Abstract
Methamphetamine dependence is a serious public health problem worldwide for which there are no approved pharmacological treatments. Psychotherapy is still the mainstay of treatment; however, relapse rates are high. The search for effective pharmacological treatment has intensified in the last decade. This review will highlight progress in pharmacological interventions to treat methamphetamine dependence as well as explore new pharmacological targets. Published data from clinical trials for stimulant addiction were searched using PubMed and summarized, as well as highlights from a recent symposium on methamphetamine pharmacotherapy presented at the ISAM 2006 meeting, including interim analysis data from an ongoing D-amphetamine study in Australia. Early pilot data are encouraging for administering D-amphetamine and methylphenidate as treatment for heavy amphetamine users. Abilify at 15 mg/day dose increased amphetamine use in an outpatient pilot study. Sertraline, ondansetron, baclofen, tyrosine, and imipramine were ineffective in proof-of-concept studies. Development of pharmacotherapy for methamphetamine dependence is still in an early stage. Data suggesting D-amphetamine and methylphenidate as effective pharmacotherapy for methamphetamine addiction will need to be confirmed by larger trials. Preclinical data suggest that use of GVG, CB1 antagonist, and lobeline are also promising therapeutic strategies.
Keywords: Methamphetamine, pharmacotherapy, bupropion, aripiprazole, methylphenidate, D-amphetamine
INTRODUCTION
Methamphetamine (MA) addiction is a serious public health problem reaching epidemic proportion worldwide. Areas especially hard hit include East and Southeast Asia, Australia, Western and Midwestern United States, and various areas of Great Britain.
Methamphetamine can be smoked, snorted, injected, or taken orally. In the brain it enters the presynaptic neurons where it exerts its main action of reversing the vesicular monoamine transporter-2 (VMAT), thus impeding the incorporation/packaging of neurotransmitters into vesicles and causing the rapid efflux of intravesicular monoamines, causing extremely high concentrations of cytosolic monoamines. In response to this rapid shift of intracellular monoamine levels, the plasmalemmal monoamines are also reversed, resulting in a dramatic dumping of these transmitters into the extracellular space. Besides its impact on monoamine transporter functions, methamphetamine also has a weak inhibitory effect on monoamine oxidase (MAO), thereby interfering with monoamine metabolism.
Short-term effects of MA include an initial “rush,” increased energy, a general sense of well-being, and decreased appetite, which typically lasts 6–8 hours. Adverse effects of MA include restlessness, insomnia, hyperthermia, and possibly convulsions. Long-term use can lead to addiction, paranoia, mood disturbances, agitation, psychosis, and cognitive impairment (1).
Following prolonged use, discontinuation of MA often results in a withdrawal syndrome including dysphoric mood, fatigue, sleep disturbances, and increased appetite (2).
Preclinical data show that multiple high-dose administrations of MA are neurotoxic, with evident damage to extrapyramidal dopamine (DA) systems in both laboratory animals and humans (3). Mechanisms for the MA toxicity are linked to excessive oxidation of DA and consequent reactive oxygen species production, initiated by MA-induced dysfunctions and abnormal trafficking of monoamine transporters and associated pathogenic management of DA sequestration and metabolism. Interestingly, this initial pathogenic response to MA appears to be reversible for up to 8 hours after drug treatment. Consequently, this and more recent data suggest that such effects are only a first phase of the MA toxicity and are likely followed by a second phase within 12–24 hours that is related to the production of striatal DA-related protein aggregations and oxidation leading to activation of immunosystems. This MA-related immunoresponse includes infusion of non-neuronal cells such as microglia and cytotoxic consequences probably linked to the production of reactive nitrogen species, similar to that caused by other DA-selective toxins such as 6OHDA and MPTP+. The effects of such an MA-initiated, 2-phased neurotoxic mechanism can be detrimental and likely compromises memory and cognitive functions short and long term, likely confounding treatment of MA addiction.
Positron emission tomography (PET) imaging studies have shown decreased dopamine D2 receptors in chronic MA users, which correlate with cognitive impairment (4). On the other hand, abstinence from MA results in some recovery of these changes with corresponding improvement of cognition (5). Similarly, nuclear magnetic imaging spectroscopy studies (6) provided evidence of neuronal damage in chronic MA users, as evidenced by changes in choline and mono- and di-phosphor esters.
PREVALENCE OF METHAMPHETAMINE ADDICTION
Globally, MA is a major health problem. The 2002 report from the United Nations Office for Drug Control and Crime Prevention noted an 11-year growing trend (1990–2000) of amphetamine-type stimulants (ATS) seizures at an annual average rate of 28% (7). Production of ATS was estimated at just over 500 tons a year; in 2000/2001, more than 40 million people used MA (8). The report also emphasized that in 2000, ATS seizures in East and Southeast Asia increased by 17%, reflecting increased production.
High rates of MA dependence have been identified in Great Britain (9,10), Japan (11,12), Australia (13–15), and many other countries (16). In Australia, amphetamines are the second most frequently used illicit drugs, after cannabis.
In Great Britain, the MA problem is a greater public health consequence than cocaine use, especially in relation to the spread of HIV. Other regions have also recently reported very dramatic increases in MA treatment admissions, including Slovakia (L. Okruhlic, personal communication, June 2004) and the Republic of South Africa (B. Meyers, personal communication, June 2004).
In the United States in 2002, the federal Office of National Drug Control Policy (17) indicated that 8 of 20 cities surveyed (Billings, MT; Denver, CO; Honolulu; Los Angeles; Memphis, TN; St. Louis, MO; Seattle, WA; and Sioux Falls, ID) consider MA as the drug of abuse associated with the “most serious consequences.” It was also stated that significant MA problems are emerging in Columbia, South Carolina, and New Orleans, Louisiana, and continuing to trend upward in Seattle and Sioux Falls. According to the Treatment Episode Data Set (TEDS) 1993–2003 report (18), primary MA treatment admissions increased from 20,776 in 1993 (1.3% of all admissions) to 116,604 in 2003 (6.3% of all admissions).
PHARMACOTHERAPY
Treatment for MA users has far-reaching health ramifications, both in terms of reducing the consequences of abusing this potent psychostimulant and in potentially reducing MA-driven behaviors that spread disease such as HIV. As a result, the development of effective treatments for MA dependence has become a pressing concern for the national and global drug abuse treatment community. The development of pharmacotherapies for the treatment of MA-related disorders is viewed as a critically important element in broadening the range of treatment options and improving therapeutic outcomes. The development of such pharmacotherapies is at an early stage.
Two approaches have been taken to achieve this goal: (1) evaluate medications that have been tested and demonstrated potential for cocaine addiction in small size, proof-of-concept trials; and (2) employ the industry model of medications development, with the goal of obtaining regulatory approval. The second strategy employs three major concepts: (1) rational targets; (2) preclinical animal models; and (3) systematic phase I–VI clinical trials.
In addition to the medications listed in Table 1, other medications currently being studied for treatment of MA dependence are bupropion, gabapentin, mirtazapine, atomoxetine, carvedilol, clonidine, peridopril, prazosin, rivastigmine, and topiramate.
TABLE 1.
