Abstract
Background
Neuroimaging studies report that individuals with posttraumatic stress disorder show abnormal responses in the amygdala and medial prefrontal cortex (mPFC)/anterior cingulate cortex (ACC) during exposure to traumatic reminders. However, neural responses arising in the early aftermath of a traumatic event have not been studied.
Methods
Twenty-two motor vehicle-collision survivors and 12 non-traumatized controls participated. Regional cerebral blood flow (rCBF) was measured using [15O]-H2O PET at rest and as subjects listened to scripts of traumatic and neutral events. Self-report measures rated emotional responses to the scripts; standardized assessments (Impact of Events-Revised) evaluated acute stress symptoms at scanning and at 3-month follow-up. Most subjects improved symptomatically.
Results
At rest, trauma subjects showed hyperperfusion in right mPFC/ACC and hypoperfusion in right amygdala compared with controls. In trauma subjects, listening to trauma-scripts versus neutral-scripts resulted in decreased flow in the right amygdala and left amygdala/perirhinal cortex, and symptom scores correlated negatively with right hippocampal flow changes. Symptom improvement at 3 months correlated negatively with rCBF changes in right perirhinal cortex and hippocampus during the trauma versus neutral script contrast. Subjective disturbance during the trauma versus neutral contrast correlated positively with rCBF changes in right amygdala and left mPFC. Functional connectivity analyses of rCBF changes during trauma versus neutral scripts demonstrated left amygdala coupling with right ACC and bilateral anterior insula, and coupling between the amygdala and contralateral hippocampus.
Conclusions
In recently traumatized subjects functional interactions between the amygdala, perirhinal cortex and ACC/mPFC that occur during exposure to traumatic reminders may underlie adaptive/recuperative processes.
Keywords: acute stress disorder, amygdala, PET, prefrontal cortex, perirhinal cortex, fear extinction, PTSD
Background
Functional neuroimaging studies in PTSD show abnormal neurophysiological responses when subjects are exposed to reminders of their traumatic experience. Research supports the involvement of the medial prefrontal cortex (mPFC), amygdala, hippocampal/parahippocampal areas and anterior insula in the pathophysiology of PTSD(1–3). However, no study has examined individuals in the acute aftermath of traumatic events to understand early functional alterations in these or other brain regions. Approximately 15% of motor vehicle collision (MVC) survivors meet DSM-IV criteria for acute stress disorder (ASD)(4) and two thirds of these individuals go on to develop posttraumatic stress disorder (PTSD)(5). Understanding alterations in cortical-limbic circuits in early stages of adaptation to trauma is critical for understanding and ameliorating posttraumatic responses.
Relative to controls PTSD subjects show exaggerated amygdala hemodynamic responses to trauma-related, aversively-conditioned or fearful-face stimuli (3, 6–9). Amygdala stimulation or activation is associated with fear responses and anxiety in humans and experimental animals(10). Additionally, studies show reduced hemodynamic activity in response to traumatic reminders in mPFC, including the anterior cingulate cortex (ACC), which are known to modulate function in the amygdala and other limbic structures during emotional processing(3, 8, 9). One model of PTSD suggests that the mPFC and hippocampus inadequately inhibit neural responses mediated through the amygdala, potentially accounting for the hyperresponsiveness of the amygdala and the hyporesponsiveness of the mPFC during fear provocation(1, 3, 11). Less is known about the adaptive, healthy response to trauma exposure, however. One study demonstrated that individuals exposed to trauma who did not develop chronic PTSD showed decreased rather than increased amygdala activity in response to traumatic reminders(12). This suggested that recovery from trauma may involve successful inhibition of amygdala reactivity.
We used [15O]-H2O PET to assess neurophysiological responses to traumatic reminders in individuals who recently experienced serious MVCs. This was the first study to investigate functional brain alterations before the diagnosis of PTSD is applicable, since PTSD cannot be diagnosed until one month post-trauma. We hypothesized that increased activity in the mPFC and reduced activity in the amygdala would occur in trauma-exposed individuals who were successfully adapting to their exposure.
Methods and Materials
Participants & Setting
Participants were 37 right-handed volunteers with no history of significant head injury or major medical illness. Twenty-five MVC subjects were recruited consecutively from a local community hospital trauma service, and from police MVC reports. All MVCs were serious with an ambulance called to the scene, evacuation to hospital of a victim (not necessarily the subject), and at least one vehicle rendered non-drivable (not necessarily the subject’s). In addition to single and multiple vehicle MVCs, one subject was a bicyclist hit by a car, one a pedestrian hit by a car, one a motorcyclist who collided with a car, and one a car driver who seriously injured a pedestrian. Twelve healthy controls (HC) free from significant lifetime traumatic events were also studied.
Exclusion criteria for all subjects were: significant closed head injuries (i.e., abnormalities on CT or MRI, neurological abnormality upon emergency room evaluation or loss of memory greater than a few seconds; subjective report of momentary memory loss after the MVC was permitted since this can occur from psychological causes); substance dependence within the previous year or any lifetime psychiatric hospitalization. Past psychiatric outpatient treatment was permitted in MVC subjects so that people vulnerable to PTSD were not excluded. Written informed consent was obtained as approved by the IRBs of all institutions involved.
There was no significant difference in age or gender between groups (table 1). No HC took psychoactive medication in the month prior to study. MVC subjects were asked to abstain from opioid analgesics, muscle relaxants and other psychoactive drugs for at least 3 days prior to scanning, but use of non-steroidal analgesics and acetaminophen was permitted. Three MVC subjects failed to comply and were dropped from participation. Results of the remaining 22 MVC subjects are reported. Of these, 11 (50%) had taken opiates and/or muscle relaxants up to 72 hours before scanning.
Table 1.
Demographics and Clinical Variables of Subject Groups [mean +/− SD (range)]
| MVC Subjects (22) | Non-trauma Controls (12) | Significance (p) | |
|---|---|---|---|
| Age | 32.5 ± 12.8 (19–63) | 34.9 ± 12.8 (19–61) | n.s. |
| Gender | 10 female/ 12 male | 5 female/ 7 male | |
| Beck Depression Score (BDI) | 7.0 ± 5.1 (0–17) | 0.4 ± 0.9 (0–3) | 0.0005 |
| Holmes and Rahe Social Readjustment Rating Scale (SD) | 170 ±127 (20–561) | 91 ± 108.5 (0–411) | 0.08 |
| Trauma History Questionnaire (sans MVC item) (SD) | 5.9 ± 4.6 (0–19) | 3.1 ± 2.5 (0–7) | 0.06 |
MVC = motor vehicle collision
Psychometric Measures
Subjects underwent the Structured Clinical Interview for Diagnosis-DSM-IV (SCID) upon enrollment. The presence of ASD was evaluated using the ASD SCID module on the scan day. Severity of posttraumatic symptoms was evaluated using the Impact of Events Scale-Revised (IES-R)(13). Mean time of IES-R administration before the scan was 4.6±3.1 (range 0–14) days. Other psychometric evaluations included the Beck Depression Inventory (BDI)(14), the Social Readjustment Rating Scale (SRRS)(15), and the Trauma History Questionnaire (THQ)(16). The impact of the event was evaluated using the Physical & Mental Health Summary Scales, Short Form (SF-36)(17). Three months after their MVC the BDI and IES-R were repeated for the MVC subjects. The Clinician Administered PTSD Scale (CAPS) also was administered to MVC subjects at 3-month follow-up.
