Abstract
The objective was to optimize and evaluate the in vivo activities of our novel bifunctional peptide inhibitor (BPI), which alters immune response in autoimmune diseases by modulating the immunological synapse formation. Previously, we have designed PLP-BPI and GAD-BPI by conjugating myelin proteolipid protein (PLP)139–151 and glutamic acid decarboxylase (GAD)208–217, respectively, with CD11a237–246 via a spacer peptide. PLP-BPI and GAD-BPI suppressed the disease progression in experimental autoimmune encephalomyelitis (EAE) and in type-1 diabetes, respectively. In this study, various PLP-BPI derivatives were synthesized and evaluated in the EAE model. Intravenous injections of PLP-BPI derivatives prevented the disease progression more efficiently than did unmodified PLP-BPI. Production of interleukin-17, a potent pro-inflammatory cytokine found commonly among MS patients, was significantly low in Ac-PLP-BPI-NH2-2-treated mice. Treatment given after the disease onset could dramatically ameliorate the disease. BPI induced anaphylactic responses at a lower incidence than PLP139–151. In conclusion, PLP-BPI derivatives can effectively suppress the disease severity and morbidity of EAE by post-onset therapeutic treatment as well as prophylactic use.
Keywords: bifunctional peptide inhibitor, immunological synapse, experimental autoimmune encephalomyelitis, proteolipid protein, anaphylactic response, interleukin-17, peptide, multiple sclerosis, autoimmune disease, therapy
INTRODUCTION
Multiple sclerosis (MS) is an organ-specific disease involving perivascular lymphocytic infiltration, leading to inflammation of white matter and subsequent injury of both white matter and underlying axons. Adaptive immune responses, generated from autoantigen-specific T cells and from antibody-producing B cells, have been described in the blood, spinal fluid, and central nervous system of patients with MS. In autoimmune diseases such as MS and type-1 diabetes, autoreactive T cells recognize self-protein fragments from myelin sheath (e.g., myelin proteolipid protein (PLP), myelin basic protein (MBP)) and glutamic acid decarboxylase (GAD)) and are believed to attack the structures in the central nervous system and the pancreatic islet β-cells, respectively. The activation of T cells, including such autoreactive T cells, is triggered by T cell/antigen-presenting cell (APC) interaction through a variety of receptor/ligand pairs called Signal-1 (TCR/MHC-antigen complex) and Signal-2 (LFA-1/ICAM-1, CD28/B7, CTLA-4/B7, etc.). The overall assembly of Signal-1, Signal-2, and other co-stimulatory signals at the interface of T cell and APC is called the “immunological synapse” (1). It has been demonstrated that the translocation and segregation of Signal-1 and Signal-2 clusters are parts of the dynamic process of immunological synapse formation (1–4).
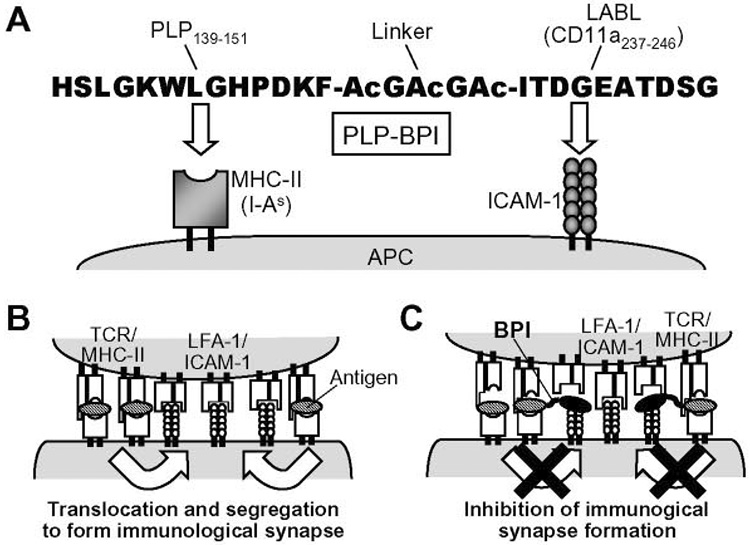
Current therapeutic approaches aim at broad modulation of the immune response with drugs that block or delete one of its fundamental components. In contrast, the hope is that suppression of MS and other autoimmune diseases can be achieved by inducing a specific adaptive immune response while preserving the capability of the immune system to fight infections. However, such antigen-specific treatments have so far eluded biomedical researchers. Thus, one of the therapeutic strategies for treating autoimmune diseases is to develop a molecule that can exclusively inhibit the activation and/or alter the responses of a specific population(s) of autoreactive T cells without affecting the other necessary and normal populations. To alter specific adaptive immune responses, we have developed a novel bifunctional peptide inhibitor (BPI) to target and inhibit the immunological synapse formation (Fig. 1). The BPI molecule is constructed by conjugating an antigenic peptide to an ICAM-1-binding peptide via a spacer peptide (Fig. 1A). In the initial stage of T-cell activation, Signal-2 and Signal-1 are formed at the central and peripheral zones at the T cell-APC interface, respectively; then, the two signals are physically translocated by exchanging their positions to form the final “immunological synapse” (Fig. 1B). Our central hypothesis is that the BPI molecules simultaneously bind to MHC-II and ICAM-1 on APC to form a bridge (tether) that prevents the translocation and segregation of Signal-1 and Signal-2 and, thereby, inhibits the immunological synapse formation (Fig. 1C). Recently, we have shown that PLP-BPI that contains PLP139–151 as antigen (5) and LABL peptide derived from αL integrin (CD11a237–246) (6, 7) can inhibit the onset and disease progression of experimental autoimmune encephalomyelitis (EAE) in mice (8), a model for human MS (9). Our group also has demonstrated that GAD-BPI that contains the type-1 diabetes-associated antigen GAD208–217 (10–12) can suppress the progression of type-1 diabetes in non-obese diabetic (NOD) mice (13). Therefore, the BPI molecules (e.g., PLP-BPI, GAD-BPI, and their derivatives) have a potential application for treating autoimmune diseases.
Fig. 1.
Sequence and target molecules of PLP-BPI (A). PLP-BPI is a linear 28-amino acid peptide, which is made of antigen epitope peptide PLP139–151 and ICAM-1-binding peptide (named LABL peptide) derived from the α-subunit of LFA-1 (CD11a237–246) connected via a linker peptide. Ac = ε-aminocaproic acid. PLP139–151 and LABL portions of PLP-BPI simultaneously bind to MHC-II (I-As) and ICAM-1, respectively, on the surface of APC. During T-cell activation, Signal-2 (LFA-1/ICAM-1) and Signal-1 (TCR/MHC-antigen complex) are initially formed at the center and around the central zone, respectively, of the T cell-APC interface, and both signals translocate each other to form a complete immunological synapse (B). BPI binds to and tethers MHC-II and ICAM-1 on APC and prevents the migration (i.e., translocation and segregation) of Signal-1 and Signal-2, which inhibits the immunological synapse formation (C).
Once activated with specific antigens, naïve CD4+ T cells begin a process of differentiation into effector T helper (Th) cell susbsets characterized by signature cytokine profiles (14). Th1 effector cells produce IFN-γ and TNF-α while Th2 ones produce IL-4, IL-5, and IL-13. Additionally, a subpopulation of memory CD4+ T cells (Th17) are activated; Th17 has been found to secrete IL-17 under the influence of IL-6, IL-23, and transforming growth factor-β (TGF-β (15, 16). IL-17, also known as cytotoxic T lymphocyte-associated antigen-8, is a 34 5 kDa disulfide-linked homodimeric glycoprotein that is produced almost exclusively by activated CD4+ memory T-cells (17, 18). IL-17 is quite proinflammatory in nature; it induces the secretion of chemokines such as monocyte chemoattractant protein-1 (MCP-1), and growth related oncogene-α (Gro-α), subsequently promoting the recruitment of monocytes and neutrophils (19, 20). Augmented expression of IL-17 in the blood and cerebrospinal fluid has been observed in patients with MS (21, 22), indicating the involvement of IL-17 in the pathogenesis of MS. In fact, administration of antibodies against IL-17 has been shown to prevent the development of EAE as well as delay the onset of paralysis once EAE had been induced (15, 23).
For clinical applications of BPI molecules, it is essential to improve their in vivo activity to achieve excellent efficacy and plasma stability as well as low toxicity. In the previous studies, BPI molecules were evaluated as a therapeutic prevention method (i.e., as a prophylactic agent) to prevent formation of the disease. In actual clinical situations, the patients would have shown the apparent signs of the disease prior to treatment; thus, it is more practical to evaluate the activity of BPI molecules to treat patients after the apparent clinical signs of the disease. In addition, we should avoid or reduce the possibility of inducing anaphylactic response, a life-threatening immediate hypersensitivity reaction caused by treatment with the antigenic peptide (e.g., PLP139–151). Therefore, this work was focused on the following four objectives: (1) to optimize PLP-BPI activity, (2) to study the “therapeutic” potentials of the BPI molecules, (3) to examine IL-17 production in BPI- and PBS-treated mice after induction of EAE in an attempt to correlate with disease progression, and (4) to examine BPI potential to induce anaphylaxis. For improved BPI activity, the PLP-BPI was chemically modified by capping the N- and C-termini and varying the length of the spacer peptide (Table 1). The in vivo activities of the BPI molecules were compared (a) in suppressing the disease by injecting BPI molecules before the disease onset (prophylactic) and (b) in reversing the disease by injecting the BPI molecules after the disease occurs (treatment). We found that the capped Ac-PLP-BPI-NH2 suppresses EAE more efficiently than the parent non-capped PLP-BPI; therapeutic application of Ac-PLP-BPI-NH2 could expeditiously reverse the disease severity. Production of IL-17, a potent proinflammatory cytokine found commonly among MS patients, was significantly decreased by Ac-PLP-BPI-NH2-2 treatment. It was also found that the incidence of anaphylactic responses is less in BPI treatment than in PLP139–151 treatment.
