smau L, Seth O, Ghisla S, Singh M, Macheroux P. A hydrophobic sequence motif common to N-hydroxylating enzymes. Trends Biochem Sci. 1998;23:56–57. doi: 10.1016/s0968-0004(97)01166-3. [DOI] [PubMed] [Google Scholar]
- 26.Krone WJ, Luirink J, Koningstein G, Oudega B, de Graaf FK. Subcloning of the cloacin DF13/aerobactin receptor protein and identification of a pColV-K30-determined polypeptide involved in ferric-aerobactin uptake. J Bacteriol. 1983;156:945–948. doi: 10.1128/jb.156.2.945-948.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrero M, de Lorenzo V, Neilands JB. Nucleotide sequence of the iucD gene of the pColV-K30 aerobactin operon and topology of its product studied with phoA and lacZ gene fusions. J Bacteriol. 1988;170:56–64. doi: 10.1128/jb.170.1.56-64.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seth O, Smau L, Welte W, Ghisla S, Stehr M, Diekmann H, Macheroux P. A reply to Dick et al. Trends Biochem Sci. 1998;23:414–415. doi: 10.1016/s0968-0004(97)01166-3. [DOI] [PubMed] [Google Scholar]
- 29.Thariath A, Socha D, Valvano MA, Viswanatha T. Construction and biochemical characterization of recombinant cytoplasmic forms of the IucD protein (lysine:N6-hydroxylase) encoded by the pColV-K30 aerobactin gene cluster. J Bacteriol. 1993;175:589–596. doi: 10.1128/jb.175.3.589-596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macheroux P, Plattner HJ, Romaguera A, Diekmann H. FAD and substrate analogs as probes for lysine N6-hydroxylase from Escherichia coli EN 222. Eur J Biochem. 1993;213:995–1002. doi: 10.1111/j.1432-1033.1993.tb17846.x. [DOI] [PubMed] [Google Scholar]
- 31.Schlenk D. Occurrence of flavin-containing monooxygenases in non-mammalian eukaryotic organisms. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;121:185–195. doi: 10.1016/s0742-8413(98)10060-9. [DOI] [PubMed] [Google Scholar]
- 32.Choi HS, Kim JK, Cho EH, Kim YC, Kim JI, Kim SW. A novel flavin-containing monooxygenase from Methylophaga sp strain SK1 and its indigo synthesis in Escherichia coli. Biochem Biophys Res Commun. 2003;306:930–936. doi: 10.1016/s0006-291x(03)01087-8. [DOI] [PubMed] [Google Scholar]
- 33.Eswaramoorthy S, Bonanno JB, Burley SK, Swaminathan S. Mechanism of action of a flavin-containing monooxygenase. Proc Natl Acad Sci U S A. 2006;103:9832–9837. doi: 10.1073/pnas.0602398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Entsch B, Van Berkel WJH. Structure and mechanism of para-hydroxybenzoate hydroxylase. The FASEB J. 1995;9:476–483. doi: 10.1096/fasebj.9.7.7737455. [DOI] [PubMed] [Google Scholar]
- 35.Poulsen LL, Ziegler DM. The liver microsomal FAD-containing monooxygenase. Spectral characterization and kinetic studies. J Biol Chem. 1979;254:6449–6455. [PubMed] [Google Scholar]
- 36.Spector T, Massey V. p-Hydroxybenzoate Hydroxylase form Pseudomonas fluorescens, Reactivity with Oxygen. J Biol Chem. 1973;247:7123–7127. [PubMed] [Google Scholar]
- 37.Spector T, Massey V. Studies of the Effector Specificity of p-Hydroxybenzoate Hydroxylase from Pseudomonas fluorescens. J Biol Chem. 1972;247:4679–4687. [PubMed] [Google Scholar]
- 38.Howell LG, Massey V. A Non-substrate Effector of p-Hydroxybenzoate Hydroxylase. Biochem Biophys Res Commun. 1970;40:887–893. doi: 10.1016/0006-291x(70)90986-1. [DOI] [PubMed] [Google Scholar]
- 39.Combes D, Monsan P. Sucrose Hydrolysis by Invertase. Characterization of Products and Substrate Inhibition. Carbohydr. Res. 1983;117:215–228. [Google Scholar]
- 40.Steennis PJ, Cordes MM, Hilkens JH, Muller F. On the interaction of para-hydroxybenzoate hydroxylase from Pseudomonas fluorescens with halogen ions. FEBS Lett. 1973;36:177–180. doi: 10.1016/0014-5793(73)80363-1. [DOI] [PubMed] [Google Scholar]
- 41.Gatti DL, Palfey BA, Lah MS, Entsch B, Massey V, Ballou DP, Ludwig ML. The Mobile Flavin of 4-OH Benzoate Hydroxylase. Science. 1994;266:110–114. doi: 10.1126/science.7939628. [DOI] [PubMed] [Google Scholar]
- 42.Dick S, Marrone L, Duewel H, Beecroft M, McCourt J, Viswanatha T. Lysine: N6-hydroxylase: stability and interaction with ligands. J Protein Chem. 1999;18:893–903. doi: 10.1023/a:1020639514998. [DOI] [PubMed] [Google Scholar]
- 43.Marrone L, Viswanatha T. Effect of selective cysteine --> alanine replacements on the catalytic functions of lysine: N6-hydroxylase. Biochim Biophys Acta. 1997;1343:263–277. doi: 10.1016/s0167-4838(97)00129-5. [DOI] [PubMed] [Google Scholar]
- 44.Holm L, Sander C. Protein structure comparison by alignment of distance matrices. J Mol Biol. 1993;233:123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]