Abstract
Focal fibrocystic change (FCC) of the breast is a rare form of FCC. Imaging presentations of focal FCC are not well known. This study aimed to analyze its MR imaging features. Eleven patients of pathology-proven focal FCC were retrospectively studied. Of the 11 patients, 7 were mass (≥5mm), 2 showed multiple foci, and 2 were focus (< 5mm). The lesion size ranged from 4mm to 12mm (mean 6.7mm). Morphologically, 3 patients were suspected as malignancy. Using kinetic enhancement curve, 8 of 13 lesions were suspected to be malignant. Overall, 9 patients (82%) were suspected for malignancy using either criterion. Using mammography, 6 of the 11 patients (55%) were diagnosed as malignancy. Breast sonography suspected malignancy in 7 patients (7/10, 70%). No statistically significant difference was found in the three diagnostic methods. In pathology, all 11 patients showed the typical pathological features of fibrocystic change, with mixed components of stromal fibrosis, cyst formation, apocrine metaplasia, adenosis, and/or focal sclerosing adenosis. In conclusion, MR imaging features of focal FCC usually present as a mass or focus lesion with rapid enhancement and washout kinetics, which mimic a malignant breast lesion and lead to unnecessary operation, especially in patients with contra-lateral malignant breast cancer.
Keywords: Fibrocystic change, Focal fibrocystic change, MR imaging, Mammography, Sonography
INTRODUCTION
Fibrocystic change (FCC) is a commonly encountered condition of the breast that affects more than half of women (1). FCC includes a wide variety of histology, including stromal fibrosis, cysts, adenosis, apocrine metaplasia, and epithelial proliferation of various degrees (2). The response of the breast tissue to the monthly changes of estrogen and progesterone levels is believed to account for the pathogenesis of FCC. Although FCC is a benign condition, it may be misdiagnosed as a malignant lesion by physical examination or imaging studies. The existence of FCC sometimes also makes it difficult to detect cancer.
FCC might appear occasionally as a focal discrete lesion mimicking a tumor in clinical and radiographic appearance. Using mammography and sonography, although the imaging appearances of some subtypes of FCC have been reported (3–5), MR imaging of FCC has been reported rarely (6). MR imaging with regard to focal FCC has not been reported at all.
The purpose of this study is to analyze the MR imaging features of focal FCC of the breast.
MATERIALS AND METHODS
Patients
In a period of 4 years from Jan. 2003 to Dec. 2006, 11 patients (39–74 years old, mean 53) of pathology-proven focal FCC were identified from a breast MRI database of over 600 patients in our institution and retrospectively studied. These patients came to our institute due to suspicion of breast tumors by other imaging modalities. Prior to the breast MRI, all patients had mammography and 10 patients had received sonographic examination of the breast. Suspicious focal lesions were identified in either imaging modality. The breast MRI study was approved by the Institutional Review Board and was HIPAA compliant. All participants gave written informed consent. Five patients had contralateral breast cancer, including 4 pure invasive ductal carcinomas and 1 invasive ductal carcinoma mixed with lobular component. The pathological evidence of focal FCC of the breast was obtained by core needle biopsy under ultrasonography guidance in 8 patients and by operation specimen after mastectomy in 3 patients.
MR Imaging Protocol
The MRI study was performed using a 1.5 T MR scanner with a phase-array bilateral breast coil (Philips Medical Systems, Cleveland, Ohio). The imaging protocol consisted of pre-contrast T1 weighted imaging and bilateral dynamic contrast-enhanced imaging. After the localizer scan to define the location of the breasts, axial and unilateral sagittal view T1-weighted pre-contrast images were acquired from the breast with suspicious lesions, using a spin echo pulse sequence with TR = 1000 ms, TE = 12 ms, FOV = 20 cm, matrix size = 256 × 256. Thirty to forty slices with 3–4 mm thickness were prescribed to cover the entire breast and part of axillary region. Following this, a 3D SPGR (RF-FAST) pulse sequence with 16 frames (repetitions) was prescribed for bilateral dynamic imaging. Thirty-two axial slices with 4 mm thickness were used to cover both breasts. The imaging parameters were TR = 8.1 ms, TE = 4.0 ms, flip angle = 20° matrix size = 256 × 128, FOV = 38 cm. The scan time was 42 sec per acquisition. The sequence was repeated 16 times for dynamic acquisitions, four pre-contrast, and 12 post-contrast sets. The contrast agent (Omniscan® 1 cc/10 lbs body weight) was manually injected at the beginning of the 5th acquisition, and was timed to finish in 12 seconds to make the bolus length consistent for all patients. Immediately following the contrast, 10 cc saline was injected to flush in all contrast medium.
Subtraction images were generated by subtracting the pre-contrast images acquired in the third frame from the post contrast enhanced images acquired in the sixth frame. The subtracted images was presented as either gray scale images or color-coded images. In color-coded images, the enhancement magnitude was presented according to the color range of 250 arbitrary units. The maximum intensity projections (MIPs) were also generated from the subtraction images. The enhancement kinetics was analyzed from manually-drawn tumor ROI (region of interest) on each imaging slice containing the lesion on subtraction images. The MIP was used as the reference for ROI drawing. The percent enhancement time course was calculated by first subtracting the mean signal intensity in the first 4 frames from each of the subsequent 12 post-contrast signal intensities, and then normalized by the mean pre-contrast signal intensity. The fitting process was carried out with software developed in-house using Matlab environment (version 6.0.0.88, The MathWorks, Inc.). The measured enhancement kinetics was analyzed using a 2-compartmental pharmacokinetic model to obtain respective vascular (red curve) and extravascular (green curve) contributions (8). The summation of these two components at each time point measured was regarded as total tumor enhancement (blue curve). Hemodynamic parameters, including Ktrans and Kep, were not analyzed in this study.