Scripts
Prior to scanning, the MVC subjects described their accident to generate a personalized traumatic-reminder script, incorporating descriptions of 3–5 sensory experiences. Scripts were tape-recorded, but not played for the subjects until the scan. A standardized trauma script was utilized from one of the MVC subject’s scripts as the trauma condition for all HCs. Subjects also described a neutral event, incorporating 3–5 sensory descriptors, of an activity in which they participated regularly. Finally, a standardized neutral script was created, which described the subject tuning a piano—an activity no one had performed. Scripts used second-person pronouns and active verb tense, and were 36–45 seconds long, with all three scripts for each subject varying from one another by no more than 1 second. Each of the three script-types was played twice in sequence during scanning.
Scanning Procedure
Mean time from MVC to scanning was 20±4.5 days (range 10–29). Regional cerebral blood flow (rCBF) was assessed using [15O]-H2O PET, and a GE Advance scanner (GE Medical Systems, Waukesha, WI; 35 slices, 4.25 mm thick; reconstructed transverse and in-plane spatial resolution was 6 and 4.25 mm FWHM, respectively). Transmission scans were obtained, followed by seven emission scans at 8-minute interscan intervals. Following I.V. bolus infusion of 10 mCi of [15O]-H2O (18), each 40 second emission scan was initiated when the rise in whole brain true-coincident radioactive events exceeded 30,000 counts-per-second above background. Head movement was restricted with a thermoplastic mask. Scans were obtained parallel to the canthomeatal line. MRI scans were obtained to define the anatomical frame-of-reference for PET analyses using a GE 1.5T scanner (SPGR sequence; voxel size 1.2×0.86×0.86 mm).
Scan sessions consisted of one resting scan, obtained as subjects rested with eyes-closed, followed by pairs of the neutral and trauma scans. Trauma-script scans were obtained last so that persistent emotional reactions would not influence the reaction to neutral scripts. The order of the personalized-neutral and standardized-neutral script pairs was counterbalanced across subjects. During the resting scan the vascular transit time (time for the [15O]-H2O bolus to travel from peripheral vein to brain) was recorded. Using this transit time, scripts were initiated such that the 15O bolus would reach the brain within 1–3 seconds after the script ended, to assay the early neural response to hearing the entire script.
Before each script subjects were instructed: “close your eyes, don’t fall asleep, and focus on the contents of the script”. After each scan subjects rated subjective disturbance from the script on a 0–100 Likert scale. At baseline and after each scan subjects used a similar scale to rate their experiences of anxiety and pain.
Data Analysis
Psychometrics
Analyses were performed in either Microsoft Excel or SPSS (Chicago, IL). The BDI, SRRS and THQ ratings were compared between the MVCs and HCs using t-tests. The THQ was analyzed without the item related to MVC involvement, to compare the groups’ trauma backgrounds prior to the index event.
Imaging
Images were preprocessed and analyzed using SPM2 (www.fil.ion.ucl.ac.uk/spm)(19). For each subject, PET images were realigned to remove interscan movement, spatially normalized into standard Montreal Neurological Institute (MNI) space using the subject’s MRI, and smoothed with an isotropic 10 mm FWHM kernel. MNI coordinates were transformed to the stereotaxic array of Talairach and Tournoux(20). Images were proportionally normalized to global blood flow using the default gray/white-matter separation estimate of 80% of the whole brain mean.
Differences in resting rCBF between the MVC and HC groups were detected using the SPM2 two-sample t-test. Changes in rCBF during trauma versus neutral provocations were identified using a random-effects ANOVA comparing the first and second trauma script exposures to the first and second standardized or personalized neutral script exposures within each group. Difference images between the personalized or standardized neutral-script image pairs and the trauma-script image pairs of rCBF changes were computed in MEDx as: (Tra1+Tra2)−(Neu1+Neu2). The specificity of resulting findings in either group was investigated post hoc by comparing the two groups in direct comparisons at voxels of relevance using a random effects model (p<.05 uncorrected). Difference images were also correlated with initial IES-R, improvement in IES-R and changes in self-rated emotional disturbance after listening to the scripts. Improvement on the IES-R was calculated as: initial IES-R minus 3-month IES-R (positive scores signified improvement). Because sex-differences in resting rCBF and task-related perfusion changes have been reported(21), gender was covaried out of all SPM correlations. Functional connectivity between flow changes in the amygdala and those in other brain areas was explored by sampling mean activity from the blood-flow-difference images within the amygdala/periamygdaloid ROI template from the WFU Pickatlas(22). This subject-wise vector was then entered as a covariate-of-interest into a voxel-wise regression analysis using the rCBF difference images.
Voxel-wise analyses were conducted in five regions-of-interest (ROIs) selected on the basis of previous literature(23, 24). Correction for multiple comparisons within these ROI was performed using the Familywise Error (FWE) adjustment within the small volume correction (SVC) option provided with SPM software(25). Five ROI templates were defined bilaterally using Pickatlas(22)(figure 1): amygdala/periamygdaloid cortex, hippocampus and insula ROI (“aal” library)(26), and the medial prefrontal conglomerate (mPFC) and ACC (Talairach Daemon library)(27). The mPFC ROI spanned from a ventral border at the gyrus rectus through the ACC and medial frontal gyrus dorsally to a horizontal plane 2 cm above the bicommissural plane. Each peak resulting from the SVC analyses was checked against the Talairach atlas(20). Local maxima at or near an edge of its respective WFU template were also checked against the full-brain map to determine whether the true local maxima were situated outside the template. In such cases the coordinates and Z-score for the true maxima were reported, with spatial extent stated as the number of contiguous voxels within the SVC template at threshold p<0.005.
Figure 1.
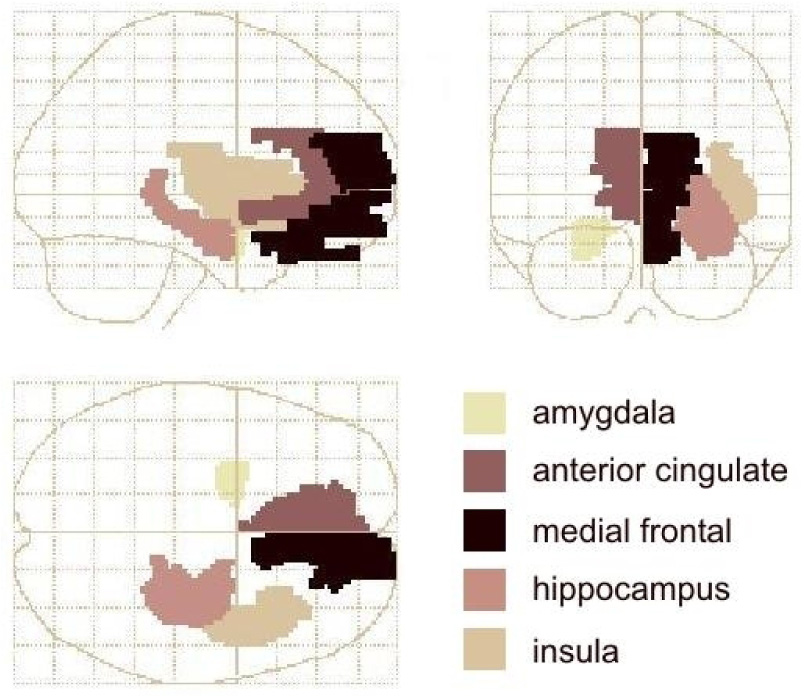
Wakeforest Pickatlas templates used for small volume correction of the SPM image analysis results, depicted in MNI space within the SPM glass brain. For clarity, only one homologue is shown here for each template pair: amygdala and anterior cingulate in the left hemisphere; medial prefrontal cortex, hippocampus and insula in the right.
