Abstract
Background
Details of the internal hippocampal structure visible at 4T allow for in-vivo volumetry of subfields. The aims of this study were: 1. To determine if Apo e4 has subfield specific effects in controls. 2. To study the influence of Apo e4 on hippocampal subfields in AD.
Methods
81 subjects (66 controls, mean age 60.8±13.6, range: 28–85 years), and 15 AD (mean age 67.5±9.3) were studied. Entorhinal cortex, subiculum, CA1, CA1-CA2 transition zone, CA3-4& dentate gyrus (CA3&DG) and total hippocampal volume were determined using a manual marking strategy.
Results
Significant effects for Apo e4 on the CA3&DG were found in the total control population (p = 0.042) and in older controls (61–85 years) (p = 0.036) but not in younger (28–60 years) controls. Significant effects for Apo e4 (p = 0.0035) on CA3&DG were also found in a subgroup of older subjects and AD subjects. AD with Apo e4 had smaller CA3&DG than AD without Apo e4 (p = 0.027).
Conclusions
These findings suggest that Apo e4 exerts a regionally selective effect on CA3&DG in normal aging and AD
1. Introduction
Apolipoprotein E (Apo e) is a major lipoprotein transporter of the brain and plays an important role in the regulation of the neuronal cholesterol metabolism (Pfrieger, 2003). There are three different isoforms: Apo e2, Apo e3 and Apo e4, which differ by the amino acid residues on sites 112 and 152. Apo e4 has arginine residues at both positions which reduce its stability and render it conformationally unstable. As a consequence, Apo e4 has a lower functionality than the other two isoforms and may even undergo potentially neurotoxic conformational changes (Mahley et al. 2006). Over the last few years, Apo e4 has been implicated as potential genetic risk factor for several neurodegenerative diseases, particularly sporadic Alzheimer’s disease (AD) but also multiple sclerosis, Parkinson’s disease or amyotrophic lateral sclerosis (Chapman et al. 2001, Bedlack et al. 2000, Blasquez et al. 2006). The molecular mechanisms by which Apo e4 promotes neurodegeneration are still not completely understood and most likely complex. In AD for example, it has been shown that Apo e4 not only enhances AD related patho-mechanisms, i.e., increases amyloid production and deposition as well as tau phosphorylation, but also exerts direct neurotoxic effects (Mahley et al. 2006) and impairs neuronal repair/maintenance mechanisms (Weisgraber and Mahley. 1996). Recent studies have also shown that compared to Apo e3, Apo e4 is associated with reduced neuronal plasticity and impaired neurogenesis (Levi et al. 2003, Levi et al. 2005, Levi 2007, Teter, 2004).
The hippocampus is a particularly interesting brain structure in the context of Apo e. It is not only affected by various neurodegenerative conditions (Morrison and Hof. 2002 Mattson et al. 1989, Joels et al. 2004, Velakoulis et al. 2006, Phillips and Reeves. 2001, Gilbert, 2004), but has also a high intrinsic neuroplasticity and is capable of neurogenesis during adulthood (Abrous et al. 2005, Klempin and Kempermann, 2007); all of these are processes which are influenced by the Apo e genotype. The hippocampus is not a homogeneous structure but consists of several subfields with distinct histological characteristics: the subiculum, the four cornu ammonis sectors (CA1-3) and the dentate gyrus. Although these subfields are functionally tightly interconnected (Duvernoy, 2005), there is evidence for a functional specialization (Rolls and Kesner, 2006, Kesner and Hopkins, 2006). Furthermore, animal models and histopathological studies suggest that different disease processes affect subfields selectively, e.g. stress affects predominantly the dentate gyrus while neuron loss in CA1 is typical for AD (West, et al. 1994 Lucassen et al. 2006). Therefore, in vivo volumetry of hippocampal subfields might yield a better distinction between different disease processes and/or allow for an earlier diagnosis than measuring global hippocampal volume loss. However, this requires that details of the internal structure of the hippocampal formation can be depicted in vivo. Recent advancements with high field MRI (3–4 Tesla), achieving superb gray/white matter contrast by exploiting increased signal sensitivity, greater dispersion of magnetization transfer effects and enhanced T1 weighting, have resulted in excellent anatomical images at sub-milimeter resolution that can be acquired within a few minutes. In this study, we used high resolution images acquired on a 4 Tesla MRI system and a manual method for subfield marking to address the following aims: 1. To determine if Apo e4 genotype has subfield specific effects in a group of healthy and cognitively intact subjects spanning an age range from 28–85 years. Based on the known actions of Apo e we expected to find an Apo e4 effect in CA1 with the Apo e4 genotype enhancing the age-associated volume loss in this subfield (Mueller et al. 2007), and/or in the dentate gyrus due to a negative impact of Apo e4 on neuroplasticity/neurogenesis (Ji et al. 2003). 2. To study the influence of Apo e4 on hippocampal subfields on AD. Based on histological studies (West et al. 1994), we expected to find a more pronounced volume loss in CA1 in AD with the Apo e4 allele than in AD without it.