Interpretation of MR Imaging Features
The MRI features were interpreted by an experienced breast radiologist based on the morphologic and enhancement kinetic descriptors defined on ACR BIRADS-MRI lexicon (7). The radiologist was not aware of the final pathology when he was interpreting the MR imaging. The morphologic criteria included mass type lesion, including focus/foci (smaller than 5mm) and mass (equal to or greater than 5 mm), and non-mass type enhancements (focal area, linear, ductal, patchy, regional, multiple regions, and diffuse enhancement). The evaluation of enhancement kinetic curve was based on the total tumor enhancement (blue curve) which was consisted of initial wash-in phase (within the first 2 minutes or when the curve starts to change slope), and the late phase (after 2 minutes or after the change of the slope). The initial wash-in phase was categorized into fast, medium, and slow. The late phase was described as persistent, plateau, and wash-out (9).
STATISTICS
Fisher’s Exact test was used to compare the diagnostic performance of different imaging modalities for focal FCC.
RESULTS
Mammography and Sonography Diagnosis
Mammography showed 6 patients with ACR category 4 lesions, 2 patients with ACR category 2 lesions, and 3 patients with category 1. Of the 10 patients receiving sonography, 7 showed ACR category 4 lesions, 1 ACR category 2, and 2 showed no significant findings.
MR Imaging Findings
Among the 11 patients, MRI identified 7 mass lesions, 2 focus lesions, and 2 multiple foci. One patient had bilateral focal fibrocystic change with one lesion in the left breast and two lesions in the right breast (Figure 1). The lesion size of focal FCC ranged from 4mm to 12mm (mean 6.7mm, 4 were 4mm, 2 were 6mm, 3 were 8mm, 1 was 1cm, and 1 was 1.2cm) in the 11 patients. Nine patients showed smooth lesion margins and 2 showed speculated margins. Kinetic enhancement curves were measured in 13 lesions of the 11 patients (a patient with bilateral focal fibrocystic change and another with multiple lesions each had two measurements taken). Eight patients (62%) showed malignant enhancement kinetics, including rapid up-slope followed by washout (N=6) (Figure 2 & Figure 3) or reaching plateau (N=2). The other 5 lesions (38%) showed benign enhancement features, slow and continuous enhancement pattern. Except for one lesion showing heterogeneous enhancement, all other lesions all showed homogeneous enhancement. The enhancement ratios of these lesions ranged from 120% to 350% (232% ± 75%). Morphologically, 3 patients were suspected as malignancy. Using kinetic enhancement curve, 8 of 13 lesions were suspected to be malignant. Overall, 9 patients were suspected for malignancy using either criterion. Table 1 showed summary of imaging findings in the 11 patients with focal FCC of the breast. Table 2 showed ACR BI-RADS lesion classifications in different imaging modalities, including mammography, sonography, and MRI, and the surgical procedures. Table 3 showed the diagnostic performance of the three imaging modalities for focal FCC. Using mammography, 6 of the 11 patients (55%) were wrongly diagnosed as malignancy. Breast sonography suspected malignancy in 7 patients (7/10, 70%) and breast MRI, with the highest false positive rate, suspected malignancy in 9 patients (9/11, 82%). However, no statistically significant difference was found in the three diagnostic methods (P > 0.05).
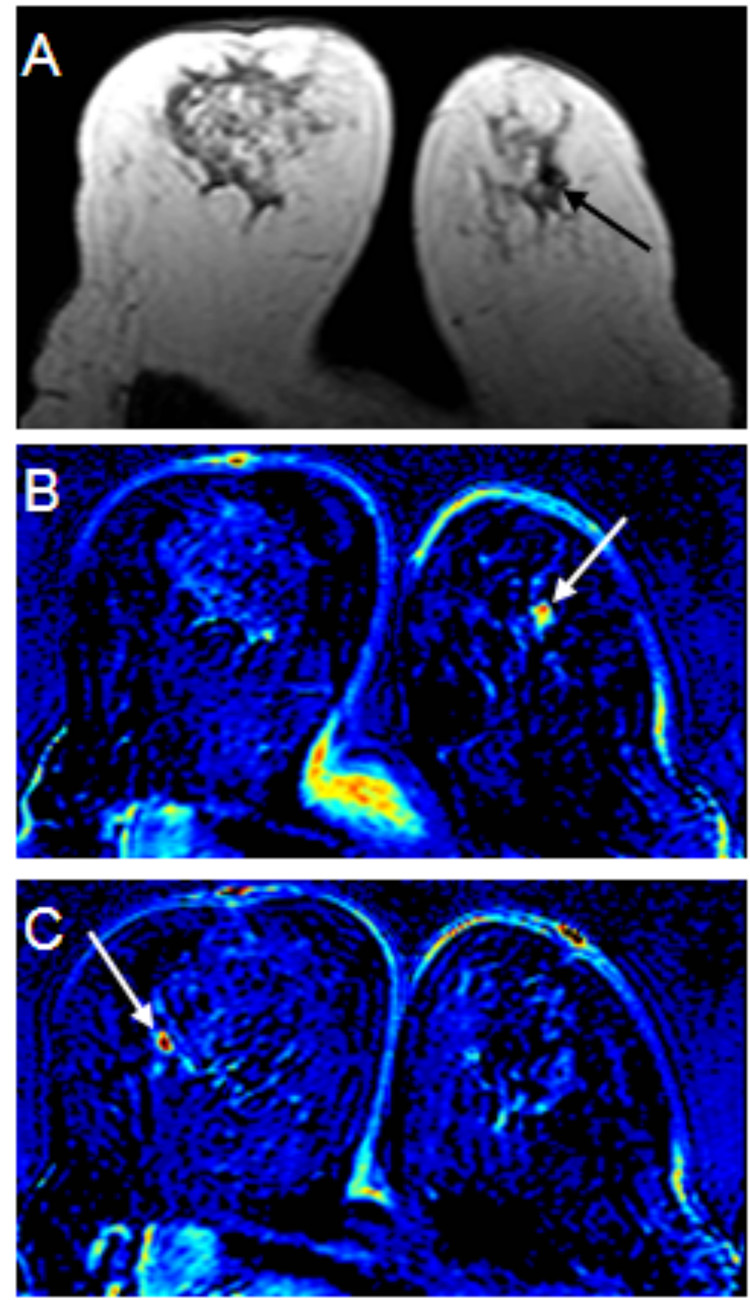
Figure 1.