Results
Psychometrics
Nine MVC subjects met DSM-IV criteria for past psychiatric conditions including social phobia (n=3), specific phobia (n=4), OCD (n=1), major depressive disorder (n=3), and alcohol abuse (n=1). Four subjects had current diagnoses of social phobia (n=2) and specific phobia (n=2). No HC met criteria for any psychiatric disorder.
Mean scores on the BDI, SRRS and THQ appear in Table 1. BDI scores were significantly higher in the MVC group than the HC group, although the mean for MVC subjects was below the threshold for mild depression. The highest scores were 14 (n=3) and 17 (n=1), in the range of mild depression. At three-month follow-up, the mean BDI score decreased to 4.5±5.3 (range 0–20) and mean improvement was significant (p<0.0005). One subject rated moderate (BDI=20) and one mild (BDI=15) depression.
On the day of scanning, 4 subjects met criteria for ASD by SCID; 2 subjects denied all ASD symptoms; others endorsed symptoms in each of the 4 ASD symptom-clusters. Initially, the mean IES-R score was 25±17 (range 0–60). Four subjects had IES-R scores below 10 and were considered psychiatrically unaffected; 12 had IES-R scores ≥23, in the clinically significant range(28). At three-month follow-up, the mean IES-R score was lower (14.±15; range 0–49; p<0.0005). Four subjects had clinically significant PTSD symptoms per IES-R. In one of these subjects the CAPS score at three-months supported a diagnosis of PTSD.
Regarding the physical impact of the MVC, nine subjects (41%) had SF-36 physical functioning scores that were more than 1 standard deviation below U.S. normative values on initial evaluation. In six subjects (27%) scores were greater than 2 standard deviations below this mean.
Imaging
The mean emotional disturbance reported by MVC subjects was 56±29 and 58±35 after the first and second personalized trauma-script scans, respectively. The HC’s disturbance scores were 45±30 and 56±32 for the standardized-trauma script pairs (no group difference). All subjects rated neutral-script scans as having zero disturbance except one MVC subject who rated one personalized neutral scan as 20% disturbing.
Resting State Group Differences
Relative to HCs, MVC subjects showed higher resting flow in a right mPFC area situated in the anterior cingulate sulcal cortex (Brodmann area [BA] 9/32)(29) and lower flow in the right amygdala (table 2).
Table 2.
Areas where blood flow differed between the motor vehicle collision group (MVC) and the healthy control (HC) group under resting conditions
| Brain Region (Brodmann Area) | Talairach Coordinates | Z-score | k* | |
|---|---|---|---|---|
| HC greater than MVC | ||||
| Right amygdala | 18, −6, −11 | 3.59 | 145 | |
| MVC greater than HC | ||||
| Right ACC/mPFC (BA 9/32) | 6, 40, 20 | 4.96 | 138 | |
Coordinates [x, y, z] correspond to the stereotaxic spatial array of Talairach and Tournoux (1988) in which each voxel is located, in millimeters, relative to the anterior commissure, with positive x= right, positive y = anterior and positive z = superior to a horizontal plane containing both the anterior and the posterior commissures.
Correction for multiple comparisons within regions was performed by Familywise Error adjustment and small volume correction.
k = cluster size and is reported for the SVC analysis thresholded at 0.005.
Trauma versus personalized- and standardized-neutral scripts
Table 3 shows results of these analyses for both MVC and HC subjects. Figure 2 shows MVC’s significant decrease in rCBF between conditions in the right amygdala. Figure 3 illustrates the mean rCBF values in right amygdala for each script condition in MVC subjects to demonstrate the specificity of the response to the trauma-script condition. Each significant rCBF difference in table 3 was analyzed post hoc for specificity between subject groups, as indicated in table 3.
Table 3.
Areas of Blood Flow Increases (↑) and Decreases (↓) During Exposure to Traumatic Scripts Relative to Neutral Scripts
| Healthy Controls | MVC Subjects | ||||||
|---|---|---|---|---|---|---|---|
| Talairach Coordinates | Z-score | k | Talairach Coordinates | Z-score | k* | ||
| Trauma Script vs. Personalized Neutral script | |||||||
| Right amygdala (↓)† | 26, −3, −17 | 3.75 | 69 | ||||
| Left amygdala/perirhinal cortexa (↓)†† | −28, 1, −27 | 4.37 | 33 | ||||
| Trauma script vs. Standardized Neutral Script | |||||||
| Left mPFC (↑) (BA 32/10) † | −22, 42, 16 | 3.96 | 45 | ||||
| Left mPFC (↑) (BA 10) † | −12, 46, −7 | 4.66 | 124 | ||||
| Right insula (↑)† | 40, 17, −9 | 3.87 | 149 | ||||
| Left hippocampus (↓)† | −28, −11, −16 | 4.03 | 101 | ||||
Correction for multiple comparisons within regions was performed by Familywise Error adjustment and small volume correction.
MVC = motor vehicle collision group
k = cluster size and is reported for the SVC analysis thresholded at 0.005.
In the whole brain analysis performed post hoc the peak voxel T-value within this cluster of similarly valenced T-values localized these coordinates to the perirhinal cortex ventral to the amygdala.
Post hoc analysis using a random effects model (p < .05 uncorrected) directly comparing MVC and HC groups revealed a significantly greater change in rCBF in the reported group in the direction indicated by the arrow at the voxels identified.
Post hoc analysis using a random effects model (p < .05 uncorrected) directly comparing MVC and HC groups revealed a trend for greater deactivation at this location in the MVC group.
Figure 2.