Table 1.
List of peptides used in the present study.
| Peptide | Sequence |
|---|---|
| PLP-BPI | HSLGKWLGHPDKF-AcGAcGAc-ITDGEATDSG |
| PLP139–151 | HSLGKWLGHPDKF |
| LABL (CD11a237–246) | ITDGEATDSG |
| PLP-polyG | HSLGKWLGHPDKF-AcGAcGAc-GGGGGGGGGG |
| Ac-PLP-BPI-NH2 | CH3CO-HSLGKWLGHPDKF-AcGAcGAc-ITDGEATDSG-NH2 |
| Ac-PLP-BPI-NH2-0 | CH3CO-HSLGKWLGHPDKF-ITDGEATDSG-NH2 |
| Ac-PLP-BPI-NH2-2 | CH3CO-HSLGKWLGHPDKF-AcGAcGAcAcGAcGAc-ITDGEATDSG-NH2 |
| Ac-LABL-PLP-NH2 | CH3CO-ITDGEATDSG-AcGAcGAc-HSLGKWLGHPDKF-NH2 |
| MBP87–99 | VHFFKNIVTPRTP |
BPI is composed of antigen epitope peptide (e.g., PLP139–151), various lengths of spacer peptide (none, Ac-G-Ac-G-Ac, or Ac-G-Ac-G-Ac-Ac-G-Ac-G-Ac; where Ac represents ε-aminocaproic acid), and LABL peptide (CD11a237–246). Some peptides are capped at both ends, i.e., N-terminal acetylated and C-terminal amidated. PLP-polyG contains deca-glycine instead of LABL peptide.
MATERIALS AND METHODS
Mice
SJL/J (H-2s) female mice were purchased from Jackson Laboratory (Bar Harbor, ME) or Charles River (Wilmington, MA) and housed under specific pathogen-free conditions at an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility at the University of Kansas. All protocols involving live mice were approved by the University’s Institutional Animal Care and Use Committee.
Peptide synthesis
The sequences of peptides used in the present study are listed in Table 1. The peptides were synthesized with 9-fluorenylmethyloxy-carbonyl-protected amino acid chemistry on appropriate PEG-PS™ resin (Applied Biosystems, Foster City, CA) using the automated peptide synthesis system (Pioneer™: PerSeptive Biosystems, Framingham, MA). Cleavage of the peptides from the resin and removal of the protecting groups from the side-chain were carried out using TFA with scavengers. The crude peptides were purified by reversed-phase HPLC using a C18 column with a gradient of solvent A (95%/5% = H2O (0.1% TFA)/acetonitrile) and solvent B (100% acetonitrile). The purity of the peptide was analyzed by analytical HPLC using an analytical C18 column. The identity of the synthesized peptide was confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.
Induction of EAE and therapeutic study
Five- to seven-wk-old SJL/J female mice were immunized subcutaneously with 200 µg PLP139–151 in a 0.2 ml emulsion comprised of equal volumes of PBS and complete Freund’s adjuvant (CFA) containing killed Mycobacterium Tuberculosis strain H37RA (at a final concentration of 4 mg/ml, Difco, Detroit, MI). The PLP/CFA was administered to regions above the shoulder and the flanks (total 4 sites; 50 µl at each injection site). In addition, 200 ng of pertussis toxin (List Biological Laboratories, Campbell, CA) was injected intraperitoneally on the day of immunization (day 0) and 2 days post immunization. Then, mice received intravenous injection of peptides (100 nmol/mouse) on indicated day(s). For the therapeutic study, mice were left untreated until the day of disease onset (determined when the mouse was scored as 1 or more for the first time). Upon disease onset, the mice received intravenous injections of peptides (100 nmol/mouse/day) for 3 consecutive days of maximum number of injections; peptide injection was discontinued once the disease score returned below 1. Disease progression was evaluated by the same observer in a blinded fashion using a clinical scoring scale ranging from 0 to 5 as follows (24): 0 – No clinical symptoms, 1 – Limp tail or waddling gait with tail tonicity; 2 – Waddling gait with limp tail (ataxia); 2.5 – Ataxia with partial paralysis of one limb; 3 – Full paralysis of one limb; 3.5 – Full paralysis of one limb with partial paralysis of the second limb; 4 – Full paralysis of two limbs; 4.5 – Full paralysis of two limbs with partial paralysis of forelimbs; 5 – Moribund or dead. Body weight was also measured daily.
Determination of IL-17 levels in serum in vivo
Blood samples were obtained from PBS- and Ac-PLP-BPI-NH2-2-treated mice on day 12 and day 35 (6 mice per group). Additionally, 6 un-primed and un-treated mice (i.e., normal mice) were also sampled. Blood samples were allowed to clot overnight at 2–8 °C before centrifuging for 20 min at 2000 × g. Serum was collected and stored at −20 °C until analysis. Enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) for IL-17 in serum were performed according to the manufacturer’s instructions. Optical density was measured at 450 nm with correction at 540 nm wavelengths in a spectrophotmetric microplate reader (Titertek Multiskan MCC/340, Flow Industries, McLean, VA).
Induction and monitoring of anaphylaxis
Mice received subcutaneous immunization with PLP139–151/CFA on day 0, and intraperitoneal injection of pertussis toxin on the day of immunization and 2 days post immunization. Four to five weeks later, the mice were divided into groups and received intravenous injection of described peptide (100 nmol/mouse). To avoid the influence of the variation in their disease severity and history, all the groups had a very similar set of mice in terms of the average highest disease score, the average cumulative disease score, and the average day of disease onset. Incidence of anaphylactic response was judged by death occurring within 30 min or the characteristic symptoms of immediate hypersensitivity such as piloerection, prostration, erythema of the tail, ears and footpads, shallow breathing, and lethargy observed within a few minutes after peptide injection. Any mice that became moribund or did not recover from severe anaphylactic symptoms were euthanized.
Proliferation assay of splenocytes isolated from peptide-treated mice
SJL/J mice were immunized with PLP139–151/CFA or MBP87–99 /CFA and pertussis toxin as above. On days 4 and 7, the PLP139–151/CFA-immunized mice were injected intravenously with PBS, PLP-BPI, or Ac-PLP-BPI-NH2 (100 nmol/mouse). The MBP87–99 /CFA-immunized mice received PBS injections only. On day 10, the mice were euthanized and lymphocytes were isolated from the spleen by centrifugation over Lymphocyte Separation Medium (MP Biomedicals, Solon, OH). The pooled splenocytes (1 × 105 cells/100 µl) were cultured in the presence or absence of antigen peptide (PLP139–151 (0.2–20 µM) and MBP87–99 (20 µM)) or concanavalin A (2 µg/ml) for 72 h at 37 °C, 5% CO2. The cell number was then determined using Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the instructions.
Statistic analysis
Statistical comparisons among the groups in clinical disease score were accomplished by calculating the average score for each mouse from the day of disease onset to the end of the study and performing a Mann-Whitney’s U test. Statistical differences in relative body weight were analyzed by calculating the average for each mouse for 10 days beginning on the day of disease onset and performing a Mann-Whitney’s U test. Statistical significance in EAE disease morbidity was determined by Cox proportional-hazards regression. Comparison in IL-17 concentration in serum was performed by one-way analysis of variance. Comparison in splenocyte proliferation was performed by Dunnet test. All analyses were carried out using StatView (SAS Institute, Inc.).
