Abstract
In order to assess the role of osteopontin (OPN) in leukocyte accumulation in inflammatory conditions, native OPN and its thrombin cleaved form (OPN+Thr) were studied in vivo using a rodent subcutaneous air pouch model (AP). Both forms of OPN induced macrophage infiltration into the AP in wild-type mice. In animals lacking CD44, macrophage numbers were significantly reduced within the cavity, but cells still accumulated along the subcutaneous lining. In animals lacking endogenous OPN, no differences were found in exogenous OPN-induced macrophage accumulation, although macrophage exhibited increased α4 integrin expression. These studies reveal that both OPN and OPN+Thr attract macrophages in vivo through CD44.
Keywords: Macrophages, Osteopontin, CD44, chemotaxis
Introduction
Osteopontin (OPN), a sialoprotein with functions in tumor cell migration, adhesion, bone remodeling and calcium metabolism, has been found to be one of the major upregulated genes in inflammatory neuropathologies such as multiple sclerosis, experimental autoimmune encephalomyelitis, Theiler’s virus infection, and simian immunodeficiency virus (SIV) encephalitis [1; 2; 3; 4]. The role of OPN in several peripheral inflammatory conditions was shown to correlate to effects on cell attachment and motility [5; 6; 7], suggesting that the upregulation of this molecule in brain pathologies may have implications on the development of an inflammatory infiltrate by influencing such functions.
The interaction of OPN with receptors on cell surface can affect myeloid cells, modulating macrophage migration as well as activation [8], affecting the differentiation, maturation, and survival of dendritic cells, as well as inducing cytokine expression [9; 10]. One important receptor for intact OPN on the cell surface is CD44v6 [6], which cooperates with β1 integrins to allow cell motility and chemotaxis in an arginine-glycine-aspartic acid (RGD)-independent manner [11]; however, thrombin can cleave OPN, shifting receptor usage to a RGD-dependent binding. Several integrins have also been identified as OPN receptors, including numerous members of the β1 and αv families [5; 12; 13; 14; 15; 16; 17], which recognize an RGD sequence that is present within the amino-terminal fragment of thrombin-cleaved OPN (OPN+Thr). These different potential interactions and usage of receptors by OPN or its cleaved form can influence different cell type migration or adherence, depending on what is expressed [5].
The modulation of different OPN receptors expression of different cell types can produce different results in terms of inflammatory response. Macrophages, which are particularly relevant in the inflammatory context, express both αv and ß1 integrins, which can be used as phagocytic receptors [18]. The expression of OPN receptors, particularly CD44v6, can be modulated by cytokines in vitro [19; 20]. In vivo, the upregulation of CD44v6 is observed in tumor-infiltrating macrophages [21], and on peripheral monocytes of monkeys that develop SIV encephalitis [22], suggesting the multifactorial character of the regulation of OPN receptor expression.
HIV infection of the brain can result in neurological disorders, linked to macrophage accumulation in the brain [23]. We have found OPN to be expressed in the brains of HIV-infected people and SIV-infected monkeys [4]. Our in vitro studies did not show chemotactic properties of OPN on monocytes, but transmigration studies revealed that OPN could inhibit the egress of transmigrated macrophages back through an endothelial layer, thus potentially leading to the accumulation of macrophages in tissue such as the brain [24].
Although we could not find in vitro chemotaxis of OPN on monocytes, OPN has been found to induce both Langerhans and dendritic cell chemotaxis in vivo [25; 26]. To further examine the in vivo chemotactic properties of OPN, we tested the ability of OPN to induce macrophage migration in a rodent air-pouch (AP) model.
Material and Methods
Mice
Breeding pairs of CD44 KO on the C57BL/6J × 129J background background [27], OPN KO on the C57BL/6 background [28], and control C57BL/6 and C57BL/6J × 129J WT mice were purchased from Jackson Laboratory (Bar Harbor, ME). The animals were maintained in The Scripps Research Institute’s specific-pathogen free animal facility according to NIH guidelines, and all experiments were performed under IACUC approval.
Air Pouch
The animals were anesthetized by halothane inhalation, and were shaved on the dorsum before a subcutaneous injection of 5 ml of sterile air. The AP was matured for a period of 3 days before the injection of the different stimuli.
AP injection
The AP cavities were injected with 2 ml of sterile saline without test substances, or sterile saline containing 7 mM of either mouse recombinant MCP-3 (Peprotech), mouse recombinant OPN (R&D Systems, Minneapolis, MN), or thrombin-treated OPN. For the thrombin treatment, 3.125 U of thrombin (Sigma-Aldrich, St.Louis, MO) was added to 1 □g of mouse recombinant OPN, and incubated for 30 minutes at 37°C. Thrombin was neutralized by the addition of hirudine (Sigma-Aldrich) at 1.3-fold higher concentration than thrombin (4.06 U for 1 □g of OPN), for another 30 minutes at 37°C. Tubes containing only thrombin were incubated for 30 minutes at 37°C in parallel, and neutralized with hirudine for injection of controls.
Western Blots
OPN concentrations were determined by Bio-Rad protein assay kit prior to thrombin digestion. The protein samples were separated by 4% to 20% SDS-PAGE and electrotransferred onto polyvinylidene difluoride membranes (Amersham Biosciences) by semi-dry transfer (Bio-Rad). The membranes were probed with anti-osteopontin polyclonal antibody (OT-7, R&D Systems) for 1 h at room temperature. The antibody was detected using anti-goat conjugated with horseradish peroxidase. The reactive proteins were visualized by means of the Pico Supersignal West Horseradish Peroxidase Detection kit (Pierce, Rockford, IL).
AP exudates
Cells in the AP cavity were harvested 24 hours after the injection of stimuli by the injection of 6 ml of ice-cold saline. Cells from individual animals were counted in the hemocytometer and pooled by group (3 mice per pool) for staining. Three or more experiments for each condition were performed for determination of surface marker expression on infiltrating cells.
FACS
Cells were resuspended in FACS buffer (PBS containing 2% FBS and 0.2% sodium azide, and stained with antibodies recognizing CD3, CD4, CD8, B220, CD11b, Gr-1, CD44, CD11c, CD80, CD86, CD62L and CD49d (BD Pharmingen, San Diego, CA). Fc receptors were blocked using CD16/CD32 blocking antibodies for 5 minutes prior to the staining. Cells were fixed using 2% paraformaldehyde, and acquired in a FACScalibur apparatus (BD Biosciences, San Jose, CA). Samples were analyzed in FlowJo Software (TreeStar, Ashland, OR).
