Abstract
Cell based therapies hold promise of repairing an injured heart, and the description of stem and progenitor cells with cardiomyogenic potential is critical to its realization. At the vanguard of these efforts are analyses of embryonic stem cells, which clearly have the capacity to generate large numbers of cardiomyocytes in vitro. Through the use of this model system, a number of signaling mechanisms have been worked out that describes at least partially the process of cardiopoiesis. Studies on adult stem and on progenitor cells with cardiomyogenic potential are still in their infancy, and much less is known about the molecular signals that are required to induce the differentiation to cardiomyocytes. It is also unclear whether the pathways are similar or different between embryonic and adult cell-induced cardiomyogenesis, partly because of the continued controversies that surround the stem cell theory of cardiac self-renewal. Irrespective of any perceived or actual limitations, the study of stem and progenitor cells has provided important insights into the process of cardiomyogenesis, and it is likely that future research in this area will turn the promise of repairing an injured heart into a reality.
Keywords: Embryonic Stem Cells, Adult Stem Cells, Cardiomyocytes, Cardiomyogenesis, Plasticity
1. Introduction
Cardiac transplantation is currently the treatment of choice for end-stage heart failure, but it is hampered by a severe shortage of donor organs and by the potential for organ rejection. Cell replacement therapy represents one promising option for myocardial repair, but this approach is currently limited by the availability of transplantable human cardiomyocytes (CMs). As a result, transplantation of non-CMs, such as skeletal muscle myoblasts and smooth muscle cells, has been proposed as alternative therapies [1–5]. This approach is however complicated by the fact that transplanted cells, which are not CMs, may have abnormal electrical coupling that could promote either conduction blocks or arrhythmias in vivo. Thus, one goal of cardiac cellular transplantation has been to find a renewable source of cardiac cells that can be used safely in human hearts.
Until a few years ago, stem cell-based therapeutics were limited to cells of bone marrow (hematopoietic and mesenchymal stem cells) and epidermal (skin) origin, but since 1999, numerous studies have described cells with cardiomyogenic potential, including some derived from the embryo (pre-implantation embryo, embryonic germ cells)(see [6,7] for references), heart [embryonic (Isl-1+), neonatal, adult (c-Kit+ or Sca-1+)] [8–12], adult bone marrow (hematopoietic and mesenchymal stem cells [13–15], multipotent adult progenitor cells [16], side population (SP) cells [17,18], cytokine mobilized cells [19], endothelial circulating progenitor cells [20], skeletal myoblasts [5,21,22], fat [23], and testes [24](also see revs. [3,25,26] for additional references). While many of these findings suggest new cell sources for the treatment of heart failure, each has its own set of limitations, and importantly for this review, no homogeneous primary cell isolate has yet shown regenerative potential in heart equivalent to the long-term repopulating hematopoietic stem cells identified from bone marrow [27–29].
The aim of this review is thus to describe current knowledge of some stem and progenitor cells with cardiomyogenic potential and discuss potential pathways that are implicated in the induction and/or differentiation of CMs. Because this field of investigation is still in its infancy, many challenges remain to be overcome, ranging from the identification and expansion of uni-, multi- or pluri-potent cells to the therapeutic application of these cells or cell derivatives in man. Since an on-going debate continues in the cardiovascular field as to whether some of the putative stem or progenitor cells with reported “cardiomyogenic potential” are genuine and whether these cells generate functional cardiac myocytes that can integrate with endogenous CMs, we have chosen to employ a very conservative definition of stem cells, one that requires an isolated cell to have not only the ability to self-renew or spontaneously differentiate into CMs in vitro and in vivo, but also one where the cells have an identifiable in vivo counterpart (i.e., resident). Moreover, we suggest that this definition is essential if we are to exploit the potential of resident cardiomyogenic stem and progenitor cells, which we believe will have the greatest therapeutic potential, as opposed to those stem cells that are artifacts of either culture (epigenetic reprogramming, fusion) or gene reprogramming (nuclear transfer or retrovirus-mediated).
2. What are Stem and Progenitor Cells?
In its simplest form, a mammalian stem cell is a special type of cell that retains the ability to self-renew (i.e., undergo cell division in an undifferentiated state) indefinitely and to differentiate into specialized cells of an embryo or adult. Differentiation is a process that involves undifferentiated cells progressing into specialized cells with restricted developmental potential. The differentiation capacity of these specialized cells in vivo to form mature cell types (i.e., the developmental potential) ultimately depends on the state of commitment of the cell and both intrinsic factors and the extra-cellular environment (niche).
Three classes of stem cells have been classified i.e., pluripotent embryonic (blastocyst or epiblast derived), adult (tissue-specific or cord blood) and cancer stem cells. Among the three, embryonic stem (ES) cells display the broadest developmental potential [7,30]. These pluripotent cells can differentiate into all cells of an embryo proper, and if implanted in utero following tetraploid aggregation, an entire ES cell derived embryo, excluding some extra-embryonic tissues, can be formed [31–33]. ES cells can also be used for the generation of chimeric animals, in which the ES cell genotype can be passed through the germline. Another pluripotent cell type includes germ cells, progenitor cells of the germ line, which also have multilineage differentiation capacity [7]. In contrast, adult stem cells possess a more restricted developmental potential and are generally considered to be multipotent to unipotent. They typically produce only cells of a closely related family, providing “new” cells in order to replenish damaged specialized cells in the adult. Both types of stem cells are capable of cell division in the undifferentiated state (self-renewal), and although ES cells readily form tumors when implanted outside the blastocyst and adult stem cells (e.g., hematopoietic, mesenchymal, neuronal) may or may not form tumors, these stem cells are unique from cancer stem cells. Cancer stem cells are by definition oncogenic, have lost the ability to prevent uncontrolled proliferation or differentiation, and are therapeutically unviable. Therapeutically viable stem cells are therefore normal units of development and tissue regeneration (for rev. see [7]). Finally, stem cells are unique from progenitor cells. Progenitor cells are products of a stem cell that generally have limited self-renewal capacity, but a very high proliferation capacity. This latter property permits the production of a large number of differentiated progeny in a relatively short period of time. The developmental potential of the progeny also tends to be restricted to a single cell lineage. Because of the tremendous capacity to divide, progenitor cell progeny may have important therapeutic potentials in an acute phase, as they would not be expected to take up long-term residence or have the potential to form tumors, like embryonic and some adult stem cells.
3. Stem Cells with Cardiomyogenic Potential
During the past few years, numerous pre-clinical studies have demonstrated that a variety of putative stem/progenitor cells can improve the cardiac function after transplantation; but because of the nature of the studies, it often remains unclear whether CM regeneration occurred through the formation of CMs or if any beneficial effects were secondary to angiogenic effects, anti-apoptotic effects, endothelial-to-mesenchymal transitions, cell fusions, or even anti-inflammatory responses [34–39]. The data however support the idea that stem and progenitor cells may indeed form CMs or CM-like cells in vitro and in vivo; however, the formation of CMs from these cells is not the rule, but instead a relatively rare event. In this section, we will review the current state of the literature, and at the end, we will describe both how and why some of the claims remain controversial.
3.1. Embryonic Stem cells and cardiomyogenesis
Pluripotent embryonic stem (ES) cells can give rise to all cells of an embryo-proper. In vitro, ES cells spontaneously form cell aggregates termed embryoid bodies (EBs), which can differentiate into a variety of cell types, including CMs (Figure 1). The hanging drop technique is extremely useful in promoting reproducible aggregation of ES cells to help direct differentiation. This technique specifically permits the formation of aggregates with a uniform size, thus limiting variations in cell numbers that can dramatically affect the developmental outcome. For example, aggregates consisting initially of 200 ES cells and induced with retinoic acid readily form neuronal cells; however, aggregates consisting of 400–800 cells in high fetal bovine serum-containing media readily produce CMs, the process of which has been extensively reviewed elsewhere [6,7].
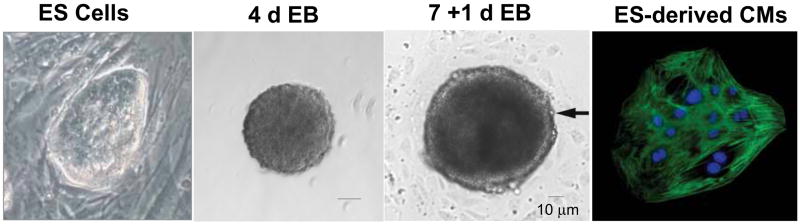
Figure 1.
ES cells readily differentiate to form CMs. A) ES cells, in an undifferentiated state, are usually grown on a layer of feeder cells and form tight colonies consisting of cells with a small cytoplasmic content. B) ES cells spontaneously aggregate to form EBs. At day 2 of aggregation, the developing EB is already spherical in shape, and between days 3–4.5, markers of primitive streak and mesoderm can already be observed. C) On days 5–7, an EB is allowed to attach, and within one day, it is characterized by the formation of an outer layer of primary endoderm cells and epithelialization of the primitive ectoderm. Between these layers, mesoderm-derived CMs are typically seen (see arrow). D) Isolated and purified ES cell derived cardiomyocytes stained with an antibody against cardiac TnT. Some images were adapted from [6].
Using the hanging drop technique, ES cells form small spheres of ~50–100 μm within two days of aggregation. Subsequently, the irregular surface of the aggregates flattens to morphologically resemble morula compaction; and by day 4–5 of aggregation, the EB is composed of an inner epiblast-like and an outer primary endoderm-like structure. The development of mesodermal cells occurs in a restricted area between these two structures (close to the dense structure of the “neuroectodermal rim”), and although unique from development, it is analogous to gastrulation-like development and axis formation, in which the rostro-caudal axis superimposed on the primitive streak of the early mouse gastrula [40].
During in vitro differentiation of ES cells to CMs via EBs, cardiac gene expression is regulated in a developmentally controlled manner [6]. As in early myocardial development, mRNAs encoding GATA4 and Nkx2.5 transcription factors appear prior to mRNAs encoding atrial natriuretic factor (ANF), myosin light chain (MLC)-2v, α-myosin heavy chain (α-MHC), β-myosin heavy chain (β-MHC), Na+-Ca2+ exchanger, and phospholamban. Sarcomeric proteins of ES cell–derived CMs are also established developmentally in the following order: titin (Z disk), α-actinin, myomesin, titin (M band), MHC, α-actin, cardiac troponin T, and M protein [41]. CMs with characteristics of fetal/neonatal rodent CMs express slow skeletal muscle troponin I isoforms and a greater proportion of β-MHC versus α-MHC, whereas CMs that more rapidly contract preferentially express cardiac troponin I and α-MHC. The process is therefore heterogeneous and cells typical of primary myocardium (early proliferating cardiac cells, pacemaker-like cells, His-, Purkinje-like cells) and working myocardium (atrial and ventricular) are formed [6].
Human ES cells also spontaneously differentiate when mechanically isolated or enzymatically dissociated from feeder layers and cultured as aggregates in suspension. Heterogeneous and cystic EBs spontaneously form under these conditions and express markers of various cell types, including those of neuronal, cardiac or pancreatic lineages (for rev. see [7]). Similarly and analogous to mouse ES cells, no single transcription factor or signaling pathway directs hES cells exclusively into a heart cell, suggesting that multiple signals and/or pathways must be involved in the commitment of pluripotent cells to cardiomyocytes. Importantly and when tested for cell-to-cell functional coupling, ES cell-derived CMs from both human and mouse integrate and functionally couple in adult myocardium [42,43].
From the pioneering work of two laboratories, we also know that ES cells progress to form CMs in vitro through intermediate progenitor cells. Using an ES cell line where the mesodermal lineage marker Brachyury gene was targeted to express GFP, Kattman et al. showed that the Flk1−(vascular endothelial growth factor receptor-2 negative) GFP+ progenitors give rise in culture to two distinct populations of Flk1+GFP+ progenitors [44]. The first corresponds to the hemangioblast, while the secondary Flk1+ cells are capable of generating CMs, vascular smooth muscle cells and endothelial cells. This latter cell population expresses Flk1 prior to cardiac markers, Nkx2.5, Gata4 and Mef2c, and these cells express either Tbx5 or Isl-1, markers of primary and secondary heart fields. Similarly, Wu et al. isolated cardiac progenitors from ES cells expressing Nkx2.5-GFP [45]. Nkx2.5 is expressed subsequent to Flk1, but the results were largely consistent with those described by Kattman et al. Essentially, these Nkx2.5-GFP+ cells were capable of generating clonal colonies with both cardiac and smooth muscle phenotypes, and these cell derivatives expressed modest levels of the stem cell markers c-Kit and Sca-1, but not endothelial cell markers. These findings suggest that endothelial and myogenic lineages may have already segregated by the time that the Nkx2.5 gene is activated. The data from these two studies thus suggest that the mammalian cardiovascular system develops from multipotential progenitors that give rise to cardiac, endothelial and vascular smooth muscle progenitors.
ES cells are definitive stem cells, albeit stem cells that may be an artifact of culture, which involves epigenetic modulation of gene expression [46]. These stem cells can unequivocally contribute to all cell lineages, including the germ line, and when appropriately introduced into the blastocyst, do not readily create tumors. The differentiated products in vitro also functionally couple with endogenous heart cells, suggesting that ES cell-derived CMs meet all criteria necessary for successful cardiac integration [26,42,47]. With that said, these cells are not optimal for therapeutic applications. First, only a few undifferentiated cell introduced in a heart may cause tumor formation; however, recent studies suggest that teratoma formation does not necessarily occur when fewer than 50,000 or 100,000 undifferentiated ES cells are transplanted [48,49]. Second, the cells may be immunologically incompatible with the host, resulting in immune reactions. Third and perhaps most importantly, ES cells undergo chromosomal rearrangements and can suffer from mutation events, particularly with long-term in vitro cultivation [7]. Until these problems can be addressed, these cell derivatives are best suited for proof-of-principle studies for therapeutics and for evaluating the mechanisms and signaling cascades necessary for formation of CMs.