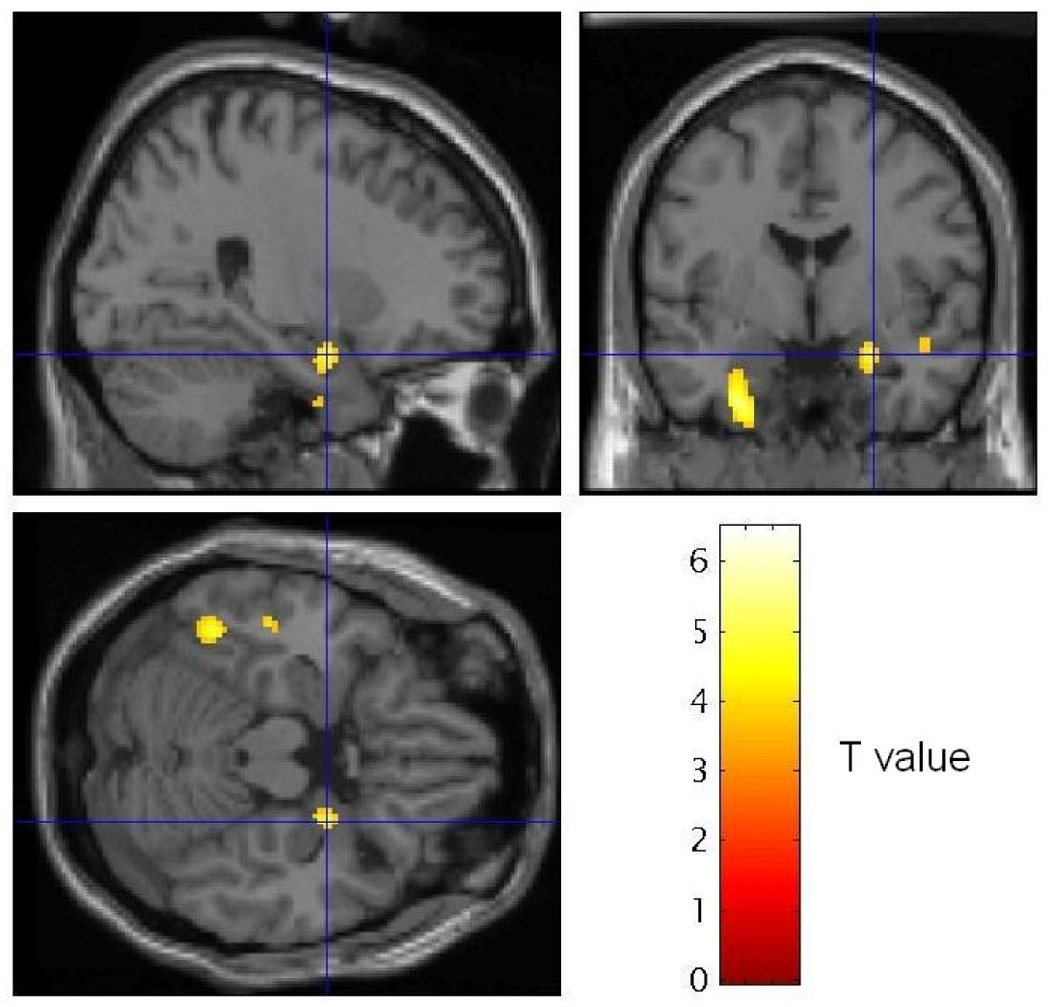
Statistical Parameteric Mapping (SPM) image sections showing voxels where normalized rCBF decreased in MVC subjects in the personalized trauma relative to neutral scripts, thresholded at T = 3.53 (p < .001). Sagittal and coronal (top row) and transverse (bottom row) sections taken at the coordinate of maximally significant deactivation in the right amygdala [26, −3, −17] are shown (coordinates shown by crosshairs are interpreted as in Table 3). An extended area of decreased blood flow also is evident in the left medial temporal cortex, located outside our a priori regions of interest. The implications of this observation will be pursued in future studies.
Figure 3.
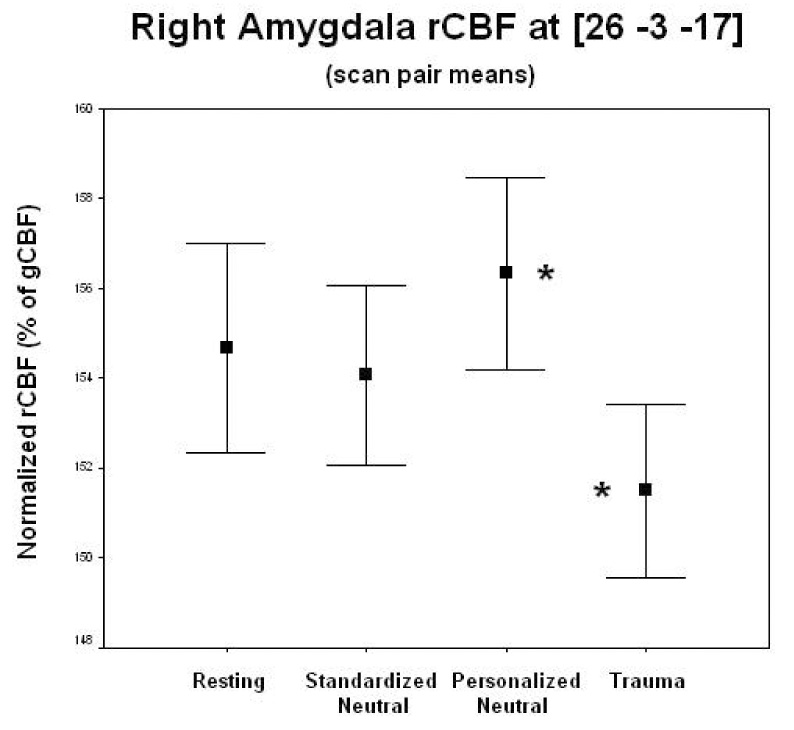
Mean normalized rCBF within each condition in the 22 MVC subjects. The rCBF values across time at the single voxel that was most significantly decreased during the trauma script versus the personalized neutral script conditions (scan pairs collapsed for all but resting scan) in the right amygdala (26 −3 −17) were used. Paired T-tests show trauma script condition significantly decreased (*) from personalized neutral script condition (T = 4.55, p = .00002). Blood flow did not differ significantly between standardized and personalized neutral script conditions. Error bars represent standard error of the mean.
Correlation of symptoms with rCBF
Correlations between rCBF and initial IES-R scores, IES-R improvement at follow-up, and subjective disturbance in MVCs are reported in table 4. These analyses were not conducted in HCs since they did not complete the IES-R. Improvement in IES-R score at three-month follow-up correlated negatively with ΔrCBF in the right perirhinal cortex (figure 4,figure 5). In the trauma versus personalized-neutral comparison anxiety ratings correlated positively with rCBF in right amygdala/claustrum in HCs (30,−4,−10; Z=3.90) and right hippocampus in MVCs (30,−18,−11; Z=3.58).
Table 4.
Significant Positive (+) and Negative (−) Correlations Between rCBF Changes and Symptom Scores of MVC Subjects in A Priori Regions-of-Interest
| Brain Region (Brodmann Area) | Talairach Coordinates | Z-score | k* | |
|---|---|---|---|---|
| Trauma script vs. Personalized Neutral script rCBF with initial IES-R score | ||||
| Right hippocampus (−) | 24, −16, −11 | 4.88 | 66 | |
| Trauma script vs. Personalized Neutral script rCBF with ΔIES-R score | ||||
| Right amygdala/perirhinal cortexa (−) | 30, −1, −25 | 3.77 | 28 | |
| Right hippocampusb (−) | 26, −12, −11 | 3.29 | 46 | |
| Trauma script vs. Personalized Neutral script rCBF with Subjective Disturbance During Scan | ||||
| Right amygdala (+) | 26, −6, −13 | 3.20 | 9 | |
| Left mPFC (BA 9/32) (+) | −12, 47, 16 | 4.28 | 159 | |
Correction for multiple comparisons within regions was performed by Familywise Error adjustment and small volume correction.
MVC = motor vehicle collision group. IES-R = Impact of Events Scale-Revised. ΔIES-R = initial IES-R score minus 3-month follow-up IES-R score.
Initial IES-R score did not correlate with any significant rCBF finding in the MVC group in the resting state.
k = cluster size and is reported for the SVC analysis thresholded at 0.005.
In the whole brain analysis performed post hoc the peak voxel T-value within this cluster of similarly valenced T-values localized these coordinates to the perirhinal cortex ventral to the amygdala.
