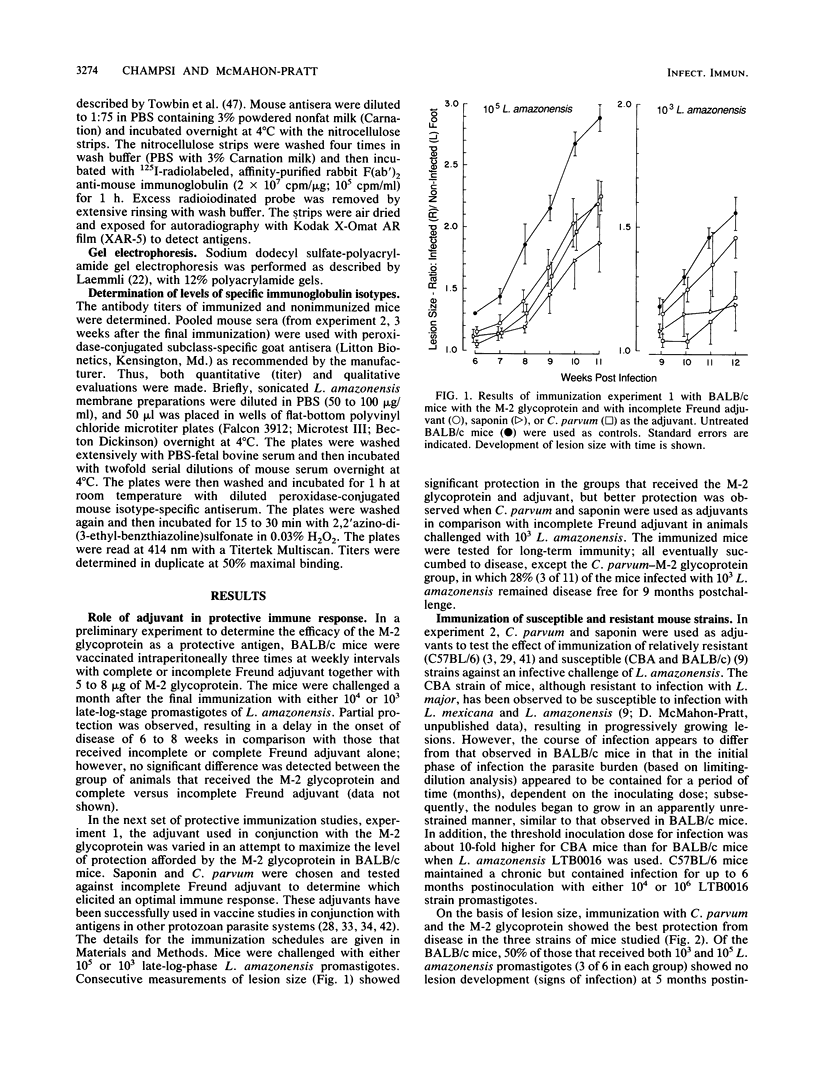
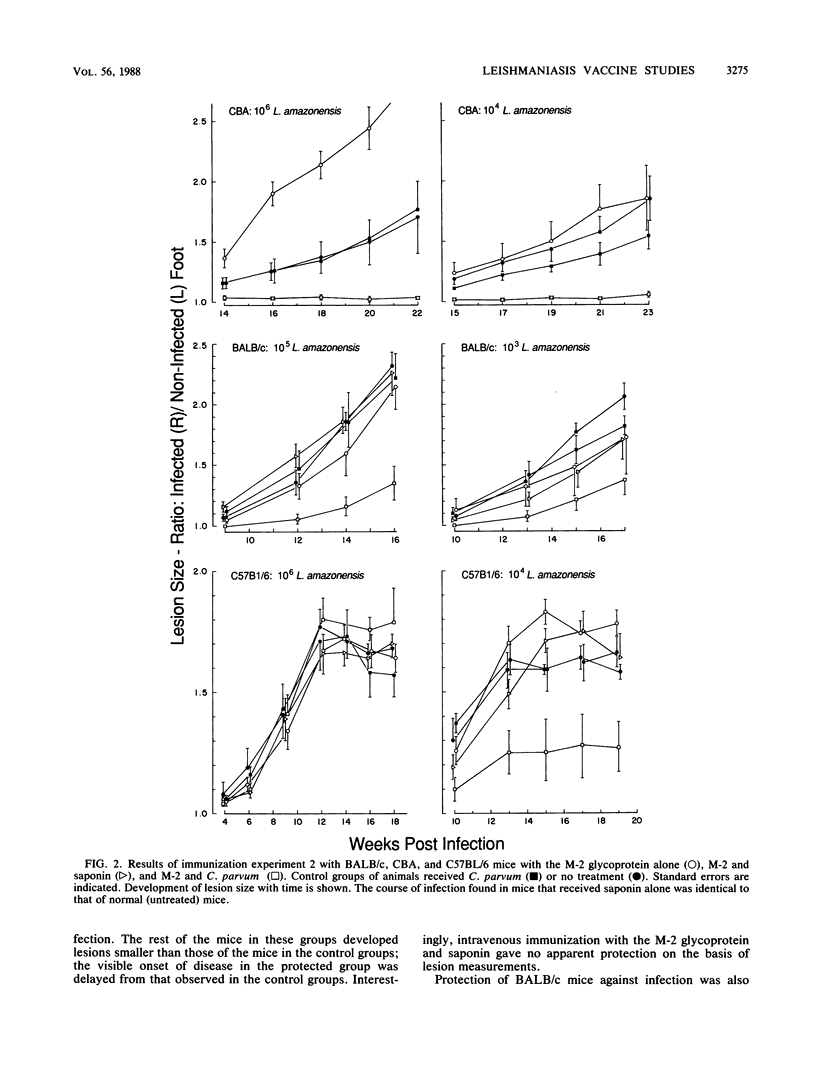
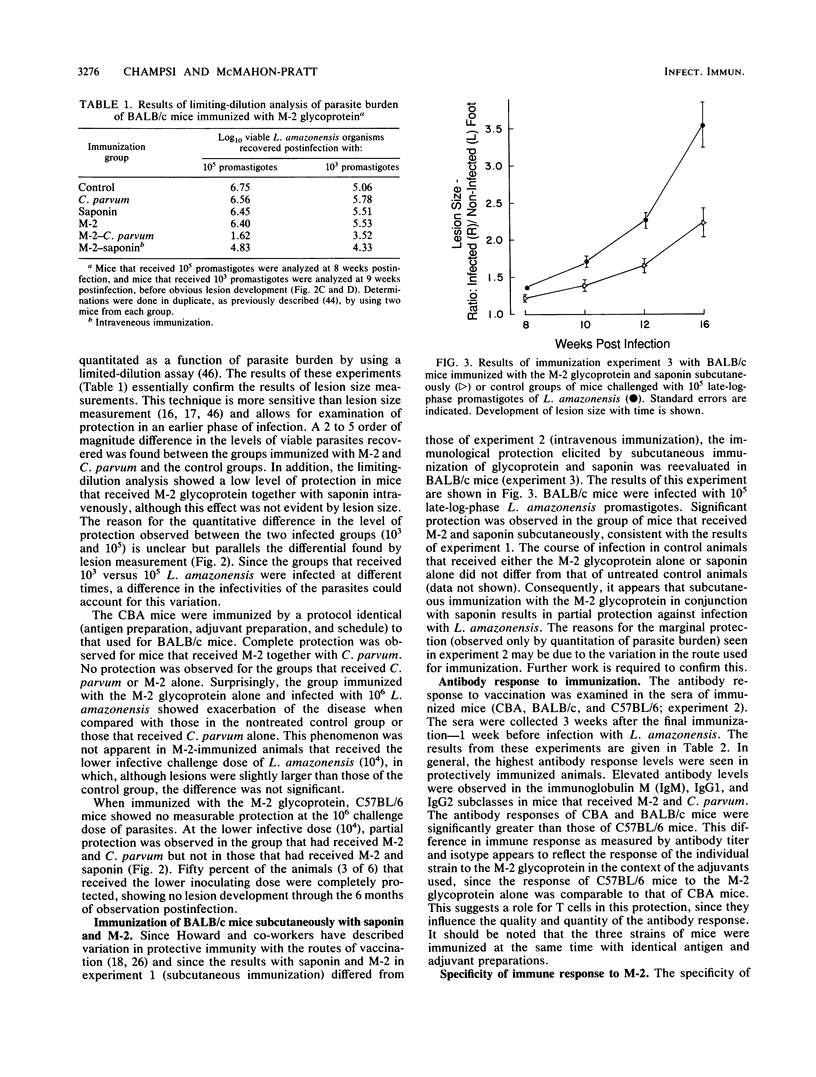
Abstract
Previous passive antibody transfer experiments have indicated that immunity to a 46-kilodalton membrane glycoprotein (M-2) of Leishmania amazonensis may protect against infection with this parasite. In the studies described in this paper, we investigated the ability of the purified M-2 molecule to elicit a protective immune response in conjunction with Freund incomplete and complete adjuvants, saponin, and Corynebacterium parvum. Both relatively susceptible (BALB/c and CBA) and resistant (C57BL/6) strains of mice were examined. C. parvum appeared to be the most effective adjuvant in the three mouse strains tested. The level of protection varied with the mouse strain, although all animals received identical preparations of antigen and adjuvant. Immunization of CBA mice with the M-2 glycoprotein and C. parvum resulted in complete protection against a challenge infection of 10(4) and 10(6) late log-phase promastigotes of L. amazonensis. In the BALB/c strain, complete protection was observed in some of the immunized animals (28 to 50%); in the rest of the mice the onset of infection was significantly delayed. Protective immunity for C57BL/6 mice was observed only at the low infecting dose (10(4) L. amazonensis organisms). The level of protection observed is reflected by increased antibody response (immunoglobulins G1 and G2) developed to the M-2 molecule. The relationship of pure T-cell (nonantibody) immunity to this protection remains to be elucidated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S., GUNDERS A. E. IMMUNITY TO LEISHMANIA MEXICANA FOLLOWING SPONTANEOUS RECOVERY FROM ORIENTAL SORE. Trans R Soc Trop Med Hyg. 1964 May;58:274–277. doi: 10.1016/0035-9203(64)90041-0. [DOI] [PubMed] [Google Scholar]
- Anderson S., David J. R., McMahon-Pratt D. In vivo protection against Leishmania mexicana mediated by monoclonal antibodies. J Immunol. 1983 Oct;131(4):1616–1618. [PubMed] [Google Scholar]
- Barral A., Petersen E. A., Sacks D. L., Neva F. A. Late metastatic Leishmaniasis in the mouse. A model for mucocutaneous disease. Am J Trop Med Hyg. 1983 Mar;32(2):277–285. doi: 10.4269/ajtmh.1983.32.277. [DOI] [PubMed] [Google Scholar]