Immunohistochemistry
After the harvesting of cells from the cavity, the skin surrounding the AP was collected and fixed in Carnoy’s fixative. After processing the tissue and embedding in paraffin, sections were obtained for immunohistochemical (IHC) procedures. IHC staining followed a basic indirect protocol using a citrate antigen retrieval method, for antibodies against F4/80, as previously described [4].
Statistical Analysis
Groups were compared by two-way ANOVA followed by Bonferroni’s post-hoc tests, using Prism software (version 4.0b, GraphPad Software, San Diego, CA).
Results
OPN attracts macrophages in vivo
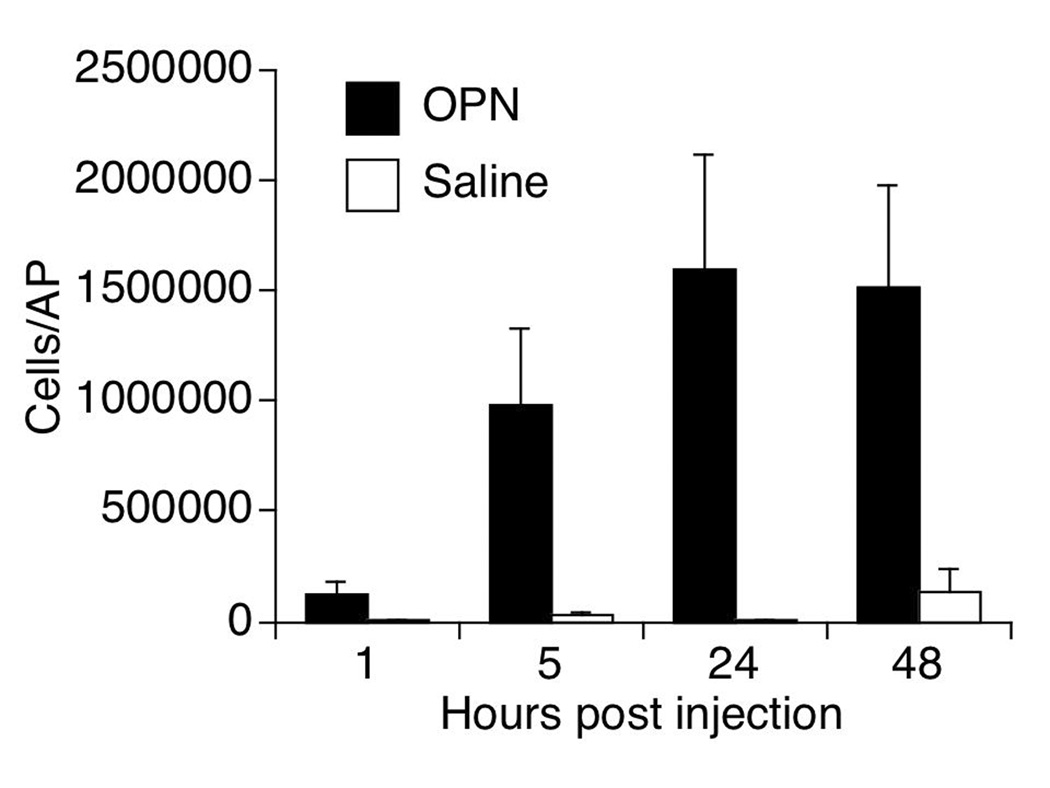
In order to establish the optimal time-point for analysis of macrophages infiltrating into the mature AP as a result of OPN, a 48-hour time course was performed using 7 mM of OPN (5 µg of OPN in 2 mls saline) in wild-type (WT) mice. The injection of OPN induced a significantly higher number of cells into the cavity in comparison to controls injected with saline at all time points analyzed (Figure 1). The largest number of infiltrating cells was obtained at 24 and 48 hrs, the majority of them being mononuclear CD11b+ cells. The 24 hr time point was selected for the analysis of macrophages in the AP.
Figure 1.
Kinetics of cell accumulation after OPN or Saline injection into AP of WT mice. Results represent the average ± SEM of absolute numbers of cells in the AP lavage of 5 animals per time-point. The 24 hour time point had the highest average number of cells, and was used for subsequent studies.
OPN chemoattractant properties are CD44 dependent
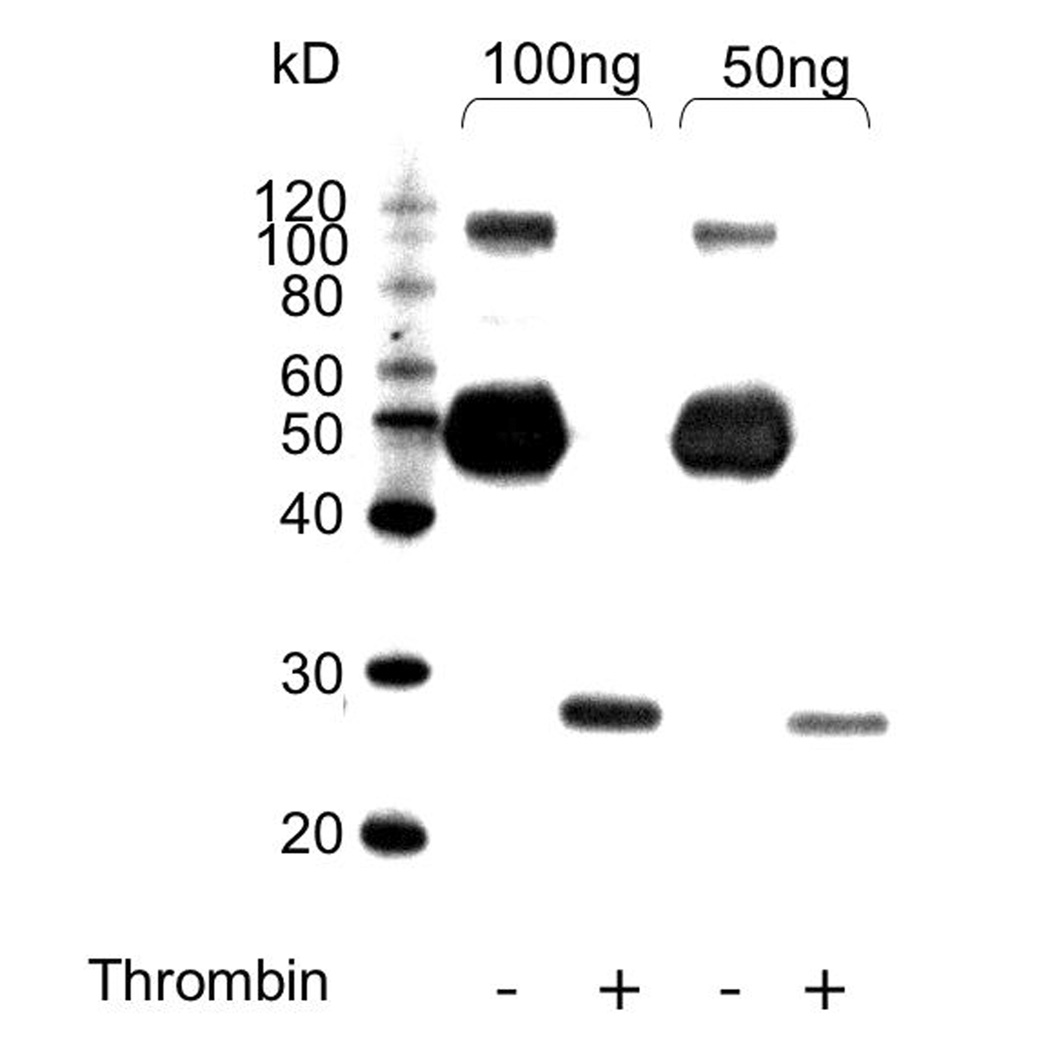