The locus of these coordinates actually localize to the amygdala/ hippocampal junction.
Figure 4.
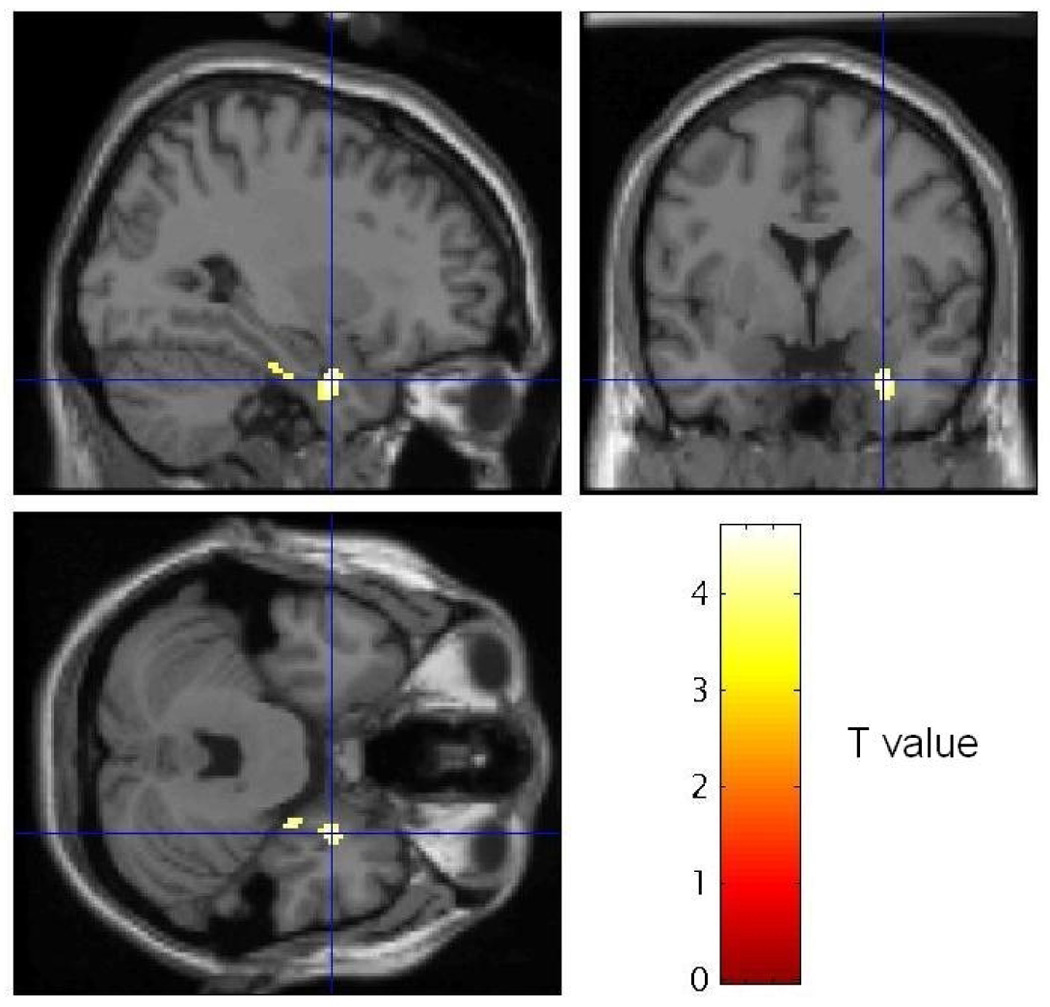
SPM image sections showing voxels where differences in rCBF in MVC subjects between personalized neutral and trauma scripts were negatively correlated with improvement on the IES-R at 3-month follow-up, thresholded at T = 3.58 (p < .001). Sagittal and coronal (top row) and transverse (bottom row) sections are shown at the maximally significant coordinate (see crosshairs) in the perirhinal cortex ventral to the right amygdala [30, −1, −25](76).
Figure 5.
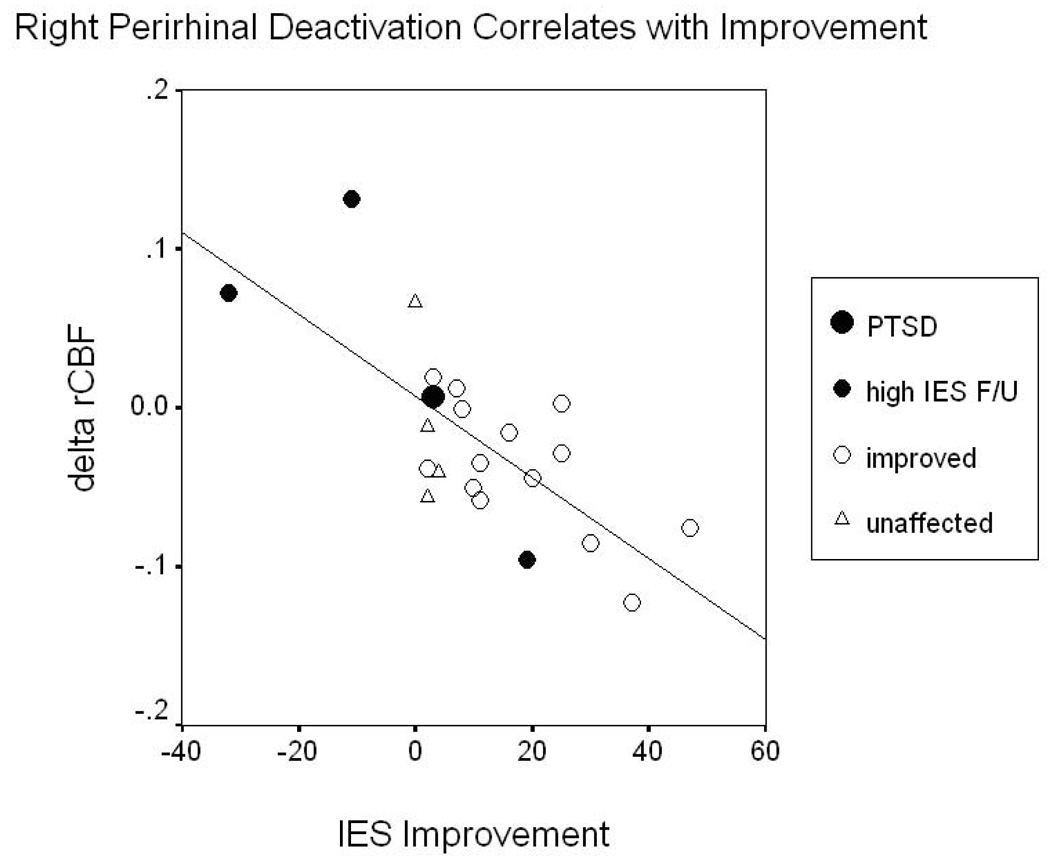
Eventual improvement on the IES-R among MVC subjects versus change in rCBF in the right perirhinal cortex between personalized-neutral and trauma script conditions. Decreased gender-adjusted blood flow in this voxel (30, −1, −25) correlated with improvement on the IES at 3 months (r = −.727, p < .0005, N = 22). Subjects fell into four groups: unaffected (N = 4; IES < 10 and IES F/U ≤ IES), shown as triangles; improved (N = 14; IES ≥ 10 and IES F/U < IES), open circles; high IES F/U (N = 3; IES > 15 and IES F/U > 40), solid small circles; and PTSD (N = 1; met diagnosis at 3 months per CAPS), solid large circle. When the four subjects with significant symptoms at follow-up (“PTSD” and “high IES F/U”) are removed, the relationship remains significant (r = −.606, p = .008, N = 18).