- CONVIT J., KERDEL-VEGAS F. DISSEMINATED CUTANEOUS LEISHMANIASIS; INNOCULATION TO LABORATORY ANIMALS, ELECTRON MICROSCOPY AND FLUORESCENT ANTIBODIES STUDIES. Arch Dermatol. 1965 May;91:439–447. doi: 10.1001/archderm.1965.01600110025007. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Human cutaneous lieshmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980 Sep 12;209(4462):1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- Convit J., Castellanos P. L., Rondon A., Pinardi M. E., Ulrich M., Castes M., Bloom B., Garcia L. Immunotherapy versus chemotherapy in localised cutaneous leishmaniasis. Lancet. 1987 Feb 21;1(8530):401–405. doi: 10.1016/s0140-6736(87)90116-4. [DOI] [PubMed] [Google Scholar]
- De Rossell R. A., Bray R. S., Alexander J. The correlation between delayed hypersensitivity, lymphocyte activation and protective immunity in experimental murine leishmaniasis. Parasite Immunol. 1987 Jan;9(1):105–115. doi: 10.1111/j.1365-3024.1987.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Debons-Guillemin M. C., Vouldoukis I., Roseto A., Alfred C., Chopin C., Ploton I., Monjour L. Inhibition in vivo of both infective Leishmania major and L. mexicana amazonensis mediated by a single monoclonal antibody. Trans R Soc Trop Med Hyg. 1986;80(2):258–260. doi: 10.1016/0035-9203(86)90030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt C. L. The present and future of vaccination for cutaneous leishmaniasis. Prog Clin Biol Res. 1980;47:259–285. [PubMed] [Google Scholar]
- Grimaldi G., Jr, David J. R., McMahon-Pratt D. Identification and distribution of New World Leishmania species characterized by serodeme analysis using monoclonal antibodies. Am J Trop Med Hyg. 1987 Mar;36(2):270–287. doi: 10.4269/ajtmh.1987.36.270. [DOI] [PubMed] [Google Scholar]
- Haidaris C. G., Bonventre P. F. Elimination of Leishmania donovani amastigotes by activated macrophages. Infect Immun. 1981 Sep;33(3):918–926. doi: 10.1128/iai.33.3.918-926.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Greenblatt C. L., Goding J. W. An amphipathic sulphated glycoconjugate of Leishmania: characterization with monoclonal antibodies. EMBO J. 1984 Oct;3(10):2301–2306. doi: 10.1002/j.1460-2075.1984.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. O., Fahey J. R. Leishmania spp.: agar plating as an alternative to limiting dilution and impression smears for the enumeration of viable parasites in tissue. Exp Parasitol. 1987 Feb;63(1):108–111. doi: 10.1016/0014-4894(87)90083-x. [DOI] [PubMed] [Google Scholar]