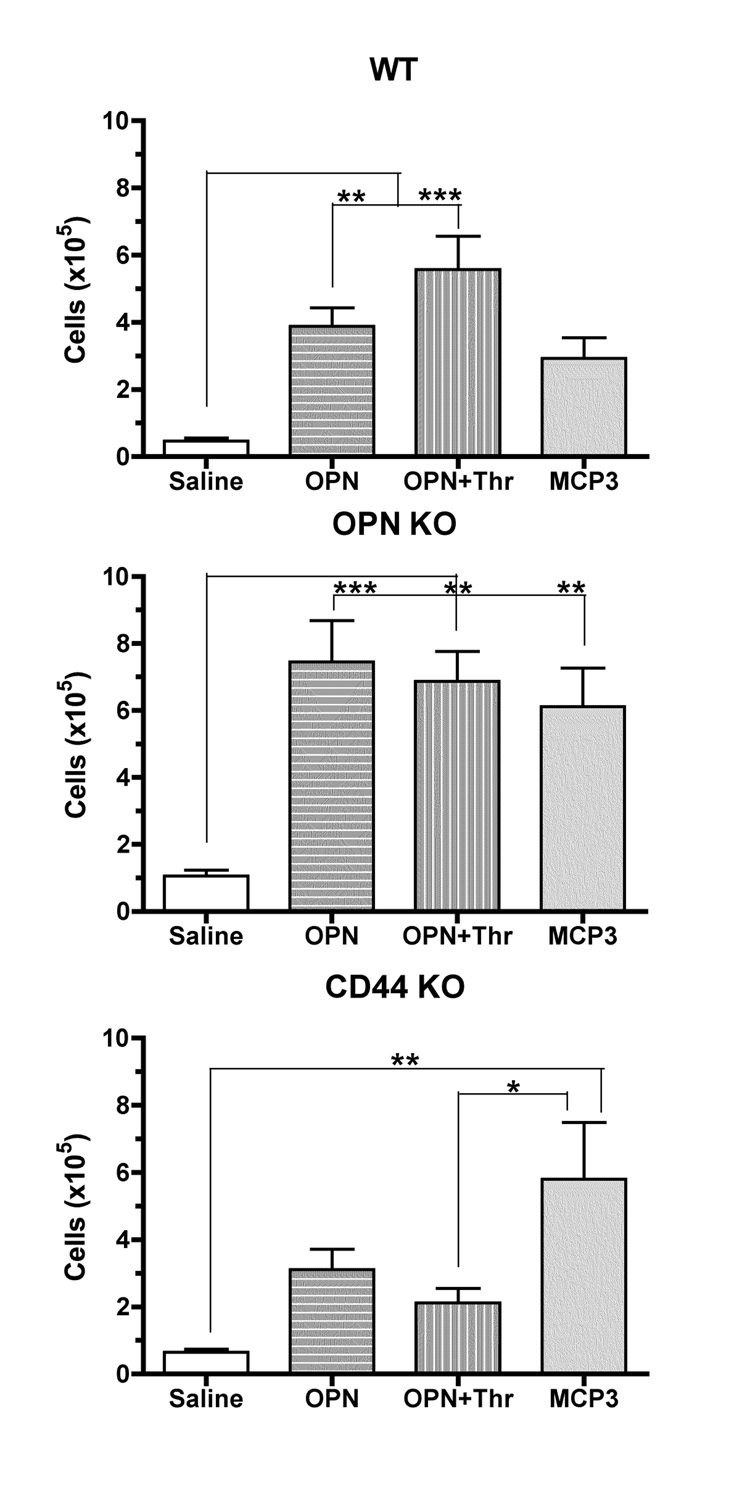
Since thrombin digestion was previously shown to influence microphage migration properties towards OPN [29], we examined the effects of native OPN and OPN+Thr on cell migration into the AP, and compared to that found for MCP-3, a largely monocyte-specific chemokine. The full ability of thrombin to digest the OPN molecule was confirmed prior to the injection of the cleaved form into the air-pouch (Figure 2). In addition to WT mice, CD44 knockout (KO) animals were used to assess the role of CD44 and its isoforms (including CD44v6), and OPN KO mice were used to assess the role of endogenous OPN in these experiments. Baseline infiltration was tested on control strains, C57BL/6Jx129/J and C57BL/6J, corresponding to the background of the CD44 and OPN knockout strains, respectively. As these two control strains provided similar results, we utilized C57BL/6Jx129/J mice as WT controls for all experiments. APs from these experimental and control strains were inoculated with the different stimuli, and cells were harvested 24 hrs later (Figure 3). When injected at equivalent molarities (7 mM), OPN, OPN+Thr and MCP-3 had similar effects on the number of cells accumulating in the AP cavities in WT and OPN KO mice. However, in mice lacking CD44, only MCP-3 induced a significant cellular influx into the AP. This suggests that CD44 expression is important for cell migration induced by OPN as well as OPN+Thr. Controls injected with thrombin plus hirudine did not show a significant cell enrichment in the airpouches in comparison to animals injected with vehicle (not shown).