Finally, the group of Yamanaka has recently shown that any adult cell can potentially be reprogrammed to form induced pluripotent stem (iPS) cells in vitro, merely through the use of retroviral mediated expression of four transcription factors (Oct4, Sox2, c-Myc, Klf4) [50,51]. Importantly, cells produced in this manner can differentiate in vitro into cell types of all three germ layers and can contribute to the germline [52]. Takahashi et al. also showed that iPS cells treated with activin A and BMP4 beat and express CM markers like cardiac troponin T, β-MHC, Mef2C and Nkx2.5 [50]. The findings of pluripotency have been re-confirmed in mouse independently by the group of R. Jaenisch and have been both confirmed and expanded in human cells by the group of J. Thompson [53,54]. Thus, all normal cells may have the capacity to be pluripotent or multipotent, and the generation of these cells may overcome many of the limitations (ethical, tumor formation, immunological incompatibility) currently associated with the generation and therapeutic applications of human ES cell lines. Ultimately, iPS cells may not prove apt for therapeutics in man, as long as retroviral sequences remain in the genome, but it is likely that this advance will play a vital role in future cell-based treatments of cardiovascular disorders.
3.2. Adult Stem Cells with Cardiomyogenic potential
3.2.1. Resident Cardiac Stem Cells
Several independent laboratories have reported cardiac stem cells based on the presence of the receptor tyrosine kinase c-Kit, stem cell antigen-1 (Sca-1), or the presence of side population (SP) cells that express multidrug-resistance transporter genes and exclude Hoechst dye (Figure 2). Cardiomyogenic progenitor cells that express the transcription factor Isl-1 have also been described [9]. Proponents of the stem cell theory of cardiac self-renewal postulate that these cells, isolated from cardiac tissues, readily differentiate into CMs. Opponents however generally ascribe these observations to experimental artifacts or cell fusion. Perhaps more importantly, opponents have suggested that some of the putative stem cell-derivatives may arise from de-differentiated adult cardiac myocytes, trans-determination or even trans-differentiation events and are thus not authentic or resident stem cells. The origin of cardiomyogenic stem cells is also open to debate, partly because hematopoietic stem cell markers such as c-Kit and Sca-1 are most prominent outside the heart, suggesting that they may originate elsewhere [55]. Although SP cells have been associated at least partially with hematopoietic stem cell (HSC) populations, others have shown that the HSCs were equally distributed in the non-SP population, suggesting that precaution should be taken with respect to the claim that SP cells represent a stem cell population [56, 57].
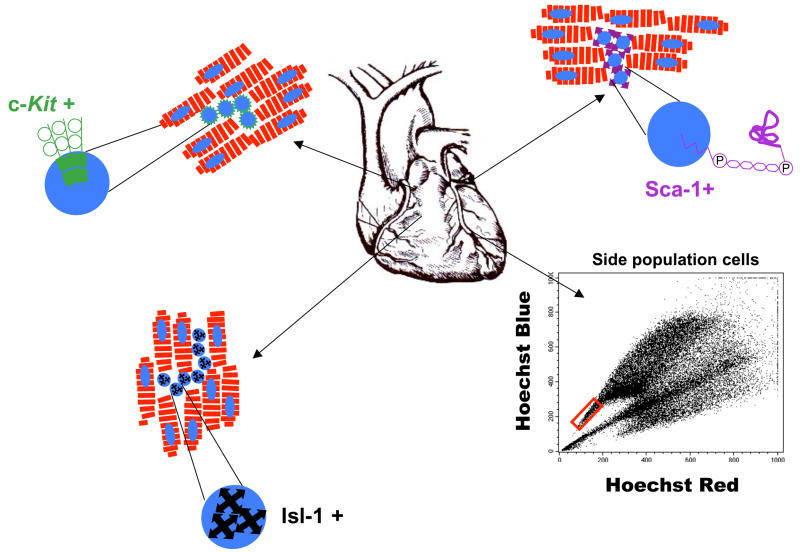
Figure 2.
Illustration of four types of putative resident cardiac stem and progenitor cells. Each of the “cardiac” stem (c-Kit+, Sca-1+ and SP cells) and progenitor (Isl-1+) cells have been described as being rather small and round. Most have been observed in interstitial spaces near to or among CMs, but all have been reported to have cardiomyogenic potential. Side population cells, which are small and identified based on the efflux of Hoechst dye, are indicated in the red box.
3.2.1.1. c-Kit cells
C-kit encodes a type III receptor tyrosine kinase that binds to stem cell factor (SCF), a product of the mouse Sl locus [58]. In the embryo, c-Kit is detectable during early organogenesis and in the lateral plate mesoderm, which gives rise to the primordial heart [59]; however, mutations in c-kit and Sl genes in mouse result primarily in defects in neural crest-derived melanocytes, hematopoietic cells, and primordial germ cells (reviewed in [60]). Beltrami et al. first reported the discovery of a c-Kit+ population of resident stem cells that could be isolated from adult rat heart and expanded in vitro under limiting dilution conditions [10]. These relatively small cells were calculated to be present at only about 1 per 100 CMs and were lineage negative (Lin−) for blood lineage markers (CD34, CD45, CD20, CD45RO and CD8). The c-Kit+ cells were also very heterogeneous, with <10% of the cells expressing Nkx.5, GATA4 and Mef2, and <0.5% of the cells expressing sarcomeric proteins. Under appropriate conditions, these cells could differentiate into CMs, smooth muscle cells and endotheial cells, and were reported to replace/repair the majority of the infarcted tissues [10]. Based on a number of criteria, the authors also indicated that repair occurred independently of cell fusion. A majority of CMs however appeared immature with either limited sarcomeric structures or the presence of stress fibers that are typical of fibroblasts or myofibroblasts. [61–63]. In addition, cultivated c-Kit cells only spontaneously contract when co-cultured with neonatal CMs, suggesting that spontaneous differentiation to CMs does not readily occur.
This pioneering study has now been extended to species other than rat. Human heart contains c-Kit+ cardiac stem cells that are reportedly self-renewing, clonogenic and multipotent. Human c-Kit+ cardiac stem cells have traits similar to those described for mouse and could repair the performance of injured heart when transplanted into immuno-deficient mice. Although direct functional coupling between CMs derived from human c-Kit+ cardiac derived cells and host myocardium was not shown, the authors confirmed that these cells took up residence in human myocardium without cell fusion through the use of a Cre Recombinase-lox system reporter system [64]. This molecular approach is really one of the few that can be used to correctly address this issue; however, questions of strain dependence and the possibility of incomplete penetrance was not considered. Separately and in collaboration with Dr. Anversa’s group, Chen et al. reported that the normal feline heart contains a population of resident c-Kit+ cardiac stem/progenitor cells that, when co-cultured with rat neonatal heart myocytes, differentiate into CMs [63].
In 2004, Messina et al. reported the isolation of undifferentiated cells from subcultures of postnatal atrial or ventricular human biopsy specimens and from murine hearts that grow as self-adherent clusters termed “cardiospheres”. These cells were clonogenic, appeared to have some properties consistent with the previously described adult cardiac stem cells, and phenotypically expressed endothelial (KDR [human]/flk-1 [mouse], CD-31) and stem cell (CD-34, c-Kit, Sca-1) markers. Marban`s group have also generated cardiospheres from human and porcine myocardial biopsy specimens. These human cardiospheres proved highly heterogeneous, and after in vitro expansion, a majority of cells expressed CD105 (endoglin), a marker that is expressed in numerous types of cells. Only subsets of these cells expressed c-Kit, CD34 and CD31 (PECAM-1); whereas most were negative for CD133 (Prominin), the multidrug resistance gene 1 (MDR1) and CD45. Using once again a co-culture system, the cells demonstrated biophysical features characteristic of CMs, and some data argued against cell fusion, even though definitive experiments were lacking. Importantly, the CMs generated with this system could restore some function to the injured heart when transplanted by intramyocardial delivery. Functional integration into the host myocardium was however only demonstrated through expression of connexin-43 [12,65].
One report however highlights some of the controversies surrounding the origin of “resident” c-Kit+ cardiac stem stem. Fazel et al. showed that the number of c-Kit+ cells increased in heart cells after myocardial infarction [66]. Using genetic tagging techniques, they were able to trace the origin of these cells back to bone marrow. This strongly suggested that these were not resident cardiac stem cells. Instead, these cells must represent a subpopulation of bone marrow cells that could be recruited to heart in response to injury. Functionally, c-Kit+ cells recruited from bone marrow established a pro-angiogenic environment in the infarct border zone that potentiated endothelial mitogenesis and facilitated the formation of a myofibroblast-rich zone that fostered tissue repair.
3.2.1.2. Sca-1 Cells
Sca-1 (stem cell antigen-1, Ly-6A/E) is an 18 kDa phosphatidylinositol-anchored protein that is a member of the Ly-6 antigen family. It is one of the most recognized HSC markers in mice, and an anti-Sca-1 antibody is routinely used to identify and isolate murine HSCs from bone marrow. Oh et al. were the first to identify a small number of Sca-1+ cardiac cells that overlapped with a side population of cells from heart. The cardiac Sca-1+ cells lacked blood cell lineage markers (CD4, CD8, B220, Gr-1, Mac-1, and TER119), c-Kit, Flt-1, Flk-1, vascular endothelial-cadherin, von Willebrand factor, and HSC markers CD45 and CD34. Moreover, these cells expressed Gata4, Mef2 and Tef-1, but not Nkx2.5 or genes that encoded cardiac sarcomeric proteins. The Sca-1+ stem/progenitor cells did not spontaneously differentiate into CMs, but following stimulation with 5-azacytidine, a DNA demethylating agent that causes pronounced epigenetic modifications, genes for Nkx2.5, α-MHC, β-MHC, and the type 1A receptor for bone morphogenetic proteins (Bmpr1a) were induced, and contraction was observed. The cells were however mononucleated and fibroblast-like in structure [11]. Subsequently, freshly isolated and undifferentiated αMHC-CreRecombinase Sca-1+ cells were introduced into infarcted hearts of ROSA26 mice to test for the possibility of cell fusion (where fusion occurs, Cre will cause recombination and the expression of LacZ found in the ROSA26 mice). In these experiments, almost 50% of the cells expressed LacZ, showing that cell fusion was a major contributor to the integration process; whereas, the remainder could be attributed to either bona fide cases of cardiomyogenesis or, alternatively, fused cells with incomplete penetrance for recombination. Conversely, Matsuura et al. reported that oxytocin, but not 5-azacytidine, induces Sca-1+ cells from the adult murine heart to differentiate into functional, spontaneously beating, immature CMs with Ca2+ transients typical to those found in heart cells [67]. The 5′-azacytidine-treated cells however developed a fibroblast-like morphology and never spontaneous contracted. In this study, both treatments up-regulated cardiac transcription factors Nkx2.5, GATA4, and MEF-2C and structural proteins for α- and β-MHC, MLC-2a, MLC-2v, and cardiac α-actin. The oxytocin-treated cells that formed CMs were few in number, but importantly, these cells had positive inotropic responses to isoproterenol via β1-adrenergic receptor signaling. Given the apparently small number of CMs generated in vitro from this stimulus, it thus appears that cardiomyogenesis is not a default pathway for these cells, and the potential to differentiate into true cardiac progenitors and CMs requires further investigation.
3.2.1.3. Side Population (SP) Cells
The ATP-binding cassette (ABC) transporter family contains 50 members that use the hydrolysis of ATP to pump toxins from cells. ABC transporters encoding multidrug resistance genes (MDRs) also efflux the DNA binding dye Hoechst 33342, which allows for the easy identification and sorting of ABC transporter-positive and transporter-negative cells by flow cytometry. In most cases, cells expressing ABC transporters comprise a very small percentage of freshly isolated cells, which appear as a “side population” (SP) of cells relative to the majority of non-SP cells. The SP cells and especially the Abcg2-dependent SP cell populations, have been associated with stem/progenitor cells and with long-term self-renewal [56]. Martin et al. showed that Abcg2 is robustly expressed in the developing heart [68]. At later states of development and in adult heart, the transporter could also be used to identify a rare cell population. Following co-culture, these cells could differentiate into α-actinin-positive cells, and in the border zone adjacent to a myocardial infarct, increased numbers of Abcg2-expressing cells have been observed. Hierlihy et al. also reported a SP cell population, which represented ~1% of the total cell number in the adult heart that was not enriched for the hematopoietic markers CD34, c-Kit, Sca-1, Flk-2, and Thy1.1 [69]. The purified myocardial SP cells from EGFP mice were also capable of forming CMs, but similarly to the c-Kit+ cells described above, only did so under co-culture conditions with primary CMs. Because these cells could readily fuse with other types of cells, it therefore remains unclear if the use of this marker is sufficient to identify a cell population that is capable of cardiomyogenesis.