Summary of Data on Published Medication Trials for Methamphetamine Dependence
| Study | Outcome | Results | Reference |
|---|---|---|---|
| Safety of intravenous MA administration during treatment with bupropion; 26 randomized participants, and 20 completers. Dosed with (0, 15, 30 mg) i.v. MA before and after randomization to BID bupropion (150 mg SR) or matched placebo | Assess safety and tolerability of MA administration during bupropion Tx. including: Dependent measures of cardiovascular effects of MA and amphetamine pharmacokinetics and peak and trough plasma concentrations of bupropion and its metabolites | Bupropion Tx. Well-tolerated with cardiovascular effects not accentuated bupropion reduced MA associated increases in B/P. Statistically significant reduction in MA-associated increases in heart rate. PK analysis revealed bupropion Tx reduced appearance of amphetamine in plasma. MA did not alter peak and trough plasma concentrations of bupropion or its metabolites | (19) |
| A comprehensive assessment of safety of i.v. methamphetamine administration during treatment with selegiline; S/B, placebo-controlled; 15, 30 mg i.v. MA for 24 randomized MA dependent participants, nine completers (N = 5 selegiline, N = 4 placebo) | Primary: Assess whether selegiline is effective in treating methamphetamine addiction secondaries included determinations of plasma levels of selegiline metabolites, evaluation of whether selegiline altered pharmacokinetics of MA or its metabolites, and whether selegiline Tx. alters subjective responses to MA | i.v. administration of moderate doses of methamphetamine was safely tolerated during Tx. with selegiline. No EKG changes, no meaningful differences in lab values between groups at screening or as result of study procedures. Selegiline did not enhance any cardiovascular changes produced by MA administration. Selegiline Tx. slightly increased MA-associated “bad effects” but did not alter any other subjective effects | (20) |
| A controlled trial of imipramine for the Tx of methamphetamine dependence; 31 subjects randomized 2-dose study of either 10 or 150 mg/day of imipramine for 180 days | Test efficacy of imipramine as a Tx. for MA dependence and establish feasibility of conducting controlled clinical trial at the clinic | Retention in Tx. was significantly longer for subjects treated with 150 mg of imipramine compared to control (median days: 33.0 vs. 10.5). No consistent differences in percentage of urine samples positive for MA, Beck Depression Inventory scores, or craving | (21) |
| Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence; 16-week, randomized, placebo-controlled, double-blind trial of 2 GABAergic medications, baclofen (20 mg tid) and gabapentin (800 mg. tid). Baclofen (n = 25), gabapentin (n = 26), placebo (n = 37), clinic thrice weekly with psychosocial counseling, complete assessments, and urine samples | To conduct a 16-week, randomized, placebo-controlled, double-blind trial of 2 GABAergic medications, baclofen and gabapentin, for the treatment of MA dependence | No statistically significant main effects for baclofen or gabapentin in reducing methamphetamine use were observed using a generalized estimating equation (GEE). A significant Tx. effect was found in post hoc analyses for baclofen relative to placebo, but not gabapentin, among participants who reported taking a higher percentage of study meds (significant Tx. group by medication adherence in GEE model of MA use) | (22) |
| Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Participants completed a 2-week, non-medication baseline, randomized to 1 of 4 conditions for 12 weeks: sertraline plus CM (n = 61), sertraline only (n = 59), matching placebo plus CM (n = 54), or matching placebo-only (n = 55). Thrice-weekly clinic visits for data collection, medication dispensing, and relapse-prevention groups | Evaluate the efficacy of sertraline (50 mg bid) and contingency management (CM) for the Tx. of MA dependency. MA urine drug screening and self-reported days of use, retention, drug craving, and mood symptoms | No statistically significant main or interaction effects for sertraline or CM in reducing methamphetamine use were observed using a generalized estimating equation (GEE), although post hoc analyses showed the sertraline-only condition had significantly poorer retention than other conditions (chi-square) (3) = 8.40, p< 0.05). Sertraline conditions produced significantly more adverse events than placebo conditions. Significantly higher proportion of participants in CM conditions achieved 3 consecutive weeks of MA abstinence than those in the non-CM conditions | (23) |
| Effects of isradipine on methamphetamine-induced changes in attention and perceptual-motor skills of cognition. Blinded, placebo-controlled, crossover designed study of cognitive effects of low and high doses of i.v. MA (15 and 30 mg, respectively) in both the presence and absence of isradipine | To determine isradipine’s effects on cognitive performance in MA-dependent individuals | i.v. D-methamphetamine produced dose-dependent increase in attention, concentration, and psychomotor performance. Isradipine, both with and without methamphetamine, had a modest effect to decrease attention | (24) |
| Effects of isradipine, a dihydropyridine-class calcium-channel antagonist, on D-methamphetamine’s subjective and reinforcing effects. Included 18 non-treatment-seeking, methamphetamine-dependent subjects aged 18–51 in this double-blind, within-subject, crossover study done in human lab; i.v. MA (0, 15, and 30 mg) was administered on 3 different days after 5 days of double-blind crossover treatment with either isradipine or matching placebo. Subjects received oral isradipine 30 mg SR at HS, plus 15 mg IR administered 2 h before MA infusion. Self-report questionnaires and MA reinforcement measured by a behavioral procedure involving choices between MA and money | To determine whether isradipine could antagonize methamphetamine’s positive subjective and reiinforcing effects in MA-dependent research subjects | Those who received isradipine second and placebo first as the pretreatment paradigm, but not vice versa, MA-induced drug liking, elation, and preference were reduced significantly by isradipine. Depending upon conditioning status, isradipine can reduce some MA-induced positive subjective and reinforcing effects associated with abuse liability in MA addicts | (25) |
| Isradipine decreases the hemodynamic response of cocaine and methamphetamine: results from 2 human laboratory studies. Examined, in 2 separate experiments of similar design conducted contemporaneously, the hemodynamic effects of cocaine or MA in the presence or absence of isradipine (total N = 31), isradipine pretreatment was provided to cocaine- or MA-dependent male and female subjects before i.v. administration of low and high doses of cocaine (0.325 or 0.650 mg/kg) or MA (15 or 30 mg), respectively, on separate test days | To study effects of cocaine and methamphetamine on hemodynamic response and for examining the potential utility of the antihypertensive and dihydropyridine-class calcium channel antagonist isradipine to block these effects | Both cocaine and methamphetamine administration produced predicted elevations in blood pressure (with peak response between 1 and 3 min after infusion). Apart from tachycardia, no arrhythmias were reported. Isradipine significantly reduced stimulant-associated increases in all measures of blood pressure except pulse pressure but tended to enhance the effects of these drugs on the heart rate | (26) |
| Kinetic and cardiovascular effects of acute topiramate dosing among non-treatment-seeking, methamphetamine-dependent individuals. Trial was a 227-day inpatient study with 10 MA-dependent individuals in a double-blind, placebo-controlled, crossover design with oral doses of topiramate (0, 100, and 200 mg) administered as pretreatment before i.v. doses of MA (0, 15, 30 mg). The 3 × 3 factorial combination of topiramate and methamphetamine resulted in a sequence of the nine treatments administered to each subject in an order determined by a 9 × 9 Latin square design | To study the effects of topiramate on the kinetic profile and hemodynamic response to MA | MA alone was associated with prototypical increases in hemodynamic response that were not altered in presence of topiramate. There were no significant kinetic interaction between topiramate and MA. There was a nonsignificant trend for topiramate to increase plasma MA level. No significant adverse events. Combination of topiramate and MA at pharmacologically relevant doses appears to be safe | (27) |
