Abstract
Endothelial monocyte-activating polypeptide II (EMAP-II) is an inflammatory cytokine with chemotactic activity. Because the dental follicle (DF) recruits mononuclear cells (osteoclast precursors) to promote the osteoclastogenesis needed for tooth eruption, it was the aim of this study to determine if EMAP-II may contribute to this recruitment. Using a DNA microarray, EMAP-II was found to be highly expressed in vivo in the DFs of day 1 to day 11 postnatal rats, with its expression elevated at days 1 and 3. Using a siRNA to knock down EMAP-II expression also resulted in a reduction in expression of CSF-1 and MCP-1 in the DF cells. Addition of EMAP-II protein to the DF cells partially restored the expression of CSF-1 and MCP-1. In chemotaxis assays using either conditioned medium of the DF cells with anti-EMAP-II antibody added or conditioned medium of DF cells with EMAP-II knocked down by siRNA, migration indexes of bone marrow mononuclear cells were significantly reduced. These results suggest that EMAP-II is another chemotactic molecule in the dental follicle involved in recruitment of mononuclear cells, and that EMAP-II may exert its chemotactic function directly by recruiting mononuclear cells and indirectly by enhancing the expression of other chemotactic molecules (CSF-1 and MCP-1).
Keywords: dental follicle, EMAP-II, microarray, chemotaxis, tooth eruption
The dental follicle (DF), a loose connective tissue sac that surrounds the unerupted tooth, provides the microenvironment for the cellular and molecular events needed for tooth eruption. The cellular events include the influx of mononuclear cells into the DF and their fusion into osteoclasts. In the first mandibular molar of rats, the peak level of mononuclear cells in the DF is reached at day 3 postnatally, the time of maximal number of osteoclasts on the surface of the bony crypt (1, 2). Parallel to the cellular events, several molecular events occur at this time. The dental follicle maximally expresses and secretes monocyte chemoattractant protein-1 (MCP-1) and colony-stimulating factor-1 (CSF-1) at day 3 postnatally to recruit mononuclear cells (3, 4, 5). Moreover, CSF-1 acts to inhibit osteoprotegerin (OPG), an osteoclastogenesis inhibitor expressed by DF, resulting in its down-regulation at day 3 (6, 7). Another molecule, receptor activator of nuclear factor-kappa B ligand (RANKL) is expressed in the DF (8). In conjunction with a stable level of RANKL, the low level of OPG at day 3 results in higher ratio of RANKL to OPG, thus creating a favorable microenvironment for mononuclear cells to differentiate and fuse into osteoclasts that resorb alveolar bone to form an eruption pathway for the tooth.
Chemokines, a superfamily of secreted proteins with low molecular weight (8-16 kDa) that regulate the migration and interactions of white blood cells, play an important role in recruiting mononuclear cells (osteoclast precursors) into sites where they differentiate into osteoclasts (9). Chemokines are categorized structurally into four subfamilies according to their number and position of conserved cysteine residues: CC, C, CXC and CX3C (10). CC denotes adjacent cysteines and CXC and CX3C denote one residue and three residues between them respectively.
One cytokine, endothelial monocyte-activating polypeptide II (EMAP-II), although it does not fall into the above chemokine subfamilies, has a domain that is structurally homologous to other chemokines (11), and acts as a chemokine to induce migration of endothelial progenitor cells via the chemokine receptor CXCR3 (12). EMAP-II was originally isolated from the supernatant of murine methylcholanthrene A fibrosarcoma cells and has been characterized as a pro-inflammatory cytokine (13). In vitro, EMAP-II modulated procoagulant activity of endothelial cells through the tissue factor, and induced migration of monocytes, as well as granulocytes and neutrophils. In vivo, injection of EMAP-II into the footpads of mice induced an acute inflammatory response characterized by tissue swelling and polymorphonuclear leukocyte infiltration (13). EMAP-II has also been reported to trigger cell apoptosis (14), and it has elevated expression in apoptotic tissues and remodeling tissue where elevated EMAP-II attracted macrophages for removal of the dead cells (15). The inflammatory effect of EMAP-II was also seen in the pathogenesis of periodontitis in which elevated EMAP-II expression was observed in aggressive periodontitis, but not in chronic periodontitis nor in normal periodontal tissue (16).