Figure 2.
OPN cleavage by thrombin. The efficiency of thrombin treatment was determined by western blot of OPN before and after digestion, with digestion leading to the lower molecular weight fragment recognized by the antibody.
Figure 3.
Cell migration induced by OPN, OPN+Thr and MCP-3into AP cavities. Mature APs were induced in WT, CD44 KO and OPN KO mice. Results represent the mean ± SEM of 4–7 experiments. *p<0.05, **p<0.01 and ***p<0.001 by two-way ANOVA followed by Bonferroni’s post-hoc tests.
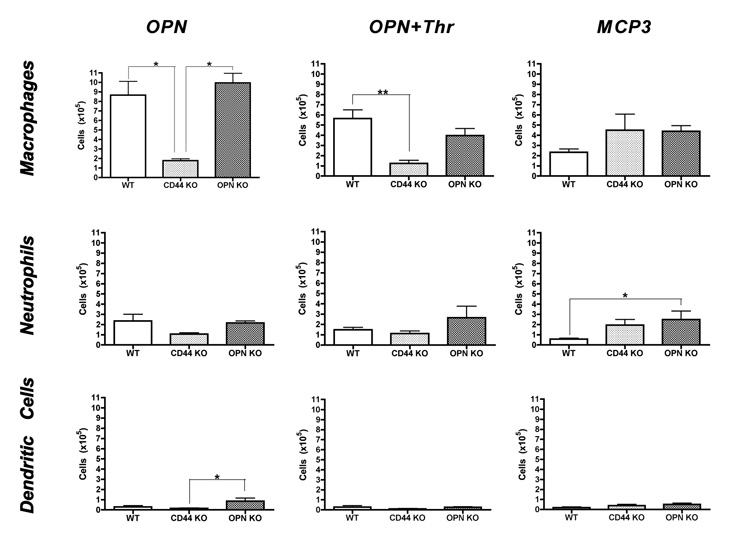
The cells recovered from the AP were predominantly CD11b+ (data not shown), indicated myeloid lineage. The cells in the exudates were then analyzed to determine the contribution of macrophages, granulocytes, and dendritic cells. Cells were stained with antibodies against CD11b and Gr1 to define macrophages (CD11b+ Gr1 low/intermediate), granulocytes (CD11b+ Gr1 high) and dendritic cells (CD11b+ CD11c+). In all cases, macrophages were the predominant cell type infiltrating the air pouch (Figure 4). Significantly fewer macrophages were present in the AP in CD44 KO in response to OPN and OPN+Thr, whereas no differences were found in response to MCP-3 (Figure 4, top). This indicates that the transudation of macrophages by OPN and OPN+Thr is primarily dependent upon CD44. Limited differences were also found for neutrophils and dendritic cells in OPN KO mice (Figure 4, middle and bottom).
Figure 4.
Macrophage, granulocyte, and dendritic cell infiltration into AP. Mature APs were induced in mice of the indicated genotype, and cell migration induced by the given agents. Cells harvested from the AP were counted and characterized by flow cytometry. Values represent average ± SEM of the same animals shown in Figure 3. *p<0.05 and **p<0.01 by two-way ANOVA followed by Bonferroni’s post-hoc tests.
Adhesion and co-stimulatory molecule expression do not explain chemoattractant properties
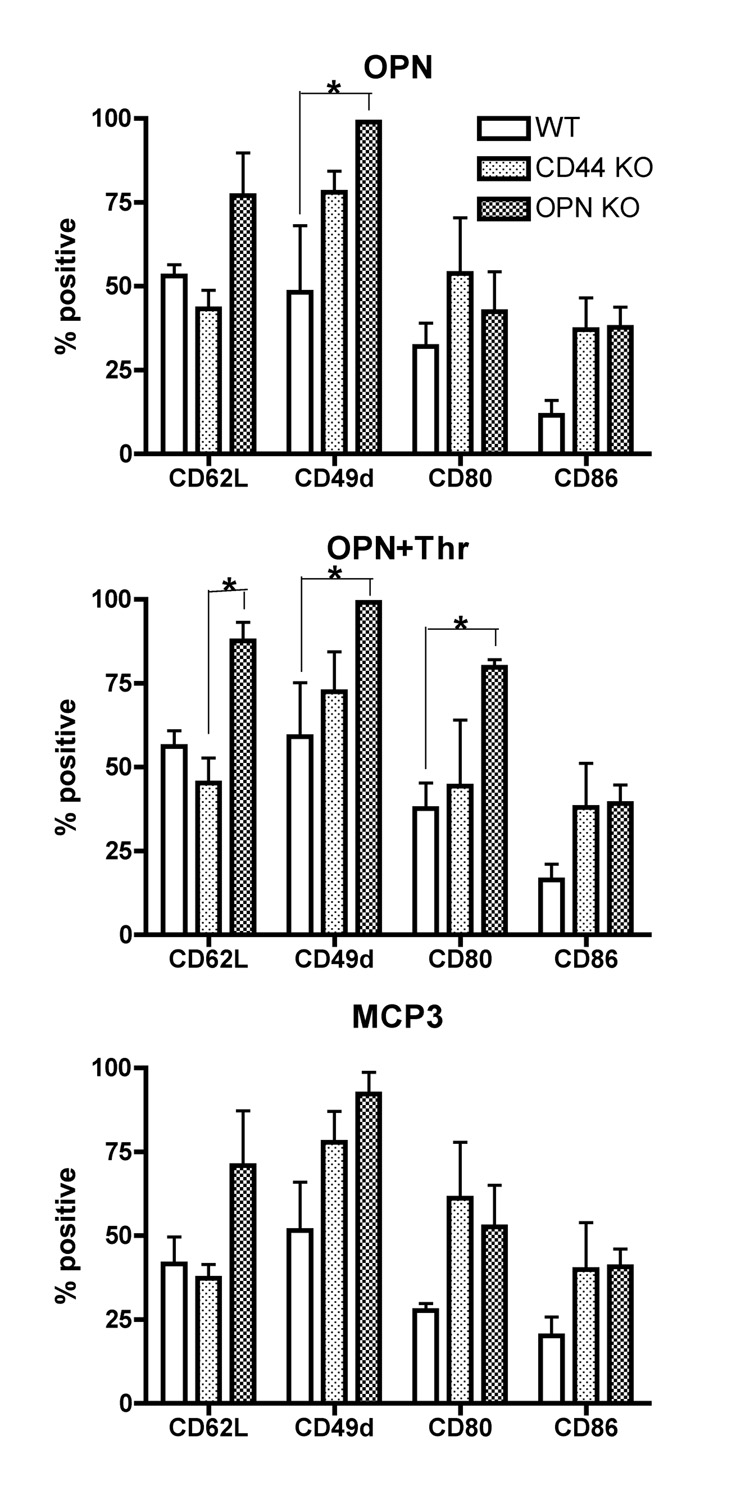
In order to characterize the macrophages in the AP in more detail, the expression of surface markers relevant for transmigration and adhesion (CD62L and CD49d) and for T cell costimulatory molecules (CD80 and CD86), were analyzed on AP macrophages (CD11b+ Gr1 low/intermediate) (Figure 5). Interestingly, in OPN KO mice, macrophages attracted to either OPN and OPN+Thr had significantly increased levels of CD49d relative to WT mice (Figure 5, top and middle). Although higher levels of CD49d were also found on macrophages attracted to MCP-3 in OPN knockouts (Figure 5, bottom), it did not reach statistical significance. Additionally, in response to OPN+Thr, the AP macrophages in OPN KO mice also had higher levels of CD80 than those in WT mice, and higher levels of CD62L than those in CD44 KO mice (Figure 5, middle).