Amygdala connectivity analyses
Results of the correlational analyses using right and left amygdala seed regions in the MVC group are presented in table 5. No significant correlations were found in the HC group.
Table 5.
Significant Positive (+) and Negative (−) Correlations Between rCBF and Amygdala Seed Regions During Trauma vs. Personalized Neutral Script Listening in MVC subjects (no region was identified where rCBF correlated significantly with amygdala flow in the healthy controls)
| MVC Subjects | ||||
|---|---|---|---|---|
| Seed Region | Brain Region (Brodmann Area) | Talairach Coordinates | Z-score | k* |
| Left Amygdala | ||||
| Right hippocampusa (+)† | 34, −13, −21 | 3.73 | 109 | |
| Right hippocampus (+)† | 42, −18, −11 | 3.98 | 109 | |
| Right ACC (BA 24/32) (+)† | 12, 36, 11 | 3.92 | 46 | |
| Right anterior insulab(+)† | 38, 11, −14 | 4.04 | 30 | |
| Left anterior insula (+) | −32, 18, 7 | 3.76 | 50 | |
| Right Amygdala | ||||
| Left hippocampus (+)† | −34, −16, −14 | 3.60 | 35 | |
Correction for multiple comparisons within regions was by Familywise Error adjustment and small volume correction.
MVC = motor vehicle collision group. ACC = anterior cingulate cortex; mPFC = medial prefrontal cortex.
k = cluster size and is reported for the SVC analysis thresholded at 0.005.
This peak was first identified as right amygdala upon SVC analysis, but post hoc analysis revealed the whole brain peak to lie in the right hippocampus at the coordinates described.
These coordinates localize to cortex along the Sylvian fissure at the junction of the insula and the temporal cortices.
Post hoc analysis using a random effects model (p < .05 uncorrected) directly comparing MVC and HC groups revealed a significantly greater correlation between rCBF in the seed region and in the reported voxels in the MVC group relative to the HC group.
Discussion
This study was the first to investigate neurophysiological responses to traumatic reminders during the acute aftermath of a traumatic event, before a diagnosis of PTSD is applicable. MVC subjects’ initial IES-R mean was above the threshold for clinical significance. Over 40% of subjects reported marked negative health effects from their MVC. At three-month follow-up only four subjects continued to show elevated IES-R scores and one of these met criteria for PTSD. Thus, most of the MVC subjects were “resilient”—they experienced a traumatic event, showed a transient stress response, and went on to recovery. These recovering trauma survivors demonstrated physiological changes in limbic and frontal regions-of-interest consistent with our hypotheses.
In the right amygdala/parahippocampal cortex MVC subjects demonstrated lower resting blood flow than non-trauma-exposed HCs. Additionally, the MVC group showed a deactivation of the bilateral amygdalae/perirhinal cortex upon hearing the trauma- compared with the personalized-neutral script and deactivation in left hippocampus in the trauma versus standardized-neutral script comparison. In general, patients with anxiety disorders(30–33) and individuals with anxious temperaments(34) show increased amygdala activity during exposure to fear- or trauma-related stimuli relative to controls. Britton et al. showed that non-trauma-exposed controls had increased rCBF with scripts describing stressful but non-traumatic events at nearly identical coordinates [−28,2,−26] to our rCBF decreases between neutral and trauma conditions [−28,1,−27](12). In contrast, and consistent with our findings, they also demonstrated that trauma-exposed subjects who did not develop PTSD showed decreased activity in the left amygdala while listening to trauma-related scripts(12). Thus, the reduction in amygdala blood flow during rest and trauma-script exposure found here may be specific to the “recovery” response to traumatic reminders following trauma in contrast to either trauma followed by pathology or non-exposure.
Greater symptom improvement over three months correlated with decreases in rCBF in the trauma- versus neutral-script comparison in right perirhinal cortex (figure 5). Perirhinal cortex is involved in multiple aspects of memory(35–41) including multimodal representations and abstractions such as the attribution of meaning(42). Lesions in perirhinal cortex in rats selectively lead to complete obliteration of fear-potentiated startle in pre-conditioned animals—an effect not seen following lesions of the prefrontal, insular or visual cortices(43). Our findings in this region may, therefore, represent an adaptive, modulatory response that terminates or prevents fear-potentiation in trauma survivors who proceed to recovery. This conceivably may involve an attenuation of fear responses to traumatic reminders, with reduced secondary neuronal atrophy from interactive effects of elevated corticosteroid secretion and repeated stress-induced over-stimulation(44, 45).
Decreased activity within the anterior hippocampus in the trauma-versus the neutral-script conditions (a contrast intended to control for declarative memory effects involved in recalling each set of events) correlated with initial and ΔIES-R scores. This may represent a process of the hippocampus being taken “off line” to suppress neurotransmission to the amygdala about the context of the traumatic event relative to that of the current experiment (traffic scene versus PET suite)(46).
The changes in right amygdala rCBF correlated positively with subjective disturbance ratings during PET scanning in the MVC subjects. This is consistent with literature demonstrating amygdala activation during fear and stress states(46), but contrasts with the deactivations/inverse correlations in right amygdala with symptoms near the time of scanning in our other analyses. Importantly, we found no correlation between subjective disturbance and IES-R scores (p≥0.2 for initial and follow-up IES-R), suggesting that these two variables assessed distinct subjective and neurobiological processes in these subjects.
Under resting conditions rCBF in the right ACC/mPFC (BA9/32) was increased in the MVCs compared with HCs. This findings appeared compatible with prior research showing that during exposure to traumatic reminders rCBF increased in ACC/mPFC regions in trauma-exposed subjects without PTSD, but either decreased or showed attenuated elevation in PTSD subjects(47, 48). These areas share extensive anatomical connections with the amygdala(33)(49), and in rats the projections from the infralimbic portion of the ACC to the amygdala have been shown to play major roles in fear extinction(50, 51). Neuroimaging studies in humans have also identified mPFC areas where physiological activity increases in direct relation to fear during extinction learning(52, 53). In our MVC subjects the reciprocal pattern of lower rCBF in the amygdala and higher rCBF in the ACC/mPFC may reflect cortical-limbic interactions involved in modulating emotional behavior either as an acute response to the mild stress associated with scanning or as a tonic change resulting from the recent traumatic exposure to prevent reactivity to emotional events in general. In either case, this increased neurophysiological activity within the ACC/mPFC, taken together with the excellent recovery shown by most of our subjects, supports our original hypothesis that enhanced activation of the ACC/mPFC coupled with attenuated activity in the amygdala after serious trauma holds positive prognostic significance.