2. Methods
2.1 Study population
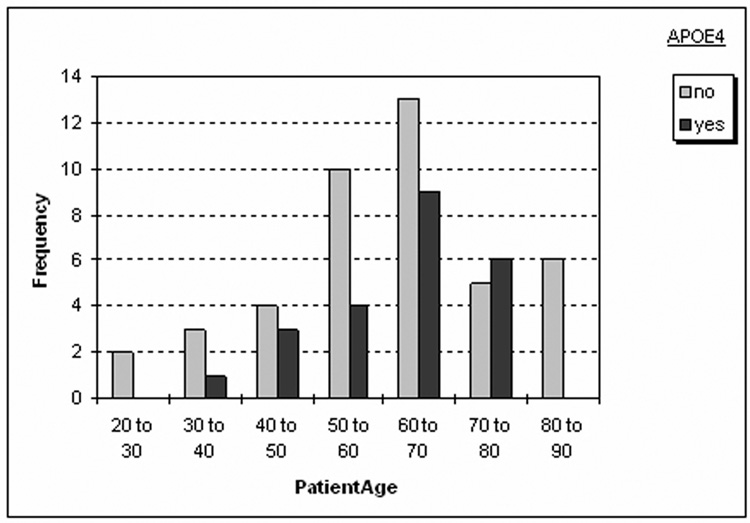
A total of 81 subjects participated in this study. Sixty-six were healthy control subjects (mean age 60.8 ± 13.6, age range: 28 – 85 years, female/male (f/m) 29/40, mean MMSE 29.4 ± 1.0) recruited from the community with flyers and advertisements in local newspapers. These subjects were recruited with the intention to build a general healthy control population for several ongoing projects in our laboratory and so no special efforts were made to achieve a perfect match between Apo e4 and non-Apo e4 subjects in the population. A subset of this population has been reported in a previous publication (Mueller et al., 2007). Exclusion criteria included any poorly controlled medical illness (untreated diabetes, hypertension, thyroid disease) and/or use of medication or recreational drugs that could affect brain function, a history of brain trauma, brain surgery or evidence for ischemic events (stroke but not white matter hyperintensities or small lacunes) and skull defects on the MRI. Normal cognitive functioning was assessed by a battery of neuropsychological tests (mini mental state examination, California Verbal Learning Test (short form), Rey-Osterrieth complex figure, Verbal Fluency, Wechsler Adult Intelligence Score (digit symbol, digit span)); emotional state and functioning in daily living were assessed with the Geriatric Depression Scale and the Functional Activities Questionnaire (cf. Table 1). Subjects, who scored −1.5 or less in more than two of these standardized tests, were excluded from the study. Twenty-three of these subjects had at least one Apo e4 allele (3/4: 19; 2/4: 2: 4/4: 2) and 43 had no Apo e4 allele (2/3: 8; 3/3: 35) (cf Figure 1). Fifteen subjects (mean age 67.5± 9.3, f/m: 6/9, mean MMSE 21.3 ± 5.0) diagnosed with Alzheimer’s disease according to the criteria by the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINDS-ADRDA) were referred from collaborating Memory Clinics (UCSF, VA Medical Center San Francisco, CPMC Sacramento). Ten had at least one Apo e4 allele (3/4: 7; 4/4: 3), the remaining five were 3/3. AD with Apo e4 allele were significantly younger than subjects without Apo e4 (p = 0.017, cf Table 1) otherwise the two AD subgroups were not different. The study was approved by the committees of human research at the University of California, San Francisco (UCSF) and VA Medical Center San Francisco, and written informed consent was obtained from all subjects or their legal representatives according to the Declaration of Helsinki.
Table 1.
Demographic and Neuropsychological Characteristics of Healthy Controls
| Group | Age | Gender f/m | Years Of Education | MMSE | Digit Symbol | Rey-Delay | Short Correct Recall | Long Correct Recall |
|---|---|---|---|---|---|---|---|---|
| Young Non-Apo e4 N = 19 |
46.8 (10.5) | 11/8 | 16.1 (2.5) | 29.4 (0.9) | 0.63 (1.06) | 0.35 (0.98) | 0.92 (0.93) | 0.50 (0.75) |
| Young Apo e4 N = 9 |
50.8 (8.1) | 6/3 | 16.4 (1.94) | 29.8 (0.4) | 0.33 (0.96) | 0.47 (0.69) | 0.94 (1.29) | 0.39 (0.70) |
| Old Non-Apo e4 N = 24 |
71.29 (7.6) | 7/17 | 17.29 (2.4) | 29.4 (1.3) | 0.83 (0.88) | 0.18 (0.81) | 0.81 (1.16) | 0.71 (0.98) |
| Old Apo e4 N = 14 |
69.2 (6.0) | 5/9 | 16.7 (2.23) | 29.1 (0.8) | 0.26 (0.91) | 0.35 (0.81) | 0.50 (1.00) | 0.54 (0.72) |
| AD Apo e4 N = 10 |
63.5 (7.5) | 2/3 | 16.5 (2.8) | 21.6 (5.7) | NA | NA | NA | NA |
| AD non Apo e4 N = 5 |
75.4 (7.6) | 4/6 | 14.3 (1.5) | 20.6 (3.7) | NA | NA | NA | NA |
All values are reported as mean and standard deviations (SD).
digit symbol, Rey delay and short correct recall and long recall of the CVLT are reported as the means and standard deviation (SD) of the standardized scores. NA, non available. AD subjects had been referred/recruited from different memory clinics and differed regarding neuropsycholgical work up.
Figure 1.
Distribution of Apo e4 carriers and non Apo e4 carriers across the age range in the control population.