| Effects of topiramate on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition in recently abstinent methamphetamine individuals. Study included 10 male and female individuals who met DSM-IV criteria for MA dependence. The effects of low (50 mg b.i.d) and high (100 mg b.i.d.) dose topiramate in both the presence and absence of low (15 mg) and high (30 mg) dose i.v. MA on cognitive performance, attention, and concentration on the Rapid Visual Information Processing task and the Digit Symbol Substitution test | To examine topiramate effects on cognitive performance and attention as an important component of understanding its potential therapeutic profile | i.v. MA enhanced cognitive performance, attention, and concentration among recently withdrawn MA addicts, an effect that had not been characterized. Topiramate cognitive effects were mixed and paradoxical, with a tendency to improve attention and concentration alone and in the presence of MA while worsening psychomotor retardation. No deleterious interaction occurred between topiramate and MA on any of these cognitive processes | (28) |
| A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Study included individuals meeting DSM-IV criteria for IV amphetamine dependence (N = 53) randomly assigned to receive aripiprazole (15 mg/day), slow-release methylphenidate (54 mg/day), or placebo for 20 weeks. Intent-to-treat analysis | Outcome measure was the proportion of amphetamine-positive urine samples | Study terminated prematurely due to unexpected results of interim analysis. Patients allocated to aripiprazole had significantly more amphetamine-positive urine samples than patients in the placebo group (odds ratio = 3.77, 95% CI = 1.55–9.18), whereas patients who received methylphenidate had significantly fewer amphetamine positive urine samples than patients who had received placebo (odds ratio = 0.46, 95% CI = 0.26–0.81) | (29) |
| Methamphetamine-amlodipine interactions: preliminary analysis. Nine subjects (6 males and 3 females) underwent 2 two-day sessons in each of which they received 30-mg release oral D-methamphetamine hydrochloride after premedication with amlodipine 20 mg or placebo | To examine the subjective and physiological effects of oral D-methamphetamine after acute premedication with amlodipine | MA produced significant increases over time in systolic and diastolic blood pressure, heart rate, ARCI, MBG. and A scores and POMS Vigor, Arousal, and Positive Mood scores. No other treatment-related differences emerged in subjective responses such as euphoria hyperactivity, with the exception of significantly higher POMS arousal scores in the amlodipine group; levels did not differ in the 2 groups | (30) |
| Methamphetamine quantitative urine concentrations during a controlled trial of fluoxetine treatment: preliminary analysis. Eight-week randomized, controlled, parallel group design with a 1-week single-blind placebo lead-in followed by 7 weeks of double-blind ?uoxetine (FLX) 40 mg per day or placebo (PLA). All subjects were DSM-IV primary MA dependent. Mean age (for n = 60) was 35, 42 (70%) were male, and 9 (15%) were HIV seropositive. Subjects had used MA an average of 7.1 years (for n = 30). Included twice a week self-report of MA use and quantitative urine MA and amphetamine concentrations. Questions regarding frequency, amount, and value of MA used, as well as craving, were addressed. Urine MA and amphetamine concentrations, measured by gas chromatography, were available for the first 30 subjects | Aim of study was to offer preliminary data to evaluate the use of quantitative urine MA concentrations as the primary outcome measure in pharmacotherapy trials in MA dependence. Attempted to answer following questions: Characteristics of quantitative urine MA levels in outpatients undergoing pharmacotherapy research. Is quantitative urine MA a valid measure of MA use? Does it correlate with self-reports of use, and with what self-reports does urine MA best correlate? | Mean urine MA concentrations at each assessment point ranged from 647 to 23,676 ng/mL, and individual sample values ranged from 0 to 336,559 ng/mL. Analysis of the relationship between urine methamphetamine levels and various self-report measures of MA use showed significant and strong correlations between MA levels and self-reported measures of MA use (nonparametric Kendall’s tau, p < .05). Correlations were significant for self-reported days, grams, and dollars worth of MA used at intake and in each of the 8 weeks of the clinical trial. Kendall’s correlations ranged from 0.33 to 0.84 and averaged .63 for these measures | (31) |
| Effects of acute topiramate dosing on methamphetamine-induced subjective mood: Tested in 10 methamphetamine-dependent individuals (3 females) whether low or high dose (15 or 30 mg. i.v.) methamphetamine-induced positive subjective effects and reinforcement can be antagonized by low or high dose (100 or 220 mg orally) topiramate using a placebo-controlled, crossover, factorial design | Hypothesis is that, mechanistically, topiramate’s therapeutic effects are due to inhibition of cortico-mesolimbic dopamine function, the primary substrate that governs the acquisition, maintenance, and reinstatement of goal-directed behavior toward seeking abused drugs | MA administration was associated with orderly, prototypical, and significant increases on measures of stimulation, euphoria, craving, and reinforcement. Some dysphoric symptoms emerged. Topiramate alone showed nonsignificant trend towards mild reductions in positive mood and reinforcement. Topiramate appeared to accentuate the appreciation of MA-induced stimulation and euphoria significantly but not for craving or reinforcement. Combination of topiramate and MA appeared to be safe and well tolerated. Few adverse events. Acute dosing with up to 200 mg topiramate appeared to enhance rather than attenuate the positive subjective effects of MA | (32) |
| Effects of naltrexone on the subjective response to amphetamine in healthy volunteers: Used the Visual Analog scales assessing subjective effects over 7 hours. Assessed effects of opioid antagonist, naltrexone’s subjective response to an oral dose of dexamphetamine (30 mg) in 12 healthy volunteers in double-blind placebo-controlled design. Study measurements included: Visual Analog scale, monitoring of blood pressure, heart rate, skin conduction, and speed of reading | To evaluate the effect of pretreatment with naltrexone on the subjective response to amphetamine | Preliminary evidence indicative that naltrexone is well tolerated in healthy human subjects and may reduce the reinforcing effects of amphetamine via modulation of the opioid system | (33) |
| Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving; 26 participants were enrolled and 20 completed (n = 10 placebo and n = 10 bupropion), parallel groups design | To assess the impact of bupropion treatment on the subjective effects produced by MA in the laboratory and to assess the effects of bupropion treatment on craving elicited by exposure to videotaped MA cues | Bupropion treatment was associated with reduced ratings of any drug effect (p < 0.02) and “high” (p < 0.02) following MA administration. There was also a significant bupropion-by-cue exposure interaction on General Craving Scale total score (p < 0.002), and on the Behavioral Intention subscale (p < 0.001). Overall, data revealed that bupropion reduced acute MA-induced subjective effects and reduced cue-induced craving | (65) |
NIDA’s pharmacotherapy division is leading the field in using the industry medication development model, with the main goal of seeking FDA approval. Starting with preclinical studies, several approaches that decrease appetitive drives for other drugs of abuse are applicable to MA. Thus, modulation of conditioned cues, priming, and stress-induced reinstatement appear to be rational approaches to the discovery and evaluation of medications for the treatment of MA dependence. Putative medications that affect one or more of these mechanisms could be tested.