RESULTS
Suppression of EAE by PLP-BPI and Ac-PLP-BPI-NH2
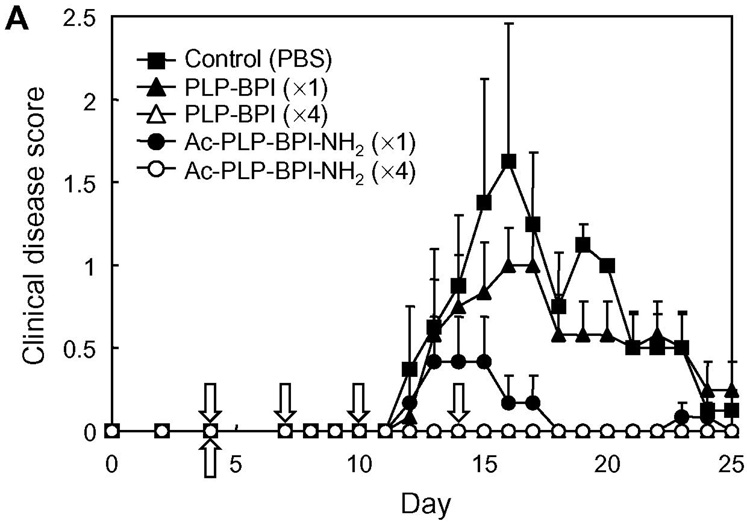
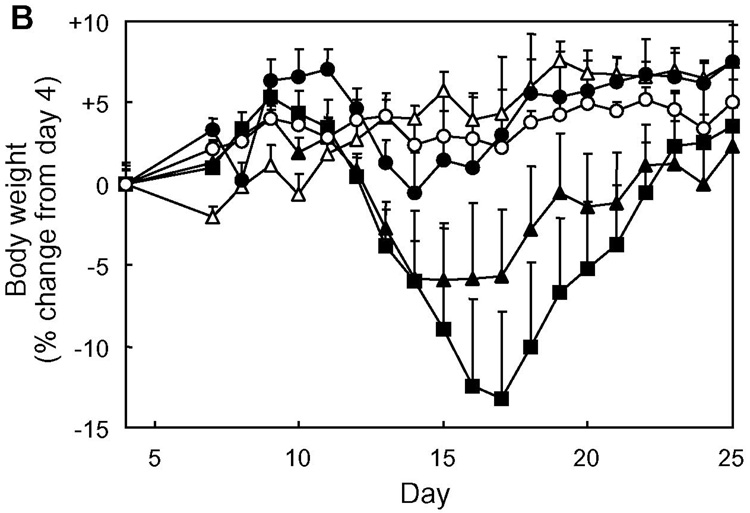
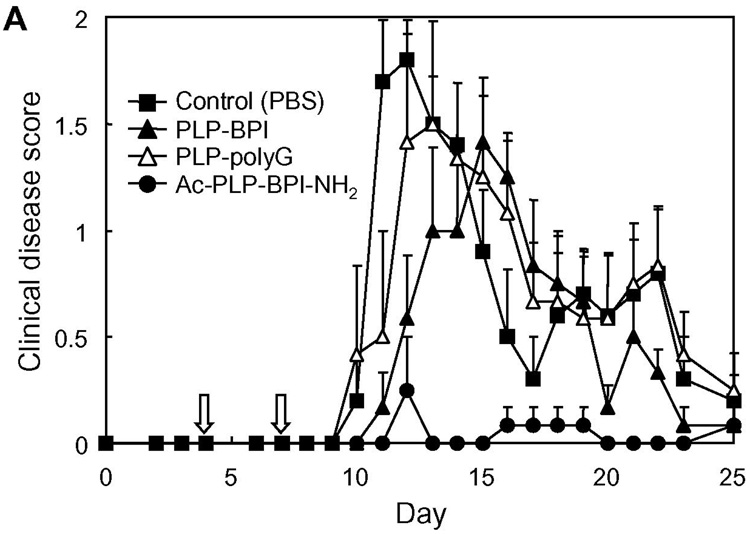
The effect of capping was first evaluated by comparing the in vivo activity of Ac-PLP-BPI-NH2 and PLP-BPI in suppressing EAE. Both molecules were given to PLP139–151/CFA-immunized mice by either intravenous injections on days 4, 7, 10, and 14, or a single intravenous injection on day 4. The results from four-time injections showed that both Ac-PLP-BPI-NH2 and PLP-BPI completely prevented the onset of EAE; the treated mice exhibited neither any clinical signs of disease nor significant loss of body weight (in vivo study-I, Fig. 2). In the case of a single injection, there was an apparent difference in the disease profile between the mice treated with Ac-PLP-BPI-NH2 and PLP-BPI; Ac-PLP-BPI-NH2 inhibited the progression of disease severity and the loss of body weight more efficiently than did PLP-BPI (Fig. 2). A similar trend between the Ac-PLP-BPI-NH2 and PLP-BPI was observed in the in vivo study-II; Ac-PLP-BPI-NH2 almost completely inhibited the disease progression by intravenous injections on days 4 and 7 while PLP-BPI injections at the same treatment schedule suppressed the EAE to only a small extent (Fig. 3). Although the EAE-suppressing activity of uncapped PLP-BPI was weak in this treatment regimen, PLP-BPI still possessed better activity to delay the progression of EAE than did PLP-polyG, which is not a “BPI”-type molecule due to its lack of LABL peptide.
Fig. 2.
In vivo suppressive activity of BPI in the mouse EAE model (in vivo study-I). SJL/J female mice were immunized subcutaneously with PLP139–151/CFA and injected intraperitoneally with pertussis toxin on days 0 and 2. Then, the mice received intravenous injection(s) of 100 nmol of the indicated peptide on days 4, 7, 10, and 14 or only on day 4. Disease progression was evaluated by (A) clinical disease scoring as described in Experimental Procedures and (B) change in body weight. The results are expressed as the mean + S.E. (n = 6). There are significant differences in clinical disease score vs. PBS; PLP-BPI (×4) (P<0.05), Ac-PLP-BPI-NH2 (×1) (P<0.05), Ac-PLP-BPI-NH2 (×4) (P<0.05) and between Ac-PLP-BPI-NH2 (×1) and PLP-BPI (×1) (P<0.05). There are significant differences in body weight vs. PBS; PLP-BPI (×4) (P<0.05), Ac-PLP-BPI-NH2 (×4) (P<0.05).
Fig. 3.
In vivo suppressive activity of BPI in the mouse EAE model (in vivo study-II). SJL/J female mice were immunized subcutaneously with PLP139–151/CFA and injected intraperitoneally with pertussis toxin on days 0 and 2. Then, the mice received intravenous injections of 100 nmol of the indicated peptide on days 4 and 7. Disease progression was evaluated by (A) clinical disease score and (B) change in body weight. The results are expressed as the mean + S.E. (n = 6). There are significant differences in clinical disease score; PBS vs. Ac-PLP-BPI-NH2 (P<0.01), PLP-BPI vs. Ac-PLP-BPI-NH2 (P<0.05), PLP-polyG vs. Ac-PLP-BPI-NH2 (P<0.05). There is significant difference in body weight between PBS and Ac-PLP-BPI-NH2 (P<0.05).
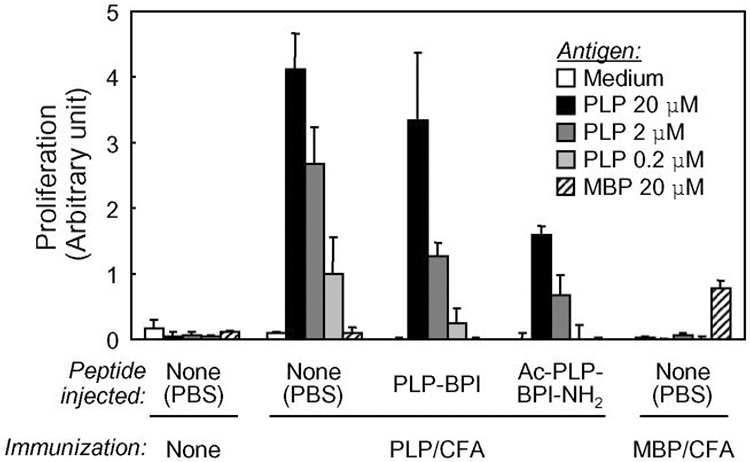
To confirm the effect of the N- and C-terminal modification of PLP-BPI on its in vivo activity, proliferation of the splenocytes isolated from the peptide-treated mice was examined upon antigen re-stimulation in vitro. Splenocytes from PLP139–151-immunized mice responded to PLP139–151 stimulation but not to MBP87–99, and splenocytes from MBP87–99-immunized mice responded to MBP87–99 stimulation but not to PLP139–151 (Fig. 4), suggesting that response of isolated splenocytes was specific to the antigen used for immunization. The response was also dose dependent. Interestingly, the splenocytes isolated from Ac-PLP-BPI-NH2-treated mice showed significantly less proliferation in re-call to PLP139–151 than those of PLP-BPI-treated and untreated mice. This suggests that intravenous injections of Ac-PLP-BPI-NH2 reduce the number of PLP-responsive populations and/or suppress the responsiveness of the populations. It was confirmed that proliferation of the splenocytes upon concanavalin A exposure was similar in all the groups tested (data not shown). It is yet to be further elucidated that the types/subpopulations of spleen cells that were activated and proliferating and the cytokine production profiles of such cells following the treatment by Ac-PLP-BPI-NH2. Neverthelss, N-terminal acetylated and C-terminal amidated Ac-PLP-BPI-NH2 proved to be more potent than the original PLP-BPI.
Fig. 4.
In vitro proliferation of splenocytes isolated from peptide-treated mice. PLP139–151/CFA- or MBP87–99 /CFA-immunized mice received intravenous injections of PBS, PLP-BPI, or Ac-PLP-BPI-NH2 (100 nmol/mouse) on days 4 and 7. On day 10, splenocytes were isolated from the mice. The pooled splenocytes in triplicate were re-stimulated with the indicated antigen peptide for 72 h at 37 °C. The cell number was determined using Cell Counting Kit-8. The results are expressed as the mean + S.D. Among the PLP139–151/CFA-immunized group, there are significant differences vs. PBS; ** P<0.01, * P<0.05, and vs. PLP-BPI; † P<0.05.