2.2. MRI acquisition
All imaging was performed on a Bruker MedSpec 4T system controlled by a Siemens Trio™ console. The following sequences, which were part of a larger research imaging and spectroscopy protocol, were acquired: 1. For the measurement of hippocampal subfields, a high resolution T2 weighted fast spin echo sequence (TR/TE: 3500/19 ms, echo train length 15, 18.6 ms echo spacing, 160° flip angle, 100% oversampling in ky direction, 0.4 × 0.4 mm in plane resolution, 2 mm slice thickness, 24 interleaved slices without gap, acquisition time 5:30 min (adapted from DeVita et al. 2003; Thomas et al. 2004), angulated perpendicular to the long axis of the hippocampal formation, 2. For the measurement of total hippocampal volume a volumetric T1-weighted gradient echo MRI (MPRAGE) TR/TE/TI = 2300/3/950 ms, 7° flip angle, 1.0 × 1.0 × 1.0 mm3 resolution, acquisition time 5.17 min and 3. For the determination of the intracranial volume (ICV), a T2 weighted turbospin echo sequence (TR/TE 8390/70 ms, 150° flip angle, 0.9 × 0.9 × 3 mm nominal resolution, 54 slices, acquisition time 3.06 min).
2.3. Postprocessing and manual marking of hippocampal subfields
The method used for subfield marking including measurement reliability has been described in detail previously (Mueller et al. 2007). The marking scheme depends on anatomical landmarks, particularly on a hypointense line probably representing myelinated fibers in the stratum moleculare,/lacunosum (Eriksson et al. 2008) which can be reliably visualized on these high resolution images (cf. Figure 2). Although the sequence used in this study provides superior resolution and thus more information about the internal structure than a clinical standard sequence, it does not allow to distinguish details on the resolution of a histological preparation and thus we do not claim that the subfield assignment actually corresponds to the histological subfields but merely provides a good approximation (cf. Figure 2). To summarize the procedure briefly: The high resolution images were re-sampled to obtain a left and a right hippocampal image on which the coronal axis was exactly perpendicular to the long axis of the hippocampus. The marking starts on the first slice on which the head of the hippocampus is no longer visible. On this slice, the hippocampal subfields, subiculum and ERC are marked manually. In addition, the ERC is marked on the two slices anterior to this starting slice and the subiculum and the hippocampal subfields are marked on the two slices posterior to the starting slice. Altogether, hippocampal subfields were marked on 5 consecutive slices, i.e. on a length of 1.0 cm in the rostral part of the hippocampus. The most medial point of the temporal cortex is chosen as medial border of the ERC and the medial end of the collateral sulcus is chosen as its lateral border. The CA1/subiculum border is determined by drawing a line perpendicular to the edge of the subiculum touching the medial border of the hippocampus. This border was chosen because it could be easily and reliably identified although by doing so, parts of the presubiculum and subiculum proper were counted towards CA1. The CA1/CA2 border is determined by dividing the line along the longest diameter of the hippocampus by two and drawing a line perpendicular to this line. A region supposed to represent mainly CA2 was marked in a square-like manner, i.e., its height at the CA1/CA2 boundary also determined its length while its overall shape was determined by the course of the outer boundary of the hippocampus and the hypointense line representing myelinated tissue in the strata moleculare/lacunosum. Although the position of CA2 using the outlined criteria showed good correspondence with the localization of CA2 in histological preparations, its volume is influenced by the width of the dorsal CA1 and it is likely to have some overlap with the dorso-medial part of CA1. Because of this, we expect that volume changes in this sector can result from changes in both subfields, i.e., CA2 and dorsal part of CA1. To reflect this “contamination” by CA1, the region was named CA1-2 transition zone (CA1-2 transition) rather than CA2. The remainder of the hippocampal formation consisting of CA3 and dentate gyrus is marked as one region (CA3&DG) because there are no reliable landmarks to distinguish between these structures. The volume of the total hippocampus was determined from the T1 image using the hippocampal masks provided by the FreeSurfer software routine (Fischl et al. 2002). These hippocampal masks were visually checked for accuracy and manually edited if necessary. The ICV was determined from the T2 weighted image using the BET program (FMRIB Image Analysis Group, Oxford University, www.fmrib.ox.ac.uk/fsl).
Figure 2.
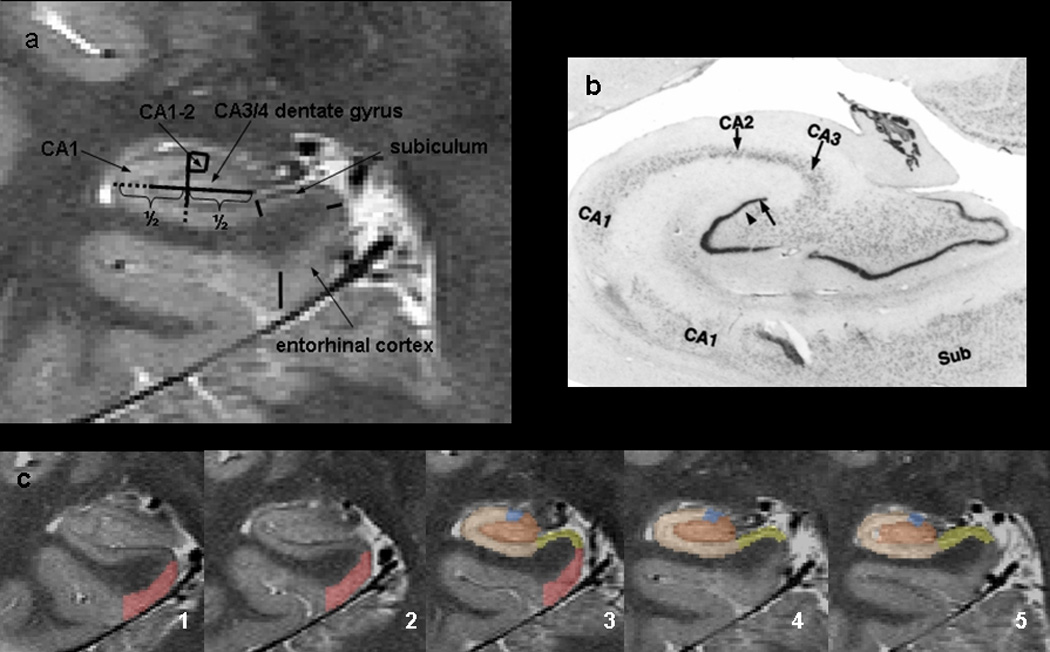