3.2.1.4. Isl-1 Cells
The LIM homeodomain transcription factor Isl-1 marks a cell population that makes a substantial contribution to the embryonic heart. Isl-1 is expressed in cells that populate the so-called anterior or secondary heart field, and Isl-1 knock out mice are deficient in most aspects of the outflow tract and the right ventricle, and suffer from a severe reduction in atrial tissue [8]. The detection of relatively few Isl-1+ cells in the postnatal heart (~500–600 in 1–5-day-old rats) led to speculation that Isl-1+ cells might be important for the reconstitution of the damaged adult heart. To address this question, Laugwitz et al. identified Isl-1+ cells in postnatal rat, mouse and human myocardium, and showed that a cardiac mesenchymal feeder layer permitted renewal of the isolated progenitor cells. These isolated cells also maintained an ability to adopt a fully differentiated CM phenotype [9]. Unfortunately, very few, if any, Isl-1+ cells have been found in adult mouse hearts, and attempts to identify Isl-1+ cells unambiguously in human hearts have proved difficult. Moreover, cell lineage tracing experiments of Isl-1+ lacZ-labeled cells failed to show an increase in Isl-1+ cells after cardiac damage, which makes it unlikely that these cells make a significant contribution to endogenous repair processes. Hence, Isl-1+ cells in the postnatal heart may represent remnants of the fetal progenitor cell population, which do not serve a dedicated function during adult life. Moreover these cells are unique from other cardiac stem/progenitor cells (c-Kit+, Sca-1+), since the Isl-1+ cells do not express these markers, at least not at this stage of development/differentiation. Because Isl-1+ cells can be expanded in culture for a limited period of time, these cells have been correctly described as cardiac progenitor cells [9]. More recently, Chien`s group have also shown that Isl-1+ cardiovascular progenitors are multipotent and can differentiate into cardiac, smooth muscle and endothelial cells from both mouse ES cells and mouse embryos [70]. Altogether, these are perhaps some of the best defined resident cardiomyogenic cells described to date, because of both the use of genetic and molecular markers and the finding in mouse that this transcription factor is critical to cardiomyogenesis.
3.2.2. Non-Resident Cardiomyogenic Stem Cells
A number of non-resident stem cell populations have been described that may have cardiomyogenic potential. Some like those from fat, only occur at a very low frequency in vitro; whereas, others from bone marrow seem to show distinct cardiomyogenic potential. Generally speaking, bone marrow stroma is a complex tissue that supports cellular differentiation and the maintenance of stem and progenitor cells (Figure 3). Included in this population are undifferentiated hematopoietic stem cells that are critical for the generation of erythroid, myeloid and lymphoid lineages; mesenchymal cells that give rise to osteoblasts, adipoblasts and chondrocytes; and endothelial cells, which may arise from hemangioblast or other endothelial cell precursors. Clinically, it is important to note that some therapeutic applications of hematopoietic stem cells (HSCs) in patients with infarcted myocardium have resulted in very different results. This is probably because the purification protocols, which may have influenced the outcome of the therapies i.e., transplanted cells may have contained different contents of “definitive” stem cells or alternatively may have contained other cells with regenerative potential (paracrine effects). The latter is quite possible because different CD expressing antigens were employed to isolate the definitive stem cell populations (i.e., cells isolated using CD133 versus CD34 may have contained unique sets of other CD expressing antigens like CD31+). Unlike blood lineages, which are actively produced in bone marrow stroma, many mesenchymal tissues, such as muscle, tendon, ligament and articular cartilage are not produced here. Instead, bone marrow actively maintains the undifferentiated state of some mesenchymal stem cells (MSCs) that can migrate and help support tissue regeneration and cell replacement. The term “marrow stromal cell” therefore implies a complex mixture of uncharacterized cells; and at least three of these have been suggested to have cardiomyogenic potential.
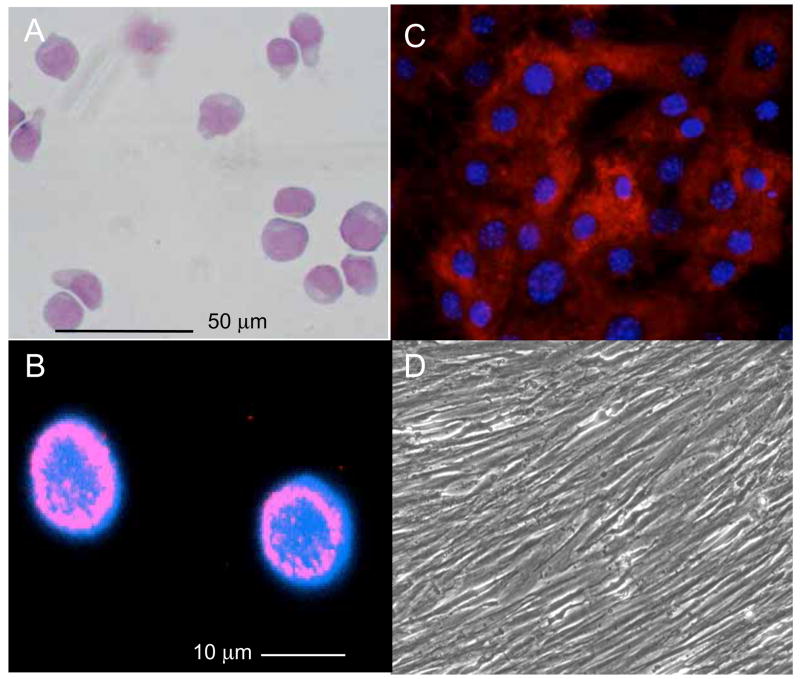
Figure 3.
Examples of Bone Marrow (Cord Blood) derived stem cells. A) CD133+ hematopoietic stem cells with a blast-like morphology (May-Gruewald fixation, Giemsa staining). B) Confocal image of Oct-3/4 positive cells that can be observed in CD133+ cells after a 3 h cultivation in non-conditioned medium (IMDM, 10 % FCS). C) Mouse MSCs with a flat morphology and stained with antibodies to CD29, a protein-tyrosine phosphatase. D) Human MSCs with an elongated/fibroblastic morphology at high density. MSCs generally express surface markers that include CD105, CD73, CD44, CD90, CD29, and CD106; however, different MSC preparations from mouse, rat and human typically display either flat morphologies or fibroblastic morphologies in vitro, indicating the degree of heterogeneity exhibited by these cells.
3.2.2.1. Hematopoietic Stem Cells (HSC)
HSCs are without question one of the very best defined populations of stem cells, but only the long-term repopulating HSC, which displays a unique set of markers (Murine: c-Kit+, Sca-1+, Lin−; Human: CD34+Thy-1+Lin−), corresponds to the resident stem cell population suitable for long-term replacement therapy [71]. The first evidence that bone marrow cells might also effect myocardial regeneration came from Bittner et al. who transplanted marrow from control mice into dystrophic DMDmdx/mdx females, and reported the presence of Y chromosome positive nuclei in CMs [72]; but Alvarez-Dolado et al. showed that marrow- derived cells could also fuse with heart cells [39]. To directly determine the ability of HSCs to promote myocardial regeneration, Orlic et al. mobilized HSCs from bone marrow to show that the “transdifferentiated cells” could repair the infarcted heart [19]. In a separate study, they isolated HSCs from mice that constitutively expressed the green fluorescence protein and used these cells to look for cardiac integration and repair [73]. Within 2 weeks of infarction, mice injected with the GFP cells had improved function, and the newly formed myocardium contained green cells that occupied up to 68% of the infarcted portion of the ventricle 9 days after transplanting the bone marrow cells. These labeled cells also expressed sarcomeric actins and myosins, troponin and several cardiac-associated transcription factors; however, the sarcomeric structures appeared disorganized. These initial observations have been followed up with a report by Rota et al., who showed that bone marrow cells engraft, survive, and grow within the spared myocardium after infarction [74]. More specifically, they reported that locally delivered bone marrow cells generate de novo myocardium composed of integrated CMs and coronary vessels, and that the cardiomyogenesis was independent of cell fusion, but dependent on close contact with endogenous CMs.
Several reports have contradicted these findings. Murry et al. reported that the HSCs do not show activation of cardiac-specific genes, and consistently, they could not detect any increase in CMs in the infarcted region of the heart [35]. Similarly, Kawada et al. showed that mobilized hematopoietic stem cells expressing GFP do not differentiate into CMs after myocardial infarction [75]. Most importantly, Balsam et al. specifically studied the ability of c-Kit-enriched bone marrow long-term reconstituting hematopoietic stem cells (Lin− c-Kit+ BM cells and c-Kit+ Thy1.1(low) Lin− Sca-1+) to regenerate myocardium in an infarct model. As discussed below, we believe that cardiomyogenic stem cells should have developmental correlates and a well-defined phenotype in an animal. These primary cell isolates when isolated to homogeneity and reintroduced into an animal can then be employed to determine a cell’s true differentiation potential. With this in mind, isolated primary HSCs from transgenic mice expressing green fluorescent protein (GFP) were isolated based on the expression of known cell markers and injected directly into ischemic myocardium of wild-type mice. Although GFP+ cells were abundantly detected in the myocardium after 10 days, by 30 days, very few cells were detectable and none of the GFP+ cells expressed cardiac tissue-specific markers. Instead, most of the cells expressed the hematopoietic marker CD45 and myeloid marker Gr-1, leading them to conclude that primary isolates of HSCs only adopt traditional haematopoietic fates[36].
3.2.2.2. Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells possess multipotent capabilities, readily proliferate in vitro and in vivo, induce angiogenesis and differentiate into osteogenic, chondrogenic, adipogenic and myogenic cells, types of the mesenchymal lineage. Animal studies and early clinical studies suggest that therapeutically delivered MSCs can improve heart function after an acute myocardial infarction (MI). More specifically, MSCs seem to improve contractility, wall thickness and decrease necrosis [76–78]. Part, if not all, of the observed therapeutic benefits are probably mediated through the release of a variety of signaling molecules, which may be anti-inflammatory, protective and angiogenic, since there is only limited data to suggest that MSCs readily form CMs. MSCs thus remain attractive as a vehicle for cell transplantation or for tissue engineering, because they can be obtained in relatively large numbers and are easily expanded in culture. In combination with their immuno-privileged status, MSCs remain a promising source for cell therapy in cardiac diseases.
In 1999, Makino et al. reported that CMs could be generated from immortalized marrow stromal cells in vitro through the addition of 5-azacytidine, a DNA demethylating agent, which induces pronounced epigenetic changes [79]. The reputed formation of CMs was based on the spontaneous contraction of cells in culture, action potentials similar to those found in fetal CMs, the expression of sarcomeric proteins, and the expression of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP). These latter two are associated with CMs; but the most predominant sarcomeric cell markers (skeletal α-actin and β-myosin heavy chain) are associated with both cardiac and skeletal muscle cells. Importantly, the published images clearly showed multinucleated cells, which are most typical of skeletal muscle. This has led many investigators to suggest that these were not authentic CMs, but more likely an abnormal muscle-like cell that in response to tissue culture and epigenetic modifications express some cardiac and skeletal markers, perhaps similar to that seen with the H9c2 cell line [80].
Subsequently, Kawada et al. transplanted genetically marked (GFP+) clonal MSCs into lethally irradiated recipient mice [75]. Following induction of myocardial infarction, mice were treated with granulocyte colony-stimulating factor, and the cardiac tissues were analyzed. In these experiments, the MSCs were not pretreated with 5-azacytidine; however, the authors were able to detect α-actinin+ GFP+ cells. They interpreted these data to mean that MSCs could in fact differentiate into CMs, even without pre-treatment with 5-azacytidine. The number of α-actinin+ GFP+ cells observed with this phenotype was very low, and they failed to discount the possibility that some of the results were due to fusion events or epigenetic changes secondary to cultivation. The incidence of α-actinin+ GFP+ cells was however much higher than that detected with transplanted HSCs (CD34− c-Kit+ Sca-1+ Lin− SP+) cells (see earlier). Interestingly, they also reported that transplantation of whole bone marrow gave an even higher number of α-actinin+ GFP+ cells in heart than labeled MSCs alone. This indicates that other cells in bone marrow, which were neither HSCs nor MSCs, might contribute to this response.
Because a majority of the transplanted MSCs differentiated into bone tissue when introduced into the bone marrow, it would also appear that the natural propensity of these cells is not to form CMs [81]; but when they do, it is likely that epigenetic modifications or trans-determination events, and not trans-differentiation events, are involved. Moreover, a recent study by Fleischmann and coworkers showed no cardiac contribution of enriched bone marrow derived MSCs. Instead, cells from whole bone marrow and MSCs adopted a mesenchymal cell fate after transplantation into cryoinjured infracted hearts that included calcifications and/or ossifications. These findings seriously questioned the biologic basis and clinical safety of using whole BM and in particular MSCs to treat non-hematopoietic disorders [82]. The ability of MSCs to form CMs is therefore still in doubt, and in almost all cases where cardiogenesis was reported, the cells had to go through a cultivation period that may have altered basic properties. There are however several other reports stating that MSCs can differentiate into CMs when injected into healthy murine heart or when co-cultured with primary CMs [83,84].
Finally, stem cells are also present in normal umbilical cord blood (UCB). The attractions of CB over bone marrow include the ease of non-invasive collection, the high proliferation and expansion potential of UCB-HSC, and the reduced graft versus host disease following transplantation [85,86]. More recently, the description of stem cell populations with non-hematopoietic potential has raised the prospect of more widespread applications for cord blood stem cells, including one where, approximately half of the human UCB-derived MSCs were reported to trans-differentiate into CMs in vitro [87].
3.2.2.3. Other Bone Marrow-Derived cells
As just described, there may be unique populations of bone marrow-derived stem cells that have the potential to contribute to myocardial cell replacement. One, the multipotent adult progenitor cell (MAPC) described by Verfaillie and co-workers showed differentiation markers of hepatocytes, endothelial cells and neurons, but there is little evidence that they contribute to heart [16,88]. When these cells were isolated from GFP-transgenic mice, MAPCs were subsequently found to be capable of multilineage hematopoietic engraftment in immunodeficient mice, but at a frequency much less than that of HSCs [89]. Although the MAPCs in vitro had an almost unlimited proliferation potential, their limited differentiation capacity relative to what was originally described in 2002 furthermore suggests that these cells are heterogeneous, highly dependent on culture conditions, and lack any significant degree of multipotency.