- Hill J. O. Modulation of the pattern of development of experimental disseminated leishmaniasis by Corynebacterium parvum. J Leukoc Biol. 1987 Feb;41(2):165–169. doi: 10.1002/jlb.41.2.165. [DOI] [PubMed] [Google Scholar]
- Hill J. O., North R. J., Collins F. M. Advantages of measuring changes in the number of viable parasites in murine models of experimental cutaneous leishmaniasis. Infect Immun. 1983 Mar;39(3):1087–1094. doi: 10.1128/iai.39.3.1087-1094.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Liew F. Y., Hale C., Nicklin S. Prophylactic immunization against experimental leishmaniasis. II. Further characterization of the protective immunity against fatal Leishmania tropica infection induced by irradiated promastigotes. J Immunol. 1984 Jan;132(1):450–455. [PubMed] [Google Scholar]
- Jaffe C. L., Bennett E., Grimaldi G., Jr, McMahon-Pratt D. Production and characterization of species-specific monoclonal antibodies against Leishmania donovani for immunodiagnosis. J Immunol. 1984 Jul;133(1):440–447. [PubMed] [Google Scholar]
- Kahl L. P., McMahon-Pratt D. Structural and antigenic characterization of a species- and promastigote-specific Leishmania mexicana amazonensis membrane protein. J Immunol. 1987 Mar 1;138(5):1587–1595. [PubMed] [Google Scholar]
- Kirkpatrick C. E., Farrell J. P. Leishmaniasis in beige mice. Infect Immun. 1982 Dec;38(3):1208–1216. doi: 10.1128/iai.38.3.1208-1216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lainson R., Shaw J. J. Leishmaniasis in Brazil: XII. Observations on cross-immunity in monkeys and man infected with Leishmania mexicana mexicana, L. m. amazonensis, L. braziliensis braziliensis, L. b. guyanensis and L. b. panamensis. J Trop Med Hyg. 1977 Feb;80(2):29–35. [PubMed] [Google Scholar]
- Lainson R. The American leishmaniases: some observations on their ecology and epidemiology. Trans R Soc Trop Med Hyg. 1983;77(5):569–596. doi: 10.1016/0035-9203(83)90185-2. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Howard J. G., Hale C. Prophylactic immunization against experimental leishmaniasis. III. Protection against fatal Leishmania tropica infection induced by irradiated promastigotes involves Lyt-1+2- T cells that do not mediate cutaneous DTH. J Immunol. 1984 Jan;132(1):456–461. [PubMed] [Google Scholar]