a. Parcellation scheme used for manual marking of subfields. As it is not possible to identify individual hippocampal layers 4 Tesla, the scheme was based on reliably recognizable anatomical landmarks even though this resulted in a part of the prosubiculum and subiculum proper being counted towards the CA1 sector. ERC, entorhinal cortex; CA1-2 transition, CA1-CA2 transition zone (cf methods in text); CA3&DG, CA3, CA4 and dentate gyrus. b. Histological preparation of hippocampal subfields c. Typical example of hippocampal subfield markings. No 1 is the most anterior slice, No 5 the most posterior slice. Slice 3, is the referred in the text as “starting” slice. Red, ERC; yellow subiculum; blue; CA1-2 transition; maroon, CA3&DG
2.4. Statistical analysis
For statistical analysis, left and right hippocampal subfield volumes and left and right total hippocampal volumes of each subject were combined. Based on the absence or presence of an Apo e4 allele, subjects were grouped into Apo e4 (Apo e 4/4, Apo e 3/4, Apo e 2/4) and non-Apo e4 (Apo e 3/3, Apo e 2/3). Multiple regression analysis were used to test the a priori hypotheses, i.e. influences of Apo e4 on CA1 and CA3&DG in healthy controls. This was in a first step done in the total population and then in a subgroup of younger subjects (28–60 years of age) and a subgroup of older subjects (61 – 85 years of age). The subdivision into a “young” and “old” group was done because we expected that a negative effect of Apo e4 on neurogenesis/neuroplasticity in CA3&DG might already be apparent in relatively young subjects. CA1 and CA3&DG were modeled as dependent variables, age, gender (in total control population only), Apo e4 carrier state (yes/no) and ICV as independent variables. To test which volumes are affected by AD and Apo e4, the 15 AD patients were combined with the group of older controls and multiple regression analyses with the subfield/hippocampal volume as dependent and age, disease state (control, AD), Apo e4 carrier state and ICV as independent variables were performed. Mann-Whitney tests were used to test for differences of CA1 and CA3&DG volumes in AD subjects with and without Apo e4. To account for differences in head size in the last analysis, all volumes were normalized using the following formula: volume norm = volume raw *1000/ICV. All statistical analyses were done in JMP6 (SAS Institute Corp.)
3. Results
3.1. Control population
Apo e4 carrier and non-Apo e4 carrier were not different regarding age or gender distribution. The analysis in the total control population showed a significant effect of Apo e4 (p = 0.042, beta = 9.450) and age (p = 0.021, beta = −0.786) on CA3&DG with Apo e4 carrier having smaller CA3&DG volumes than non-Apo e4 carrier irrespective of age (cf. Table 1). There was a significant negative effect of age (p = 0.0002, beta = −1.694) on CA1 volumes confirming the findings of a previous study (Mueller et al. 2007), but no significant effect of Apo e4 on CA1. We also tested for an interaction between age and Apo e4 on CA3&DG and CA1 but found no significant effects. There were no significant effects of age, Apo e4 carrier state or gender on any of the other subfields or total hippocampal volume. Significant effects for age (p = 0.016, beta = −2.35) and Apo e4 carrier state (p = 0.036, beta = 13.62) on CA3&DG volume but not for age on CA1 volume were also found when the analysis was restricted to the subgroup of old subjects (n = 38, f/m: 12/26, Apoe e4/non-Apo e4: 14/24). Again, there were no significant effects for the interaction of age and Apo e4 on CA3&DG and none of the other subfields or total hippocampal volume did show significant Apo e 4 effects. When the analysis was restricted to the subgroup of young subjects (n = 28, f/m: 17/11, Apo e4/non-Apo e4: 9/19), none of the subfields showed significant effects for age or Apo e4 carrier state or their interaction. A protective effect for Apo e2 has been reported (Corder et al. 1994). Therefore, the whole analysis was repeated and Apo e2 carriers were excluded; the findings remained unchanged (data not presented). A table with the detailed results of the regression analyses can be found as Supplementary Material.
3.2. Apoe e4 carrier state and Alzheimer’s Disease
When the combined group was analyzed (old controls and AD), there were significant effects for disease state for ERC (p = 0.0006, beta = −28.043), subiculum (p = 0.0011, beta = −21.093), CA1 (p = 0.0005, beta = −31.077), CA1-2 transition (p < 0.0001, beta = −3.663) and total hippocampal volume (p = 0.0025, beta = −0.449) which were all smaller in AD subjects than in old control subjects. There was no significant disease state effect for CA3&DG. However, there was a significant effect for Apo e4 carrier state on CA3&DG (Apoe e4 carrier state p = 0.0035, beta = 18.740). A significant effect for Apo e4 carrier state and also for age was also found for the total hippocampal volume (age p = .0.049, beta = −0.034; Apo e4 carrier state p = 0.0277, beta = 0.301). Finally, the comparison of the normalized CA1 and normalized CA3&DG volumes between AD with and without Apo e4 showed that CA3&DG (p = 0.027, AD with Apo e4: 124.9 ± 26.8; AD without Apo e4: 159.0 ± 29.6) but not CA1 (p = 0.46, AD with Apo e4: 160.9 ± 44.9; AD without Apo e4: 180.2 ± 32.6) was significantly smaller in AD with Apo e4 than in AD without Apo e4. In an exploratory analysis, we also tested for differences between other subfields or total hippocampus but none of them was significant.