Figure 5.
Surface markers on macrophages obtained from APs. Mature APs were induced in mice of the indicated genotype, and cell migration induced by the given agents. Cells were harvested from the AP cavities 24 hours after the injection of various stimuli, and CD11b+ Gr1low macrophages were characterized for the expression of functional surface markers by flow cytometry. The Y-axis corresponds to the percentage of positive cells for surface markers indicated in the X-axis. Positive cells were determined by the expression of markers above the baseline levels determined upon the staining with isotype controls. Values represent average ± SEM of the same animals shown in Figure 3. * p<0.05 by two-way ANOVA followed by Bonferroni’s post-hoc tests.
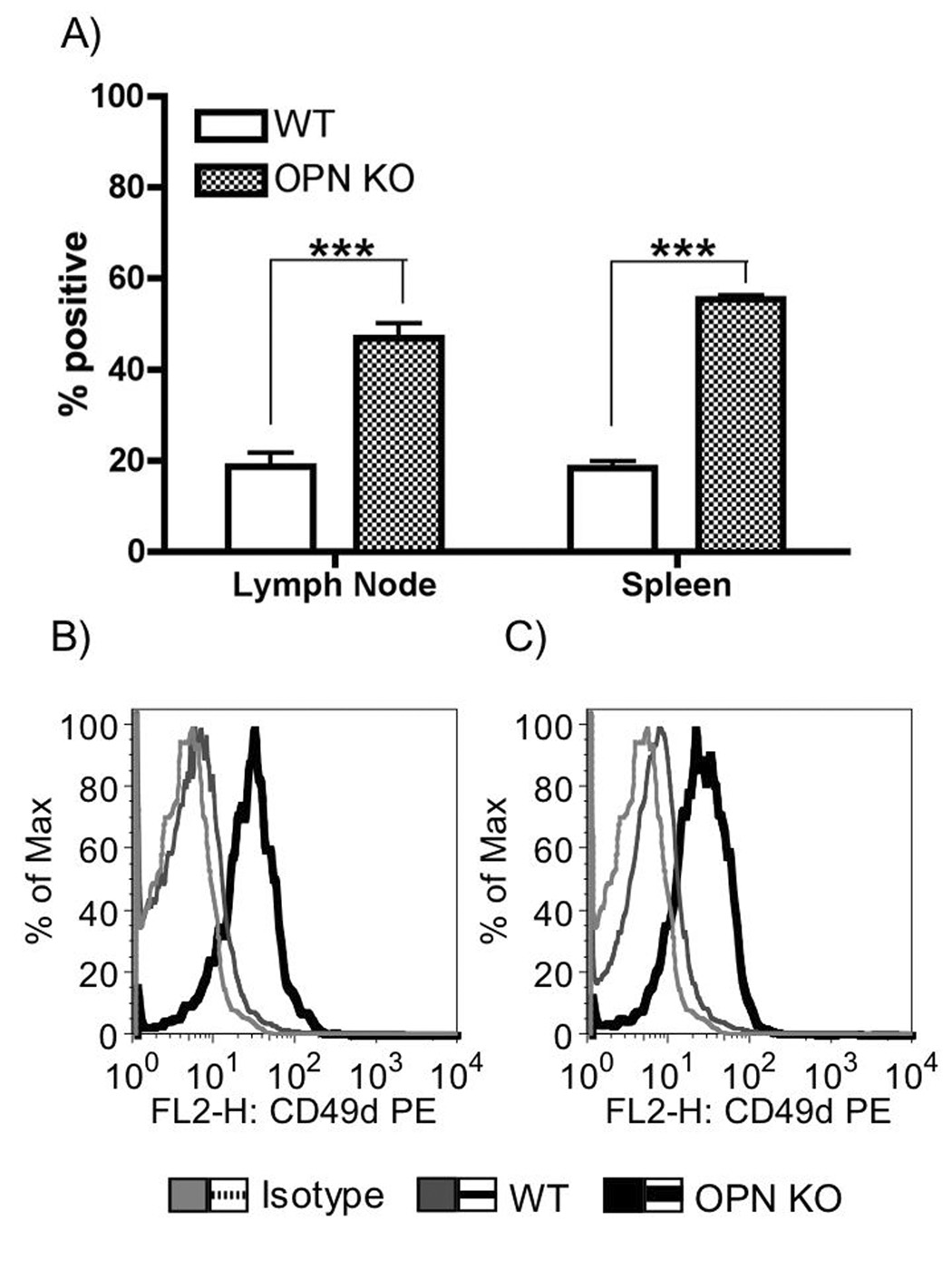
The increased CD49d in OPN KO mice was intriguing given that CD49d is the alpha chain of the α4b1 homing receptor (also known as VLA-4), an osteopontin receptor [17]. In order to assess whether this is a general property of OPN-deficient macrophages, we examined macrophages from lymph nodes and spleens of WT and OPN KO mice. Indeed, CD49d was increased on macrophages from both lymphoid organs in OPN KO (Figure 6).