Evaluation of various Ac-PLP-BPI-NH2 derivatives
To further optimize the BPI activity, we studied the effect of linker length of BPI and the effect of reversing the positions of PLP and LABL in BPI. Ac-PLP-BPI-NH2-0 does not contain a linker peptide (i.e., PLP and LABL are directly linked to each other) and Ac-PLP-BPI-NH2-2 contains a linker twice as long (Ac-G-Ac-G-Ac-Ac-G-Ac-G-Ac) as that of Ac-PLP-BPI-NH2 (Ac-G-Ac-G-Ac). Ac-LABL-PLP-NH2 is another derivative that contains a regular length linker (Ac-G-Ac-G-Ac), but the positions of PLP and LABL peptides are reversed (Table 1). In vivo EAE-suppressing activity of these peptides was evaluated using one (on day 4) and two (on days 4 and 7) intravenous injection(s). All the Ac-PLP-BPI-NH2 derivatives completely suppressed the EAE onset when two injections were employed (in vivo study-III, Fig. 5A). Also, these peptides efficiently but not completely inhibited the progression of the disease severity with a single injection (Fig. 5B). The derivatives with different lengths of linkers (Ac-PLP-BPI-NH2-0 and Ac-PLP-BPI-NH2-2) were similarly more effective in suppressing EAE than the parent Ac-PLP-BPI-NH2; however, Ac-LABL-PLP-NH2 has slightly lower potency. Therefore, it was very difficult to conclude which is the best derivative based solely on this screening study.
Fig. 5.
In vivo suppressive activity of Ac-PLP-BPI-NH2 derivatives in mouse EAE model (in vivo study-III). PLP139–151/CFA-immunized mice received intravenous injections of 100 nmol of the indicated peptide on days 4 and 7 (A) or only on day 4 (B). The results are expressed as the mean + S.E. (n = 5–10). Statistical analyses were not performed for the two injection experiment. For the single injection experiment, there are significant differences vs. PBS; Ac-PLP-BPI-NH2-0 (P<0.01), Ac-PLP-BPI-NH2 (P<0.01), Ac-PLP-BPI-NH2-2 (P<0.01).
IL-17 serum levels in SJL/J mice in vivo
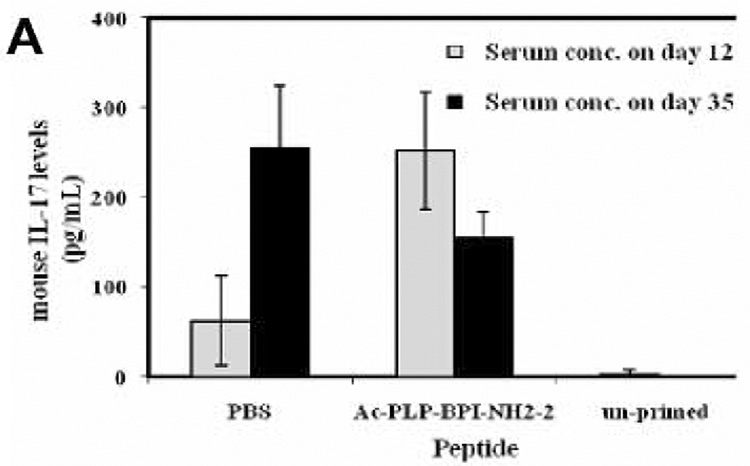
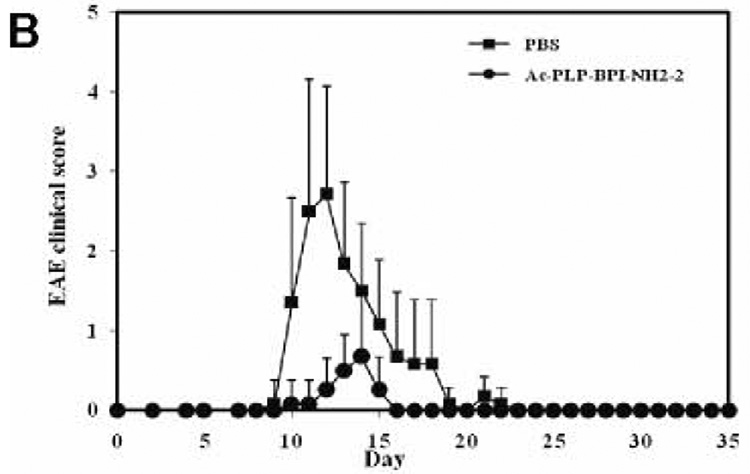
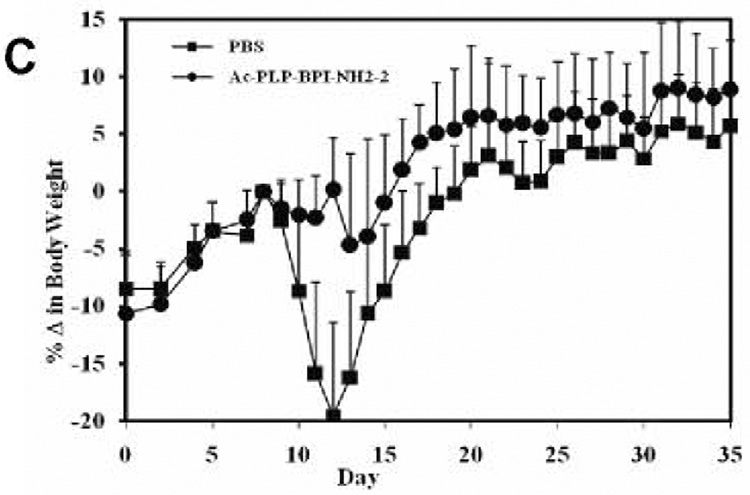
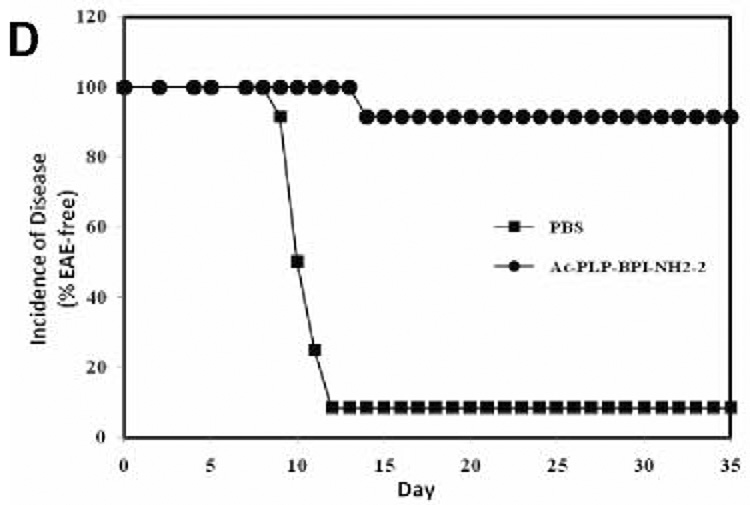
To examine the effect of BPI treatment on IL-17 production, we determined serum IL-17 levels in mice after induction of EAE and treatment with BPI or PBS. We anticipated that serum IL-17 levels among the PBS-treated mice would be significantly high at the peak of the disease (days 12–17) compared to either BPI-treated or un-primed mice. However, mice receiving Ac-PLP-BPI-NH2-2 had unexpectedly significantly high (p<0.001) level of IL-17 at the peak of the disease (i.e., day 12, when PBS-treated mice exhibited severe EAE symptoms) compared to the mice receiving PBS alone (Fig. 6). Ac-PLP-BPI-NH2-2 treated mice showed more than 90-fold increase in the IL-17 production whereas mice receiving PBS had an almost 23-fold increase when compared to un-primed mice. In contrast to the IL-17 levels observed on day 12, mice receiving Ac-PLP-BPI-NH2-2 showed a significant decrease (p<0.05) in IL-17 serum level on day 35. Conversely, PBS-treated mice showed a significant increase (p<0.001) in IL-17 serum concentration at day 35 compared to that observed on day 12. At the end of the study (day 35), mice receiving Ac-PLP-BPI-NH2-2 had significantly lower (p<0.05) IL-17 serum concentration than did PBS-treated mice. It was confirmed that un-primed and un-treated mice had significantly lower (p<0.001) IL-17 serum concentration than either Ac-PLP-BPI-NH2-2-or PBS-treated mice.
Fig. 6.
Production of IL-17 in serum in vivo. SJL/J female mice were immunized subcutaneously with PLP139–151/CFA and injected intraperitoneally with pertussis toxin on days 0 and 2. Then, the mice received intravenous injections of 100 nmol of Ac-PLP-BPI-NH2-2 or PBS on days 4, 7, and 10. (A) Serum concentration of IL-17 was determined on day 12 and 35 in Ac-PLP-BPI-NH2-2-treated, PBS-treated, and un-primed mice using Elisa kit. Data is represented as mean ± S.D. (6 mice per group). Disease progression was evaluated by (B) EAE clinical disease score, (C) percent change in body weight, and (D) incidence of disease. Results are expressed as mean ± S.D. (12 mice per group). There were significant differences between Ac-PLP-BPI-NH2-2- and PBS-treated groups in clinical EAE score (p<0.001, through days 12–17), body weight (p<0.05, through 12–25), and incidence of disease (p<0.001).