However, in the left mPFC another anterior cingulate sulcal cortex area (BA 9/32) was identified where rCBF correlated positively with subjective disturbance during the trauma-script versus neutral-script comparison. This area (x=−12, y=47, z=16) was separated from the area where resting CBF was higher in MVC subjects than controls (x=6, y=40, z=20) by a distance greater than the spatial resolution of the image data, suggesting these were distinct regions. The left mPFC region was within the vicinity of areas where physiological activity increased in subjects with a history of depression in direct relation to depressive symptoms induced via catecholamine and serotonin depletion(54–56). Both subject groups demonstrated flow increases in separate regions of the left mPFC in the trauma-versus standardized-neutral script contrast. The preclinical literature shows that emotional expression is inhibited by some mPFC regions, but facilitated or enhanced in other mPFC regions(57). For example, in contrast to the inhibitory role of the infralimbic cortex on fear expression described above, prelimbic cortex neuronal activity enhances fear expression mediated through the amygdala(58–60). Because hemodynamic activity predominantly reflects local synaptic activity, functional imaging assessments have limited capability to distinguish such functional relationships. Thus, the rCBF findings in the mPFC may reflect activation either in response to the experience of such emotions in order to modulate their expression, or to enhance the fear/distress response.
Amygdala Connectivity
The amygdala connectivity analyses identified several regions that were functionally associated with left amygdala, specific to the MVC subjects, including the right hippocampus, right ACC and bilateral anterior insula. Right amygdala activity was associated with left hippocampal activity. The implicated “pregenual” region of the ACC and the anterior (agranular) insula share extensive reciprocal, anatomical connections with the amygdala(29, 61). The anterior insula has been shown to participate in the modulation of contextual fear(62, 63) and lesions there reduce fear reactivity to contextual stimuli, but do not affect conditioned stimulus acquisition or response extinction(63). Anterior insular areas also participate in modulating peripheral responses to stress, including heart rate and blood pressure(61, 64), and have been implicated in processing emotional stimuli and experience(65, 66).
Amygdala connectivity analyses additionally implicated the hippocampus. Projections from the hippocampal formation to the amygdala have been specifically associated with spatial contextual conditioning(67, 68). Lesioning these projections in rats prevents fear conditioning to spatial locations where aversive stimulation previously occurred(68–71). Thus, our amygdala functional connectivity findings of trauma exposed subjects comparing trauma-script versus neutral-script conditions confirms previous literature involving fear processing. The specificity of our amygdala connectivity findings to the MVCs may be due to fact that while the (standardized) trauma-script did induce subjective disturbance, it did not involve a contextually-significant, fearful memory for those subjects. Alternatively, the smaller number of HC subjects may have limited the power of those analyses.
Limitations
The smaller n for the HC group may have led to the absence of comparable findings to the MVC group in several analyses. In addition, the MVC group differed from the HC group by having more psychiatric diagnoses currently and historically, potentially contributing to baseline neurophysiological differences. Interestingly, only one of the four MVC subjects with elevated symptoms on follow-up had past or active psychiatric diagnoses on enrollment and this was not the subject who developed PTSD.
Some MVC subjects had taken opiates and/or muscle relaxants as recently as 3 days before scanning. These subjects had taken such agents only since the MVC and were unlikely to have developed physical dependence. Nevertheless, drug exposure over 72 hours before scanning may conceivably have produced confounding neurophysiological and cerebrovascular effects on PET measures.
Anxiety ratings during scanning were non-significantly higher in MVC compared to HC subjects (p=0.10), while resting pain ratings did not differ significantly (p=0.2). When anxiety was covaried from the imaging analysis, the peak Z-scores corresponding to resting rCBF differences between groups decreased only slightly in mPFC (from 4.96 to 4.70) and amygdala (from 3.59 to 3.28), suggesting that state anxiety alone did not account for these differences.
MVC subjects had significantly higher initial BDI scores than controls. The items endorsed by the MVC subjects (e.g., increased effort in work activities, sleep problems, fatigue, excessive worry, decreased sexual interest) may have reflected pain and physical impairment rather than depression per se. They generally denied depressed mood or anhedonia. Two subjects had clinically significant BDI scores at follow-up. The highest BDI scorer was the individual diagnosed with PTSD and the second highest also had a clinically significant IES-R score at follow-up. Previous research has suggested a role of depression in ASD and PTSD(72–74) and these disorders may be neurophysiologically related.
Finally, responses to each script-type was imaged only twice to reduce effects of habituation and slow termination of arousal states(75). Consequently, we selected PET with the bolus administration technique because of its superior sensitivity for providing valid and reliable CBF measures to events that happen only one or two times over a short interval(62). Under such conditions BOLD-fMRI is less sensitive since it depends upon imaging similar events repeatedly to permit signal averaging across many trials.
In summary, our data showed that trauma-recovering subjects studied in the acute aftermath of exposure were distinguished from non-trauma controls by reduced rCBF in mesiotemporal limbic structures and increased flow in mPFC regions under multiple conditions. They suggest an adaptive process in recovering individuals that involves deactivation of perirhinal cortex, perhaps as a means of terminating or preventing fear-potentiating associations. The next step in further characterizing these findings would be to prospectively compare a trauma-exposed population who develop PTSD versus those who do not.
Acknowledgments
This research was funded by NIMH extramural grant # U01 MH63397-01A1 awarded at the Uniform Services University of the Health Sciences; and the intramural NIMH IRP, Section on Neuroimaging in Mood and Anxiety Disorders.
CSTS Neuroimaging Study Group includes: Carol Fullerton, Brian Crowley, Richard Epstein, Miriam Gerber, Monica Grover, John Russotto, Juliana Tiongson.
Nursing staff at Suburban Hospital in Bethesda, Maryland and the Montgomery County Police Department, Records Division have been instrumental in the collection of the data presented here. Special thanks to Mr. Shawn Donnelly, social worker at Suburban Hospital, for his work identifying potential research participants.