Figure 6.
CD49d expression on macrophages in lymphoid organs. A) Graph showing mean ± SEM of 4 experiments. ***p<0.001, two-way ANOVA followed by Bonferroni’s post-hoc test. B and C) Representative histogram overlays of macrophages staining from B) Lymph nodes and C) Spleen. Light grey – isotype control. Grey – C57BL/6J wild-type. Black – OPN KO.
Macrophage migration towards OPN, but not adhesion, is affected by the absence CD44
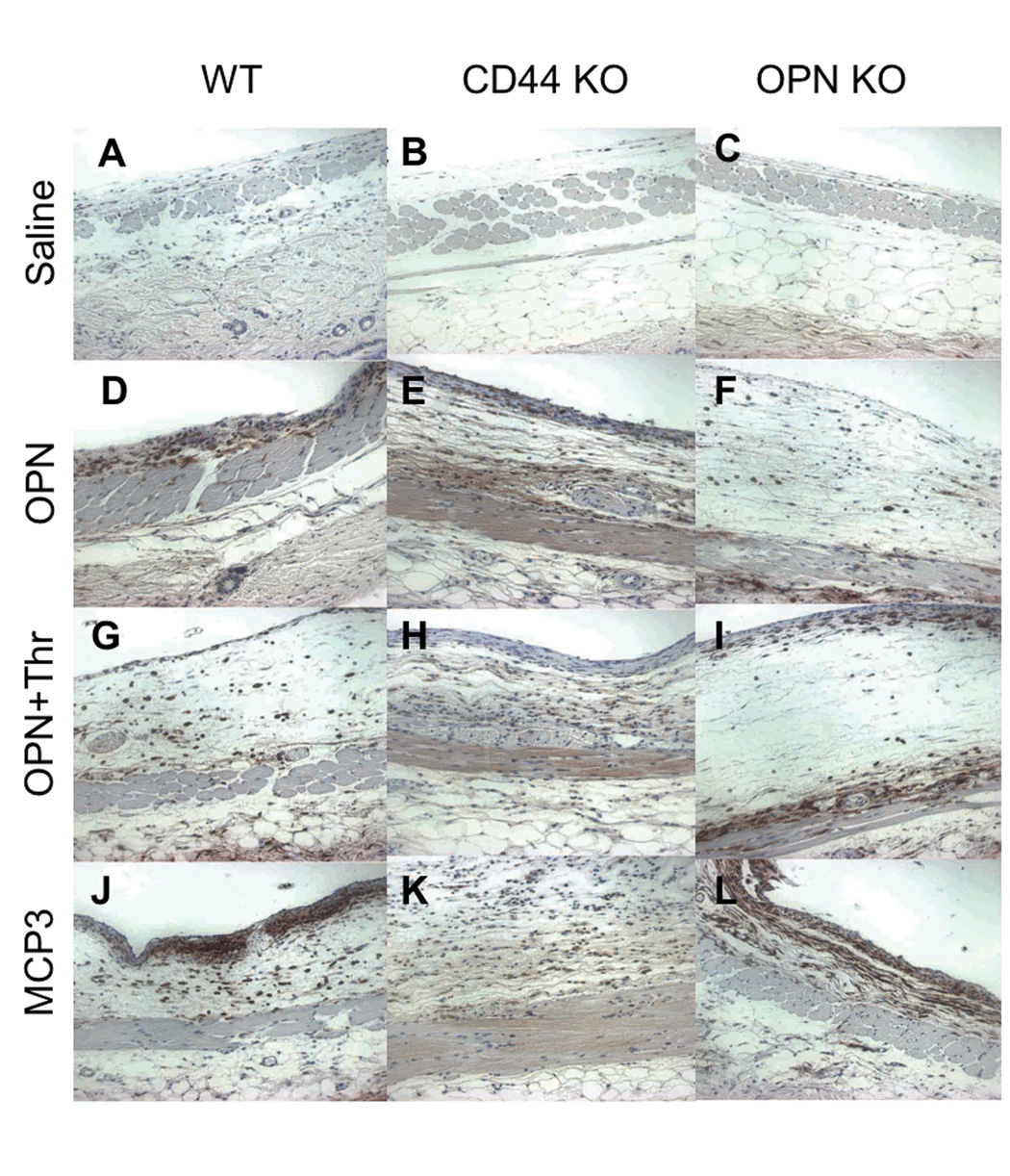
We also examined the lining of the AP from the above experiments. Histological assessment revealed that all stimuli (OPN, OPN+Thr, and MCP-3) induced cell adherence on the lining of the AP cavity (Figure 7). Although there were fewer cells within the AP, CD44 KO animals exhibited a much more robust cell accumulation on the AP lining (Figures 7E, 7H, 7K).
Figure 7.
Cell adherence on pouch lining. Histological analysis of the air pouch surrounding lining after injection of stimulant agents was performed on paraffin-fixed sections immunostained for detection of F4/80+ cells, corresponding to macrophages. A, B and C correspond to tissue from saline injected AP; D, E, F are from OPN injected AP; G, H, I are samples from OPN+Thr injected AP; J, K, L are from MCP-3 injected AP. A, D, G, J are sections from AP lining from B6 mice; B, E, H, K from CD44 KO mice; and C, F, I, L from OPN KO mice.
Discussion
In the present study we addressed the capacity of OPN and its thrombin-cleaved form to induce accumulation of macrophages. Both forms of OPN, when injected into the AP, were able to attract infiltrating immune cells. However, the migration of cells into the lumen was in large part dependent on the presence of CD44. This was specific to OPN, as MCP-3 did not show a dependence on CD44. For all stimuli, the absence of CD44 resulted in a large number of cells remaining attached to the AP lining.