4. Discussion
There were two major findings of this study: 1. Apo e4 was associated with smaller CA3&DG volumes in the whole control population and in the subgroup of old controls but not in the subgroup of young controls. Although total hippocampal volumes tended to be smaller in Apo e4 carriers compared to non-Apo e4 carriers, these differences were not significant. 2. AD with the Apo e4 allele had significantly smaller CA3&DG than AD without the Apo e4 allele. Furthermore, AD patients had smaller ERC, subiculum, CA1, CA1-2 transition volumes and consequently also total hippocampal volumes but not CA3&DG volumes than age-matched controls. Taken together, these results suggest that Apo e4 exerts a regional selective effect on CA3&DG in cognitively normal subjects and AD.
The first major finding was that Apo e4 showed a regional selective effect on CA3&DG in cognitively normal Apo e4 carriers who had smaller CA3&DG volumes than non-Apo e4 carriers. Previous neuroimaging studies assessing the influence of Apoe e 4 on hippocampal volume in cognitively healthy subjects in cross-sectional studies had inconsistent results. For example, Lind et al. (2006) studied a non demented population of 60 subjects between 49–79 years and found significantly smaller right hippocampal volumes in Apo e4 carriers compared to non-Apo e4 carriers which were also associated with lower performance in hippocampal related memory tasks. Similarly, Den Heijer et al (2002) found bilaterally reduced hippocampal volumes in 60 – 90 years old cognitively normal elderly Apo e4 carriers which persisted even after exclusion of subjects with evidence for mild memory impairment. In contrast, Jack et al. (1998) studying 125 cognitively normal elderly controls (mean age 80 years), Reiman et al. (1998) studying 33 middle aged controls (50 –60 years), Cohen et al (2001) studying 25 elderly women with increased risk for AD either due to advanced age or a first degree relative with AD and finally Jak et al (2007) studying 52 cognitively normal elderly subjects (63–92 years) all found that, although hippocampal volumes tended to be smaller in Apo e4 carriers compared to non-Apo e4 carriers, these differences did not reach statistical significance. There are several possible explanations for the inconsistent finding of an Apo e4 effect on total hippocampal volume in cognitively normal subjects in these neuroimaging studies, e.g. different definitions for cognitively normal or non-demented and thus different frequencies of subjects with preclinical AD in the study population, different age ranges of the study population and, since the effect of Apo e4 seems to be dose-dependent, different frequencies of Apo e4 homozygotes in the study population. In our study, the effect of Apo e4 seemed to be restricted to CA3&DG, none of the other subfields was significantly affected. Although CA3&DG represents a relatively large part of the total hippocampus and Apo e4 carriers had smaller total hippocampal volumes than non-Apo e4 carriers, this difference was not statistically significant. This suggests that subfield measurements might be more sensitive to detect subtle effects on the hippocampus, particularly if these effects are regionally selective.
The effect of Apo e4 on CA3&DG was not in all age groups equally present. Apo e4 carrier state was associated with a significant effect on CA3&DG in the total population and in the subgroup of older subjects but not in the subgroup of young Apo e4 carriers although they tended to have smaller CA3&DG than young non-Apo e4 carriers. A power analysis showed that given the number of young subjects and using the error estimates from the regression analysis we had only a 15% power to detect significant difference between young Apo e4 carriers and non-Apo e4 carriers at a significance level alpha = 0.05. This suggests that, although a subtle effect of Apo e4 in young subjects cannot be excluded, the effect of Apo e4 on CA3&DG probably accumulates over life time so that it only becomes manifest as a volume reduction detectable by in vivo imaging at an higher age. An alternative explanation is that Apo e4 renders CA3&DG more vulnerable towards non-Apo e4 related insults occurring later in life, e.g. increased oxidative stress or amyloid deposition. Interestingly, age had also a significant effect on CA3&DG in the total population and in the subgroup of older subjects but only if Apo e4 was included in the model (data not represented). The interaction between Apo e4 carrier state and age however was not significant, indicating, that age and Apo e4 carrier state act additively but independently on the CA3&DG volume. This is in contrast to the age effect on CA1 which was only observed in the whole population and was independent from the inclusion of Apo e4 carrier state in the model.
Our findings of Apo e4 effects on CA3&DG in older subjects are in good agreement with findings in animal and autopsy studies. Studies in transgenic mice for example have shown that while there is no difference in DG synaptic spine density between young Apo e3 and Apo e4 mice, synaptic spine density is significantly reduced in old Apo e4 mice compared to wild-type or Apo e3 mice (Cambon et al., 2000, Ji et al., 2003). A similar reduction of synaptic spine density in the DG region in Apo e4 carriers compared to Apo e3 carriers has also been found in an autopsy study of elderly cognitively normal humans (Ji et al., 2003). Based on the similarities, it is tempting to speculate that the age related CA3&DG volume loss in our study reflects the age related loss of synaptic connectivity/neuroplasticity described in these studies. However it cannot be excluded that the volume loss in CA3&DG is caused by other negative effects associated with Apo e4 carrier state, e.g. reduced neurogenesis (Levi et al., 2005), reduced protection against extrinsic or intrinsic neurotoxic insults, e.g. amyloid deposition (Horsburgh et al., 1999, Buttini et al., 1999, Levi and Michaelson, 2007) or a combination thereof.