Therapeutic application of Ac-PLP-BPI-NH2
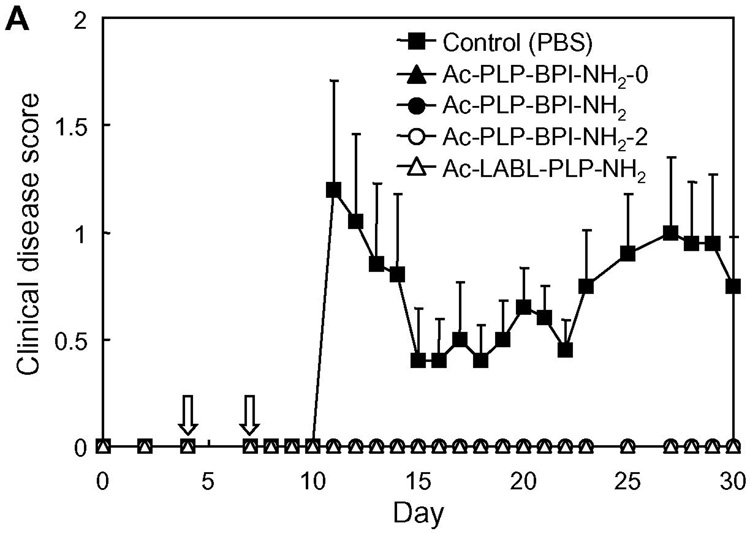
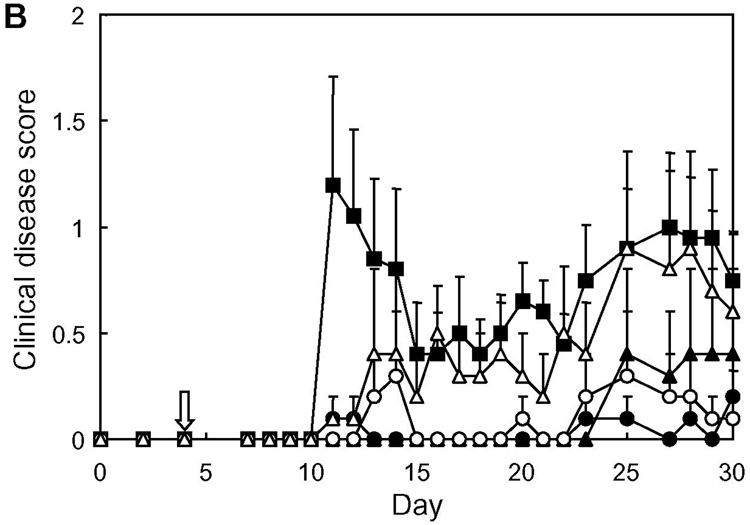
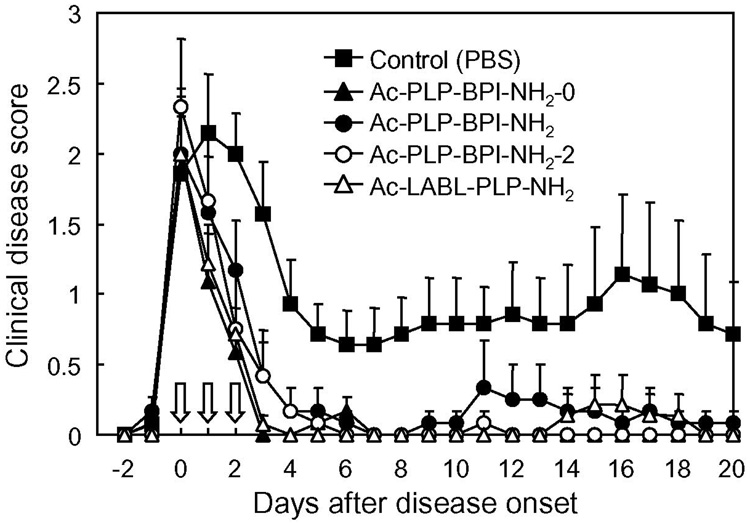
When considering clinical applications of BPI to treat autoimmune diseases, it is important to examine the “therapeutic” potentials of Ac-PLP-BPI-NH2. In this case, the Ac-PLP-BPI-NH2 derivatives were intravenously injected to individual mice upon disease onset (defined as a score of 1 or more). The day of disease onset varied among the mice from day 9 to 15 after immunization with PLP139–151/CFA. Fig. 7 shows the profile of clinical disease score following the day of starting treatment (in vivo study-IV). Surprisingly, the mice treated with BPI recovered from the disease very quickly; the effectiveness of BPI injection can be seen after only a single injection (i.e., at 1 day after injection), and the clinical disease score returned to a normal level (0.5 or less) within 2–3 days in almost all the BPI-treated mice. On the contrary, PBS-treated mice stayed sick for a longer period of time while a spontaneous decrease in disease score was observed; the remission and relapse found in PBS-treated mice is one of the characteristics of EAE and MS. The average number of days from disease onset to remission was 12.3 days for PBS-treated mice and 2–3 days for all the Ac-PLP-BPI-NH2 derivatives-treated groups. In addition, the apparent relapse of the disease was observed in most of PBS-treated mice but in very few of the BPI-treated groups. Thus, BPI can significantly ameliorate the EAE even when the treatment is carried out after the onset of disease and can suppress the possible relapse thereafter. This strongly suggests the potential therapeutic applications of BPI. Again, it was very difficult to see the difference in therapeutic activity of the tested Ac-PLP-BPI-NH2 derivatives because they are all very potent in this experiment.
Fig. 7.
In vivo therapeutic activity of Ac-PLP-BPI-NH2 derivatives in mouse EAE model (in vivo study-IV). PLP139–151/CFA-immunized mice received intravenous injections of the indicated peptide (100 nmol/mouse/day) starting on the day of disease onset as described in Experimental Procedures. The results are expressed as the mean + S.E. (n = 6–7). There are significant differences vs. PBS; Ac-PLP-BPI-NH2-0 (P<0.01), Ac-PLP-BPI-NH2 (P<0.05), Ac-PLP-BPI-NH2-2 (P<0.05), Ac-LABL-PLP-NH2 (P<0.01).
Anaphylactic reactions following BPI injection
Lethal anaphylactic reaction is one of the most problematic issues in treating autoimmune diseases with antigen-related peptides (25–29). We have previously shown that PLP-BPI has a relatively lower possibility of inducing anaphylaxis compared with the original antigenic peptide PLP139–151 (8). Thus, we examined the potentials of Ac-PLP-BPI-NH2 and other derivatives in inducing anaphylactic response in comparison with PLP139–151. Table 2 shows the incidence of anaphylactic reactions in PLP139–151/CFA-immunized mice that received the peptide challenge on day 27 (Experiment A) or on day 32 (Experiment B). Ac-PLP-BPI-NH2 and its derivatives induced relatively lower incidence of lethal anaphylaxis than did PLP139–151. Interestingly, Ac-PLP-BPI-NH2-2 caused the least incidence of anaphylaxis among the peptides tested. While further studies need to be done to specify the influence of spacer length, BPI with a long linker may be the best molecule to minimize the adverse effect of peptide treatment. Taken together, although anaphylaxis was not completely suppressed, it is suggested that BPI is a safer molecule than the parental antigenic peptide (i.e., PLP139–151) alone in terms of inducing anaphylactic reactions.
Table 2.
Incidence of anaphylactic response upon peptide challenge.
| Experiment | Peptide challenged | Incidence of anaphylaxis |
|---|---|---|
| A | PLP | 13/17 (76.5%) |
| Ac-PLP-BPI-NH2 | 7/16 (43.8%) | |
| B | PLP | 9/11 (81.8%) |
| Ac-PLP-BPI-NH2-0 | 7/11 (63.6%) | |
| Ac-PLP-BPI-NH2 | 7/11 (63.6%) | |
| Ac-PLP-BPI-NH2-2 | 5/11 (45.5%) | |
| Ac-LABL-PLP-NH2 | 7/11 (63.6%) | |
PLP139–151/CFA-immunized mice received intravenous injection of the indicated peptide on day 27 (Experiment A) and day 32 (Experiment B). Incidence of anaphylactic response was determined as described in Experimental Procedures.
DISCUSSION
For treatment of MS and other autoimmune diseases, it is hoped that antigen-specific therapies can be developed that will control only those immune responses involved in a particular autoimmune disease without suppressing the general immune response, thus leaving the remaining functions of the immune system intact. Recently, the formation of the immunological synapse has been gaining much attention as a central mechanism of regulating immune responses. Although the detailed functions in a series of complicated immunological events are still under debate and investigation, the formation of the immunological synapse is an important process during T-cell activation. Initially, T cells and APC contact each other through LFA-1/ICAM-1 adhesion molecules that congregate at the central cell-cell contact region, while TCR/MHC-antigen complexes are found peripherally in a ring shape in the nascent synapse. As T-cell activation proceeds, the clusters of both molecular complexes interchange the positions into a bull's eye pattern, where the TCR/MHC-antigen complexes (Signal-1) form central supramolecular activation clusters surrounded by LFA-1/ICAM-1 (Signal-2) as a member of peripheral supramolecular activation clusters (1–4). To modulate the immunological synapse formation, we have designed a novel peptide molecule called BPI that targets particularly the translocation and segregation of the Signal-1 and Signal-2 clusters (Fig. 1). So far, we have developed two different versions of BPI, PLP-BPI and GAD-BPI (8, 13); they consist of the MS-associated antigen PLP139–151 and the type-1 diabetes-associated antigen GAD208–217, respectively, as antigen epitope parts of BPI.