Xaymardan et al. have however described a unique population of bone marrow cells that when grown in FGF2- and VEGF-containing media generated aggregates of spontaneously beating cells [90]. Supplementation of the culture media with platelet-derived growth factor (PDGF)-AB furthermore shortened the time to α-myosin heavy chain expression. Interestingly, PDGF-AB treatment could also stimulate the formation of bone marrow-derived CM-like cells. These cells were however disorganized bundles of myocardium that did not improve function from bone marrow cells in the endogenous heart. Although many conclusions were based on the expression of marker genes and not function, these experiments were performed without co-culture thus indicating that the expression of cardiac genes from bone marrow cells can in fact be elicited merely through the addition of growth factors. Pallante et al. subsequently described a unique cell population in bone marrow that was positive for the pluripotency markers Oct4, nanog and Dppa3, negative for the hematopoietic stem cell markers CD34, CD45, and Sca-1, and showed variable levels of CXCR4+/− and c-Kit+/− [91]. In this study, the authors focused on the mechanism by which bone marrow cells could differentiation into CMs. PDGF-AB and the PDGF receptor alpha were both implicated in CM differentiation, and the loss of differentiation that occurs with aging could be restored by PDGF-AB supplementation. They furthermore showed the importance of the niche environment, and suggested that the PDGF pathway may be essential to the translation of bone marrow cell-mediated cardiomyogenesis.
Finally, circulating endothelial progenitor cells (EPCs) mobilized from bone marrow have been described with some cardiomyogenic potential. Badorff et al. showed that human peripheral blood mononuclear cells cultured for 3 days in endothelial cell medium with growth factors formed EPCs. When these cells were co-cultured with neonatal rat cardiomyocytes, they expressed α-sarcomeric actinin, cardiac troponin I, atrial natriuretic peptide, and myocyte enhancer factor 2; however, the cells only had a partially developed sarcomeric structure. Based on this information and the apparent formation of functional gap junctions, the authors concluded that these cells trans-differentiated to form cardiomyocytes [92]. Koyanagi et al. subsequently showed that Wnt11, but not Wnt3A, augments cardiomyogenic differentiation of circulating endothelial progenitor cells [20]. In 2007, they showed that gamma-secretase-dependent notch activation is required for cardiac gene expression in human EPCs. Notch activation either through co-culture with cardiomyocytes or gamma secretase activation is thought to induce the expression of non-canonical Wnt proteins [93].
4. Cell Plasticity and Putative Cardiomyogenic Stem Cells
We have now cited numerous examples of resident and non-resident cells with cardiomyogenic potential. Most of these analyses have been based on the isolation of heterogeneous populations of cells and subsequent cultivation or co-cultivation in vitro. Primarily, the expression of cardiac-associated gene markers have been used to indicate cardiomyogenic potential, and in some few cases, direct functional coupling has been shown [25,26]. With respect to molecular signaling processes responsible for cardiomyogenesis and the potential for epigenetic modifications, the question thus arises as to whether these putative cardiomyogenic cells are stem cells fated to the cardiomyogenic lineage or are other processes involved, like the so-called trans-differentiation events. Clearly numerous studies have now challenged the dogma that cells differentiate only according to a hierarchical model; however, normal developmental processes that involve both reversible hierarchical or lateral changes have been observed in both stem and non-stem cell populations, which could account for some changes reported in vitro (Figure 4).
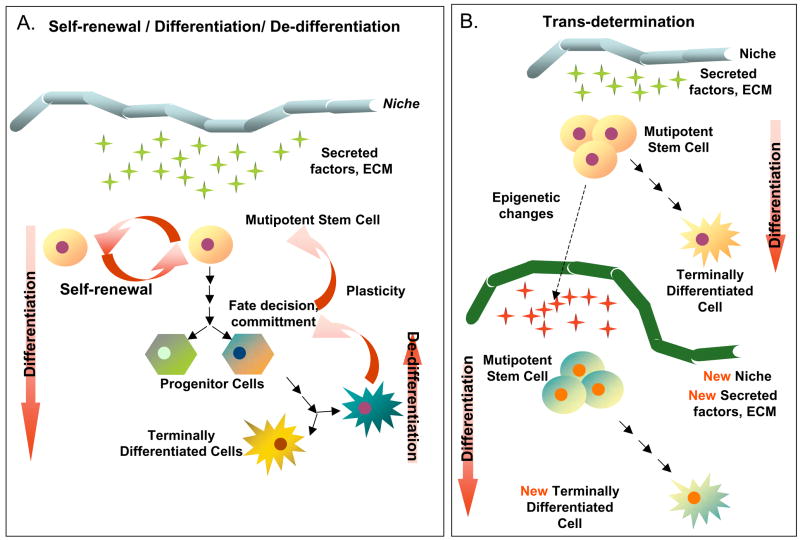
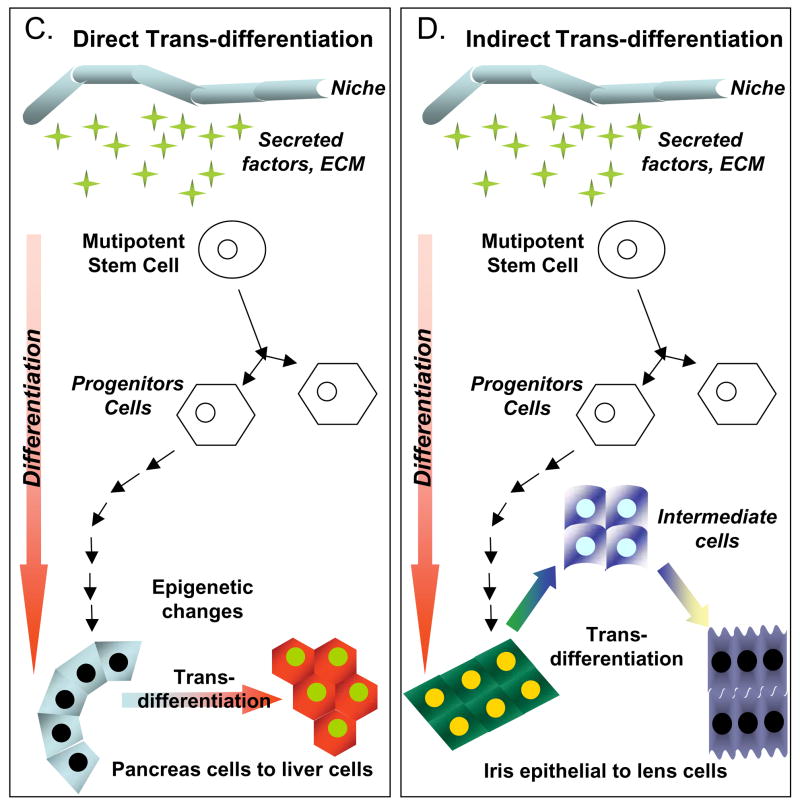
Figure 4.
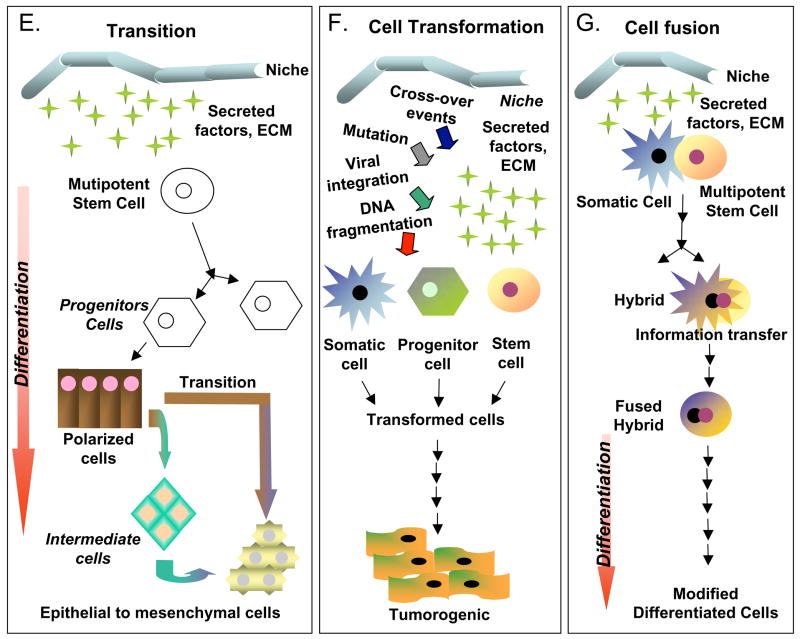
Illustrative examples of cell plasticity that might account for some of the differentiation events attributed to cardiomyogenic stem cells. A) Stem cells, which intrinsically self-renew, can differentiate into a single or multiple types of progeny, depending on their developmental potential. The differentiation process may not however be unidirectional, as there is some evidence, particularly in amphibians, to suggest that cell regression to a less differentiated state (de-differentiation) can occur. B) Trans-determination involves a “lineage switch” that can occur when stem cells are placed into a “new niche” environment. Such a switch is thought to be due primary to changes in local signaling and perhaps secondary to epigenetic modifications. C) Direct trans-differentiation is a lateral movement that permits a cell (stem or differentiated) to alter its fate and produce different progeny. Importantly, direct trans-differentiation should occur without cell division. D) Indirect trans-differentiation may involve a step of de-differentiation, with or without cell division. This process results in altered plasticity and permits phenotypic or fate changes in the progeny. E) Cell transitions, such as an Epithelial-to-Mesenchymal transition (EMT), involve the loss of cell polarity and the acquisition of migratory capabilities. Transitions such as these occur naturally during many stages of development, including gastrulation. F) Cell transformations are generally not considered by many stem cell researchers; however, the cultivation of cells in vitro can lead to chromosomal rearrangements, epigenetic changes and other DNA modifications. If severe, this can cause the cells to assume stem cell-like characteristics (immortality), but such cells are more analogous to forms of cancer, and therefore are completely inappropriate for therapeutic testing. G) Cell fusion events are common occurrences both in vitro (co-cultivation) and in vivo, in which adult cells may gain or show altered potentials, merely through the sharing of cytoplasmic factors (signaling molecules and transcription factors), after joining. These events can lead to pronounced reprogramming changes that lead to an altered cell phenotype. Although “cell-specific” markers and proteins may be expressed following fusion, these cells are rarely normal, and are not indicative of cell plasticity.
Developmentally, investigators have shown that tissue stem cells are quite capable of acquiring the identity of other stem cells just by placing the cells into a “new niche” environment [94–96]. In most of these studies the fate of marked cells introduced into a new environment was analyzed to determine if new cell types or lineages could be identified. Such a lineage switch, which has been defined as “trans-determination”, is one of several pathways that stem cells could potentially use to generate cells out of the “classic hierarchic model”, where a strict forward progression occurs linearly [97].
A second pathway that accounts for altered stem cell plasticity could involve normal cell transitions. Epithelial cells have different functions on their basal and apical sides, and this polarity is maintained through cell-cell and cell-extracellular matrix interactions. In contrast, mesenchymal cells often exist without direct cell-cell contacts and/or defined cell polarity. An epithelial-to-mesenchymal transition (EMT) occurs when epithelial cells lose their polarity and become mesenchymal. EMT plays a role in many stages of development, including gastrulation, in which the embryonic epithelium gives rise to the mesoderm, and in delamination of the neural crest, which produces a population of highly mobile cells that migrate to and are incorporated into many different tissues [98–100]. Alternatively, when a mesenchymal cell migrates to a new location, the cells may revert to their original epithelial phenotype through a process known as mesenchymal-epithelial transition (MET) [100]. Thus, the defining property of cells that undergo EMT is the ability to separate from neighboring cells and penetrate into and through surrounding tissues. Importantly, EMT processes identified through developmental studies also seem to be involved in critical steps of tumor metastasis, and it is likely that transitions also occur when in vivo-derived cells are transferred in vitro [100,101].
A third pathway, trans-differentiation, classically refers to differentiated cells changing directly (laterally) into another form of differentiated cell, ideally without the need for cell division or de-differentiation. Trans-differentiation in vivo has been observed in the conversion of white to brown adipocytes and in endothelial cells that reportedly became vascular smooth muscle cells [102,103]. A series of in vitro trans-differentiation events have also been described including central nervous system stem cells differentiating into muscle cells, and skeletal muscle cells differentiating into hematopoietic cells [104,105]. Moreover, cells derived from blood or bone marrow have been reported to trans-differentiate into muscle [106,107], neural cells [108,109], hepatocytes [96,110], and myocardial cell [13,14,19]; however, these latter experiments have been questioned due to numerous possible interpretations. The first of these possibilities includes de-differentiation. Cellular de-differentiation is required for functional regeneration in salamanders, and newts in particular generate stem-cell like blastema cells from differentiated cells that may either renew themselves or differentiate into specialized cells [111]. Adult CMs also have some potential for de-differentiation in vitro [112,113]. Secondly, fusion events can affect the fate of stem cells [37,114]. Fusion of male myocytes with embryonal carcinoma cells activates the inactived X-chromosome, while fusion of bone marrow and liver cells generates hepatocytes in mice [115–117]. Many trans-differentiation events reported with hematopoietic, mesenchymal and neural lineages have now been ascribed to cell fusion events [106,108,109,118–121]. Thus, adult stem cells may gain differentiation potential by fusion with other cells, which can result in reprogramming to a specific lineage or dedifferentiation to a more pluripotent state, depending on the cell type [38]. Finally, transformation or spontaneous immortalization (post-senescent fibroblasts) events may occur spontaneously in vitro, secondary to mutations or chromosomal rearrangements, which can give the cells properties sometimes associated with self-renewal [122,123].