In our experiments we did not systemically stimulate the immune system, thus the cells brought into the AP, either into the lumen, or adhered on the AP lining, were not pre-activated. Therefore, our results represent a parallel to the immediate effects of local upregulation of OPN that can occur in inflamed sites. The upregulation of OPN gene can be observed as early as seven hours following tissue injury [30]. The result of this upregulation on unstimulated cells, as modeled here, can be crucial to understand the initial steps of the development of an inflammatory process. Furthermore, tissue damage may be accompanied by the local increase on thrombin levels, which is a modifier of the OPN molecule that may affect the properties of the molecule on cells.
Differences in the capability of OPN versus OPN+Thr in attracting macrophages to the site were previously observed in animals with arthritis, but not in control animals [29], suggesting that a pre-activation status may be crucial, perhaps altering the expression of surface molecules necessary for response and migration. In our experiments we found that in the AP both OPN and OPN+Thr can induce cell migration, and that in both cases it is CD44 dependent. It is possible that the CD44 binding site for OPN, or OPN’s ability to trigger signals through this binding that facilitate migration, were not affected by the thrombin digestion. The decreased number of macrophages in the exudate from CD44 KO animals was not due to global migration defects, since CD44 expression was not a requirement to mediate migration induced by MCP-3. On the other hand, adhesion properties were globally changed in CD44 KO animals, as with all stimuli, a larger number of cells were attached to the subcutaneous lining.
CD44 is an important molecule for adhesion and migration through interaction with hyaluronic acid at the extracellular matrix, and in some inflammatory models CD44 expression correlates with severity of lesions [31; 32]. In addition, at least one isoform of CD44 has been shown to be a receptor for OPN [11]. This suggests a role for CD44 on OPN-mediated cell accumulation at early stages of inflammatory response.
In vitro studies using cultured RAW 264.7 macrophage-like cells have revealed that macrophages in which OPN gene was silenced were deficient for several functions, including migration [33]. In our in vivo model, the absence of endogenous expression of OPN by macrophages did not interfere with the migration towards OPN or MCP-3, since both WT and OPN KO animals responded to stimuli with cell migration. However, we observed an increased expression of CD49d in OPN KO animals. This could be due to a compensatory increase to facilitate migration, or to a higher availability of the molecule on cell surface in the absence of the endogenous ligand OPN.
The ability of OPN to induce macrophage chemotaxis is controversial. Our study, as well as those of others [34; 35] indicate that OPN is an effective, and in some cases key, inducer of macrophage infiltration in vivo. However our previous studies [24] and those of others [29; 35; 36; 37] report divergent effects of OPN in vitro. This can be attributed to experimental paradigms, including the cell type examined (monocytes, peritoneal macrophages, dendritic cells), and properties of the OPN itself, as distinct roles are played by native OPN versus thrombin-cleaved OPN [29], as well as intracellular versus extracellular OPN [37]. Furthermore, we note that some studies utilize bacterial-produced as opposed to mammalian cell-derived OPN. In addition to pronounced differences in post-translational modifications in this heavily modified protein, the issue of endotoxin contribution to the measured OPN effects has been raised, and some of the in vitro effects of OPN have been linked to such contamination [38].
Our studies reveal that OPN is a macrophage attractant in an in vivo model for cell migration, where transudation, but not adhesion, was specifically mediated by CD44. The property of the OPN molecule in inducing cell migration into the air-pouch cavity was not essentially altered by thrombin cleavage of OPN. This suggests that the cleavage site does not alter the molecular domains responsible for attraction in vivo, and that the exposure of a cryptic epitope by thrombin-cleavage [29] does not play a major role in this model. Overall, in the absence of CD44, macrophage transmigration into the air-pouch cavity towards OPN stimuli was impaired, although adhesion functions were not altered. Taken together, our results offer insights on the in vivo mechanisms by which OPN upregulation in inflamed tissues can contribute to the cell accumulation and perpetuation of the inflammatory process.
Acknowledgements
We thank Dr. Toshimitsu Uede (Institute of Immunological Sciences, Hokkaido University, Sapporo, Japan) for interesting discussions and suggestions and Dr. Momtchilo Russo (Biomedical Science Institute, University of Sao Paulo, Sao Paulo, Brazil) for insights and discussion. MCGM performed experiments, participated on discussions and wrote the manuscript, MP, DDW and DH performed experiments. HSF participated on discussions, wrote the manuscript and obtained funding. This work was supported by NIH grants NS045534, MH073490 and MH062261. This is manuscript #19528 of The Scripps Research Institute.
REFERENCES
- 1.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 2.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 3.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: Attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J Immunol. 2002;168:2096–2099. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 4.Roberts ES, Zandonatti MA, Watry DD, Madden LJ, Henriksen SJ, Taffe MA, Fox HS. Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol. 2003;162:2041–2057. doi: 10.1016/S0002-9440(10)64336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liaw L, Skinner MP, Raines EW, Ross R, Cheresh DA, Schwartz SM, Giachelli CM. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J Clin Invest. 1995;95:713–724. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 7.Weber GF, Cantor H. The immunology of Eta-1/osteopontin. Cytokine Growth Factor Rev. 1996;7:241–248. doi: 10.1016/s1359-6101(96)00030-5. [DOI] [PubMed] [Google Scholar]
- 8.Weber GF, Zawaideh S, Hikita S, Kumar VA, Cantor H, Ashkar S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J Leukoc Biol. 2002;72:752–761. [PubMed] [Google Scholar]
- 9.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, Cantor H. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawamura K, Iyonaga K, Ichiyasu H, Nagano J, Suga M, Sasaki Y. Differentiation, maturation, and survival of dendritic cells by osteopontin regulation. Clin Diagn Lab Immunol. 2005;12:206–212. doi: 10.1128/CDLI.12.1.206-212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katagiri YU, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K, Chikuma S, Yagita H, Okumura K, Murakami M, Saiki I, Chambers AF, Uede T. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59:219–226. [PubMed] [Google Scholar]