In our recent studies, we have demonstrated that PLP-BPI can inhibit the disease progression and incidence in in vivo mouse EAE, a model for human MS. PLP-BPI has better activity in suppressing EAE than do other peptides, including VP2-BPI (a BPI with an epitope peptide of Theiler’s encephalomyelitis virus capsid protein (VP274–86), which is known to bind to MHC-II (I-As) in SJL/J mice (30)), PLP-BPIsLABL (PLP-BPI with a scrambled sequence of LABL), and the unlinked mixture of PLP139–151 and LABL. These data suggest the significance of a unique structure in PLP-BPI such as (a) the presence of both PLP139–151 and LABL peptides and (b) covalent linking of these two peptides in the same molecule. In a parallel study, our group also has designed GAD-BPI and demonstrated that it can suppress type-1 diabetes in NOD mice (13). MHC-II (I-Ag7) and ICAM-1 molecules on the surface of isolated B cells are co-localized in the presence of GAD-BPI but not the unlinked mixture of GAD208–217 peptide and LABL, suggesting simultaneous binding of GAD-BPI to its target molecules, I-Ag7 and ICAM-1.
As to mechanistic aspect of PLP-BPI, we found that CD4+CD25+TGF-β+, CD4+CD25+IL-4+, and CD4+CD25+IL-10+ T cells were significantly increased in the lymphocyte population isolated from the spleen of PLP-BPI-treated mice; in addition, CD4+CD25+IFN-γ+ cells were also significantly induced by the PLP-BPI treatment (8). As in PLP-BPI, we showed that GAD-BPI enhanced the production of IL-4 as well as IFN-γ; the IL-4 was produced by a rare population of NOD T cells bearing the NK-marker DX5 (CD4+DX5+ T cells) (13). Therefore, taken together, it is possible that PLP-BPI treatment altered the characteristics of cytokine producers from “strictly Th1-like” phenotype to “balanced Th1- plus Th2-like” cells.
Elevated levels of IL-17 have been demonstrated in the cerebrospinal fluid and in brain lesions of patients with MS (31). IL-17 level has been found to correlate with disease activity (22), suggesting that IL-17 plays an important role in the pathogenesis of MS. It has also been suggested that IL-17 induces EAE in animals (32). In the present study, we compared IL-17 production in serum among Ac-PLP-BPI-NH2-2-treated, PBS-treated, and un-primed mice. Unexpectedly, we found increased production of IL-17 at the peak of the disease in animals receiving Ac-PLP-BPI-NH2-2 compared to PBS-treated ones. Based on the fact that mice receiving Ac-PLP-BPI-NH2-2 had lower EAE clinical scores, lower EAE incidence, and minimal loss in body weight (Fig. 6), the only plausible explanation to this observation could be that IL-17 response in this group was specific to the antigen used for immunization. To induce EAE, mice were primed with PLP139–151, which is also a constituent of Ac-PLP-BPI-NH2-2 used to suppress EAE. Presumably, a subpopulation of memory CD4+ T cells induced during the priming process recognizes the PLP139–151 fragment either in intact Ac-PLP-BPI-NH2-2 or produced as a result of degradation of Ac-PLP-BPI-NH2-2, which consequently lead to increased proliferation and thus increased production of IL-17. Since Ac-PLP-BPI-NH2-2 effectively suppressed EAE by blocking the formation of the immunological synapse, further proliferation of autoreactive CD4+ T cells as well as production of IL-17 were inconsequential. Hence the absence of exacerbation of EAE was observed in mice receiving Ac-PLP-BPI-NH2-2 as demonstrated by lower incidence of EAE, lower EAE clinical scores, and minimal loss in body weight. More importantly, there was significant decrease in IL-17 production at the end of the study (day 35) in Ac-PLP-BPI-NH2-2-treated animals; this further supports our earlier believe that the initial IL-17 response was primarily specific to the antigenic peptide used for priming. In contrast, IL-17 production was significantly increased among PBS-treated animals on day 35, being consistent with increased inflammation and maybe an impending relapse, i.e., one of characteristic features of MS. Further insights into the fate of IL-17 would be gained through studies carried out for periods much longer than 35 days.
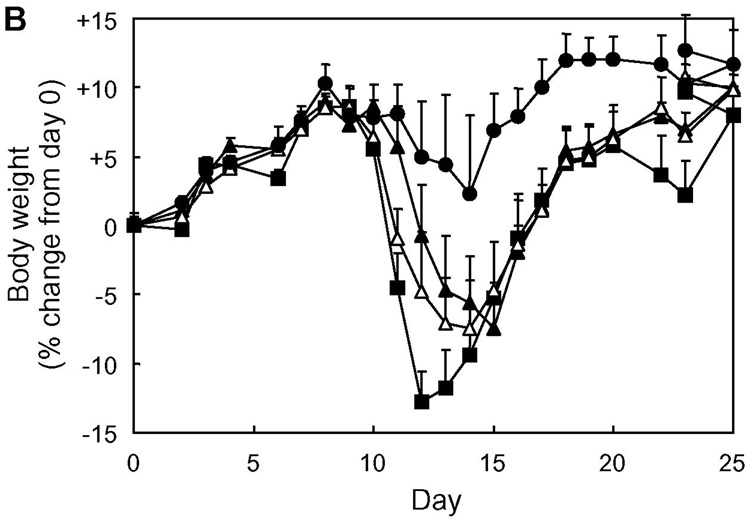
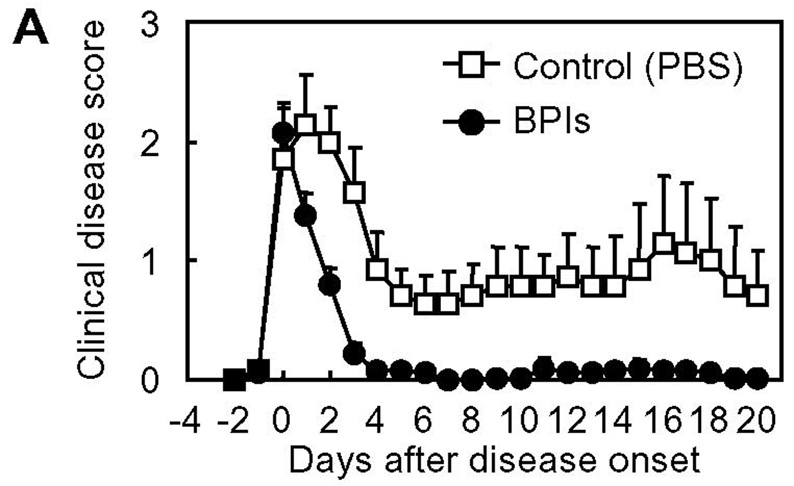
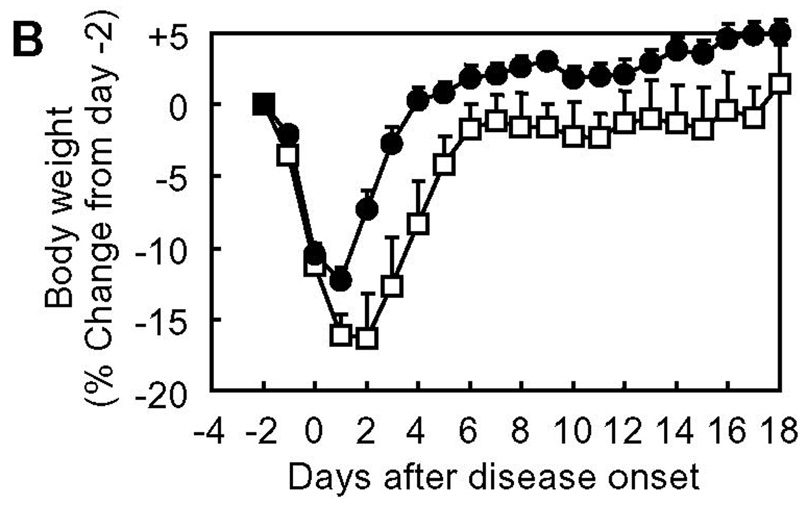
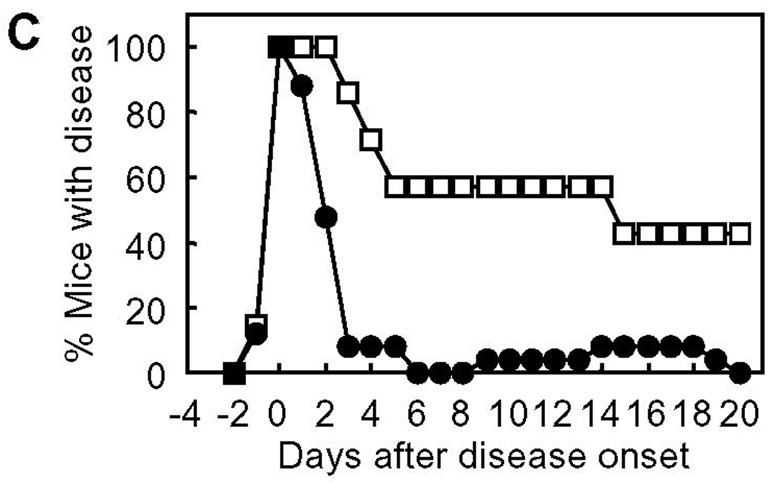
For clinical development of therapeutic agents for autoimmune diseases, one of the most important concerns is whether they are really effective in “therapeutic” applications. In many studies, disease-suppressing activity of compounds of interest is tested by dosing the compounds prior to the manifestation of clinical signs of disease; treatment(s) are carried out during the period between induction of disease and disease onset, at the same time as the induction of disease, and/or before the induction of disease (33–38). These regimens may be regarded as “prophylactic” treatment, not a genuine “therapeutic” application. This is also the case for our previous studies. We have examined the in vivo EAE-suppressing activity of PLP-BPI by injecting the molecule as early as day 4 through day 14; thus, most of the injections made fall in the period between subcutaneous PLP139–151/CFA-immunization on day 0 (i.e., induction of disease) and the disease onset (typically around day 9–13). However, in clinical situations, BPI injection to patients is unlikely to be started until the patients exhibit apparent clinical signs of the disease. Therefore, as an example of a practical and reasonable timing of starting BPI treatment, we gave BPI injection(s) to mice upon disease onset to examine the in vivo therapeutic effect of BPI molecules (Fig. 7). It is interesting to find that the injections of Ac-PLP-BPI-NH2 and its derivatives (as combined as BPIs in Fig. 8) after apparent disease onset can reverse the disease severity very quickly. Most of the BPI-treated mice became free of apparent clinical disease signs and stopped losing body weight within a few days, and never showed a relapse of the disease that was observed in the untreated group at a later time period. These data suggest that BPI is effective in suppressing EAE when injected after disease onset and therefore can be a promising “therapeutic” agent for autoimmune diseases.