The second major finding was that AD with the Apo e4 allele had significantly smaller CA3&DG volumes than AD without the Apo e4 allele. Apo e4 enhances the negative effects of amyloid on neurons (Mahley et al. 2006) and is associated with impaired neuronal repair processes (Weisgraber and Mahley, 1996), therefore, we had assumed that its effect would be most prominent in regions with high intrinsic vulnerability to AD. Autopsy studies found CA1 to be the most severely affected hippocampal subfield in AD (West et al, 1994, Roessler et al., 2002; Price et al., 2001; Fukutani et al., 1995) and therefore we had expected to find smaller CA1 volumes in AD with Apo e4 than in AD without the allele, but that was not the case. One reason for this might be that we did not have enough statistical power to detect an effect on CA1 since the number of AD subjects in our study, particularly the number of non-Apo e4 AD subjects, was small. A power analysis based on the subjects in the analysis showed that we only had a power of 12% to detect CA1 volume differences of CA1between the Apo e4 carriers and non-carriers. This might indicate that either there is truly no effect of Apo e4 on CA1 or that our marking method is not sensitive enough to detect it. An alternative explanation for the absence of an effect of Apo e4 on CA1 could be that other effects overshadowed the Apo e4 effect. For example, it could be possible that the combined pathological effects of AD and aging (AD without Apo e4 were older than AD with Apo e4) on this subfield and consequently loss of neuropil and neurons are so severe at this advanced stage of the disease that additional negative effects due to Apo e4 genotype are obscured.
It is particularly interesting that it was CA3&DG, the only subfield which did not show an AD effect, which was smaller in AD with Apo e4. It has been demonstrated in autopsy studies of AD patients that the human hippocampus reacts with increased neurogenesis and formation of new, immature neurons in the DG and CA1 to the pathological processes in AD (Jin et al., 2004). Since efficient neurogenesis requires an intact cholesterol metabolism, it seems plausible that those compensatory processes could be adversely affected by Apo e4 carrier state. However, such a general effect of Apo e4 would not explain the regional selectivity of the effect observed in our study. A recent study in transgenic mice offers an intriguing explanation for this regional preference. Levi and co-workers (2007) found that conditions favoring neurogenesis are associated with a selective accumulation of intraneuronal Apo e in the DG in Apo e3 and Apo e4 mice. In the case of Apo e4 but not of Apo e3 mice, they also observed a regionally selective increase of intraneuronal soluble amyloid beta in the DG which was associated with signs of increased apoptosis and reduced neuronal density. A mechanism like this would be consistent with the fact that Apo e4 had a regional selective effect on CA3&DG in AD, although this is of course highly speculative. Additional studies in a larger population of AD subjects and if possible correlations with autopsy findings will be necessary to confirm the subfield specific findings of Apo e4 on CA3&DG respectively lack thereof on CA1 in this preliminary study..
This study has several limitations: 1. The sample size of the AD group, particularly the group of non-Apo e4 AD subjects was small. It will be necessary, to validate the findings regarding AD and Apo e4 in a larger study. 2. The study was cross-sectional in design and thus we cannot exclude cohort effects or that some controls were in the early stages of AD or another dementing disease associated with hippocampal atrophy. Furthermore, since the subjects were not particularly recruited for a study on Apo e4 effects, the Apo e4 and non Apo e4 groups were not optimally matched and thus we might have missed Apo e4 effects. 3. Hippocampal subfields were only marked in a relatively small region of the anterior hippocampus. Furthermore, except for the hypointense line, the boundaries between the subfields were based on arbitrarily defined rules and compromises were made to facilitate consistent marking (cf methods section). Therefore, we cannot exclude that we missed Apo e4 effects with a regional preference for regions not included into the marked section, e.g. for the head or tail, or Apo e4 effects restricted to the ventral aspect of CA1 or subiculum/presubiculum. Nonetheless, the total volume of the hippocampal cross-section on which the hippocampal subfield were marked was highly correlated with the volume of the total hippocampus (Pearson correlation coefficient r = 0.76, p <0.0001). Therefore, we think that volume changes in this section are representative for hippocampal volume losses in diseases which are likely to affect the whole length of the hippocampus, as for example AD, aging or Apo e4.
In conclusion, these preliminary findings suggest that Apo e4 carrier state exerts a regionally selective effect on hippocampal subfields which is restricted to CA3&DG. This effect becomes more manifest with increasing age. In addition to this, we also found evidence of a regionally selective effect of Apo e4 on CA3&DG in AD. These effects are consistent with findings in animal and autopsy studies which describe a negative effect of Apo e4 allele in the dentate gyrus. The fact that aging, Apo e4 carrier state and AD show regionally selective effects on hippocampal subfields suggests that measurements of hippocampal subfields might be more sensitive to distinguish between different processes affecting the hippocampus than measurements of total hippocampal volume.
Supplementary Material
Table 2.