- 12.Nasu K, Ishida T, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Expression of wild-type and mutated rabbit osteopontin in Escherichia coli, and their effects on adhesion and migration of P388D1 cells. Biochem J. 1995;307(Pt 1):257–265. doi: 10.1042/bj3070257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denda S, Reichardt LF, Muller U. Identification of osteopontin as a novel ligand for the integrin alpha8 beta1 and potential roles for this integrin-ligand interaction in kidney morphogenesis. Mol Biol Cell. 1998;9:1425–1435. doi: 10.1091/mbc.9.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu DD, Lin EC, Kovach NL, Hoyer JR, Smith JW. A biochemical characterization of the binding of osteopontin to integrins alpha v beta 1 and alpha v beta 5. J Biol Chem. 1995;270:26232–26238. doi: 10.1074/jbc.270.44.26232. [DOI] [PubMed] [Google Scholar]
- 15.Miyauchi A, Alvarez J, Greenfield EM, Teti A, Grano M, Colucci S, Zambonin-Zallone A, Ross FP, Teitelbaum SL, Cheresh D, et al. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J Biol Chem. 1991;266:20369–20374. [PubMed] [Google Scholar]
- 16.Yokosaki Y, Matsuura N, Sasaki T, Murakami I, Schneider H, Higashiyama S, Saitoh Y, Yamakido M, Taooka Y, Sheppard D. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem. 1999;274:36328–36334. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 17.Bayless KJ, Meininger GA, Scholtz JM, Davis GE. Osteopontin is a ligand for the alpha4beta1 integrin. J Cell Sci. 1998;111(Pt 9):1165–1174. doi: 10.1242/jcs.111.9.1165. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JD, Hess KL, Cook-Mills JM. CD44, alpha(4) integrin, and fucoidin receptor-mediated phagocytosis of apoptotic leukocytes. J Leukoc Biol. 2003;74:810–820. doi: 10.1189/jlb.0303092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braumuller H, Gansauge S, Ramadani M, Gansauge F. CD44v6 cell surface expression is a common feature of macrophages and macrophage-like cells - implication for a natural macrophage extravasation mechanism mimicked by tumor cells. FEBS Lett. 2000;476:240–247. doi: 10.1016/s0014-5793(00)01737-3. [DOI] [PubMed] [Google Scholar]
- 20.Marroquin CE, Downey L, Guo H, Kuo PC. Osteopontin increases CD44 expression and cell adhesion in RAW 264.7 murine leukemia cells. Immunol Lett. 2004;95:109–112. doi: 10.1016/j.imlet.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Mytar B, Baran J, Gawlicka M, Ruggiero I, Zembala M. Immunophenotypic changes and induction of apoptosis of monocytes and tumour cells during their interactions in vitro. Anticancer Res. 2002;22:2789–2796. [PubMed] [Google Scholar]
- 22.Marcondes MC, Burdo TH, Watry DD, Fox HS. Increased CD44v6 Expression Levels on Monocytes Precede the Development of Encephalitis in SIV-Infected Rhesus Macaques. J Inf Dis. 2008;197:1567–1576. doi: 10.1086/588002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burdo TH, Wood MR, Fox HS. Osteopontin prevents monocyte recirculation and apoptosis. J Leukoc Biol. 2007;81:1504–1511. doi: 10.1189/jlb.1106711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauer S, Hauser IA, Obermuller N, Holzmann Y, Geiger H, Goppelt-Struebe M. Synergistic induction of osteopontin by aldosterone and inflammatory cytokines in mesangial cells. J Cell Biochem. 2008;103:615–623. doi: 10.1002/jcb.21433. [DOI] [PubMed] [Google Scholar]
- 26.Weiss JM, Renkl AC, Maier CS, Kimmig M, Liaw L, Ahrens T, Kon S, Maeda M, Hotta H, Uede T, Simon JC. Osteopontin is involved in the initiation of cutaneous contact hypersensitivity by inducing Langerhans and dendritic cell migration to lymph nodes. J Exp Med. 2001;194:1219–1229. doi: 10.1084/jem.194.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, Wakeham A, Shahinian A, Catzavelos C, Rak J, Furlonger C, Zakarian A, Simard JJ, Ohashi PS, Paige CJ, Gutierrez-Ramos JC, Mak TW. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–2233. [PubMed] [Google Scholar]
- 28.Rittling SR, Matsumoto HN, McKee MD, Nanci A, An XR, Novick KE, Kowalski AJ, Noda M, Denhardt DT. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J Bone Miner Res. 1998;13:1101–1111. doi: 10.1359/jbmr.1998.13.7.1101. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto N, Sakai F, Kon S, Morimoto J, Kimura C, Yamazaki H, Okazaki I, Seki N, Fujii T, Uede T. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J Clin Invest. 2003;112:181–188. doi: 10.1172/JCI17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Wu W, Dentchev T, Zeng Y, Wang J, Tsui I, Tobias JW, Bennett J, Baldwin D, Dunaief JL. Light damage induced changes in mouse retinal gene expression. Exp Eye Res. 2004;79:239–247. doi: 10.1016/j.exer.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Katoh S, Matsubara Y, Taniguchi H, Fukushima K, Mukae H, Kadota J, Matsukura S, Kohno S. Characterization of CD44 expressed on alveolar macrophages in patients with diffuse panbronchiolitis. Clin Exp Immunol. 2001;126:545–550. doi: 10.1046/j.1365-2249.2001.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sano N, Kitazawa K, Sugisaki T. Localization and roles of CD44, hyaluronic acid and osteopontin in IgA nephropathy. Nephron. 2001;89:416–421. doi: 10.1159/000046113. [DOI] [PubMed] [Google Scholar]
- 33.Nystrom T, Duner P, Hultgardh-Nilsson A. A constitutive endogenous osteopontin production is important for macrophage function and differentiation. Exp Cell Res. 2007;313:1149–1160. doi: 10.1016/j.yexcr.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 34.Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol. 1998;152:353–358. [PMC free article] [PubMed] [Google Scholar]
- 35.Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschop MH, Bruemmer D. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117:2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka K, Morimoto J, Kon S, Kimura C, Inobe M, Diao H, Hirschfeld G, Weiss JM, Uede T. Effect of osteopontin alleles on beta-glucan-induced granuloma formation in the mouse liver. Am J Pathol. 2004;164:567–575. doi: 10.1016/s0002-9440(10)63146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu B, Suzuki K, Goldberg HA, Rittling SR, Denhardt DT, McCulloch CA, Sodek J. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol. 2004;198:155–167. doi: 10.1002/jcp.10394. [DOI] [PubMed] [Google Scholar]
- 38.Konno S, Hoshi T, Taira T, Plunkett B, Huang SK. Endotoxin contamination contributes to the in vitro cytokine-inducing activity of osteopontin preparations. J Interferon Cytokine Res. 2005;25:277–282. doi: 10.1089/jir.2005.25.277. [DOI] [PubMed] [Google Scholar]