New molecular entities and targets that fit the above profiles include CRF-1, dopamine D3, cannabinoid CB1 antagonists, and glutamate site modulators, which all show promising preclinical animal data.
The role of corticotropin-releasing factor (CRF) in drug addiction and the rationale for development of CRF-1 receptor antagonists as treatments for drug dependence have been extensively reviewed (34–36). Interestingly, in rat models of stress-induced relapse or reinstatement of drug use, CRF-1 antagonists have been shown to block footshock-induced reinstatement of responding for cocaine (37,38), heroin (38,39), and alcohol (40). These data suggest possible efficacy of CRF-1 antagonists in counteracting the widely acknowledged ability of stress to trigger relapse to multiple drugs that have addicting properties. Such efficacy in multiple drug addiction disorders would be beneficial because abuse and addiction to a single compound are less common than polydrug abuse and addiction. Multiple pharmaceutical companies have been working toward the development of CRF-1 antagonists for the treatment of depression and/or anxiety.
Dopamine D3 receptor ligands as potential treatments for drug abuse also have been the subject of several recent reviews (41–44). These receptors were cloned in 1990 (45) and have been of particular interest to drug abuse researchers, in part because they are selectively located in brain regions that are affected by drug abuse, and they are up-regulated in the brains of cocaine overdose fatalities (46). Agonists of these receptors produce behavioral effects in rodents that do not resemble effects of stimulants (47) but are perceived as cocaine-like by rodents and primates in that they will substitute for cocaine in self-administration paradigms (48,49). The potency of compounds to activate D3 receptors is related to their ability to decrease cocaine self-administration in rats, suggesting the involvement of these receptor types in cocaine drug-taking (50). In addition, dopamine D3 partial agonists have been shown to block the behaviorally activating effects of cues that have been paired with cocaine in rats, suggesting potential usefulness in blocking relapse following contact with environmental cues associated with drug use (51).
Dopamine D3 antagonists also have been reported to block nicotine-primed reinstatement of nicotine self-administration in rats (52) as well as cocaine-primed cocaine seeking in rats (53,54). A D3 antagonist has been reported to dose-dependently block footshock-induced reinstatement of cocaine self-administration in rats (55), overall suggesting a potential role for D3 antagonists in preventing the three triggers of relapse. A D3 antagonist has been shown to block enhancement of electrical brain stimulation reward by cocaine (56), and D3 antagonists have been reported to block both the acquisition and expression of nicotine (57), cocaine, and heroin (58) conditioned place preference in rats. Taken together, results from different laboratories using different behavioral endpoints and different compounds suggest that both dopamine D3 partial agonists and D3 antagonists may be useful treatments and may be effective for polysubstance addiction.
Evidence that cannabinoid-1 (CB-1) receptor antagonists may prove useful in treating drug addiction disorders has been the subject of 2 recent reviews (59,60). Particularly notable in these reviews is the ability of CB-1 receptor antagonists to modulate the pharmacology of THC, nicotine, cocaine, MA, opiates, and ethanol. These observations have generated a high level of interest in this class of compounds. Unlike compounds that block the ability of stress to trigger drug-seeking behavior in animal models of relapse, CB-1 antagonists act either by blocking the subjective/rewarding effects of drugs like THC or by blocking the ability of conditioned cues to promote reinstatement of drug-seeking behavior in animals extinguished from drug self-administration. Taken together, results suggest a role for the cannabinoid system for polysubstance addiction.
Reported interactions of virtually all drugs of abuse with glutamatergic systems in brain provide strong rationale for the pursuit of several related biochemical targets. Tzchentke and Schmidt (61) have reviewed glutamatergic mechanisms in addiction, emphasizing a role for glutamate in stimulating dopamine systems related to reward and a dopamine-independent role for glutamate in altering the effects of conditioned stimuli on behavior. It has been proposed that the hallmark of addiction, an unmanageable motivation to take drugs, results from pathological changes in prefrontal accumbens glutamate transmission (62).
There are data supporting a role for both group I and group II metabotropic glutamate receptors in addiction, which have been reviewed by Kenny and Markou (63). A rationale for pursuing mGluR5 antagonists as addiction treatments is supported by the results of mGluR5 knockout studies (64) and by reported effects of the mGluR5 antagonist MPEP on self-administration of cocaine, nicotine, and alcohol (65,66). Additionally, a rationale for pursuing mGluR2/3 agonists is suggested by the efficacy of LY379268 in rat models of cue-induced relapse to cocaine (67) and heroin (68). Two other potentially promising mechanisms of glutamate modulation for addiction treatment are AMPA receptor antagonism (69,70) and NAALADASE inhibition (71).
Other pharmacological targets for stimulants addiction, for which a rationale is in earlier stages of development, include Orexin-A receptor antagonists (72,73), Opioid receptor-like 1 agonists (74,75), and muscarinic M5 receptor ligands (76,77). It is anticipated that as research tools and potential medications are developed, additional data evaluating these targets will become available to guide decisions for further development.
Vigabatrin is a GABA transaminase inhibitor, which leads to a marked elevation of GABA levels. It has been shown to be very effective in animal models of cocaine and MA self-administration and in primate PET imaging studies to block dopamine release (78). Early open-label pilot data in cocaine and MA actively using addicted patients showed promising results in facilitating abstinence (79). Vigabatrin has been reported to cause visual field defects following prolonged use, which may or may not be an issue in its development for addiction treatment. Safety and proof-of-concept trials are planned to further clarify this issue.
The unique effect of MA on the VMAT2 makes lobeline a candidate for testing. Indeed preclinical studies showed that lobeline blocks methamphetamine self-administration (80).
Methamphetamine has been shown to affect a number of cognitive processes. Attempting to get individuals with cognitive deficits to learn new cognitive skills can be time consuming and difficult. Thus, an alternate approach would be to develop medications for the treatment or normalization of cognitive processes affected by MA abuse, in order to enhance the therapeutic process. For example, amphetamine abusers have problems with extra-dimensional set shifting on neuropsychology tests. Drugs affecting the frontal cortex dopaminergic system, D-1 agonists, and 5-HT 6 antagonists can reverse this deficit. Another viable approach that involves learning could be to facilitate the extinction of conditioned cues. D-Cycloserine and other medications may facilitate this process. A third approach would be to pharmacologically modulate strategic thinking. Nootropic agents to improve cognitive functions may have such a capability. Modafinil has multiple effects on cognition, including an ability to increase strategic thinking.
Atypical antipsychotics, especially aripiprazole, may have a role in reducing craving, as has been shown for cocaine in comorbid schizophrenics, and for the treatment of MA-induced psychosis. Preliminary results from a Finnish randomized 3-arm study (n = 53) showed that methylphenidate treatment (54 mg/day) was associated with significantly decreased use of amphetamine, while aripiprazole treatment (15 mg/day) showed significantly increased use of amphetamine, when compared with placebo. This effect could be dose dependent—lower doses of aripiprazole in a relapse prevention study may not have the same effect; however, these preliminary data suggest that caution should be used in prescribing atypicals to methamphetamine- and amphetamine-dependent patients.
D-Amphetamine
The increase in amphetamine use and dependence (81,82) has resulted in greater demands on health services (83,84), but few effective therapies have been identified and there is an urgent need for evidence-based treatments (85). Maintenance treatment approaches have proven very effective for opioid dependence, with methadone (86) and buprenorphine (87) the major therapeutic agents. The model of opioid maintenance treatment has been adopted for amphetamine users in the U.K. using dexamphetamine (88–93) and it has also been tried in Australia (94,95).