Cells cultivated in vitro appear particularly susceptible to the acquisition of new or modified characteristics, some of which occur secondary to epigenetic modifications, which may mimic, but be unique from normal cell processes. A classic example of how epigenetic modifications can alter cell fates can be traced back to the discovery of Myo D, an essential transcription factor implicated in the formation of skeletal myoblasts [124]. In these experiments, stable myoblast cell lines could be isolated after a brief exposure of 10T1/2 mouse fibroblasts to 5-azacytidine, similar to what we described earlier for several types of cardiomyogenic “stem cells” [125]. Separately, Myo D could activate muscle-specific genes in pigment, nerve, fat, liver, and fibroblasts, showing how abnormal expression of a single transcription factor can elicit cell plasticity in vitro [126]. Thus, stem cells that have been cultivated in vitro for several passages prior to transplantation and/or treated with signaling molecules or transcription factors may no longer have their original properties [127].
Similarly, cells may react differently when implanted in control versus a pathological situation, just because the niche environment is altered [128]. Special attention should therefore be paid to the homogeneity or clonal properties of transplanted cells as well as the evidence of whether cell fusion has occurred, ideally through the use of genetic markers that separately label donor from host cells. Co-culture techniques of putative stem cells with differentiated cells can clearly result in some cell fusion but may also be susceptible to cell-to-cell connections via nanotubes, which may make a stem cell appear to take on characteristics of the others cells in culture [129]. To date, many cardiovascular researchers have failed to consider these alternative interpretations, which may account for some of the reported observations [97,111,130].
The question is therefore how to best define a “true” resident stem cell from a stem cell that may be an artifact of in vitro cultivation (epigenetic modifications, trans-determination, trans-differentiation, transition, cell fusion, etc), a transformation event or one induced by retroviral transfer of transcription factors [50,51]. Because several of these in vitro artifacts occur spontaneously, we argue that a putative stem cell with cardiomyogenic potential needs to be defined not only on its ability to self-renew and differentiate into CMs, but also on its ability to be identified and isolated to homogeneity from a tissue, either through lineage tracing or the presence of a unique set of markers (e.g., cell surface, molecular). In the strictest sense, therefore, cardiomyogenic stem cells should have developmental correlates and a well-defined phenotype in an animal, and thus far, no single cell surface marker has proven sufficient for the isolation of a stem cell to homogeneity. Consistent with this definition, Weissman et al. proposed that the final decision of a resident stem cell’s potency and authenticity should be made based on the isolation of a putative stem cell from the tissue of interest, and where possible, a single cell should be re-introduced in vivo without an in vitro step to determine its potential to self-renew and differentiate [27].
Based on our criteria and separately on the notion that cardiomyogenic stem cells must produce offspring that can functionally couple with CMs in vivo, we would argue that only a very few stem or progenitor cells with cardiomyogenic potential have been adequately described thus far [25,42,47]. By far the best example comes from embryonic stem cells, which can spontaneously form CMs in the absence of co-culture; however and since this is a heterogeneous system, some of the non-CMs that form spontaneously may release factors that are critical to cardiomyogenesis. Secondarily, the Isl-1+ cells also appear to be authentic cardiomyogenic progenitors. The absence of the right heart in Isl-1 knockout animals attests to its critical role; and through the use of other molecular markers, the findings meet many of the developmental standards outlined by Braun and Martire [55]. The stem cell status of the remaining cardiomyogenic cells described above must therefore continue to be questioned, at least for the moment, until questions associated with epigenetic phenomena can be resolved. While our definition may appear overly restrictive, cardiovascular researchers who claim to have identified a novel stem cell population with cardiomyogenic potential must be willing to perform well designed experiments with molecular and genetic markers to address the Weissman paradigm. In its absence, critiques and concerns about such claims will always be forthcoming, and our ability to elucidate signaling pathways of cardiogenesis will continue to be compromised. Practically, however, it has proven extremely difficult to definitively discern stem and progenitor cells with therapeutic potential from other cells with in vitro differentiation potential, including those that may have undergone transformation or epigenetic modifications. To adequately address this issue, appropriate cell surface markers and antibodies must first become available that permit the isolation of primary cell populations of putative stem cells to homogeneity. Until this is achieved, most findings with cardiomyogenic stem cells will continue to be subject to open interpretation by both proponents and opponents of the stem cell theory of cardiac self-renewal.
5. Stem Cell Differentiation and Signaling Implicated in Cardiomyogenesis
Because many of the pathways associated with adult stem cell differentiation to CMs may be related to epigenetic phenomena and/or factors transferred between cells during co-culture conditions or secondary to changes in the niche environment, the mechanisms responsible for cardiomyogenesis are still difficult to assess in vitro; but the reproducible induction of cardiomyogenesis from EBs has been exploited to identify and test factors that might be capable of improving the differentiation process of ES cells to CMs. The success of the ES system was however complicated initially by the role of serum, which, by analogy, is similar to current problems with the cardiomyogenic stem cells. We do, however, predict as with the ES cells that these shortcomings will be overcome as the model systems are more fully understood.
Irrespective, ES cell-based and a few adult stem cell-based studies have already implicated multiple peptide growth factors or cytokines in the process of differentiation (Figure 5) and cardiomyogenesis (Figure 6). More specifically, members of the interleukin-6 family, the transforming growth factor β (TGF-β) superfamily (TGFs and bone morphogenetic proteins, BMPs), the fibroblast growth factor family (FGFs), and the Notch and Wnt signaling pathways can either activate or inhibit cardiomyogenesis in vitro. Several other molecules, including PDGFs, retinoic acid (RA), and prodynorphins have also been implicated.
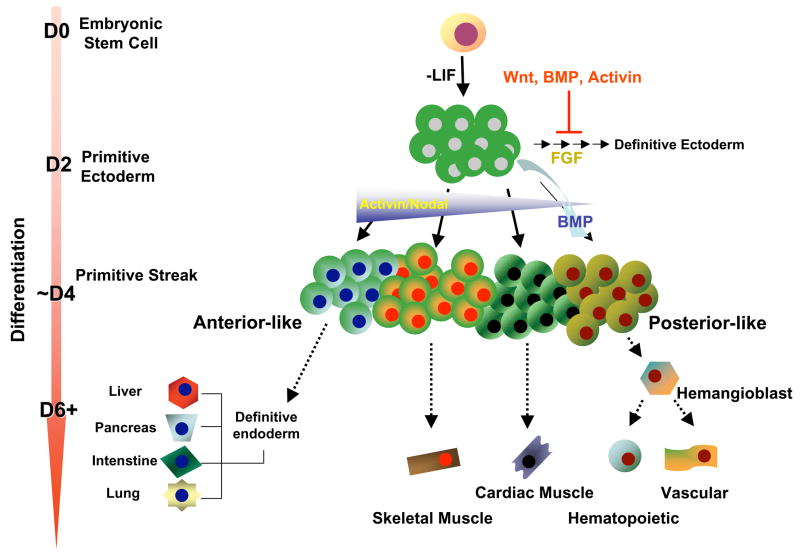
Figure 5.
Possible scheme for ES cell differentiation and the role of specific factors in the formation of primitive ectoderm and primary germ layers. A hypothetical primitive streak consisting of both posterior and anterior populations is shown, the populations of which are based on information from mouse. FGF is shown to play a role in neural induction; whereas Wnt, BMP, and activin are all implicated as inhibitors of the early stages of this pathway. BMP4 functions to induce posterior mesoderm, while a gradient of activin/nodal signaling is necessary for other fate decisions. At low concentrations of activin, more posterior-like populations are generated; whereas at high concentrations, it induces endoderm, consistent with an anterior primitive streak-derived population. This figure has been adapted from [30].
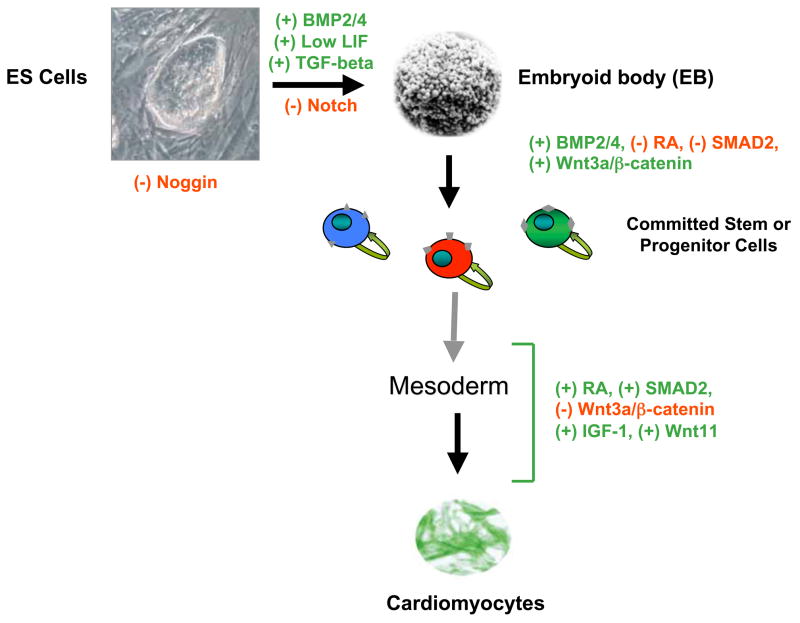
Figure 6.
Common signaling pathways implicated in the generation of CMs from ES cells. Members of the interleukin-6 family (LIF), transforming growth factor β (TGF-β) superfamily (TGF-βs, BMPs), Notch, and Wnt (Wnt3a, Wnt11) signaling pathways can either activate (+) or inhibit (−) cardiomyogenesis in vitro. Some of the signaling molecules (e.g., RA, Wnt3a, SMAD2) also show a temporal dependency in their effects on cardiomyogenesis.
5.1. Interleukin-6 Family Members
Members of the Interleukin (IL)-6 family include IL-6, IL-11, oncostatin M (OSM), Leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), cardiotrophin 1 (CT1). LIF, in particular, has been associated with the maintenance of the undifferentiated state of ES cells and cardiomyogenesis. More specifically in ES cells, LIF acts through a receptor complex composed of a low affinity LIF receptor (LIFR) and gp130 receptor molecules [131]. Gp130 receptor complexes activate a wide range of effector molecules, including signal transducer and activator of transcription (STAT) 1, 3 and 5 transcription factors, insulin receptor substrate (IRS) proteins, the tyrosine phosphatase SHP-2, mitogen-activated protein kinases (MAPK), extracellular regulated kinases (ERK) 1 and 2, phosphoinositol-3 kinase and the Src family tyrosine kinases, Hck, Btk and Fes. Neither the LIFR nor the gp130 molecule have any intrinsic protein kinase domain, but each associates constitutively with the JAK family of non-receptor cytoplasmic protein tyrosine kinases, and binding of LIF and formation of the GP130 receptor complex rapidly activates JAK tyrosine kinases, which promotes phosphorylation of the LIFR and gp130 on tyrosine residues. In response to phosphorylation, SH2-domain containing molecules (e.g., STATs and SHP-2) are recruited to the complex and phosphorylated at tyrosine residues by JAK tyrosine kinases. STATs subsequently dimerize, translocate to the nucleus, and bind to DNA to direct gene(s) transcription.
LIF has also been implicated in hematopoiesis, neuroectoderm formation, bone development, acute inflammation and, more recently, ES cell cardiomyogenesis. The latter seems to be associated primarily through its effects on parietal endoderm differentiation, but several publications suggest more direct effects [132,133]. More specifically, ES cells deprived of LIF differentiate to form EBs with a capacity to form CMs; however, if LIF is present during the first 4 days of aggregation, it inhibits mesoderm formation and the commitment toward CMs. In contrast, the addition of LIF to developing EBs after this time frame, promotes the differentiation of CMs. Different doses of LIF also produce distinctly different effects. Low doses of LIF in LIF-deficient and LIF receptor–deficient ES cells rescue the onset of cardiac differentiation; whereas, higher doses of LIF attenuate CM differentiation [132,133]. The relevance of LIF has recently been expanded to show that LIF together with BMP-2 downstream signaling components or cell type specific precommitment may facilitate the effects of ES cell-based therapies for post-MI myocardial repair and regeneration.[48]. Thus, LIF signaling is not only important for the maintenance of undifferentiated ES cells, but it plays a critical role in the induction and survival of CMs.
5.2. TGF-β Superfamily Members
The TGF-β superfamily consists primarily of TGF-β/activin, BMP/glial cell-derived neurotropic factor (GDNF) and nodal signaling molecules [134]. Each group transmit signals from the membrane to the nucleus by binding to a heteromeric complex of serine/threonine kinase receptors known as TGF-β type I (TβRI) and type II (TβRII) receptors. The type I receptor, also known as activin receptor-like kinase (ALK), acts downstream of the type II receptor and propagates the signal through downstream mediators, known as SMAD (small mother against decapentaplegic) molecules, a class of proteins that function as intracellular signal transducers [135]. The eight known SMAD proteins can also be classified into three functional classes: (i) receptor-regulated SMADs (SMAD1, 2, 3, 5, and 8), (ii) co-mediator SMAD (SMAD4), and (iii) inhibitory SMADs (SMAD6 and 7). TGF-β and nodal signaling activate primarily SMAD2 and 3 phosphorylation through activin receptor-like kinases (ALK)-4, -5, and –7, while SMADs 1, 5 and 8 are phosphorylated by BMP activation of ALKs-1, -2, -3, and -6 [135–137].