This research was supported by extramural grant number # U01 MH63397-01A1 from the NIMH awarded at the Uniform Services University of the Health Sciences and the intramural NIMH IRP, Mood Imaging Branch.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: None of the authors reported any biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 2.Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- 3.Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, et al. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 4.DSM-IV. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Fourth ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 5.Harvey AG, Bryant RA. The relationship between acute stress disorder and posttraumatic stress disorder: a prospective evaluation of motor vehicle accident survivors. J Consult Clin Psychol. 1998;66:507–512. doi: 10.1037//0022-006x.66.3.507. [DOI] [PubMed] [Google Scholar]
- 6.Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- 7.Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162:1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- 8.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 10.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bremner JD. Effects of traumatic stress on brain structure and function: relevance to early responses to trauma. J Trauma Dissociation. 2005;6:51–68. doi: 10.1300/J229v06n02_06. [DOI] [PubMed] [Google Scholar]
- 12.Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 13.Weiss DS, Marmar CR. The Impact of Event Scale-Revised. In: Wilson JP, Keane TM, editors. Assessing psychological trauma and PTSD. New York: The Guilford Press; 1997. [Google Scholar]
- 14.Beck AT, Ward CH, Mendelssohn MJ, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 15.Slusarcick AL, Ursano RJ, Fullerton CS, Dinneen MP. Life events in health care providers before and during Persian Gulf War deployment: The USNS comfort. Military Medicine. 1999;164:675–682. [PubMed] [Google Scholar]
- 16.Green BL. Trauma history questionnaire. In: Stamm BH, Varra EM, editors. Measurement of stress, trauma and adaptation. Lutherville: Sidran Press; 1996. pp. 366–368. [Google Scholar]
- 17.Ware JE, Snow KK, Kosinski M. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 18.Fox PT, Mintun MA. Noninvasive functional brain mapping by change-distribution analysis of averaged PET images of H215O tissue activity. J Nucl Med. 1989;30:141–149. [PubMed] [Google Scholar]
- 19.Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 20.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. 1988 [Google Scholar]
- 21.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 22.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 23.Jatzko A, Schmitt A, Kordon A, Braus DF. [Neuroimaging findings in posttraumatic stress disorder: review of the literature] Fortschr Neurol Psychiatr. 2005;73:377–391. doi: 10.1055/s-2004-830120. [DOI] [PubMed] [Google Scholar]
- 24.Liberzon I, Phan KL. Brain-imaging studies of posttraumatic stress disorder. CNS Spectr. 2003;8:641–650. doi: 10.1017/s109285290000883x. [DOI] [PubMed] [Google Scholar]
- 25.Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res. 2003;12:419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- 26.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 27.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ursano RJ, Fullerton CS, Kao TC, Bhartiya VR. Longitudinal assessment of posttraumatic stress disorder and depression after exposure to traumatic death. J Nerv Ment Dis. 1995;183:36–42. doi: 10.1097/00005053-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 30.Fredrikson M, Furmark T. Amygdaloid regional cerebral blood flow and subjective fear during symptom provocation in anxiety disorders. Ann N Y Acad Sci. 2003;985:341–347. doi: 10.1111/j.1749-6632.2003.tb07092.x. [DOI] [PubMed] [Google Scholar]
- 31.Pissiota A, Frans O, Fernandez M, von Knorring L, Fischer H, Fredrikson M. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur Arch Psychiatry Clin Neurosci. 2002;252:68–75. doi: 10.1007/s004060200014. [DOI] [PubMed] [Google Scholar]
- 32.Pissiota A, Frans O, Michelgard A, Appel L, Langstrom B, Flaten MA, et al. Amygdala and anterior cingulate cortex activation during affective startle modulation: a PET study of fear. Eur J Neurosci. 2003;18:1325–1331. doi: 10.1046/j.1460-9568.2003.02855.x. [DOI] [PubMed] [Google Scholar]
- 33.Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, et al. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry. 2001;158:1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 34.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 35.Buffalo EA, Reber PJ, Squire LR. The human perirhinal cortex and recognition memory. Hippocampus. 1998;8:330–339. doi: 10.1002/(SICI)1098-1063(1998)8:4<330::AID-HIPO3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 36.Pihlajamaki M, Tanila H, Hanninen T, Kononen M, Mikkonen M, Jalkanen V, et al. Encoding of novel picture pairs activates the perirhinal cortex: an fMRI study. Hippocampus. 2003;13:67–80. doi: 10.1002/hipo.10049. [DOI] [PubMed] [Google Scholar]
- 37.Bonda E, Petrides M, Evans A. Neural systems for tactual memories. J Neurophysiol. 1996;75:1730–1737. doi: 10.1152/jn.1996.75.4.1730. [DOI] [PubMed] [Google Scholar]
- 38.de Zubicaray GI, McMahon K, Wilson SJ, Muthiah S. Brain activity during the encoding, retention, and retrieval of stimulus representations. Learn Mem. 2001;8:243–251. doi: 10.1101/lm.40301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J Neurosci. 2002;22:523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose M, Haider H, Weiller C, Buchel C. The role of medial temporal lobe structures in implicit learning: an event-related FMRI study. Neuron. 2002;36:1221–1231. doi: 10.1016/s0896-6273(02)01105-4. [DOI] [PubMed] [Google Scholar]
- 41.Henke K, Mondadori CR, Treyer V, Nitsch RM, Buck A, Hock C. Nonconscious formation and reactivation of semantic associations by way of the medial temporal lobe. Neuropsychologia. 2003;41:863–876. doi: 10.1016/s0028-3932(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 42.Taylor KI, Moss HE, Stamatakis EA, Tyler LK. Binding crossmodal object features in perirhinal cortex. Proc Natl Acad Sci U S A. 2006;103:8239–8244. doi: 10.1073/pnas.0509704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosen JB, Hitchcock JM, Miserendino MJ, Falls WA, Campeau S, Davis M. Lesions of the perirhinal cortex but not of the frontal, medial prefrontal, visual, or insular cortex block fear-potentiated startle using a visual conditioned stimulus. J Neurosci. 1992;12:4624–4633. doi: 10.1523/JNEUROSCI.12-12-04624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joels M, Krugers H, Karst H. Stress-induced changes in hippocampal function. Prog Brain Res. 2007;167:3–15. doi: 10.1016/S0079-6123(07)67001-0. [DOI] [PubMed] [Google Scholar]
- 45.Patel R, McIntosh L, McLaughlin J, Brooke S, Nimon V, Sapolsky R. Disruptive effects of glucocorticoids on glutathione peroxidase biochemistry in hippocampal cultures. J Neurochem. 2002;82:118–125. doi: 10.1046/j.1471-4159.2002.00948.x. [DOI] [PubMed] [Google Scholar]
- 46.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abus-erelated PTSD: A PET investigation. Am J Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 49.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A Role for the Human Dorsal Anterior Cingulate Cortex in Fear Expression. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 53.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, et al. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry. 2004;61:765–773. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- 55.Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60:93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Hasler G, Fromm S, Carlson PJ, Luchenbaugh DA, Weldek T, Geraci M, et al. Neural response to catecholamine depletion in unmedicated, remitted subjects with major depressive disorder and healthy subjects. Arch Gen Psychiatry. doi: 10.1001/archpsyc.65.5.521. (In press.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 60.Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 62.Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007;27:6313–6319. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgan MA, LeDoux JE. Contribution of ventrolateral prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Neurobiol Learn Mem. 1999;72:244–251. doi: 10.1006/nlme.1999.3907. [DOI] [PubMed] [Google Scholar]
- 64.Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol. 2000;523(Pt 1):259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 66.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 67.Gray JA, McNaughton N. The neuropsychology of anxiety: reprise. Nebr Symp Motiv. 1996;43:61–134. [PubMed] [Google Scholar]
- 68.Phillips RG, LeDoux JE. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. J Neurosci. 1995;15:5308–5315. doi: 10.1523/JNEUROSCI.15-07-05308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 70.Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- 71.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 72.Blanchard EB, Hickling EJ, Taylor AE, Loos W. Psychiatric morbidity associated with motor vehicle accidents. J Nerv Ment Dis. 1995;183:495–504. doi: 10.1097/00005053-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 73.Harvey AG, Bryant RA. Predictors of acute stress following motor vehicle accidents. J Trauma Stress. 1999;12:519–525. doi: 10.1023/A:1024723205259. [DOI] [PubMed] [Google Scholar]
- 74.O'Donnell ML, Creamer M, Pattison P. Posttraumatic stress disorder and depression following trauma: understanding comorbidity. Am J Psychiatry. 2004;161:1390–1396. doi: 10.1176/appi.ajp.161.8.1390. [DOI] [PubMed] [Google Scholar]
- 75.Osuch EA, Benson B, Geraci M, Podell D, Herscovitch P, McCann UD, et al. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol Psychiatry. 2001;50:246–253. doi: 10.1016/s0006-3223(01)01107-6. [DOI] [PubMed] [Google Scholar]
- 76.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. 2nd ed. San Diego: Elsevier Academic Press; 2004. [Google Scholar]