49 year-old woman with focal fibrocystic change in the bilateral breasts. Sonography showed small hypoechoic lesions in the bilateral breasts. Core biopsy of these lesions (one in the left and two in the right breast) showed fibrocystic change. No malignancy was found. The patient’s pre-contrast T1 weighted axial image (figure 1A) shows a small hypointense area suspicious of a lesion in the left breast (black arrow). A post-contrast subtracted color coded images (figure 1B & 1C) demonstrate small enhanced lesions in the both breasts (white arrows). The enhancement kinetic curve of the small lesion in the left breast reveals rapid up-slope followed by washout (not shown). The right breast lesion, however, shows benign enhancement features (not shown). The patient then received lumpectomy for the left breast lesion after sonography-guided wire localization. Pathologic examination showed fibrocystic change with stromal fibrosis and diffuse sclerosing adenosis.
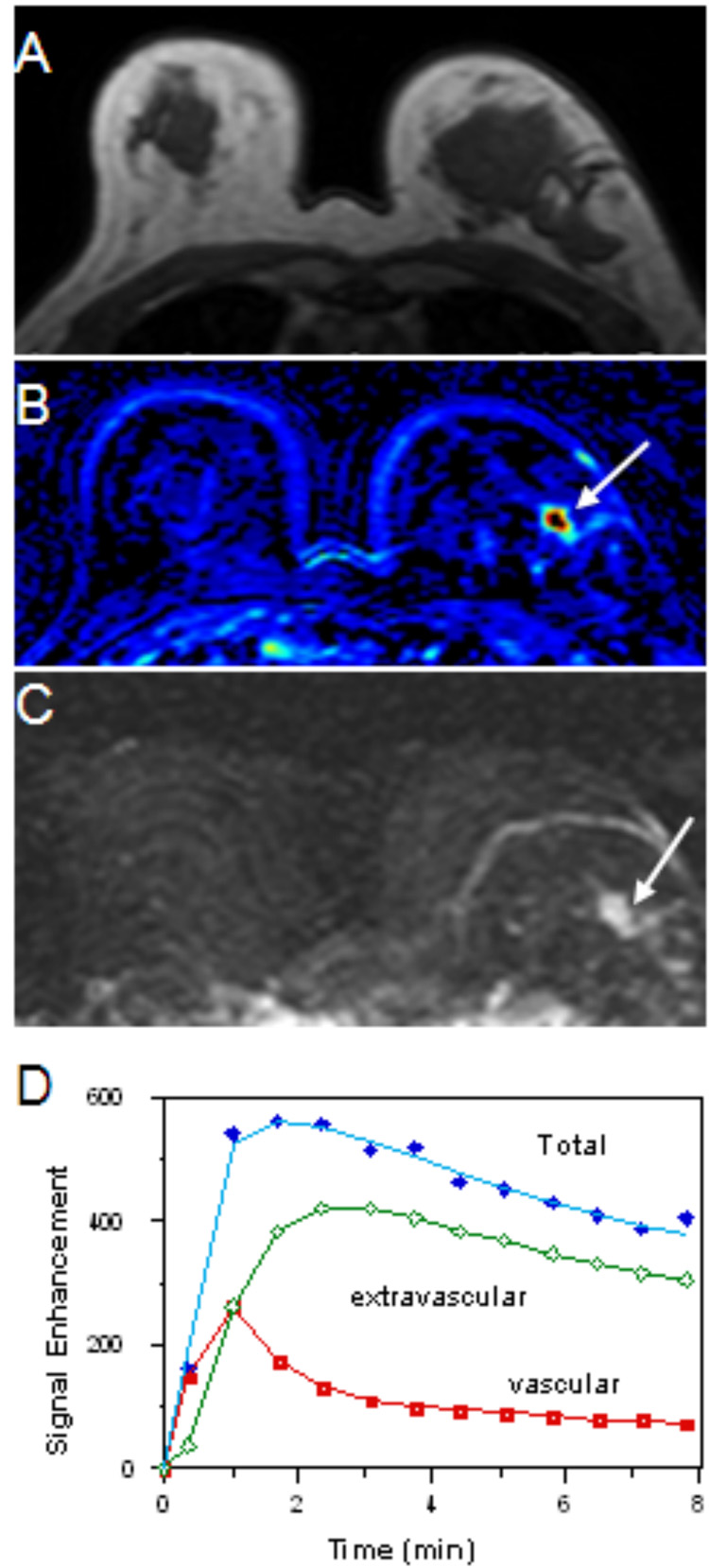
Figure 2.