Members of the transforming growth factor-β superfamily (TGF-β and BMP2) specifically up-regulate transcripts encoding cardiac specific transcription factors (Nkx2.5, MEF2C) and transcripts to Brachyury, a mesoderm-specific lineage marker that is vital for the formation and differentiation of posterior mesoderm and for axial development in all vertebrates [138]. In vivo, bone morphogenic protein (BMP) signaling promotes vertebrate cardiomyogenesis, and it is required to generate mesoderm/cardiac cells from mouse teratocarcinoma stem cells and mouse ES cells in culture [139–142]. EBs stimulated with these growth factors show an increased potential for cardiac differentiation concomitant with a significant increase in beating areas and enhanced myofibrillogenesis. Disruption of the TGF-β/BMP signaling pathways in vitro by a latency-associated peptide and/or noggin also prevents differentiation of ES cells to CMs. Yuasa et al. however found that the BMP antagonist noggin is transiently and strongly expressed in the heart-forming region during gastrulation, and that it induces mesendoderm to establish conditions conducive to cardiogenesis [143]. They also suggested that pretreatment of ES cells with noggin could induce cardiomyogenesis; however, we have been unable to reproduce these findings (unpublished data), suggesting that there may be ES cell line variation. Finally, activin shows dose dependency on the formation of cell lineages. More specifically, Laflamme et al. generated highly purified CMs from human ES cells using a readily scalable system for directed differentiation that relies on BMP4 and high levels of activin A [30,144].
Downstream of TGF-β and BMP, SMAD signaling is directly involved with cardiopoiesis. Brown et al. showed that the Smad1/4 complex cooperates with GATA4 to activate Nkx2.5 gene transcription, which suggested a vital role for these factors in the specification of cardiac progenitors [145]. Using a cell line that over-expressed noggin, Monzen et al were also able to show that co-over-expression of Smad1 and Smad4 restored the ability of a noggin over-expressing P19 cell line to differentiate into CMs [146]. More recently, Prall et al. have shown Smad1-dependent negative feedback pathway that regulates heart progenitor specification and proliferation [147]. Kitamura et al. reported the unique role of SMAD2 in embryonic stem cells-derived cardiomyogenesis [148]. SMAD2 was shown to be biphasically activated in both early and late phases during differentiation. Activation of SMAD2 in the early phase augmented the induction of endoderm and mesoderm, which promoted cardiomyogenesis, whereas the activation of SMAD2 in the late phase inhibits CM formation.
Cripto, a critical co-factor for nodal and antagonist for activin signaling, plays critical roles during embryogenesis and has been implicated in promoting the growth and spread of tumors. ES cells lacking Cripto selectively lose the ability to form beating CMs [149,150]. Because some critical cardiac transcription factor transcripts (Nkx2.5, GATA4, and MEF-2C ) are still present in ES cells after the loss of Cripto-1, it has been suggested that Cripto-1 may act as a master gene regulator for progression of mesoderm to form functional myocardium. Parisi et al. subsequently showed that the timing of initiation and the duration of Cripto signaling are crucial for priming differentiation of ES cells into CMs. Failure to activate Cripto signaling at an early time point resulted in ES cells adopting a neural fate. More importantly, the induction of Cripto activates the SMAD2 pathway, and over-expression of activated forms of type I receptor ActRIB compensated for the lack of Cripto signaling in promoting cardiomyogenesis. Finally, Nodal antagonists inhibited Cripto-regulated cardiomyogenesis, thus showing that the Nodal/Cripto/Alk4 pathway is involved in this process.[151]. Taken all together, these data show that the TGF-β superfamily, either alone or in conjunction with other signaling pathways, plays an important role during differentiation and onset to precardiac mesoderm lineages.
5.3. Wnt Signaling
Wnt proteins (or Wg/Wingless) belong to a family of highly conserved secreted signaling molecules that control cellular processes such as embryonic induction, cell fate specification, the generation of cell polarity and are critical to vertebrate cardiomyogenesis Wnts trigger signaling pathways after binding to a membrane receptor complex, but the recruitment of proteins in the cytoplasm depends on which Wnt pathway is activated. The canonical Wnt pathway exerts its effects through β-catenin/TCF/Lef factors; whereas, the non-canonical pathway is PKC-dependent, involves G-proteins, the transmembrane protein strabismus, phospholipase C, c-Jun Kinase (JNK), Rho family GTPases, Ca2+/calmodulin-dependent protein kinase II [152–154].
Moreover, Wnt proteins have been classified into two groups, depending on their functional activities [155]. The Wnt1 group (Wnt1, Wnt3A and Wnt8A) transduces signal through the canonical pathway; whereas, the Wnt5A group (Wnt4, Wnt5A and Wnt11) acts mostly through the non-canonical pathway [152,154]. The Wnt5A group can also act as a dominant negative of the Wnt1 class protein, suppressing the β-catenin mediated signaling. Moreover, canonical Wnt inhibitors, such as Dickkopf1 (Dkk1), Crescent and Chibby (Cby), have been dubbed as cardiac inducers, acting to promote cardiac-specific gene expression in the posterior lateral plate mesoderm [156]. More specifically, Cby is expressed in ES cells and is gradually down-regulated during differentiation, but up-regulated during CM specification. Knockdown of Cby however leads to an inhibition of mesoderm/endoderm differentiation, while over-expression of Cby led to a 2-fold increase in CM formation, thus indicating that Cby facilitates cardiomyogenesis [157].
In contrast, canonical Wnt activators may have different effects on cardiogenesis [20,158]. Although the addition of Wnt3a has been suggested to induce CM differentiation of embryonic carcinoma cells, Wnt3a seems to enhance the early stages of differentiation to mesoderm lineages, but it has a repressive effect during later stages of differentiation to CMs [156,159]. Wnt11, a member of the non-canonical Wnt pathway, has been suggested to promote CM differentiation through activation of the non-canonical Wnt signaling pathway via JNK and PKC, while inhibiting the canonical signaling pathway through β-catenin. Specifically, an approximate 2-fold increase in the number of CMs was observed when Wnt11 was added beginning after 4 days of differentiation [160].
The cell surface κ-opioid receptor has also been reported to be involved in cardiac differentiation from ES cells. & kappa; opioid receptor agonists promote GATA4 and Nkx-2.5 gene expression and trigger subcellular redistribution of protein kinase C (PKC ) isozymes in ES cells. Moreover, opioid receptor antagonists suppressed the nuclear increase of PKC-α, PKC-β1 and PKC-β2 isozymes, which decreased the number of EBs with synchronously beating clusters. This led Ventura et al. to suggest that κ opioid-activated GATA4/Nkx-2.5 expression and PKC activation might be a primary signal transduction step in ES cell cardiomyogenesis; however, others have suggested that some of these effects may in fact be secondary to effects on the non-canonical Wnt signaling pathway [161,162]. Irrespective, there is now very good evidence from differentiation models of cardiomyogenesis to implicate Wnt signaling pathways in this process.
5.4. Notch Signaling
Notch signaling plays a crucial role in many developmental processes including cardiogenesis. There are four Notch proteins (Notch-1 to -4) and five ligands (Delta-like-1, -3 and -4, and Jagged-1 and -2) which are expressed on the cell surface in mammals, which control cell fate decisions through cell-cell interaction [163]. Although it has been reported that Notch signaling can cross-talk with other pathways involving BMP, TGF- β or Wnt proteins, the interactions among these pathways in cardiomyogenesis remain ill-defined, even if implicated both in ES cell and EPC differentiation [164, 165,93].
Activation of Notch signaling pathway inhibits cardiac differentiation and, conversely, inhibition of Notch in heart promotes myogenesis, suggesting that Notch has suppressive effects on cardiac differentiation [166]. Indeed, Nemir et al. have shown that Notch signaling is a critical regulator of CM differentiation. Inhibition of Notch signaling in ES cells with a dominant negative mutant of recombination signal sequence-binding protein Jkappa (RBP-J), a key downstream transcription factor of Notch signaling, enhances cardiomyogenesis; whereas, forced expression of the Notch intracellular domain facilitates differentiation into neuroectoderm [167]. Schroeder et al. however showed that RBP-J-deficient ES cells gave rise to CMs, endothelial cells, and primitive and definitive hematopoietic cells. This suggested that RBP-J-mediated signals were not in fact required for generation of these cell types. Unexpectedly, cardiomyogenesis derived from RBP-J-deficient ES cells was also increased, and repression of the cardiogenic pathway was restored by expressing RBP-J in RBP-J-deficient ES cells [168]. Altogether, these data indicate that Notch signaling via RBP-J play an important role in the specification of myocardial cell fates; however, its time of activation and the mode of inhibition must influence the final fate decisions.
Fischer et al. have also generated Hey-deficient ES cell line and shown that loss of Hey1/2 leads to the elevation of ANF, GATA4 and GATA6 mRNA levels, while forced expression of Hey strongly represses expression of the GATA4 and 6 [169]. Moreover, Srivastava’s group have shown that Delta-1, a ligand of Notch, is a target of a micro RNA that regulates differentiation of cardiac progenitor cells, indicating that Notch signaling might play a role in differentiation of cardiac progenitor cells [170]. Forced expression of Notch1 in the ventricular cells also inhibits the expression of cardiac muscle markers, sarcomeric myosin heavy chain and α-actin, but increases the expression of conduction cell markers, HNK-1 and SNAP-25, suggesting that Notch signaling also regulates the cardiac cell type specification [166].
5.5. Other Signaling Pathways
A number of additional growth factors and receptors have been shown to affect CM differentiation. As we have seen, PDGF-AB promotes cardiomyogenesis of Oct4 positive bone marrow cells, but in ES cells both PDGF-BB and sphingosine 1 phosphate increase the number of EBs with beating areas and the expression of myosin heavy chain [171]. FGF2 also regulates early development of pericardial mesoderm and promotes cardiac differentiation from ES cells; however, deletion of FGF receptor 1 impairs CMs differentiation from ES cells [172–174]. Insulin and insulin-like growth factor I (IGF-I) are also essential for mesoderm and heart formation, and IGF-I promotes the proliferation of CMs differentiated from ES cells [171,175,176]. The binding of insulin and IGF to its receptors can also activate phosphoinositide 3-kinase (PI3K); however, addition of a PI3K inhibitor either abolishes cardiac commitment or reduces CMs number and beating area in ES cell-derived EBs [177–179].
Moreover, nitric oxide (NO), which is generated by NO synthetase (NOS), facilitates cardiac differentiation. Two forms of NOS, inducible NOS (inos) and endothelial NOS (enos), are present during the early stage of heart development, and their expression declines in the ventricle at 14.5 days after embryonic formation, but not in the atrium [180]. Similar and in ES cells, NO generation is at high levels during early stages of differentiation, but stops when CMs switch to postnatal-like stages. NOS inhibitors can also introduce a significant delay in cardiac differentiation from mES cells [180]; but through the use of SNAP, a NO donor chemical, the differentiation of CMs from ES cells can be augmented, and the proportion of CMs greatly increased [181].
Nanomolar concentrations of retinoic acid (RA) significantly inhibit the development of beating clusters within EBs if administered during the first 5 days of EB formation; however, administration of RA between days 5 and 7 not only produces the opposite effects, it causes an increase in the number of contracting EBs. Interestingly, RA mediated induction of cardiomyoblast differentiation preferentially leads to a ventricular cell type within EBs cultivated in high serum conditions [182,183]. Paradoxically, depletion of RA in low serum conditions enhances cardiomyogenic differentiation [182].
Finally, studies on retinoblastoma protein (Rb)-deficient ES cells also showed LEK1 to be a pivotal cardiogenic factor [184,185]. In Rb-deficient CMs, beating activity in EBs is delayed and the expression of GATA, MEF2 and Nkx-2.5 cardiogenic factors and myocardium-specific genes are downregulated, suggesting a critical role of LEK1 protein in early stages of mesoderm-derived cell lineage specification and differentiation. Restoring the structural interaction of Rb with LEK1 induced a transcriptional program priming ES cells toward a cardiac fate.