- Mayrink W., Williams P., da Costa C. A., Magalhães P. A., Melo M. N., Dias M., Oliveira Lima A., Michalick M. S., Ferreira Carvalho E., Barros G. C. An experimental vaccine against American dermal leishmaniasis: experience in the State of Espírito Santo, Brazil. Ann Trop Med Parasitol. 1985 Jun;79(3):259–269. doi: 10.1080/00034983.1985.11811917. [DOI] [PubMed] [Google Scholar]
- McColm A. A., Bomford R., Dalton L. A comparison of saponin with other adjuvants for the potentiation of protective immunity by a killed Plasmodium yoelii vaccine in the mouse. Parasite Immunol. 1982 Sep;4(5):337–347. doi: 10.1111/j.1365-3024.1982.tb00445.x. [DOI] [PubMed] [Google Scholar]
- McElrath M. J., Kaplan G., Nusrat A., Cohn Z. A. Cutaneous leishmaniasis. The defect in T cell influx in BALB/c mice. J Exp Med. 1987 Feb 1;165(2):546–559. doi: 10.1084/jem.165.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E. Leishmania tropica major in mice: vaccination against cutaneous leishmaniasis in mice of high genetic susceptibility. Aust J Exp Biol Med Sci. 1983 Feb;61(Pt 1):11–25. doi: 10.1038/icb.1983.2. [DOI] [PubMed] [Google Scholar]
- Mitchell G. H., Richards W. H., Voller A., Dietrich F. M., Dukor P. Nor-MDP, saponin, corynebacteria, and pertussis organisms as immunological adjuvants in experimental malaria vaccination of macaques. Bull World Health Organ. 1979;57 (Suppl 1):189–197. [PMC free article] [PubMed] [Google Scholar]
- Petersen E. A., Neva F. A., Oster C. N., Bogaert Diaz H. Specific inhibition of lymphocyte-proliferation responses by adherent suppressor cells in diffuse cutaneous leishmaniasis. N Engl J Med. 1982 Feb 18;306(7):387–392. doi: 10.1056/NEJM198202183060702. [DOI] [PubMed] [Google Scholar]
- Pratt D. M., David J. R. Monoclonal antibodies recognizing determinants specific for the promastigote state of Leishmania mexicana. Mol Biochem Parasitol. 1982 Nov;6(5):317–327. doi: 10.1016/0166-6851(82)90064-0. [DOI] [PubMed] [Google Scholar]
- Pratt D. M., David J. R. Monoclonal antibodies that distinguish between New World species of Leishmania. Nature. 1981 Jun 18;291(5816):581–583. doi: 10.1038/291581a0. [DOI] [PubMed] [Google Scholar]
- Rodrigues M. M., Xavier M. T., Previato L. M., Barcinski M. A. Characterization of cellular immune response to chemically defined glycoconjugates from Leishmania mexicana subsp. amazonensis. Infect Immun. 1986 Jan;51(1):80–86. doi: 10.1128/iai.51.1.80-86.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Sacks D. L., Scott P. A., Asofsky R., Sher F. A. Cutaneous leishmaniasis in anti-IgM-treated mice: enhanced resistance due to functional depletion of a B cell-dependent T cell involved in the suppressor pathway. J Immunol. 1984 Apr;132(4):2072–2077. [PubMed] [Google Scholar]