Fig. 8.
In vivo therapeutic activity of BPI in mouse EAE model: summary of in vivo study-IV. The data of Ac-PLP-BPI-NH2 derivatives in in vivo study-IV were combined as BPIs to investigate the therapeutic effect of BPI treatment as (A) clinical disease score, (B) change in body weight, and (C) morbidity rate of disease. The results are expressed as the mean + S.E. (n = 7 for control group and n = 25 for BPI-treated group). There are significant differences between BPI- and PBS-treated groups in clinical disease score (P < 0.01), body weight (P < 0.05), and morbidity rate (P < 0.01).
Another important issue of concern with peptide-based therapeutics is the anaphylactic response, a life-threatening immediate hypersensitivity reaction caused by injections of the antigen-related peptide(s). Anaphylactic reactions have been observed with several peptides (25–29) that were evaluated for treating autoimmune diseases. Due to hypersensitivity reactions in patients during phase II clinical trials, development of an MS-targeted peptide drug has been suspended (39, 40). Previously, we found that intravenous injection of PLP-BPI at 4–5 wk post immunization causes anaphylactic reaction to a much lower number of mice than does PLP139–151. In the present study, we confirmed that the N- and C-terminal-modified form of PLP-BPI (Ac-PLP-BPI-NH2) also induces anaphylaxis at lower incidence than PLP139–151 (Table 2, Experiment A). Furthermore, we performed a similar experiment with Ac-PLP-BPI-NH2 derivatives to examine the effect of spacer length of BPI on the rate of inducing anaphylactic reactions. Interestingly, we found that among the Ac-PLP-BPI-NH2 derivatives tested, BPI with a relatively long linker (Ac-PLP-BPI-NH2-2) appears less aggressive in causing lethal anaphylaxis (Table 2, Experiment B). The lower incidence of anaphylaxis in BPI is presumably due to (a) the presence of LABL peptide in BPI to inhibit LFA-1/ICAM-1 interactions at the site of antigen recognition or (b) the addition of another peptide moiety at the N- or C-terminal to the parental allergic peptide PLP139–151. Involvement of LFA-1/ICAM-1-mediated heterotypic aggregation of activated T cells to mast cells is implicated in augmenting mast cell degranulation and histamine release (41). However, the contribution of the presence of LABL peptide is unlikely to be significant since PLP-BPI and PLP-BPIsLABL with a scrambled LABL sequence had a similar incidence in inducing anaphylactic reactions (data not shown). Another group has demonstrated successful reduction of anaphylaxis-inducing rate by addition of extra sequences to the C-terminal of an antigenic peptide (29). Although the lethal anaphylaxis was not completely avoided in Ac-PLP-BPI-NH2 or its derivatives under the current experimental conditions, it would be possible to dissociate the disease-protective effect of BPI from the allergic side reactions by optimizing the dose, injection route, formulation, and/or treatment schedule (42).
It is also important to improve the in vivo activity of BPI in order to accomplish its clinical application. For this purpose, we designed Ac-PLP-BPI-NH2 with capped N- and C-termini; this type of modification could improve peptide stability in vivo due to the suppression of degradation by carboxy- and amino-peptidases (43). It is not yet clear whether the acetyl group on the N-terminus of PLP peptide and/or the amide group on the C-terminus of LABL peptide contribute to binding properties of BPI to the respective receptors. Nonetheless, Ac-PLP-BPI-NH2 was more efficient in suppressing the disease progression than unmodified PLP-BPI (Fig. 2 and Fig. 3). It is possible that Ac-PLP-BPI-NH2 has a better bioavailability than the parent PLP-BPI; thus, fewer injections would be needed for Ac-PLP-BPI-NH2 to reverse the disease compared to the parent PLP-BPI. Next, Ac-PLP-BPI-NH2 was further modified by changing the linker length to optimize the distance between PLP139–151 and LABL; this modification was aimed at optimizing the simultaneous binding of PLP139–151 and LABL peptide to MHC-II and ICAM-1, respectively, on the surface of APC. Our initial design of linker peptide (Ac-G-Ac-G-Ac) was based on modeling BPI interaction with both I-Ag7 and domain-1 of ICAM-1. LABL and GAD208–217 peptides were docked to the domain-1 of ICAM-1 and I-Ag7, respectively, using X-ray structural information from previous studies (44–47). The distance between the N-terminal of LABL peptide and the C-terminal of GAD208–217 peptide was estimated, and the number of aminocaproic acid (Ac) and glycine (G) residues was determined. Influences of linker structure bridging two functional groups were clearly suggested before (48). Therefore, while the treatment is antigen-specific in any of the PLP-BPI derivatives, their overall efficiency in affecting the immunological synapse formation could be different from one another. Unfortunately, because all the Ac-PLP-BPI-NH2 derivatives were very active in vivo in suppressing EAE, we could not differentiate the activities of the derivatives with various linker lengths. Thus, it seems very difficult to perform an in vivo screening study under the current experimental conditions.
In conclusion, Ac-PLP-BPI-NH2 could be a promising potential therapeutic and prophylactic agent for MS although further improvements in its activity and safety concerns are necessary. Establishment of an in vitro assay that allows efficient screening of best BPI molecules is strongly desired for further improvement of BPI activities. Other versions of BPI molecules could be developed for many autoimmune diseases in which antigenic epitopes are identified.
ACKNOWLEDGMENT
The authors would like to express their grateful appreciation to Nancy Harmony for proofreading the manuscripts.
Footnotes
This work is financially supported by NIH (R01-AI-063002) and National MS Society to T.J.S. and a postdoctoral fellowship to N.K. from the American Heart Association, Heartland Affiliate.