6. Conclusions
Only a few years ago, the idea of a static heart with limited repair capacity was the accepted dogma in the cardiovascular field. The study of stem cells has however revolutionized this and other fields of clinical investigation to the point where the idea of cell based repair, either with endogenous or exogenous cell sources, is now accepted as a new reality. This paradigm shift has occurred very swiftly, partly because of the rapid progress in the field, but also because of great expectations on the part of lay persons and researchers. With change also comes controversy, and the stem cell theory of cardiac self-renewal is not without its detractors. We see merit in the arguments of both the opponents and proponents of this theory, but we also argue that there are experiments that can be performed to resolve these issues. Paramount among these will be the identification and isolation of primary cells with putative cardiomyogenic potential, but before this can be done, appropriate cell surface markers and antibodies must become available. Until this is achieved, most findings with cardiomyogenic stem cells will continue to be subject to open interpretation. The future is however bright and if successful, then cell-based repair has the potential to improve the health and well-being of tens of millions of people.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murry CE, Whitney ML, Reinecke H. Muscle cell grafting for the treatment and prevention of heart failure. J Card Fail. 2002;8:S532–41. doi: 10.1054/jcaf.2002.129268. [DOI] [PubMed] [Google Scholar]
- 2.Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, et al. Myoblast transplantation for heart failure. Lancet. 2001;357:279–80. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 3.Dowell JD, Rubart M, Pasumarthi KB, Soonpaa MH, Field LJ. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc Res. 2003;58:336–350. doi: 10.1016/s0008-6363(03)00254-2. [DOI] [PubMed] [Google Scholar]
- 4.Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–83. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs JR, Nasseri BA, Vacanti JP, Fauza DO. Postnatal myocardial augmentation with skeletal myoblast-based fetal tissue engineering. Surgery. 2006;140:100–7. doi: 10.1016/j.surg.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res. 2002;91:189–201. doi: 10.1161/01.res.0000027865.61704.32. [DOI] [PubMed] [Google Scholar]
- 7.Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–78. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 8.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 11.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 13.Orlic D. Adult BM stem cells regenerate mouse myocardium. Cytotherapy. 2002;4:521–5. doi: 10.1080/146532402761624674. [DOI] [PubMed] [Google Scholar]
- 14.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant. 2003;7 Suppl 3:86–8. doi: 10.1034/j.1399-3046.7.s3.13.x. [DOI] [PubMed] [Google Scholar]
- 15.Kodama H, Inoue T, Watanabe R, Yasuoka H, Kawakami Y, Ogawa S, et al. Cardiomyogenic potential of mesenchymal progenitors derived from human circulating CD14+ monocytes. Stem Cells Dev. 2005;14:676–86. doi: 10.1089/scd.2005.14.676. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 17.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, et al. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–41. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyanagi M, Haendeler J, Badorff C, Brandes RP, Hoffmann J, Pandur P, et al. Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J Biol Chem. 2005;280:16838–42. doi: 10.1074/jbc.M500323200. [DOI] [PubMed] [Google Scholar]
- 21.Reinecke H, Poppa V, Murry CE. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol. 2002;34:241–9. doi: 10.1006/jmcc.2001.1507. [DOI] [PubMed] [Google Scholar]
- 22.Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci U S A. 2003;100:7808–11. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Planat-Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia-Verdugo JM, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–9. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 24.Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 25.Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annu Rev Physiol. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- 26.Rubart M, Field LJ. Cell-based approaches for cardiac repair. Ann N Y Acad Sci. 2006;1080:34–48. doi: 10.1196/annals.1380.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 28.Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–6. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- 29.Wagers AJ, Christensen JL, Weissman IL. Cell fate determination from stem cells. Gene Ther. 2002;9:606–12. doi: 10.1038/sj.gt.3301717. [DOI] [PubMed] [Google Scholar]
- 30.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–55. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 31.Beddington RS, Robertson EJ. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989;105:733–7. doi: 10.1242/dev.105.4.733. [DOI] [PubMed] [Google Scholar]
- 32.Nagy A, Gocza E, Diaz EM, Prideaux VR, Ivanyi E, Markkula M, Rossant J. Embryonic stem cells alone are able to support fetal development in the mouse. Development. 1990;110:815–21. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- 33.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90:8424–8. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wollert KC, Drexler H. Cell-based therapy for heart failure. Curr Opin Cardiol. 2006;21:234–9. doi: 10.1097/01.hco.0000221586.94490.d2. [DOI] [PubMed] [Google Scholar]
- 35.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 36.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 37.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 38.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–73. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 40.Weitzer G. Embryonic stem cell-derived embryoid bodies: an in vitro model of eutherian pregastrulation development and early gastrulation. Handb Exp Pharmacol. 2006:21–51. [PubMed] [Google Scholar]
- 41.Guan K, Furst DO, Wobus AM. Modulation of sarcomere organization during embryonic stem cell-derived cardiomyocyte differentiation. Eur J Cell Biol. 1999;78:813–823. doi: 10.1016/S0171-9335(99)80032-6. [DOI] [PubMed] [Google Scholar]
- 42.Rubart M, Pasumarthi KB, Nakajima H, Soonpaa MH, Nakajima HO, Field LJ. Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circ Res. 2003;92:1217–24. doi: 10.1161/01.RES.0000075089.39335.8C. [DOI] [PubMed] [Google Scholar]
- 43.Rubart M, Field LJ. Cardiac repair by embryonic stem-derived cells. Handb Exp Pharmacol. 2006:73–100. [PMC free article] [PubMed] [Google Scholar]
- 44.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–32. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–50. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 46.Buehr M, Smith A. Genesis of embryonic stem cells. Philos Trans R Soc Lond B Biol Sci. 2003;358:1397–402. doi: 10.1098/rstb.2003.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubart M, Soonpaa MH, Nakajima H, Field LJ. Spontaneous and evoked intracellular calcium transients in donor-derived myocytes following intracardiac myoblast transplantation. J Clin Invest. 2004;114:775–83. doi: 10.1172/JCI21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajasingh J, Bord E, Hamada H, Lambers E, Qin G, Losordo DW, Kishore R. STAT3-dependent mouse embryonic stem cell differentiation into cardiomyocytes: analysis of molecular signaling and therapeutic efficacy of cardiomyocyte precommitted mES transplantation in a mouse model of myocardial infarction. Circ Res. 2007;101:910–8. doi: 10.1161/CIRCRESAHA.107.156786. [DOI] [PubMed] [Google Scholar]
- 49.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. Faseb J. 2007;21:1345–57. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 52.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 53.Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–81. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 54.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 55.Braun T, Martire A. Cardiac stem cells: paradigm shift or broken promise? A view from developmental biology. Trends Biotechnol. 2007;25:441–7. doi: 10.1016/j.tibtech.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morita Y, Ema H, Yamazaki S, Nakauchi H. Non-side-population hematopoietic stem cells in mouse bone marrow. Blood. 2006;108:2850–6. doi: 10.1182/blood-2006-03-010207. [DOI] [PubMed] [Google Scholar]
- 58.Witte ON. Steel locus defines new multipotent growth factor. Cell. 1990;63:5–6. doi: 10.1016/0092-8674(90)90280-r. [DOI] [PubMed] [Google Scholar]
- 59.Orr-Urtreger A, Avivi A, Zimmer Y, Givol D, Yarden Y, Lonai P. Developmental expression of c-kit, a proto-oncogene encoded by the W locus. Development. 1990;109:911–23. doi: 10.1242/dev.109.4.911. [DOI] [PubMed] [Google Scholar]
- 60.Fleischman RA. From white spots to stem cells: the role of the Kit receptor in mammalian development. Trends Genet. 1993;9:285–90. doi: 10.1016/0168-9525(93)90015-a. [DOI] [PubMed] [Google Scholar]
- 61.Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, et al. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;19:10440–10445. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–71. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X, Wilson RM, Kubo H, Berretta RM, Harris DM, Zhang X, et al. Adolescent feline heart contains a population of small, proliferative ventricular myocytes with immature physiological properties. Circ Res. 2007;100:536–44. doi: 10.1161/01.RES.0000259560.39234.99. [DOI] [PubMed] [Google Scholar]
- 64.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 66.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–77. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–91. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 68.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–75. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 69.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–43. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 70.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 71.Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509–38. doi: 10.1146/annurev.med.54.101601.152334. [DOI] [PubMed] [Google Scholar]
- 72.Bittner RE, Schofer C, Weipoltshammer K, Ivanova S, Streubel B, Hauser E, et al. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol (Berl) 1999;199:391–6. doi: 10.1007/s004290050237. [DOI] [PubMed] [Google Scholar]
- 73.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 74.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–8. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–7. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 76.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 77.Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–93. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- 78.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H2670–6. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 79.Makino S, Fukuda K, Miyoshi S, Konishi R, Kodama H, Pan J, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Scultz G. Morphological, biochemical and electrophysiological characterisation of a clonal cell (H9c2) line from rat heart. Circulation Research. 1991;69:1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 81.Fukuda K, Fujita J. Mesenchymal, but not hematopoietic, stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction in mice. Kidney Int. 2005;68:1940–3. doi: 10.1111/j.1523-1755.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 82.Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–9. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 83.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 84.Rangappa S, Entwistle JW, Wechsler AS, Kresh JY. Cardiomyocyte-mediated contact programs human mesenchymal stem cells to express cardiogenic phenotype. J Thorac Cardiovasc Surg. 2003;126:124–32. doi: 10.1016/s0022-5223(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 85.Piacibello W, Sanavio F, Severino A, Garetto L, Dane A, Gammaitoni L, Aglietta M. Ex vivo expansion of cord blood progenitors. Vox Sang. 1998;74 Suppl 2:457–62. doi: 10.1111/j.1423-0410.1998.tb05456.x. [DOI] [PubMed] [Google Scholar]
- 86.Gluckman E, Locatelli F. Umbilical cord blood transplants. Curr Opin Hematol. 2000;7:353–7. doi: 10.1097/00062752-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 87.Nishiyama N, Miyoshi S, Hida N, Uyama T, Okamoto K, Ikegami Y, et al. The significant cardiomyogenic potential of human umbilical cord blood-derived mesenchymal stem cells in vitro. Stem Cells. 2007;25:2017–24. doi: 10.1634/stemcells.2006-0662. [DOI] [PubMed] [Google Scholar]
- 88.Verfaillie CM. Multipotent adult progenitor cells: an update. Novartis Found Symp. 2005;265:55–61. [PubMed] [Google Scholar]
- 89.Serafini M, Dylla SJ, Oki M, Heremans Y, Tolar J, Jiang Y, et al. Hematopoietic reconstitution by multipotent adult progenitor cells: precursors to long-term hematopoietic stem cells. J Exp Med. 2007;204:129–39. doi: 10.1084/jem.20061115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xaymardan M, Tang L, Zagreda L, Pallante B, Zheng J, Chazen JL, et al. Platelet-derived growth factor-AB promotes the generation of adult bone marrow-derived cardiac myocytes. Circ Res. 2004;94:E39–45. doi: 10.1161/01.RES.0000122042.51161.B6. [DOI] [PubMed] [Google Scholar]
- 91.Pallante BA, Duignan I, Okin D, Chin A, Bressan MC, Mikawa T, Edelberg JM. Bone marrow Oct3/4+ cells differentiate into cardiac myocytes via age-dependent paracrine mechanisms. Circ Res. 2007;100:e1–11. doi: 10.1161/01.RES.0000253487.02398.85. [DOI] [PubMed] [Google Scholar]
- 92.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, et al. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–32. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 93.Koyanagi M, Bushoven P, Iwasaki M, Urbich C, Zeiher AM, Dimmeler S. Notch signaling contributes to the expression of cardiac markers in human circulating progenitor cells. Circ Res. 2007;101:1139–45. doi: 10.1161/CIRCRESAHA.107.151381. [DOI] [PubMed] [Google Scholar]
- 94.Maves L, Schubiger G. Cell determination and transdetermination in Drosophila imaginal discs. Curr Top Dev Biol. 1999;43:115–51. doi: 10.1016/s0070-2153(08)60380-4. [DOI] [PubMed] [Google Scholar]
- 95.Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, et al. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- 96.Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, et al. Liver from bone marrow in humans. Hepatology. 2000;32:11–6. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- 97.Martin-Rendon E, Watt SM. Stem cell plasticity. Br J Haematol. 2003;122:877–91. doi: 10.1046/j.1365-2141.2003.04576.x. [DOI] [PubMed] [Google Scholar]
- 98.Nieto MA. The early steps of neural crest development. Mech Dev. 2001;105:27–35. doi: 10.1016/s0925-4773(01)00394-x. [DOI] [PubMed] [Google Scholar]
- 99.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120:1351–83. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 100.Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–6. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 101.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 102.Cinti S. Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ. J Endocrinol Invest. 2002;25:823–35. doi: 10.1007/BF03344046. [DOI] [PubMed] [Google Scholar]
- 103.Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90:1189–96. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- 104.Galli R, Borello U, Gritti A, Minasi MG, Bjornson C, Coletta M, et al. Skeletal myogenic potential of human and mouse neural stem cells. Nat Neurosci. 2000;3:986–91. doi: 10.1038/79924. [DOI] [PubMed] [Google Scholar]