Dexamphetamine acts by increasing synaptic concentrations of the monoamines dopamine, noradrenaline, and serotonin. In low oral doses it is used therapeutically in the treatment of narcolepsy and attention deficit/hyperactivity disorder (ADHD) without evidence of long-term harm (96,97). Dexamphetamine also has a less pronounced central effect than MA (98).
Maintenance programs using dexamphetamine have reported many positive outcomes, such as reductions in illicit amphetamine use and injecting and improvements in general health. In addition, the availability of such programs has increased the number of users presenting to services as well as increasing retention in treatment. Importantly, studies have found that the incidence of side effects, including psychotic symptoms, is low. However, the validity of most of these studies has been limited by factors such as small sample sizes, no control groups, and self-reported measures of illicit amphetamine use (99).
In contrast, the present study is a randomized, double-blind, placebo-controlled trial that involves supervised daily dosing of the medication. The formulation of dexamphetamine being used is sustained-release, enabling efficient once-daily dosing. Moreover, the use of hair analysis in addition to self-report methods provides an objective and quantifiable means of assessing changes in illicit amphetamine use.
AIMS AND HYPOTHESES
The main objective of this study was to assess the effectiveness of dexamphetamine maintenance for the treatment of amphetamine dependence, and any benefits over current best available treatment. The primary hypothesis is that dexamphetamine can be used safely for the treatment of amphetamine dependence and will result in improved treatment retention and greater reductions in illicit amphetamine use compared with placebo in the context of usual standards of care.
DESIGN
The study is a randomized, double-blind placebo-controlled trial carried out on an outpatient basis. To date, 30 dependent amphetamine users have been randomized to receive dexamphetamine or placebo. Recruitment for this study is ongoing, and consequently the results presented here are preliminary analyses only.
Potential subjects underwent a screening and enrollment process before commencing treatment. A number of instruments were administered to screen for dependence on amphetamines and other drugs and severe depression. Subjects provided a urine sample to establish recent use of amphetamines and females were tested for pregnancy. Eligible subjects were given a complete clinical assessment by a medical officer, including a medical and psychiatric history and examination, and blood samples were taken.
The study period included an initial stabilization period of up to 14 days, with an initial dose of 20 mg/day dexamphetamine to a maximum of 110 mg/day. Subjects were monitored each day with respect to vital signs, reviewed for withdrawal symptoms or adverse effects of the study medication, and other parameters such as sleep and craving were measured. Following stabilization, subjects continued a daily treatment with dexamphetamine or placebo for a period of 3 months, at the dosage determined for that individual during the stabilization phase of the trial. Medication was administered daily at Drug and Alcohol Services South Australia (DASSA) pharmacies or at community pharmacies, and dosing was supervised throughout the trial to minimize the risk of diversion. Subjects were monitored at least fortnightly during the maintenance phase. This consisted of regular clinical assessments by a medical officer, counseling appointments, and research assessments every month. Research measures included vital signs, withdrawal symptoms and craving, drug effects, mental and physical health, sleeping patterns and assessment of side effects or adverse events. At the end of the maintenance period, subjects were tapered off the medication over 1 month in order to minimize any withdrawal symptoms experienced. Subjects were monitored clinically at least fortnightly during this period and a research assessment was carried out at the end of the month. Subjects were followed up after the withdrawal period with a final research assessment 2 months after completing treatment. At this time, subjects were told whether they received dexamphetamine or placebo.
Hair samples were taken at 3 time-points (enrollment, at the end of maintenance, and at follow-up) to test for the presence of amphetamines. Subjects were reimbursed $20 for time and travel expenses involved in attending each research assessment.
DRUG INFORMATION
Dexamphetamine sulfate was used in a slow-release oral medication (Spansule®) capsule form. It is available in 5-, 10-, or 15-mg doses. Each Spansule sustained-release capsule is prepared so that an initial dose is released immediately and the remaining medication released gradually over a prolonged period. The slow-release formulation may prevent an acute stimulant effect and therefore be superior to immediate release with once-daily dosing. The active medication was reencapsulated in an opaque capsule with glucose as the exipient, and the placebo was produced by using the same capsules filled with glucose.
PRELIMINARY RESULTS
Sample Characteristics
We looked for differences in demographics or drug use between the active and placebo groups. The majority of subjects in both groups were male, aged between 30 and 33 years. Both groups started using amphetamines at a median age of 21 years. The length of regular use before starting the trial was the only variable to reach statistical significance, with subjects in the dexamphetamine group having used regularly for significantly less time than placebo (3 ± 6.4 years vs. 8.5 ± 5.3 years, p < 0.05).
Subjects were predominantly unemployed, with small percentages having tertiary education, and only half had received prior treatment for amphetamine dependence. Most were intravenous users and reported an average use of 4–5 times per week. Subjects had on average used one other illicit drug in the previous month, primarily cannabis 50% (n = 15) and ecstasy 10% (n = 3). Only one subject had used cocaine and one had used heroin. Licit drugs were more commonly reported, with 87% of the sample using tobacco (n = 26), 60% using alcohol (n = 18), and 33% using benzodiazepines (n = 10).
Survival Analysis
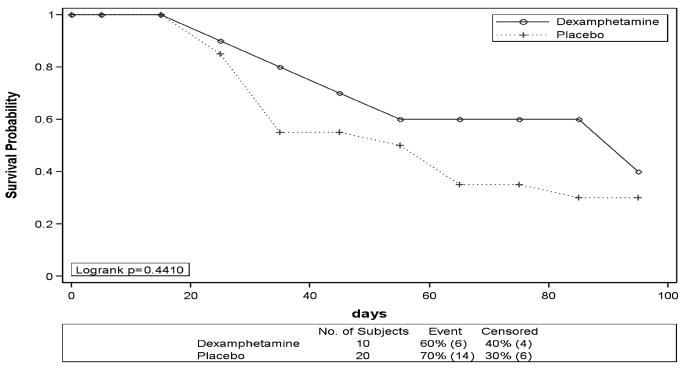
To compare subject retention in the 2 groups, a life-table analysis was performed. In the analysis, failure was defined as the event of a subject dropping out of the study. Right censoring of the data occurred at 100 days. Results from the analysis are shown in Figure 1.
FIGURE 1.
Life-table survival function estimates.
Subject retention was not significantly different between placebo and dexamphetamine groups (p = 0.441), although the trends were suggestive of dexamphetamine improving retention in treatment.