74 year-old woman with focal fibrocystic change of the left breast. Sonography of the left breast showed a 1.2cm hypoechoic lesion in the left breast. Core biopsy of the lesion showed fibrocystic change. Pre-contrast T1weighted axial image (figure 2A) does not show any suspicious lesion in either breast. The patient’s post-contrast subtracted color-coded image (figure 2B) demonstrates a strongly enhanced lesion in the left breast (white arrow). A maximal intensity projection (MIP) image (figure 2C) clearly depicts the lesion (white arrow). The enhancement kinetic curve of the lesion shows a rapid up-slope followed by washout (figure 2D), a feature suspicious for malignancy. Note that using a 2-compartmental pharmacokinetic model for measuring enhancement kinetics, the summation of the vascular and extravascular components at each time point measured was regarded as total tumor enhancement. The patient received lumpectomy after sonography-guided wire localization. Pathologic examination showed fibrocystic change with prominent stromal fibrosis, ductal hyperplasia, and a few foci of atypical ductal hyperplasia.
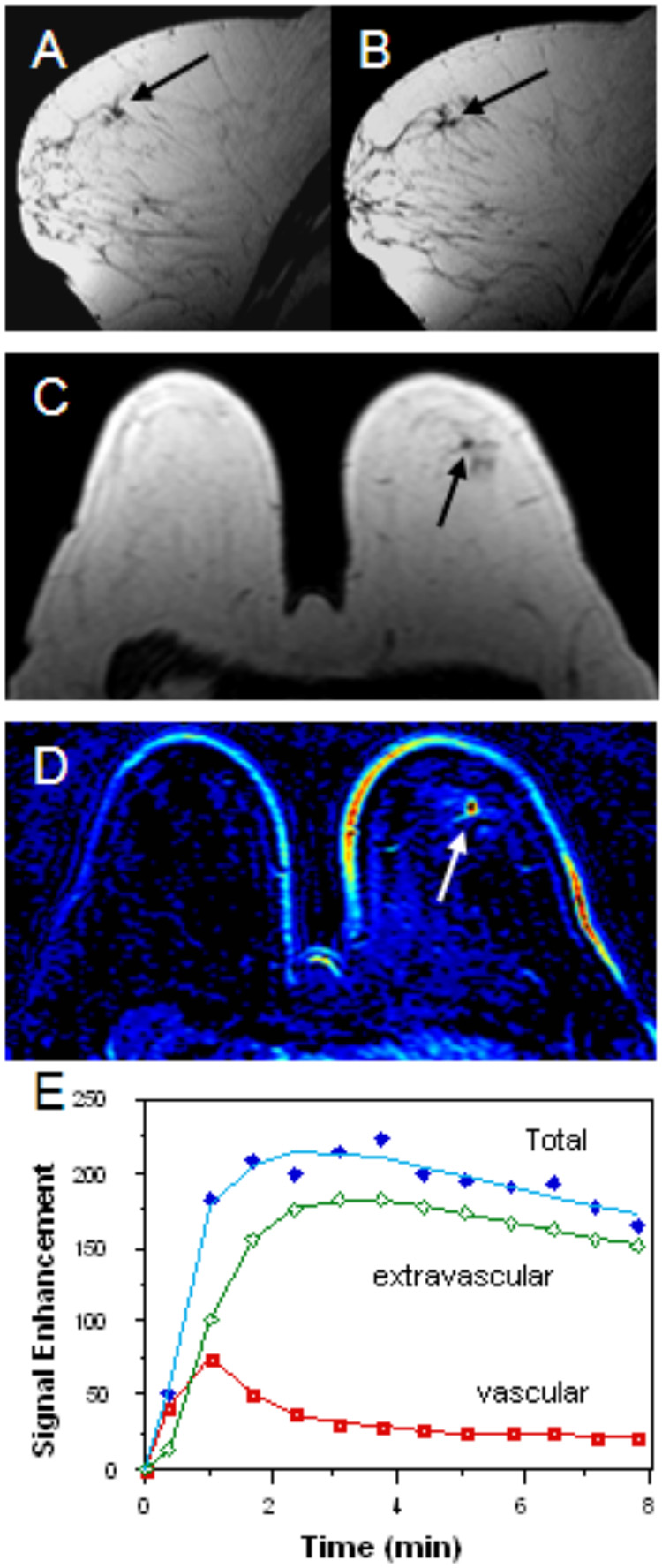
Figure 3.
61 year-old woman with focal fibrocystic change of the left breast. Mammography of the left breast showed a small mass lesion with speculated margin. Sonography of the left breast showed a 6mm hypoechoic lesion in the left breast. Pre-contrast T1 weighted sagittal images (figure 3A & 3B) shows a small speculated hypointense area suspicious of a lesion in the left breast (black arrows). Pre-contrast T1 weighted axial images (figure 3C) also showed a small hypointense lesion (black arrow). The patient’s post-contrast subtracted color coded image (figure 3D) demonstrates an enhanced lesion in the same location (white arrow). An enhancement kinetic curve of the lesion reveals rapid up-slope followed by washout (figure 3E). Pathologic examination showed focal area of fibrocystic change with stromal fibrosis, cyst formation, mild ductal hyperplasia and intraductal pappilomatosis.
Table 1.