Mean Volumes and SD of Total Hippocampus and Subfields in ccmm in Healthy Controls and Alzheimer's Disease
| Region | Total Control Population | Young Controls | Old Controls | AD | ||||
|---|---|---|---|---|---|---|---|---|
| Non-Apo e4 N = 43 |
Apo E4 N = 23 |
Non-Apo e4 N = 19 |
Apo E4 N = 9 |
Non-Apo e4 N = 24 |
Apo E4 N = 14 |
Non-Apo e4 N = 5 |
Apo E4 N = 10 |
|
| ERC | 205.5 (50.4) | 197.4 (55.7) | 221.3 (58.7) | 205.4 (54.1) | 193.0 (39.7) | 192.2 (58.1) | 120.4(34.6)# | 152.4 (56.5)# |
| Subiculum | 199.1 (36.1) | 192.0 (31.1) | 194.3 (37.9) | 195.6 (29.7) | 202.9 (35.0) | 189.6 (32.8) | 146.6 (28.2)# | 159.8 (504)# |
| CA1 | 346.9 (52.4) | 346.5 (50.0) | 371.7 (54.4) | 364.7 (28.6) | 327.2 (42.1) | 334.8 (57.9) | 287.2 (58.4)# | 261.3 (62.8)# |
| CA1-2 transition | 21.4 (6.0) | 19.2 (4.8) | 21.4 (7.2) | 17.8 (3.3) | 21.4 (5.1) | 20.1 (5.5) | 14.4 (2.3)# | 12.5 (2.6)# |
| CA3&DG | 236.2 (36.4)* | 215.9 (35.2) | 239.6 (35.6) | 223.6 (20.7) | 233.5 (37.5)* | 211.0 (42.1) | 253.0 (49.8)º | 205.0 (45.4) |
| Total Hippocampus | 5676.2 (726.5) | 5563.3 (826.3) | 5684.1 (749.5) | 5812.0 (623.3) | 5670.0 (723.9) | 5403.5 (919.8) | 5037.6 (747.8)# | 4351.0 (1379.3)# |
ERC, entorhinal cortex; CA1-2 transition, CA1-CA2 transition zone. SD, standard deviation
p <0.05 Apo e4 compared to non-Apo e4 of the same age group;
p <0.05 AD (Apo e4 and non Apo e 4 combined) compared to controls
p< 0.05 AD non Apo e4 with AD Apo e4 using Mann Whitney test using volumes normalized to intracranial volume
Acknowledgement
The study was supported by grant RO1 AG010897 and P01 AG12435 to Dr. M. W. Weiner and the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: None of the authors has any actual or potential conflicts of interest.
References
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Bedlack RS, Strittmatter WJ, Morgenlander JC. Apolipoprotein E and neuromuscular diseases. Arch Neurol. 2000;57:1561–1565. doi: 10.1001/archneur.57.11.1561. [DOI] [PubMed] [Google Scholar]
- Blasques L, Otaegui D, Saenz A, Paisan-Ruiz, Emparanza JI, Riuz-Martinez J, Moreno F, Marti-Masso JF, Lopez de Munain A. Apolipoprotein E e4 allele in familial and sporadic Parkinson’s disease. Neuroscience Letter. 2006;406:235–239. doi: 10.1016/j.neulet.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Buttini M, Orth M, Bellosta S, Akeefe H, Pitas RE, Wyss-Coray T, Mucke L, Mahley RW. Expression of human apolipoprotein E3 or E4 in the brains of Apoe −/− mice: Isoform specific effects on neurodegeneration. J Neuroscience. 1999;19:4867–4880. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambon K, Davies HA, Stewart MG. Synaptic loss is accompanied by an increase in synaptic area in the dentate gyrus of ages human apolipoprotein E4 transgenic mice. Neuroscience. 2000;97:685–692. doi: 10.1016/s0306-4522(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Chapman J, Korczyn AD, Karussis DM, Michaelson DM. The effects of APOE genotype on age of onset and progression of neurodegenerative diseases. Neurology. 2001;57:1482–1485. doi: 10.1212/wnl.57.8.1482. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Small C, Lalonde F, Friz J, Sunderland T. Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology. 2001;57:2223–2228. doi: 10.1212/wnl.57.12.2223. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Rimmler JB, Locke PA, Conneally PM, Schmader KE, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Den Heijer T, Oudkerk M, Launer J, van Dujin CM, Hofman A, Breteler MMB. Hippocampal, amydgalar and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- De Vita E, Thomas DL, Roberts S, Parkes HG, Turner R, Kinchesh P, Shmueli K, Yousry TA, Ordidge RJ. High resolution MRI of the brain at 4.7 Tesla using fast spin echo imaging. Br J Radiol. 2003;76:631–637. doi: 10.1259/bjr/69317841. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. Functional anatomy, vascularization and serial sections with MRI. Third edition. Berlin, Heidelberg, New York: Springer Verlag; 2005. The human hippocampus. [Google Scholar]
- Eriksson SH, Thom M, Bartlett PA, Symms MR, McEvoy AW, Sisodiya SM, Duncan JS. Propeller MRI visualizes detailed pathology of hippocampal sclerosis. Epilepsia. 2008;49:33–39. doi: 10.1111/j.1528-1167.2007.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Halsegrove C, van der Kouwe A, Killiany R, Kennedy D, Klavenes S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fukutani Y, Kobayashi K, Nakamura I, Watanabe K, Isaki K, Cairns NJ. Neurons, intracellular and extracellular neurofibrillary tangles in subdivisions of the hippocampal cortex in normal ageing and Alzheimer’s disease. Neurosci Lett. 1995;200:57–60. doi: 10.1016/0304-3940(95)12083-g. [DOI] [PubMed] [Google Scholar]