Self-Reported Methamphetamine Use
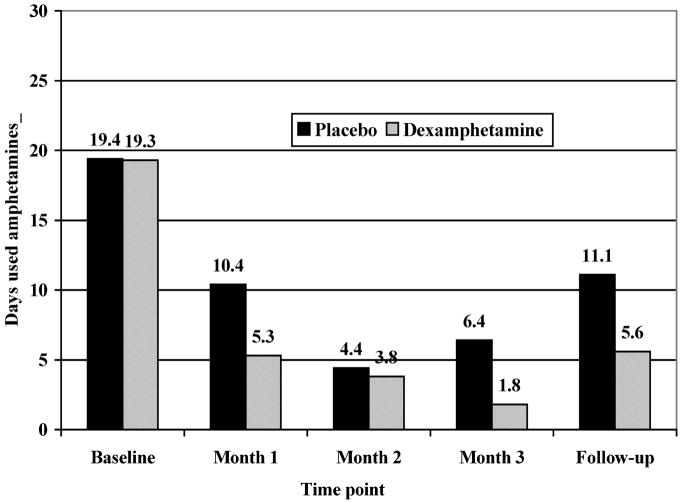
At each research assessment, subjects were asked how many days they had used MA in the previous month. The mean number of days is presented in Figure 2 at each time point for both dexamphetamine and placebo groups. The treatment phase (3 months of maintenance) is highlighted within the rectangle.
FIGURE 2.
Number of days used amphetamines in previous month.
Number of days used at baseline was almost identical between groups. There were significant reductions in self-reported methamphetamine use between baseline and follow-up (p = 0.0006 for dexamphetamine and p = 0.0047 for placebo). Although the mean number of days used at the end of the maintenance period and at follow-up was much lower for the dexamphetamine group, differences were not significant. This may be due to small sample sizes.
Hair Analysis: Methamphetamine Use
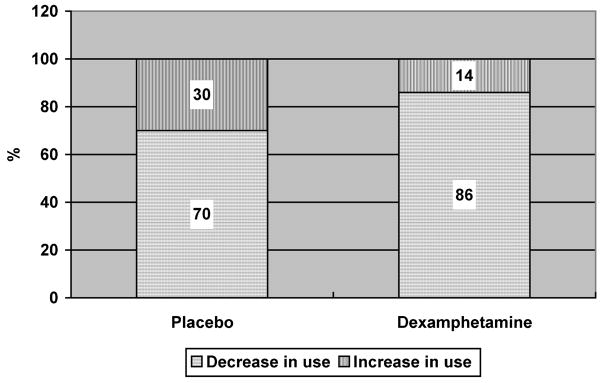
Figure 3 presents the results of analyses of hair samples taken from subjects at baseline and follow-up. Data are only shown for subjects for whom hair samples were taken and tested at both time-points. For the dexamphetamine group (n = 7) there was a decrease in MA use from baseline to follow-up in 6 cases (86%) and an increase in 1 case (14%). For the placebo group (n = 10) there was a decrease in MA use from baseline to follow-up in 7 cases (70%) and an increase in 3 cases (30%). Sample sizes were too small to enable significance testing.
FIGURE 3.
Methamphetamine use between baseline and follow-up.
CONCLUSIONS
Progress in MA pharmacotherapy is in an early stage; multiple failed trials in proof-of-concept are shown in Table 1. The results of the interim analysis of White’s D-amphetamine study have demonstrated the feasibility and validity of implementing a maintenance pharmacotherapy program for amphetamine users, with trends toward better retention in treatment and decreased MA use among the dexamphetamine group. In addition, there were no adverse events associated with dexamphetamine. Recruitment for this study is ongoing. The results of the interim analysis of methylphenidate by Tiihonen suggest that it is an effective treatment for reducing i.v. drug use in patients with severe amphetamine dependence. This study is also ongoing. These data suggest that, compared to other medications tested, direct dopamine agonists that might substitute for MA are showing promise as future candidate medications. This is also supported by preclinical data showing that dopamine agonists block MA-induced dopamine receptor changes and neurotoxicity.
Considering the long list of candidate medications that are currently being tested or are in the pipeline, we believe that the next few years will be very promising for finding effective medications to treat methamphetamine addiction.
Contributor Information
Ahmed Elkashef, affiliated with the Clinical Medical Branch, Division of Pharmacotherapies and Medical Consequences of Drug Abuse, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, 6001 Executive Boulevard, Room 4151, Bethesda, MD 20892 (E-mail: ae8a@nih.gov).
Frank Vocci, affiliated with the Division of Treatment Research and Development, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, 6001 Executive Boulevard, Room 4133, Bethesda, MD 20892.
Glen Hanson, affiliated with the Department of Pharmacology and Toxicology, University of Utah.
Jason White, affiliated with the Pharmacotherapies Research Unit, Drug and Alcohol Services South Australia, Discipline of Pharmacology, University of Adelaide.
Wendy Wickes, affiliated with the Pharmacotherapies Research Unit, Drug and Alcohol Services South Australia, Discipline of Pharmacology, University of Adelaide.
Jari Tiihonen, affiliated with the Department of Forensic Psychiatry, University of Kuopio, Niuvanniemi Hospital, FI-70240 Kuopio, Finland.
References
- 1.Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- 2.Newton T, Kalechstein A, Duran S, Vansluis N, Ling W. Methamphetamine abstinence syndrome: preliminary findings. Am J Addict. 2004;13:248–255. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- 3.Hanson GR, Bush L, Keefe KA, Alburges ME. Distinct responses of basal ganglia substance P systems to low and high doses of methamphetamine. J Neurochem. 2002;82:1171–1178. doi: 10.1046/j.1471-4159.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 5.Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- 6.Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- 7.United Nations Office for Drug Control and Crime Prevention. ODCCP Studies on drugs and crime control: global illicit drug trends 2002. New York: United Nations Publications; 2002. [Google Scholar]
- 8.United Nations Office on Drugs and Crime. Ecstasy and amphetamines, global survey. New York: United Nations Publications; 2003. [Google Scholar]
- 9.Klee H. A new target for behavioural research—amphetamine misuse. Brit J Addict. 1992;87:439–446. doi: 10.1111/j.1360-0443.1992.tb01944.x. [DOI] [PubMed] [Google Scholar]
- 10.Klee H. Amphetamine misusers in contemporary Britain: the emergence of a hidden population. In: Klee H, editor. Amphetamine misuse: international perspectives on current trends. Amsterdam, The Netherlands: Harwood Academic Publishers; 1997. pp. 19–34. [Google Scholar]
- 11.Suwaki H. Methamphetamine abuse in Japan. NIDA Res Monogr. 1991;115:84–98. [PubMed] [Google Scholar]
- 12.Suwaki H, Fukui S, Konuma K. Methamphetamine abuse in Japan: its 45 year history and the current situation. In: Klee H, editor. Amphetamine misuse: international perspectives on current trends. Amsterdam, The Netherlands: Harwood Academic Publishers; 1997. pp. 199–214. [Google Scholar]
- 13.Hando J, Hall W. HIV risk-taking behavior among amphetamine users in Sydney, Australia. Addiction. 1994;89:79–85. doi: 10.1111/j.1360-0443.1994.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 14.Hando J, Hall W. Patterns of amphetamine use in Australia. In: Klee H, editor. Amphetamine misuse: international perspectives on current trends. Amsterdam, The Netherlands: Harwood Academic Publishers; 1997. pp. 81–110. [Google Scholar]
- 15.Makkai T, McAllister I. Patterns of drug use in Australian society. Canberra: Australian Government Printing Services; 1993. [Google Scholar]
- 16.Klee H, editor. Amphetamine misuse: international perspectives on current trends. Amsterdam, The Netherlands: Harwood Academic Publishers; 1997. [Google Scholar]
- 17.Office of National Drug Control Policy. Pulse check. [accessed March 18, 2005]; http://www.whitehousedrugpolicy.gov/publications/drugfact/pulsechk/apr02/index.html.