Summary of Imaging Findings in the 11 Patients with Focal Fibrocystic Change of the Breast
| Age | Mammography | Sonography | MRI morphology | MRI kinetics |
|---|---|---|---|---|
| 49 | 2×3 cm mass & 9mm nodular opacity | R: 6mm & 7mm hypoechoic nodules; L: 8×7mm hypoechoic nodule |
LT: one focus RT: two foci |
LT: type 1a RT: type 2 |
| 61 | mass with spiculated margins | hypoechoic mass | 6mm speculated mass | Type 1a |
| 49 | NSF | 8mm hypoechoic lesion | 8mm mass | Type 1b |
| 46 | 1cm masses | 6mm hypoechotic cyst; 3mm cyst |
6mm speculated mass | Type 1b |
| 59 | 4mm opacity | 4mm hypoechoic lesion | 4mm focus | Type 1a |
| 74 | 4.5cm branching calcification | 1.2 cm mass, hypoechoic |
1.2cm mass | Type 1a |
| 39 | 1.5cm and 5mm masses | 7×6×8mm hypoechoic mass | Three foci | Type 2 |
| 39 | NSF | 8mm hypoechoic nodule | 8mm mass | Type 2 |
| 47 | NSF | NSF | 8mm | Type 1a |
| 64 | Benign calcification | NSF | 1cm mass | Type 2 |
| 60 | Ill-defined density | N/A | 4mm focus | Type 1a |
NSF: no significant findings; N/A: not available or not performed; Kinetics: type 1a, rapid up-slope followed by washout; type; 1b, rapid up-slope and reaching the plateau; type 2, moderate or slow up-slope with continuous enhancement
Table 2.
ACR BIRADS Lesion Classifications in Different Imaging Modalities & Surgery
| Age | Mammo. BIRADS | Sonography BIRADS | MRI BIRADS | Lesion laterality | Final Surgical Procedure |
|---|---|---|---|---|---|
| 49 | ACR 4 | ACR 4 | ACR 4 | Bilateral FCC | Lt: Lumpectomy Rt: Core biopsy only |
| 61 | ACR 4 | ACR 4 | ACR 4 | Lt FCC | Lumpectomy |
| 49 | ACR 1 | ACR 4 | ACR 4 | Rt FCC | Core biopsy only |
| 46 | ACR 4 | ACR 2 | ACR 4 | Rt FCC | Core biopsy only |
| 59 | ACR 4 | ACR 4 | ACR 4 | Rt FCC | Core biopsy only |
| 74 | ACR 2 | ACR 4 | ACR 4 | Lt FCC | Lumpectomy |
| 39 | ACR 4 | ACR 4 | ACR 3 | Lt FCC Rt IDC |
Bil: Mastectomy |
| 39 | ACR 1 | ACR 4 | ACR 4 | Lt FCC Rt IDC |
Lt: Core biopsy only Rt: Lumpectomy |
| 47 | ACR 1 | ACR 1 | ACR 4 | Lt FCC Rt IDC |
Bil: Mastectomy |
| 64 | ACR 2 | ACR 1 | ACR 3 | Rt FCC Lt IDC |
Rt: Mastectomy Lt: Lumpectomy |
| 60 | ACR 4 | N/R | ACR 4 | Rt FCC Lt: IDC + ILC |
Bil: Mastectomy |
Table 3.
Diagnostic Performance of Mammography, Sonography, and MRI for Focal Fibrocystic Change of the Breast
| Mammography (N=11) | Sonography (N=10) | MRI (N=11) | |
|---|---|---|---|
| ACR BI-RADS 1–3 (correct diagnosis) | 5/11 (45%) | 3/10 (30%) | 2/11 (18%) |
| ACR BI-RADS 4–5 (over-diagnosis) | 6/11 (55%) | 7/10 (70%) | 9/11 (82%) |
No significant difference in the correct diagnosis of focal FCC between mammography and MRI (P = 0.36), and between sonography and MRI (P = 0.64). Note: MRI diagnosis was based on the combination of both morphological and kinetic features.
Surgical Procedures
Of the 12 lesions of focal FCC in 11 patients, 4 received mastectomy, 3 received lumpectomy, and 5 received core needle biopsy. For the 5 patients undergoing biopsy, clips were placed in the biopsy sites after the procedures under sonography guidance. Three lesions underwent lumpectomy due to the concerns of suspicious malignant enhancement kinetic pattern of early wash-in and late washout (N=3), tumor size > 5mm (N=2), strong enhancement (N=1), speculated margin (N=1), irregular margin (N=1), and co-existence of atypical ductal hyperplasia (N=1). In 5 patients with contra-lateral breast cancer, in addition to the surgical removal of the tumors (3 mastectomies and 2 lumpectomies), 4 of the 5 patients also received mastectomy of the breast affected by focal FCC. These 4 patients consisted of 2 patients due to suspicious enhancement kinetics by MRI (one also with suspicious malignancy by mammography) (Figure 4), 1 patient of multiple enhanced lesions diagnosed as ACR category 3 lesion but both mammography and sonography showed category 4, and 1 patient due to pathology of a previous biopsy showing focal FCC mixed with atypical lobular hyperplasia. Five breast sides, not receiving surgical resection of the FCC after biopsy, were followed up by the outpatient department. Two of these 5 lesions had follow-up breast MRI studies and showed complete disappearance of the enhanced lesion noted in the previous MRI, hence proving the correct localization and biopsy of the suspicious lesions. Another three lesions had follow-up mammography which showed no definite abnormality.