REFERENCES
- 1.Grakoui A, Bromley S, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: A molecular machine controlling T-cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 2.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 3.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 4.Dustin ML. The immunological synapse. Arthritis. Res. 2002;4:S119–S125. doi: 10.1186/ar559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuohy VK, Lu Z, Sobel RA, Laursen RA, Lees MB. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J. Immunol. 1989;142:1523–1527. [PubMed] [Google Scholar]
- 6.Yusuf-Makagiansar H, Makagiansar IT, Hu Y, Siahaan TJ. Synergistic inhibitory activity of α- and β-LFA-1 peptides on LFA-1/ICAM-1 interaction. Peptides. 2001;22:1955–1962. doi: 10.1016/s0196-9781(01)00546-0. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf-Makagiansar H, Makagiansar IT, Siahaan TJ. Inhibition of the adherence of T-lymphocyte to epithelial cells by a cyclic peptide derived from inserted domain of lymphocyte function-associated antigen-1. Inflammation. 2001;25:203–214. doi: 10.1023/a:1011044616170. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi N, Kobayashi H, Gu L, Malefyt T, Siahaan TJ. Antigen-specific suppression of experimental autoimmune encephalomyelitis by a novel bifunctional Peptide inhibitor. J. Pharmacol. Exp. Ther. 2007;322:879–886. doi: 10.1124/jpet.107.123257. [DOI] [PubMed] [Google Scholar]
- 9.Bigazzi PE. Animal models of multiple sclerosis. Clin. Immunol. Immunopathol. 1995;77:3. doi: 10.1016/0090-1229(95)90129-9. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Purdy LE, Rabinovitch S, Jevnikar AM, Elliott JF. Major DQ8-restricted T-cell epitopes for human GAD65 mapped using human CD4, DQA1*0301, DQB1*0302 transgenic IA(null) NOD mice. Diabetes. 1999;48:469–477. doi: 10.2337/diabetes.48.3.469. [DOI] [PubMed] [Google Scholar]
- 11.Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366:72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 12.Chao CC, Sytwu HK, Chen EL, Toma J, McDevitt HO. The role of MHC class II molecules in susceptibility to type I diabetes: identification of peptide epitopes and characterization of the T cell repertoire. Proc. Natl. Acad. Sci. USA. 1999;96:9299–9304. doi: 10.1073/pnas.96.16.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray SJ, Oney S, Page EJ, Kratochvil A, Hu Y, Makagiansar TI, Brown CJ, Kobayashi N, Siahaan JT. Suppression of type-1 diabetes in NOD mice by bifunctional peptide inhibitor: modulation of the immunological synapse formation. Chemical Biology & Drug Design. 2007;70:227–236. doi: 10.1111/j.1747-0285.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins 1986. J Immunol. 2005;175:5–14. [PubMed] [Google Scholar]
- 15.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 17.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 18.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino H, Lotvall J, Skoogh BE, Linden A. Neutrophil recruitment by interleukin-17 into rat airways in vivo. Role of tachykinins. Am J Respir Crit Care Med. 1999;159:1423–1428. doi: 10.1164/ajrccm.159.5.9806008. [DOI] [PubMed] [Google Scholar]
- 20.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Reboul P, He Y, Jolicoeur FC, Pelletier JP. Modulation of TIMP-1 synthesis by antiinflammatory cytokines and prostaglandin E2 in interleukin 17 stimulated human monocytes/macrophages. J Rheumatol. 2001;28:712–718. [PubMed] [Google Scholar]
- 21.Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1995;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 22.Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology. 2008 doi: 10.1111/j.1365-2567.2008.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasan M, Gienapp IE, Stuckman SS, Rogers CJ, Jewell SD, Kaumaya PT, Whitacre CC. Suppression of experimental autoimmune encephalomyelitis using peptide mimics of CD28. J. Immunol. 2002;169:2180–2188. doi: 10.4049/jimmunol.169.4.2180. [DOI] [PubMed] [Google Scholar]
- 25.Pedotti R, Mitchell D, Wedemeyer J, Karpuj M, Chabas D, Hattab EM, Tsai M, Galli SJ, Steinman L. An unexpected version of horror autotoxicus: anaphylactic shock to a self-peptide. Nat. Immunol. 2001;2:216–222. doi: 10.1038/85266. [DOI] [PubMed] [Google Scholar]
- 26.Pedotti R, Sanna M, Tsai M, DeVoss J, Steinman L, McDevitt H, Galli SJ. Severe anaphylactic reactions to glutamic acid decarboxylase (GAD) self peptides in NOD mice that spontaneously develop autoimmune type 1 diabetes mellitus. BMC Immunol. 2003;4:2. doi: 10.1186/1471-2172-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu E, Moriyama H, Abiru N, Miao D, Yu L, Taylor RM, Finkelman FD, Eisenbarth GS. Anti-peptide autoantibodies and fatal anaphylaxis in NOD mice in response to insulin self-peptides B:9–23 and B:13–23. J. Clin. Invest. 2002;110:1021–1027. doi: 10.1172/JCI15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith CE, Eagar TN, Strominger JL, Miller SD. Differential induction of IgE-mediated anaphylaxis after soluble vs. cell-bound tolerogenic peptide therapy of autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9595–9600. doi: 10.1073/pnas.0504131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu E, Moriyama H, Abiru N, Paronen J, Devendra D, Finkelman FD, Eisenbarth GS. Preventing peptide-induced anaphylaxis: addition of C-terminal amino acids to produce a neutral isoelectric point. J. Allergy Clin. Immunol. 2004;114:607–613. doi: 10.1016/j.jaci.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 30.Gerety SJ, Karpus WJ, Cubbon AR, Goswami RG, Rundell MK, Peterson JD, Miller SD. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. V. Mapping of a dominant immunopathologic VP2 T cell epitope in susceptible SJL/J mice. J. Immunol. 1994;152:908–918. [PubMed] [Google Scholar]
- 31.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 32.Steinman L. Optic neuritis, a new variant of experimental encephalomyelitis, a durable model for all seasons, now in its seventieth year. J Exp Med. 2003;197:1065–1071. doi: 10.1084/jem.20030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fridkis-Hareli M, Santambrogio L, Stern JN, Fugger L, Brosnan C, Strominger JL. Novel synthetic amino acid copolymers that inhibit autoantigen-specific T cell responses and suppress experimental autoimmune encephalomyelitis. J. Clin. Invest. 2002;109:1635–1643. doi: 10.1172/JCI15402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stern JN, Illes Z, Reddy J, Keskin DB, Fridkis-Hareli M, Kuchroo VK, Strominger JL. Peptide 15-mers of defined sequence that substitute for random amino acid copolymers in amelioration of experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 2005;102:1620–1625. doi: 10.1073/pnas.0409022102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falk K, Rotzschke O, Santambrogio L, Dorf ME, Brosnan C, Strominger JL. Induction and suppression of an autoimmune disease by oligomerized T cell epitopes: enhanced in vivo potency of encephalitogenic peptides. J. Exp. Med. 2000;191:717–730. doi: 10.1084/jem.191.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luca ME, Kel JM, van Rijs W, Wouter Drijfhout J, Koning F, Nagelkerken L. Mannosylated PLP(139–151) induces peptide-specific tolerance to experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2005;160:178–187. doi: 10.1016/j.jneuroim.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson LB, Murtaza A, Hafler BP, Sette A, Kuchroo VK. A T cell receptor antagonist peptide induces T cells that mediate bystander suppression and prevent autoimmune encephalomyelitis induced with multiple myelin antigens. Proc. Natl. Acad. Sci. U. S. A. 1997;94:9279–9284. doi: 10.1073/pnas.94.17.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuchroo VK, Greer JM, Kaul D, Ishioka G, Franco A, Sette A, Sobel RA, Lees MB. A single TCR antagonist peptide inhibits experimental allergic encephalomyelitis mediated by a diverse T cell repertoire. J. Immunol. 1994;153:3326–3336. [PubMed] [Google Scholar]
- 39.Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, Martin R. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 40.Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L The Altered Peptide Ligand in Relapsing MS Study Group. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nat. Med. 2000;6:1176–1182. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]
- 41.Inamura N, Mekori YA, Bhattacharyya SP, Bianchine PJ, Metcalfe DD. Induction and enhancement of Fc(epsilon)RI-dependent mast cell degranulation following coculture with activated T cells: dependency on ICAM-1- and leukocyte function-associated antigen (LFA)-1-mediated heterotypic aggregation. J. Immunol. 1998;160:4026–4033. [PubMed] [Google Scholar]
- 42.Lichtenegger FS, Kuerten S, Faas S, Boehm BO, Tary-Lehmann M, Lehmann PV. Dissociation of experimental allergic encephalomyelitis protective effect and allergic side reactions in tolerization with neuroantigen. J. Immunol. 2007;178:4749–4756. doi: 10.4049/jimmunol.178.8.4749. [DOI] [PubMed] [Google Scholar]
- 43.Fletcher MD, Campbell MM. Partially Modified Retro-Inverso Peptides: Development, Synthesis, and Conformational Behavior. Chem. Rev. 1998;98:763–796. doi: 10.1021/cr970468t. [DOI] [PubMed] [Google Scholar]
- 44.Casasnovas JM, Stehle T, Liu J-H, Wang J-H, Springer TA. A dimeric crystal structure for the N-terminal two domains of intercellular adhesion molecule-1. Proc. Natl. Acad. Sci. USA. 1998;95:4134–4139. doi: 10.1073/pnas.95.8.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bella J, Kolatkar PR, Marlor CW, Greve JM, Rossmann MG. The structure of the two amino-terminal domains of human ICAM-1 suggests how it functions as a rhinovirus receptor and as an LFA-1 integrin ligand. Proc. Natl. Acad. Sci. USA. 1998;95:4140–4145. doi: 10.1073/pnas.95.8.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corper AL, Stratmann T, Apostolopoulos V, Scott CA, Garcia KC, Kang AS, Wilson IA, Teyton L. A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science. 2000;288:505–511. doi: 10.1126/science.288.5465.505. [DOI] [PubMed] [Google Scholar]
- 47.Xu CR, Yusuf-Makagiansar H, Hu Y, Jois SD, Siahaan TJ. Structural and ICAM-1-docking properties of a cyclic peptide from the I-domain of LFA-1: An inhibitor of ICAM-1/LFA-1-mediated T-cell adhesion. J. Biomol. Struct. Dyn. 2002;19:789–799. doi: 10.1080/07391102.2002.10506785. [DOI] [PubMed] [Google Scholar]
- 48.Fethiere J, Tsuda Y, Coulombe R, Konishi Y, Cygler M. Crystal structure of two new bifunctional nonsubstrate type thrombin inhibitors complexed with human alpha-thrombin. Protein Sci. 1996;5:1174–1183. doi: 10.1002/pro.5560050620. [DOI] [PMC free article] [PubMed] [Google Scholar]