- 18.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. DASIS Series: S-22, DHHS Publication No. (SMA) 04–3946. DHHS, (US) Department of Health and Human Services; Rockville, MD: 2004. Treatment Episode Data Set (TEDS). Highlights—2002. National admissions to substance abuse treatment services. [Google Scholar]
- 19.Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology (Berl) 2005;182:426–435. doi: 10.1007/s00213-005-0102-8. [DOI] [PubMed] [Google Scholar]
- 20.Newton TF, De La Garza R, 2nd, Fong T, Chiang N, Holmes TH, Bloch DA, Anderson A, Elkashef A. A comprehensive assessment of the safety of intravenous methamphetamine administration during treatment with selegiline. Pharmacol Biochem Behav. 2005;82:704–711. doi: 10.1016/j.pbb.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Galloway G, Newmeyer J, Knapp T, Stalcup SA, Smith D. A controlled trial of imipramine for the treatment of methamphetamine dependence. J Subst Abuse. 1996;13:493–497. doi: 10.1016/s0740-5472(96)00154-7. [DOI] [PubMed] [Google Scholar]
- 22.Heinzerling KG, Shoptaw S, Peck JA, Yang X, Liu J, Roll J, Ling W. Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85:177–184. doi: 10.1016/j.drugalcdep.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Shoptaw S, Hubert A, Peck J, Yang X, Liu J, Dang J, Roll R, Shapiro B, Rotheram-Fuller E, Ling W. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85:12–18. doi: 10.1016/j.drugalcdep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Johnson BA, Roache JD, Ait-Daoud N, Wallace C, Wells LT, Wang Y. Effects of isradipine on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition. Psychopharmacology (Berl) 2005;178:296–302. doi: 10.1007/s00213-004-1998-0. [DOI] [PubMed] [Google Scholar]
- 25.Johnson BA, Roache JD, Ait-Daoud N, Wallace C, Wells L, Dawes M, Wang Y. Effects of isradipine, a dihydropyridine-class calcium-channel antagonist, on D-methamphetamine’s subjective and reinforcing effects. Int J Neuropsychopharmacol. 2005;8:203–213. doi: 10.1017/S1461145704005036. [DOI] [PubMed] [Google Scholar]
- 26.Johnson BA, Wells LT, Roache JD, Wallace C, Ait-Daoud N, Wang Y. Isradipine decreases the hemodynamic response of cocaine and methamphetamine: results from two human laboratory studies. Am J Hypertens. 2005;18:813–822. doi: 10.1016/j.amjhyper.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Johnson BA, Wells LT, Roache JD, Wallace CL, Ait-Daoud N, Dawes M, Liu L, Wang XQ, Javors MA. Kinetic and cardiovascular effects of acute topiramate dosing among non-treatment-seeking, methamphetamine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:455–461. doi: 10.1016/j.pnpbp.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson BA, Roache JD, Ait-Daoud N, Wells LT, Wallace CL, Dawes MA, Liu L, Wang XQ. Effects of topiramate on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition in recently abstinent methamphetamine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:123–130. doi: 10.1016/j.pnpbp.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiihonen J, Kuoppasalmi K, Fohr J, Tuomola P, Kuikanmaki O, Vorma H, Sokero P, Haukka J, Meririnne E. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164:160–162. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- 30.Batki SL, Bui L, Mendelson J, Benowitz N, Bradley JM, Jones RT, Deluchi P, Jacob P., III Methamphetamine-amlodipine interactions: preliminary analysis. Poster session College on Problems of Drug Dependency (CPDD); 2002. [Google Scholar]
- 31.Batki SL, Moon J, Delucchi K, Bradley M, Hersh D, Smolar S, Mengis M, Lefkowitz E, Sexe D, Morello L, Evenhart T, Jones RT, Jacob P., 3rd Methamphetamine quantitative urine concentrations during a controlled trial of fluoxetine treatment: preliminary analysis. Annual NY Acad Sci. 2000;909:260–263. doi: 10.1111/j.1749-6632.2000.tb06688.x. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BA, Roache JD, Ait-Daoud N, Wells LT, Wallace CL, Dawes MA, Liy L, Wang XQ. Effects of acute topiramate dosing on methamphetamine-induced subjective mood. Int J Neuropsychopharmacol. 2007;10:85–98. doi: 10.1017/S1461145705006401. [DOI] [PubMed] [Google Scholar]
- 33.Jayaram-Lindstrom N, Wennberg P, Hurd J. Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. Poster session 2004 CPDD; [DOI] [PubMed] [Google Scholar]
- 34.Koob GF. Stress, corticotrophin-releasing factor, and drug addiction. Ann N Y Acad Sci. 1999;897:27–45. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- 35.Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- 36.Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- 37.Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress-and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:4429–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaham Y, Erb S, Leung S, Buczek Y. CP-154–526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- 39.Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- 41.Sokoloff P, Le FB, Perachon S, Bordet R, Ridray S, Schwartz JC. The dopamine D3 receptor and drug addiction. Neurotox Res. 2001;3:433–441. doi: 10.1007/BF03033202. [DOI] [PubMed] [Google Scholar]
- 42.Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joyce JN, Millan MJ. Dopamine D3 receptor antagonists as therapeutic agents. Drug Discov Today. 2005;10:917–925. doi: 10.1016/S1359-6446(05)03491-4. [DOI] [PubMed] [Google Scholar]
- 44.Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- 45.Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 46.Mash DC. D3 receptor binding in human brain during cocaine overdose. Mol Psychiatry. 1997;2:5–6. [PubMed] [Google Scholar]
- 47.Geter-Douglass B, Katz JL, Alling K, Acri JB, Witkin JM. Characterization of unconditioned behavioral effects of dopamine D3/D2 receptor agonists. J Pharmacol Exp Ther. 1997;283:7–15. [PubMed] [Google Scholar]
- 48.Acri JB, Carter SR, Alling K, Geter-Douglass B, Kijkstra D, Wikstrom H, Katz JL, Witkin JM. Assessment of cocaine-like discriminative stimulus effects of dopamine D3 receptor ligands. Eur J Pharmacology. 1995;281(2):R7–R9. doi: 10.1016/0014-2999(95)00411-d. [DOI] [PubMed] [Google Scholar]
- 49.Spealman RD. Dopamine D3 receptor agonists partially reproduce the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 1996;278:1128–1137. [PubMed] [Google Scholar]
- 50.Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P. D3 receptor test in vitro predicts decreased cocaine self-administration in rats. Neuroreport. 1997;8:2373–2377. doi: 10.1097/00001756-199707070-00054. [DOI] [PubMed] [Google Scholar]
- 51.Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- 52.Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003;28:1272–1280. doi: 10.1038/sj.npp.1300183. [DOI] [PubMed] [Google Scholar]