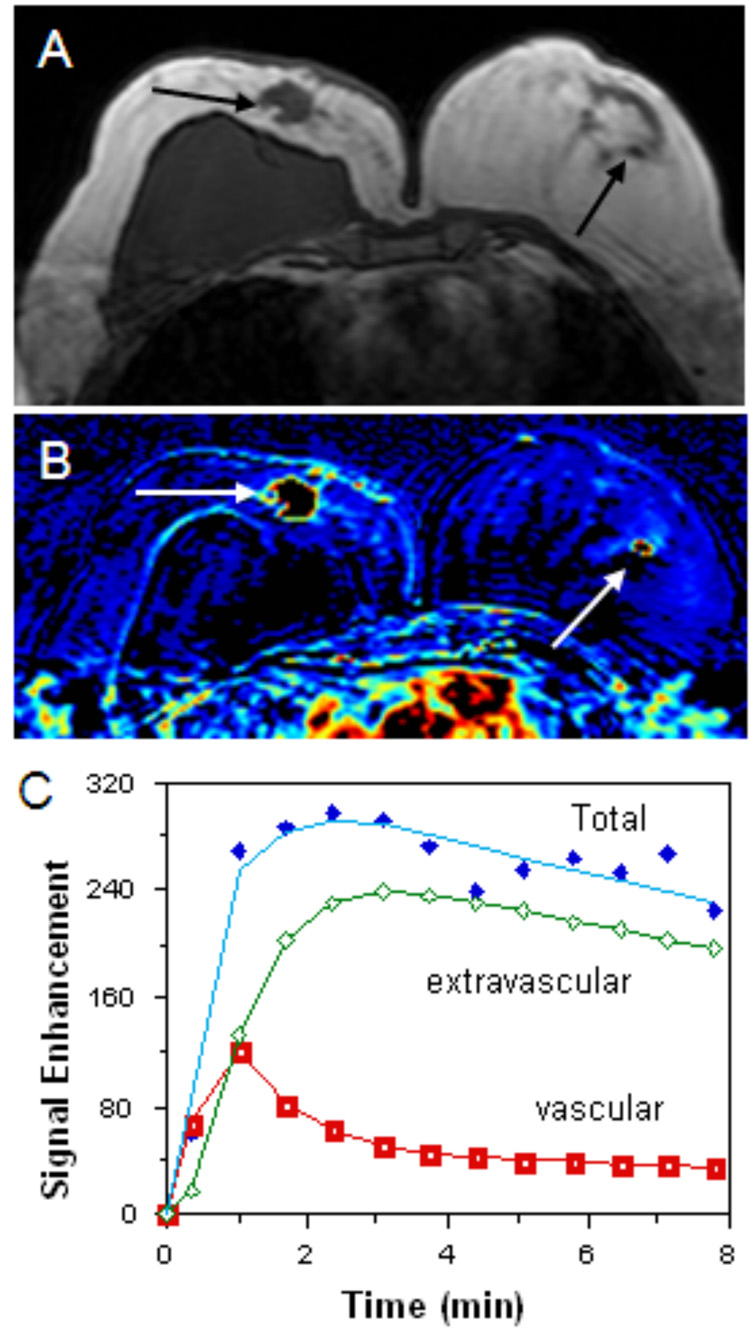
Figure 4.
47 year-old woman with invasive ductal carcinoma in the right breast and focal fibrocystic change in the left breast. Sonography and mammography did not show any suspicious lesions in the left breast. A pre-contrast T1 weighted axial image (figure 4A) shows a suspicious large hypointense lesion in the right breast (black arrow) and a tiny hypointense area in the left breast (black arrow). The patient’s post-contrast subtracted color coded image (figure 4B) demonstrates enhanced lesions in the same locations well (white arrows). An enhancement kinetic curve of the small lesion in the left breast reveals rapid up-slope followed by washout (figure 4C), a feature suspicious for malignancy. Subsequent bilateral mastectomy showed invasive ductal carcinoma in the right breast. Small focal area of fibrocystic change in the left breast was noted but no malignancy was identified.
Pathological Diagnosis
When reviewing the removed breast specimen, the pathologist was aware of the location of the suspected lesion detected by MRI and slice-by-slice examination of the mastectomy specimen was performed. Three patients received lumpectomy of the focal FCC after wire localization of the lesion with sonography. Before the sonography-guided wire localization procedure, the breast radiologist had reviewed the MRI report, checked the location of the enhanced lesion, and correlated it with the sonographic finding. In pathology, all 12 lesions of focal FCC in 11 patients showed the typical pathological features of fibrocystic change, with mixed components of stromal fibrosis, cyst formation, apocrine metaplasia, adenosis, and/or focal sclerosing adenosis. One patient showed a pathology feature of diffuse sclerosing adenosis, 4 patients showed concomitant ductal hyperplasia (DH), 2 showed atypical DH, 1 showed atypical LH, and 2 intra-ductal pappilomatosis.
DISCUSSION
Although most of the FCC patients show typical clinical symptoms and require no further work-up, some patients may need imaging studies when suspicion arises in the nature of the palpated lump. Mammography cannot reliably diagnose FCC due to wide variation of imaging findings. Using mammography, FCC might mimic breast cancer when it is presented as a discrete mass or density. The sonographic features of FCC are also not specific enough to differentiate FCC from other breast lesions. Shetty et al. studied 60 pathologically-proven focal FCC and found that a significant number 46.6% presented as solid masses, 15% presented as heterogeneously echogenic lesion, and 13.3% presented as cysts (10). About half (46.4%) of the masses were classified as sonographically indeterminate and 50% of the masses were classified sonographically benign.