- Scott M. T., Snary D. Protective immunisation of mice using cell surface glycoprotein from Trypanosoma cruzi. Nature. 1979 Nov 1;282(5734):73–74. doi: 10.1038/282073a0. [DOI] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Sher A. B lymphocytes are required for the generation of T cells that mediate healing of cutaneous leishmaniasis. J Immunol. 1986 Aug 1;137(3):1017–1021. [PubMed] [Google Scholar]
- Scott P., Pearce E., Natovitz P., Sher A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987 Jul 1;139(1):221–227. [PubMed] [Google Scholar]
- Scott P., Sacks D., Sher A. Resistance to macrophage-mediated killing as a factor influencing the pathogenesis of chronic cutaneous leishmaniasis. J Immunol. 1983 Aug;131(2):966–971. [PubMed] [Google Scholar]
- Shaw J. J., Lainson R. Leishmaniasis in Brazil: X. Some observations of intradermal reactions to different trypanosomatid antigens of patients suffering from cutaneous and mucocutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1975;69(3):323–335. doi: 10.1016/0035-9203(75)90127-3. [DOI] [PubMed] [Google Scholar]
- Sljivić V. S., Watson S. R. The adjuvant effect of Corynebacterium parvum: T-cell dependence of macrophage activation. J Exp Med. 1977 Jan 1;145(1):45–57. doi: 10.1084/jem.145.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher N., Swindell R., Crowther D. Effects of repeated Corynebacterium parvum and BCG therapy on immune parameters: a weekly sequential study of melanoma patients. I. Changes in non-specific (NK, K and T cell) lymphocytotoxicity, peripheral blood counts and delayed hypersensitivity reactions. Clin Exp Immunol. 1979 May;36(2):227–236. [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Marchand M., Boon T., Louis J. A. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985 Sep;7(5):545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr G. W., James K. Effect of Corynebacterium parvum on the class and subclass of antibody produced in the response of different strains of mice to sheep erythrocytes. Immunology. 1975 Mar;28(3):431–442. [PMC free article] [PubMed] [Google Scholar]