- 105.Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci U S A. 1999;96:14482–6. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–30. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 107.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–4. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 108.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–9. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 109.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–82. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 110.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–70. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 111.Morrison JI, Loof S, He P, Alestrom P, Collas P, Simon A. Targeted gene delivery to differentiated skeletal muscle: a tool to study dedifferentiation. Dev Dyn. 2007;236:481–8. doi: 10.1002/dvdy.21019. [DOI] [PubMed] [Google Scholar]
- 112.Eppenberger ME, Hauser I, Baechi T, Schaub MC, Brunner UT, Dechesne CA, Eppenberger HM. Immunocytochemical analysis of the regeneration of myofibrils in long-term cultures of adult cardiomyocytes of the rat. Developmental Biology. 1988;130:1–15. doi: 10.1016/0012-1606(88)90408-3. [DOI] [PubMed] [Google Scholar]
- 113.Eppenberger-Eberhardt M, Flamme I, Kurer V, Eppenberger HM. Reexpression of α-smooth muscle actin isoform in cultured adult rat cardiomyocytes. Developmental Biology. 1990;139:269–278. doi: 10.1016/0012-1606(90)90296-u. [DOI] [PubMed] [Google Scholar]
- 114.Vassilopoulos G, Russell DW. Cell fusion: an alternative to stem cell plasticity and its therapeutic implications. Curr Opin Genet Dev. 2003;13:480–5. doi: 10.1016/s0959-437x(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 115.Takagi N, Yoshida MA, Sugawara O, Sasaki M. Reversal of X-inactivation in female mouse somatic cells hybridized with murine teratocarcinoma stem cells in vitro. Cell. 1983;34:1053–62. doi: 10.1016/0092-8674(83)90563-9. [DOI] [PubMed] [Google Scholar]
- 116.Mise N, Sado T, Tada M, Takada S, Takagi N. Activation of the inactive X chromosome induced by cell fusion between a murine EC and female somatic cell accompanies reproducible changes in the methylation pattern of the Xist gene. Exp Cell Res. 1996;223:193–202. doi: 10.1006/excr.1996.0073. [DOI] [PubMed] [Google Scholar]
- 117.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–4. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 118.Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo [see comments] Science. 1999;283:534–7. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 119.Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, et al. Generalized potential of adult neural stem cells. Science. 2000;288:1660–3. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- 120.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 121.Lagasse E, Shizuru JA, Uchida N, Tsukamoto A, Weissman IL. Toward regenerative medicine. Immunity. 2001;14:425–36. doi: 10.1016/s1074-7613(01)00123-6. [DOI] [PubMed] [Google Scholar]
- 122.Kopper L, Hajdu M. Tumor stem cells. Pathol Oncol Res. 2004;10:69–73. doi: 10.1007/BF02893458. [DOI] [PubMed] [Google Scholar]
- 123.Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–6. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 124.Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, Lassar AB. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–11. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- 125.Lassar AB, Paterson BM, Weintraub H. Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell. 1986;47:649–56. doi: 10.1016/0092-8674(86)90507-6. [DOI] [PubMed] [Google Scholar]
- 126.Weintraub H, Tapscott SJ, Davis RL, Thayer MJ, Adam MA, Lassar AB, Miller AD. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci U S A. 1989;86:5434–8. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frisen J. Stem cell plasticity? Neuron. 2002;35:415–8. doi: 10.1016/s0896-6273(02)00798-5. [DOI] [PubMed] [Google Scholar]
- 128.Tosh D, Slack JM. How cells change their phenotype. Nat Rev Mol Cell Biol. 2002;3:187–94. doi: 10.1038/nrm761. [DOI] [PubMed] [Google Scholar]
- 129.Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–41. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 130.Morrison JI, Loof S, He P, Simon A. Salamander limb regeneration involves the activation of a multipotent skeletal muscle satellite cell population. J Cell Biol. 2006;172:433–40. doi: 10.1083/jcb.200509011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Burdon T, Stracey C, Chambers I, Nichols J, Smith A. Suppression of SHP-2 and ERK signaling promotes self-renewal of mouse embryonic stem cells. Dev Biol. 1999;210:30–43. doi: 10.1006/dbio.1999.9265. [DOI] [PubMed] [Google Scholar]
- 132.Bader A, Al-Dubai H, Weitzer G. Leukemia inhibitory factor modulates cardiogenesis in embryoid bodies in opposite fashions. Circ Res. 2000;86:787–794. doi: 10.1161/01.res.86.7.787. [DOI] [PubMed] [Google Scholar]
- 133.Bader A, Gruss A, Hollrigl A, Al-Dubai H, Capetanaki Y, Weitzer G. Paracrine promotion of cardiomyogenesis in embryoid bodies by LIF modulated endoderm. Differentiation. 2001;68:31–43. doi: 10.1046/j.1432-0436.2001.068001031.x. [DOI] [PubMed] [Google Scholar]
- 134.de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev. 2004;15:1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 135.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 136.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 137.Wang H, Tsang BK. Nodal signaling and apoptosis. Reproduction. 2007;133:847–53. doi: 10.1530/REP-07-0053. [DOI] [PubMed] [Google Scholar]
- 138.Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, Puceat M. Stem cell differentiation requires a paracrine pathway in the heart. Faseb J. 2002;16:1558–66. doi: 10.1096/fj.02-0072com. [DOI] [PubMed] [Google Scholar]
- 139.Zaffran S, Frasch M. Early signals in cardiac development. Circ Res. 2002;91:457–69. doi: 10.1161/01.res.0000034152.74523.a8. [DOI] [PubMed] [Google Scholar]
- 140.Schneider MD, Gaussin V, Lyons KM. Tempting fate: BMP signals for cardiac morphogenesis. Cytokine Growth Factor Rev. 2003;14:1–4. doi: 10.1016/s1359-6101(02)00053-9. [DOI] [PubMed] [Google Scholar]
- 141.Johansson BM, Wiles MV. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Molecular and Cellular Biology. 1995;15:141–51. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, et al. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci U S A. 2002;99:2878–83. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, et al. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23:607–11. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- 144.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 145.Brown CO, 3rd, Chi X, Garcia-Gras E, Shirai M, Feng XH, Schwartz RJ. The cardiac determination factor, Nkx2-5, is activated by mutual cofactors GATA-4 and Smad1/4 via a novel upstream enhancer. J Biol Chem. 2004;279:10659–69. doi: 10.1074/jbc.M301648200. [DOI] [PubMed] [Google Scholar]
- 146.Monzen K, Shiojima I, Hiroi Y, Kudoh S, Oka T, Takimoto E, et al. Bone morphogenetic proteins induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4. Mol Cell Biol. 1999;19:7096–7105. doi: 10.1128/mcb.19.10.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, et al. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–59. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kitamura R, Takahashi T, Nakajima N, Isodono K, Asada S, Ueno H, et al. Stage-specific role of endogenous Smad2 activation in cardiomyogenesis of embryonic stem cells. Circ Res. 2007;101:78–87. doi: 10.1161/CIRCRESAHA.106.147264. [DOI] [PubMed] [Google Scholar]
- 149.Xu C, Liguori G, Adamson ED, Persico MG. Specific arrest of cardiogenesis in cultured embryonic stem cells lacking Cripto-1. Dev Biol. 1998;196:237–247. doi: 10.1006/dbio.1998.8862. [DOI] [PubMed] [Google Scholar]
- 150.Xu C, Liguori G, Persico MG, Adamson ED. Abrogation of the Cripto gene in mouse leads to failure of postgastrulation morphogenesis and lack of differentiation of cardiomyocytes. Development. 1999;126:483–494. doi: 10.1242/dev.126.3.483. [DOI] [PubMed] [Google Scholar]
- 151.Parisi S, D’Andrea D, Lago CT, Adamson ED, Persico MG, Minchiotti G. Nodal-dependent Cripto signaling promotes cardiomyogenesis and redirects the neural fate of embryonic stem cells. J Cell Biol. 2003;163:303–14. doi: 10.1083/jcb.200303010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 153.Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, Moon RT. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol. 2003;161:769–77. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–5. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 155.Du SJ, Purcell SM, Christian JL, McGrew LL, Moon RT. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol Cell Biol. 1995;15:2625–34. doi: 10.1128/mcb.15.5.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yamashita JK, Takano M, Hiraoka-Kanie M, Shimazu C, Peishi Y, Yanagi K, et al. Prospective identification of cardiac progenitors by a novel single cell-based cardiomyocyte induction. Faseb J. 2005;19:1534–6. doi: 10.1096/fj.04-3540fje. [DOI] [PubMed] [Google Scholar]
- 157.Singh AM, Li FQ, Hamazaki T, Kasahara H, Takemaru K, Terada N. Chibby, an antagonist of the Wnt/beta-catenin pathway, facilitates cardiomyocyte differentiation of murine embryonic stem cells. Circulation. 2007;115:617–26. doi: 10.1161/CIRCULATIONAHA.106.642298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nakamura T, Sano M, Songyang Z, Schneider MD. A Wnt- and beta -catenin-dependent pathway for mammalian cardiac myogenesis. Proc Natl Acad Sci U S A. 2003;100:5834–9. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, et al. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–90. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Terami H, Hidaka K, Katsumata T, Iio A, Morisaki T. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Commun. 2004;325:968–75. doi: 10.1016/j.bbrc.2004.10.103. [DOI] [PubMed] [Google Scholar]
- 161.Ventura C, Zinellu E, Maninchedda E, Maioli M. Dynorphin B is an agonist of nuclear opioid receptors coupling nuclear protein kinase C activation to the transcription of cardiogenic genes in GTR1 embryonic stem cells. Circ Res. 2003;92:623–9. doi: 10.1161/01.RES.0000065169.23780.0E. [DOI] [PubMed] [Google Scholar]
- 162.Ventura C, Zinellu E, Maninchedda E, Fadda M, Maioli M. Protein kinase C signaling transduces endorphin-primed cardiogenesis in GTR1 embryonic stem cells. Circ Res. 2003;92:617–22. doi: 10.1161/01.RES.0000065168.31147.5B. [DOI] [PubMed] [Google Scholar]
- 163.Fleming RJ. Structural conservation of Notch receptors and ligands. Semin Cell Dev Biol. 1998;9:599–607. doi: 10.1006/scdb.1998.0260. [DOI] [PubMed] [Google Scholar]
- 164.Herpin A, Cunningham C. Cross-talk between the bone morphogenetic protein pathway and other major signaling pathways results in tightly regulated cell-specific outcomes. Febs J. 2007;274:2977–85. doi: 10.1111/j.1742-4658.2007.05840.x. [DOI] [PubMed] [Google Scholar]
- 165.Katoh M. Networking of WNT, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesis. Stem Cell Rev. 2007;3:30–8. doi: 10.1007/s12015-007-0006-6. [DOI] [PubMed] [Google Scholar]
- 166.Chau MD, Tuft R, Fogarty K, Bao ZZ. Notch signaling plays a key role in cardiac cell differentiation. Mech Dev. 2006;123:626–40. doi: 10.1016/j.mod.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nemir M, Croquelois A, Pedrazzini T, Radtke F. Induction of cardiogenesis in embryonic stem cells via downregulation of Notch1 signaling. Circ Res. 2006;98:1471–8. doi: 10.1161/01.RES.0000226497.52052.2a. [DOI] [PubMed] [Google Scholar]
- 168.Schroeder T, Fraser ST, Ogawa M, Nishikawa S, Oka C, Bornkamm GW, et al. Recombination signal sequence-binding protein Jkappa alters mesodermal cell fate decisions by suppressing cardiomyogenesis. Proc Natl Acad Sci U S A. 2003;100:4018–23. doi: 10.1073/pnas.0438008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fischer A, Klattig J, Kneitz B, Diez H, Maier M, Holtmann B, et al. Hey basic helix-loop-helix transcription factors are repressors of GATA4 and GATA6 and restrict expression of the GATA target gene ANF in fetal hearts. Mol Cell Biol. 2005;25:8960–70. doi: 10.1128/MCB.25.20.8960-8970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kwon C, Han Z, Olson EN, Srivastava D. MicroRNA1 influences cardiac differentiation in Drosophila and regulates Notch signaling. Proc Natl Acad Sci U S A. 2005;102:18986–91. doi: 10.1073/pnas.0509535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sachinidis A, Gissel C, Nierhoff D, Hippler-Altenburg R, Sauer H, Wartenberg M, Hescheler J. Identification of plateled-derived growth factor-BB as cardiogenesis-inducing factor in mouse embryonic stem cells under serum-free conditions. Cell Physiol Biochem. 2003;13:423–9. doi: 10.1159/000075130. [DOI] [PubMed] [Google Scholar]
- 172.Antin PB, Yatskievych T, Dominguez JL, Chieffi P. Regulation of avian precardiac mesoderm development by insulin and insulin-like growth factors. J Cell Physiol. 1996;168:42–50. doi: 10.1002/(SICI)1097-4652(199607)168:1<42::AID-JCP6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 173.Kawai T, Takahashi T, Esaki M, Ushikoshi H, Nagano S, Fujiwara H, Kosai K. Efficient cardiomyogenic differentiation of embryonic stem cell by fibroblast growth factor 2 and bone morphogenetic protein 2. Circ J. 2004;68:691–702. doi: 10.1253/circj.68.691. [DOI] [PubMed] [Google Scholar]
- 174.Dell’Era P, Ronca R, Coco L, Nicoli S, Metra M, Presta M. Fibroblast growth factor receptor-1 is essential for in vitro cardiomyocyte development. Circ Res. 2003;93:414–20. doi: 10.1161/01.RES.0000089460.12061.E1. [DOI] [PubMed] [Google Scholar]
- 175.Morali OG, Jouneau A, McLaughlin KJ, Thiery JP, Larue L. IGF-II promotes mesoderm formation. Dev Biol. 2000;227:133–45. doi: 10.1006/dbio.2000.9875. [DOI] [PubMed] [Google Scholar]
- 176.McDevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J Mol Cell Cardiol. 2005;39:865–73. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Siddle K, Urso B, Niesler CA, Cope DL, Molina L, Surinya KH, Soos MA. Specificity in ligand binding and intracellular signaling by insulin and insulin-like growth factor receptors. Biochem Soc Trans. 2001;29:513–25. doi: 10.1042/bst0290513. [DOI] [PubMed] [Google Scholar]
- 178.Klinz F, Bloch W, Addicks K, Hescheler J. Inhibition of phosphatidylinositol-3-kinase blocks development of functional embryonic cardiomyocytes. Exp Cell Res. 1999;247:79–83. doi: 10.1006/excr.1998.4309. [DOI] [PubMed] [Google Scholar]
- 179.Sauer H, Rahimi G, Hescheler J, Wartenberg M. Role of reactive oxygen species and phosphatidylinositol 3-kinase in cardiomyocyte differentiation of embryonic stem cells. FEBS Lett. 2000;476:218–23. doi: 10.1016/s0014-5793(00)01747-6. [DOI] [PubMed] [Google Scholar]
- 180.Bloch W, Fleischmann BK, Lorke DE, Andressen C, Hops B, Hescheler J, Addicks K. Nitric oxide synthase expression and role during cardiomyogenesis. Cardiovasc Res. 1999;43:675–84. doi: 10.1016/s0008-6363(99)00160-1. [DOI] [PubMed] [Google Scholar]
- 181.Kanno S, Kim PK, Sallam K, Lei J, Billiar TR, Shears LL., 2nd Nitric oxide facilitates cardiomyogenesis in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12277–81. doi: 10.1073/pnas.0401557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wobus AM, Guan K, Jin S, Wellner MC, Rohwedel J, Ji G, et al. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol. 1997;29:1525–1539. doi: 10.1006/jmcc.1997.0433. [DOI] [PubMed] [Google Scholar]
- 183.Hidaka K, Lee JK, Kim HS, Ihm CH, Iio A, Ogawa M, et al. Chamber-specific differentiation of Nkx2.5-positive cardiac precursor cells from murine embryonic stem cells. Faseb J. 2003;17:740–2. doi: 10.1096/fj.02-0104fje. [DOI] [PubMed] [Google Scholar]
- 184.Papadimou E, Menard C, Grey C, Puceat M. Interplay between the retinoblastoma protein and LEK1 specifies stem cells toward the cardiac lineage. EMBO J. 2005;24:1750–61. doi: 10.1038/sj.emboj.7600652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Puceat M. Rb and LEK1: A “Pas de Deux” in Cardiogenesis. Cell Cycle. 2005;4:1030–32. doi: 10.4161/cc.4.8.1905. [DOI] [PubMed] [Google Scholar]