Although MRI has been proved to be very useful for detecting small, multiple or obscured breast lesions with high sensitivity when mammography and sonography cannot make the definite diagnosis, the drawback of this imaging modality is its low specificity. Both benign and malignant breast lesions can share common morphological and kinetic features. Since MRI is increasingly used to screen young high risk women, diagnostic ambiguity will become more common due to the high incidence of fibrocystic breast seen in this age group. A variety of benign breast conditions may mimic a malignant lesion, which decreases the specificity of breast MRI. They included benign proliferative breast disease, fibroadenoma, intraductal papilloma, granular cell tumor, pseudoangiomatous stromal hyperplasia, fat necrosis, mastitis, inflammatory granuloma, epidermal inclusion cyst, and benign intra-mammary lymph node (11). When suspicion occurs, careful correlation of MR imaging findings with sonography and mammography is needed. Detailed physical examination of the suspected breast and trace-back of the patient’s past history are also important to exclude some benign lesions. These strategies will definitely help to decrease the unnecessary biopsy rate.
Focal FCC is occasionally encountered in the daily clinical practice, yet its MR imaging features are not well known. In this study, MR imaging features in 11 patients with focal FCC were analyzed. Although the patient number was small, they were recruited from a breast MRI database of over 600 patients in a 4-year period. Therefore, we believe large scale studies focusing on MR imaging of focal FCC are difficult to obtain. Understanding MR imaging features of this benign condition is very important to exclude malignancy, avoid unnecessary biopsy, and decrease patients' anxiety. Studies specifically reporting MR imaging of fibrocystic breast are very few and the case numbers are limited. The fibrocystic breast has a wide spectrum of morphologic and kinetic features on MRI. Maurice et al. reported that it most often presents as a mass or as a non-mass like regional enhancing lesion with benign enhancement kinetics (6). In their study, 6 of 14 patients presented a mass (N=5) or focus (N=1) lesion. Morphologically, two of these 6 patients were suspected to have malignancies due to irregular tumor margins. Using enhancement kinetics criteria, 3 of these 6 patients were suspected to have a malignancy due to their presentation as initial rapid-upslope and late-phase plateau. With either criterion, 5 patients in total were suspected for malignancy. In our study, we focused on MR imaging of focal FCC only. Morphologically, 3 patients were suspected as malignancy. Using kinetic enhancement curve, 8 of 13 lesions were suspected to be malignant. Overall, 9 patients were suspected for malignancy using either criterion. From Maurice et al and our study, morphological criteria have less false positive diagnoses of malignant lesions than of kinetic criteria. This finding was comparable to the study of Goto et al., which concluded that the sensitivity and specificity of the morphologic criteria were significantly higher than those of the enhancement patterns based on a study of 144 malignant and 60 benign breast lesions (12). When compared with mammography and sonography, breast MRI had even worse diagnostic performance (55%, 70%, 82% false positive rate, respectively). This fact further supports the unreliability of using the pattern of enhancement kinetics in diagnosing breast malignancy.
All 11 patients in our study had focal FCC smaller than 1.5cm. Liberman L et al. studied how the lesion size impacted on the positive predictive value (PPV) of biopsy in MRI-detected breast lesions. It was found that the frequency of malignancy increased significantly with lesion size. Lesions less than 5 mm in size had a 3% chance of being malignant. Lesions 5–9 mm and lesions 10–14 mm had respective malignancy rates of 17% and 25% (13). They therefore concluded that biopsy is rarely necessary for lesions smaller than 5 mm because of their low (3%) likelihood of cancer.
Five of our patients had concomitant contra-lateral malignancy leading to 4 mastectomies of the focal FCC in the affected breast. Yabuuchi H et al. analyzed the incidentally detected lesions on DCE-MR imaging in patients for breast-conserving therapy. They found 48 incidental lesions in 299 patients prepared for breast-conserving therapy. Of these 48 lesions, 25 lesions were proved to be benign and 23 were malignant. It was found that lesions larger than 10 mm tended to be malignant, and lesions equal or smaller than 5 mm tended to be benign. Lesions with early enhancement peak tended to be malignant, whereas those with persistent enhancement tended to be benign (14). Using their criteria, 3 of the 5 patients of focal FCC with concomitant cancer in our study were diagnosed as having malignancies. A recently published multiple-center study using MRI in evaluation of 969 women diagnosed with breast cancer showed that MRI detected 3.1% (30/969) of clinically and mammographically occult breast cancer in the contralateral breast. Although the negative predictive value of MRI was 99%, the estimated positive predictive value of a positive MRI examination was only 21%. A biopsy was performed on the basis of a positive MRI finding in 121 women, and only 30 of whom had specimens that were positive for cancer (24.8%). A total of 91 of 969 women (75.2%) with a positive MRI finding underwent a biopsy that detected a benign lesion (15).
Fibrocystic change encompasses a group of histological changes which are characterized by various combinations of cysts, fibrous overgrowth, and epithelial proliferation. Some of these changes are entirely innocuous while others are associated with increased risk of subsequent carcinoma. Two of our patients showed atypical ductal hyperplasia and one showed atypical lobular hyperplasia in the focal FCC lesion. Dupont et al. have studied the relationship between fibrocystic change and the relative risk of developing subsequent invasive carcinoma (16). It was stated that FCC will not have increased risk for cancer if only cyst, apocrine metaplasia, sclerosing adenosis, fibrosis and mild hyperplasia (more than 2 but less than 4 cells thick) exist. There was slightly increased risk (1.5 to 2X) if moderate or florid hyperplasia was found. There was moderately increased risk (5X) if atypical hyperplasia (AH), regardless of ductal or lobular, was found in the FCC.