- Gilbert ME. Alterations in synaptic transmission and plasticity in area CA1 of adult hippocampus following developmental hypothyroidism. Brain Dev Brain Res. 2004;148:11–18. doi: 10.1016/j.devbrainres.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW. Cholesterol homeostatis and function in neurons of the central nervous system. Cell Mol Life Sci. 2003;60:1158–1171. doi: 10.1007/s00018-003-3018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh K, Kelly S, McCulloch J, Higgins GA, Rosese AD, Nicoll JAR. Increased damage in apolipoprotein E-deficient mice following global ischemia. Neuroreport. 1999;10:837–841. doi: 10.1097/00001756-199903170-00031. [DOI] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, O’Brien PC, Waring SC, Tangalos EG, Smith GE, Ivnik RJ, Thibodeau SN, Kokman E. Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer’s disease. Ann Neurol. 1998;43:303–310. doi: 10.1002/ana.410430307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Houston WS, Nagel BJ, Corey-Bloom J, Bondi MW. Differential cross-sectional and longitudinal impact of APOE genotype on hippocampal volumes in nondemented older adults. Dement Geriatr Cogn Disord. 2007;23:382–389. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Gong Y, Gan W, Beach T, Holtzman DM, Wisniewski T. Apolipoprotein E isoform specific regulation of dendtritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122:305–315. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XQ, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, Verkuyl M, Lucassen PJ, Krugers HJ. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hopkins RO. Mnemonic functions of the hippocampus: A comparison between animals and humans. Biol Psychol. 2006;73:3–18. doi: 10.1016/j.biopsycho.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Klempin F, Kempermann G. Adult hippocampal neurogenesis and aging. Eur Arch Psychiatry Clin Neurosci. 2007 doi: 10.1007/s00406-007-0731-5. [DOI] [PubMed] [Google Scholar]
- Levi O, Jongen-Relo AL, Feldon J, Roses AD, Michaelson DM. Apo E4 impairs hippocampal plasticity isoform specifically and blocks the environmental stimulation of synaptogenesis and memory. Neurobiol Dis. 2003;3:273–282. doi: 10.1016/s0969-9961(03)00045-7. [DOI] [PubMed] [Google Scholar]
- Levi O, Jongen-Relo AL, Feldon J, Michaelson DM. Brain area and isoform-specific inhibition of synaptic plasticity by apo E4. J Neurol Sci. 2005;229–230:241–448. doi: 10.1016/j.jns.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Levi O, Michaelson DM. Environmental enrichement stimulates neurogenesis in apolipoprotein E3 and neuronal apoptosis in apolipoprotein E4 transgenic mice. J Neurochem. 2007;100:202–210. doi: 10.1111/j.1471-4159.2006.04189.x. [DOI] [PubMed] [Google Scholar]
- Levi O, Dolev I, Belinson H, Michaelson DM. Intraneuronal amlyloid-β plays a role in mediating the synergistic pathological effects of apoE4 and environmental stimulation. J Neurochem, epub in advance. 2007 doi: 10.1111/j.1471-4159.2007.04810.x. [DOI] [PubMed] [Google Scholar]
- Lind J, Larson A, Persson J, Ingvar M, Nilsson LG, Baeckman L, Adolfsson R, Cruts M, Sleegers K, Van Broeckhoven C, Nyberg L. Reduced hippocampal volume in non-demented carriers of the apolipoprotein E ϵ4: Relation to chronological age and recognition memory. Neuroscience Letters. 2006;396:23–27. doi: 10.1016/j.neulet.2005.11.070. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Heine VM, Muller MB, van der Beek EM, Wiegant VM, De Kloet ER, Joels M, Fuchs E, Swaab DF, Czeh B. Stress, depression and hippocampal apoptosis. CNS Neurol Disord Dru Targets. 2006;5:531–546. doi: 10.2174/187152706778559273. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Nat Acad Sci. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Guthrie PB, Kater SB. Intrinsic vulnerability of hippocampal pyramidal neurons. Prog Clin Res. 1989;317:333–351. [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Selective vulnerability of corticocortical neurons and hippocampal circuits in aging and Alzheimer’s disease. Prog Brain Res. 2002;136:467–486. doi: 10.1016/s0079-6123(02)36039-4. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Stables L, Du AT, Schuff N, Truran D, Cashdollar N, Weiner MW. Measurements of hippocampal subfields and age related changes with high resolution MRI at 4T. Neurobiol Aging. 2007;28:719–726. doi: 10.1016/j.neurobiolaging.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LL, Reeves T. Interactive pathology following traumatic brain injury modifies hippocampal palsticity. Restor Neurol Neurosci. 2001;19:213–235. [PubMed] [Google Scholar]
- Price JL, Ko A, Wade MJ, Tsou SK, McKeel D, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer Disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Uecker A, Caselli RJ, Lewis S, Bandy D, de Leon M, De Sancti S, Convir A, Osborne D, Weaver A, Thibodeau SN. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer’s Disease. Ann Neurol. 1998;44:288–291. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function and empirical tests of the theory. Progress Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Rössler M, Zarski M, Bohl J, Ohm TG. Stage dependent and sector specific neuronal loss in hippocampus during Alzheimer’s disease. Acta Neuropath. 2002;103:363–369. doi: 10.1007/s00401-001-0475-7. [DOI] [PubMed] [Google Scholar]
- Teter B. ApoE-dependent plasticity in Alzheimer’s disease. J Mol Neurosci. 2004;23:167–179. doi: 10.1385/JMN:23:3:167. [DOI] [PubMed] [Google Scholar]
- Thomas DL, De Vita E, Roberts S, Turner R, Yousry TA, Ordidge RJ. High resolution fast spin echo imaging of the human brain at 4.7T: Implementation and sequence characteristics. Mag Reson Med. 2004;51:1254–1264. doi: 10.1002/mrm.20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgraber KH, Mahley RW. Human apolipoprotein E: The Alzheimer’s disease connection. FASEB J. 1996;10:1485–1494. doi: 10.1096/fasebj.10.13.8940294. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, Smith D, Brewer W, Proffitt T, Desmond P, Pantelis Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first episode psychosis and ultra-high risk individuals. Arch Gen Psychiatry. 2008;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.