- 53.Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- 54.Gilbert JG, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, Peng XQ, Xi ZX. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking rats. Psychopharmacology (Berl) 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 receptor ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- 58.Ashby CR, Paul M, Gardner EL, Heidbreder CA, Hagan JJ. Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 2003;48(3):154–156. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- 59.Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312:875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- 60.Beardsley PM, Thomas BF. Current evidence supporting a role of cannabinoid CB1 receptor (CB1R) antagonists as potential pharmacotherapies for drug abuse disorders. Behav Pharmacol. 2005;16(5–6):275–296. doi: 10.1097/00008877-200509000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8:373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- 62.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25(5):265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 64.Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 65.Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine- and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- 66.Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- 67.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16:1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- 69.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Backstrom P, Hyytia P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–565. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- 71.Slusher BS, Thomas A, Paul M, Schad CA, Ashby CR., Jr Expression and acquisition of the conditioned place preference response to cocaine in rats is blocked by selective inhibitors of the enzyme N-acetylated-alpha-linked-acidic dipeptidase (NAALADASE) Synapse. 2001;41:22–28. doi: 10.1002/syn.1056. [DOI] [PubMed] [Google Scholar]
- 72.Bourtrel B, Kenny PI, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role of r hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 74.Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172(2):170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ciccocioppo R, Economidou D, Fedeli A, Massi M. The nociceptin/orphanin FQ/NOP receptor system as a target for treatment of alcohol abuse: a review of recent work in alcohol-preferring rats. Physiol Behav. 2003;79:121–128. doi: 10.1016/s0031-9384(03)00112-4. [DOI] [PubMed] [Google Scholar]
- 76.Basile AS, Fedorova I, Zapata A, Liu X, Shippenberg T, Duttaroy A, Yamada M, Wess J. Deletion of the M5 muscarinic acetylcholine receptor attenuates morphine reinforcement and withdrawal but not morphine analgesia. Proc Natl Acad Sci USA. 2002;99:11452–11457. doi: 10.1073/pnas.162371899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fink-Jensen A, Fedorova I, Wortwein G, Woldbye DP, Rasmussen T, Thomsen M, Bolwig TG, Knitowski KM, McKinzie DL, Yamada M, Wess J, Basile A. Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J Neurosci Res. 2003;74:91–96. doi: 10.1002/jnr.10728. [DOI] [PubMed] [Google Scholar]
- 78.Dewey SL, Chaurasia CS, Chen CE, Volkow ND, Clarkson FA, Porter SP, Straughter-Moore RM, Alex-off DL, Tedeschi D, Russo NB, Fowler JS, Brodie JD. GABAergic attenuation of cocaine-induced dopamine release and locomotor activity. Synapse. 1997;25:393–398. doi: 10.1002/(SICI)1098-2396(199704)25:4<393::AID-SYN11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 79.Brodie JD, Figueroa E, Laska EM, Dewey SL. Safety and efficacy of gamma-vinyl GABA (GVG) for the treatment of methamphetamine and/or cocaine addiction. Synapse. 2005;55:122–125. doi: 10.1002/syn.20097. [DOI] [PubMed] [Google Scholar]
- 80.Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT. Lobeline attenuates d-methamphetamine self-administration in rats. J Pharmacol Exp Ther. 2001;298:172–179. [PubMed] [Google Scholar]
- 81.Australian Crime Commission. Australian illicit drug report 2001–2002. Canberra: Australian Crime Commission; 2003. [Google Scholar]
- 82.Australian Institute of Health and Welfare. Drug Statistics Series No. 16. Canberra: AIHW; 2005. National Drug Strategy Household Survey: detailed findings. [Google Scholar]
- 83.Topp L, Darke S. The applicability of the dependence syndrome to amphetamine. Drug Alcohol Depend. 1997;48:113–118. doi: 10.1016/s0376-8716(97)00111-7. [DOI] [PubMed] [Google Scholar]
- 84.Vincent N, Shoobridge J, Ask A, Allsop S, Ali R. Physical and mental health problems in amphetamine users from metropolitan Adelaide, Australia. Drug Alcohol Rev. 1998;17:187–195. doi: 10.1080/09595239800186991. [DOI] [PubMed] [Google Scholar]
- 85.Srisurapanont M, Jarasuraisin N, Kittiratanapaiboon P. Treatment for amphetamine dependence and abuse. Cochrane Library. Vol. 2. Oxford: Update Software; 2003. [Google Scholar]
- 86.Ward J, Mattick R, Hall W. Key issues in methadone maintenance treatment. Sydney: University of New South Wales Press; 1992. [Google Scholar]
- 87.Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Systematic Review. 2002:CD002025. doi: 10.1002/14651858.CD002025. [DOI] [PubMed] [Google Scholar]
- 88.Charnaud B, Griffiths V. Levels of intravenous drug misuse among clients prescribed oral dexamphetamine or oral methadone: a comparison. Drug Alcohol Depend. 1998;52:79–84. doi: 10.1016/s0376-8716(98)00052-0. [DOI] [PubMed] [Google Scholar]
- 89.Fleming P, Roberts D. Is the prescription of amphetamine justified as a harm reduction measure? J Royal Soc Health. 1994;114:127–131. doi: 10.1177/146642409411400303. [DOI] [PubMed] [Google Scholar]
- 90.Klee H, Wright S, Carnwath T, Merrill J. Role of substitute therapy in the treatment of problem amphetamine use. Drug Alcohol Rev. 2001;20:417–429. [Google Scholar]
- 91.McBride AJ, Sullivan G, Blewett AE, Morgan S. Amphetamine prescribing as a harm reduction measure: a preliminary study. Addict Res. 1997;5:95–112. [Google Scholar]
- 92.Pates R, Coombes N, Ford N. A pilot programme in prescribing dexamphetamine for amphetamine users. J Subst Misuse. 1996;1:80–84. [Google Scholar]
- 93.White R. Dexamphetamine substitution in the treatment of amphetamine abuse: an initial investigation. Addiction. 2000;95:229–238. doi: 10.1046/j.1360-0443.2000.9522299.x. [DOI] [PubMed] [Google Scholar]
- 94.Shearer J, Wodak A, Mattick RP, Van Beek I, Lewis J, Hall W, Dolan K. Pilot randomized controlled study of dexamphetamine substitution for amphetamine dependence. Addiction. 2001;96:1289–1296. doi: 10.1046/j.1360-0443.2001.96912898.x. [DOI] [PubMed] [Google Scholar]
- 95.Sherman JP. Dexamphetamine for “speed” addiction. Med J Aust. 1990;153:306. doi: 10.5694/j.1326-5377.1990.tb136930.x. [DOI] [PubMed] [Google Scholar]
- 96.Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100:662–666. doi: 10.1542/peds.100.4.662. [DOI] [PubMed] [Google Scholar]
- 97.Paterson R, Douglas C, Hallmayer J, Hagan M, Krupenia Z. A randomised, double-blind, placebo-controlled trial of dexamphetamine in adults with attention deficit hyperactivity disorder. Aust N Z J Psychiatry. 1999;33:494–502. doi: 10.1080/j.1440-1614.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 98.Iwanami A, Sugiyama A, Kuroki N, Toda S, Kato N, Nakatani Y, Horita N, Kaneko T. Patients with methamphetamine psychosis admitted to a psychiatric hospital in Japan. A preliminary report. Acta Psychiatr Scand. 1994;89:428–432. doi: 10.1111/j.1600-0447.1994.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 99.Bradbeer TM, Fleming PM, Charlton P, Crichton JS. Survey of amphetamine prescribing in England and Wales. Drug Alcohol Rev. 1998;17:299–304. doi: 10.1080/09595239800187131. [DOI] [PubMed] [Google Scholar]