Several limitations existed in our study. 1) We defined focal FCC as a discrete lesion found in MR imaging. Therefore it was different from that which was usually defined from a clinician's perspective as “a condition in which there are palpable lumps in the breast.” This formed a selection bias of our study. 2) Most FCC patients do not come for MRI studies. Hence our study cannot reflect the spectra of MR imaging features for FCC. 3) The spatial resolution of MRI protocol used in this study was suboptimal; which needs to be improved to enhance diagnostic accuracy of small tumor foci. This imaging protocol was set in year 2002. The relatively low spatial resolution was selected to cover the whole breast, also to reach a temporal resolution of 42 sec, which was critical in analysis of enhancement kinetics. 4) Subtraction was used to obtain enhancement maps, which was susceptible to patient motion. We have moved the study to a 3.0T scanner, and our current protocol has improved spatial resolution, as well as fat-suppression. Post-contrast enhanced images can be directly evaluated without subtraction; thus less prone to the motion problem. 5) One major difficulty of breast MRI is to spatially correlate MRI findings with pathological findings. Although MRI-guided biopsy has become available, it is much more complicated compared to mammogram or ultrasound guided biopsy; thus was not used in this study.
In conclusion, from our small number of patients studied, it was found that more than half (62%) of focal FCC showed rapid enhancement and washout kinetics, which mimic a malignant breast lesion and usually lead to unnecessary operation, especially in patients with contra-lateral malignant breast cancer. Compared with mammography and sonography, breast MRI had even worse diagnostic performance due to high frequency of the malignant enhancement kinetics pattern happening in focal FCC and was mistaken as a malignant breast lesion.
Acknowledgement
This study was supported in part by NIH/NCI R01 CA90437, CA121568 and California Breast Cancer Research Program # 9WB-0020.
REFERENCES
- 1.Wu C, Ray RM, Lin MG, et al. A case- control study of risk factors for fibrocystic breast conditions. Am J Epidemiol. 2004;160:945–960. doi: 10.1093/aje/kwh318. [DOI] [PubMed] [Google Scholar]
- 2.Love SM, Gelman RS, Silen W. Fibrocystic “disease” of the breast—a nondisease? N Engl J Med. 1982;307:1010–1014. doi: 10.1056/NEJM198210143071611. [DOI] [PubMed] [Google Scholar]
- 3.DiPiro PJ, Gulizia JA, Lester SC, et al. Mammography and sonographic appearances of nodular adenosis. AJR Am J Roentgenol. 2000;175:31–34. doi: 10.2214/ajr.175.1.1750031. [DOI] [PubMed] [Google Scholar]
- 4.Harvey SC, Denison CM, Lester SC, et al. Fibrous nodules found at large-core needle biopsy of the breast: imaging features. Radiology. 1999;211:535–540. doi: 10.1148/radiology.211.2.r99ma42535. [DOI] [PubMed] [Google Scholar]
- 5.Warner JK, Kumar D, Berg WA. Apocrine metaplasia: mammography and sonographic appearances. AJR Am J Roentgenol. 1998;170:1375–1379. doi: 10.2214/ajr.170.5.9574619. [DOI] [PubMed] [Google Scholar]
- 6.Maurice AAJ, van den Bosch, et al. Magnetic resonance imaging characteristics fibrocystic change of the breast. Investigative Radiology. 2005 July;Volume 40(7):436–441. doi: 10.1097/01.rli.0000167123.26334.c8. [DOI] [PubMed] [Google Scholar]
- 7.BI-RADS® – MRI. American College of Radiology (ACR) Breast Imaging Reporting and Data System Atlas (BI-RADS® Atlas) First Edition. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 8.Su MY, Yu HJ, Carpenter PM, McLaren CE, Nalcioglu O. Pharmacokinetic parameters analyzed from MR contrast enhancement kinetics of multiple malignant and benign breast lesions detected in the same patients. Technol Cancer Res Treat. 2005;4(3):255–263. doi: 10.1177/153303460500400305. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser WA, Zeitler E. MR imaging of the breast: fast imaging sequences with and without Gd-DTPA. Preliminary observations. Radiology. 1989 Mar;170(3 Pt 1):681–686. doi: 10.1148/radiology.170.3.2916021. [DOI] [PubMed] [Google Scholar]
- 10.Shetty MK, Shah YP. Sonographic findings in focal fibrocystic changes of the breast. Ultrasound Q. 2002 Mar;18(1):35–40. doi: 10.1097/00013644-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Iglesias A, Arias M, Santiago P, Rodríguez M, Mañas J, Saborido C. Benign breast lesions that simulate malignancy: magnetic resonance imaging with radiologic-pathologic correlation. Curr Probl Diagn Radiol. 2007 Mar–Apr;36(2):66–82. doi: 10.1067/j.cpradiol.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Goto M, Ito H, Akazawa K, et al. Diagnosis of breast tumors by contrast-enhanced MR imaging: comparison between the diagnostic performance of dynamic enhancement patterns and morphologic features. J Magn Reson Imaging. 2007 Jan;25(1):104–112. doi: 10.1002/jmri.20812. [DOI] [PubMed] [Google Scholar]
- 13.Liberman L, Mason G, Morris EA, Dershaw DD. Does size matter? Positive predictive value of MRI-detected breast lesions as a function of lesion size. Am J Roentgenol. 2006 Feb;186(2):426–430. doi: 10.2214/AJR.04.1707. [DOI] [PubMed] [Google Scholar]
- 14.Yabuuchi H, Kuroiwa T, Kusumoto C, Fukuya T, Ohno S, Hachitanda Y. Incidentally detected lesions on contrast-enhanced MR imaging in candidates for breast-conserving therapy: correlation between MR findings and histological diagnosis. J Magn Reson Imaging. 2006 Apr;23(4):486–492. doi: 10.1002/jmri.20532. [DOI] [PubMed] [Google Scholar]
- 15.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007 Mar 29;356(13):1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 16.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985 Jan 17;312(3):146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]