In our previous study using a chemokines and receptors DNA microarray, EMAP-II was identified as being expressed in the DF cells (17). Because of its high expression level in the DF cells and its chemotactic effect on mononuclear cells found in other studies (13, 18), it was the aim of this study to characterize the expression patterns of EMAP-II in rat DF in vivo and to determine its possible role in recruiting mononuclear cells for osteoclastogenesis, as well as its effect on the expression of other chemotactic molecules in the DF.
Material and methods
Dental follicle isolation and cell culture
Rats (Harlan Sprague-Dawley) were maintained in the School of Veterinary Medicine at Louisiana State University in compliance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC). DFs were isolated surgically from the first mandibular molars of the rats at postnatal days 1, 3, 5, 7, 9 and 11 for RNA isolation. DF cells were obtained by trypsinizing the DFs of the first mandibular molars of 5- to 6-d-old rats and culturing them in minimum essential medium (MEM) (Sigma-Aldrich, St. Louis, MO, USA) plus 10% (V/V) newborn calf serum, 1 mM sodium pyruvate, 1% penicillin/streptomycin and 0.2% fungizone as previously reported (19). DF cells of passages 6 to 9 were used for the experiments.
RNA isolation
Total RNA was isolated from the DFs or DF cells using Tri-Reagent (Molecular Research Center, Cincinnati, OH, USA). As controls, total RNA was also isolated from spleen and bone (tibia of hind limb without marrow) of 6 to 8 month old rats using the Tri-Reagent with pestle grinding. The RNA was then treated with DNase I (Ambion, Austin, TX, USA) to remove possible DNA contaminations. Total RNA was quantified by a spectrophotometer at an absorbance (A) of 260 nm, and RNA purity was confirmed by an A260/A280 ratio of 2.0 or higher.
DNA microarray analysis
DNA microarrays were performed as in our previous study (17). Briefly, total RNA (1-1.5 μg) from the DFs of rats aged 1 – 11 days was labeled with biotin-16-UTP (Roche Applied Science, Indianapolis, IN, USA) to generate complementary RNA (cRNA) using TrueLabeling-AMPTM 2.0 kit (SuperArray Bioscience, Frederick, MD, USA) per manufacturer’s instructions. Then, 4 μg of the labeled cRNA was hybridized to a rat chemokines and receptors oligo-DNA microarray containing 113 genes (SuperArray Bioscience) in a hybridization oven at 60°C for 18 h, followed by two washes at the same temperature. Next, the array was blocked in blocking buffer at room temperature for 40 min, incubated with alkaline phosphatase-conjugated streptavidin for 10 min, and washed four times. Finally, the array was incubated at room temperature with a CDP-Star chemiluminescent substrate for 5 min.
The image was acquired after 20 min of exposure using a FluoChemTM 8800 image system (Alpha Innotech Corp., San Leandro, CA, USA). The gene expression data were obtained and analyzed using software GEArray Expression Analysis Suite (SuperArray Bioscience). The gene expression level was expressed as the ratio of EMAP-II gene to the internal control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene.
RT-PCR and real-time RT-PCR
To detect gene expression, 2 μg total RNA was reverse transcribed by M-MLV Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) to synthesize the first strand cDNA per the manufacturer’s instructions. The reverse transcription was performed at 37°C for 1 h, followed by 10 min incubation at 70°C to inactivate the reverse transcriptase.
Real-time RT-PCR was used to analyze the expression of EMAP-II, CSF-1 and MCP-1 in the DF cells. The primers were designed for the following genes based on their mRNA sequences in GenBank: 5′-CCTGTCGACGTATCACGCCT-3′ and 5′-CCCACATCGACTTCCTCCAC-3′ for EMAP-II (GenBank accession No. NM_053757); 5′-CGAGGTGTCGGAGCACTGTA-3′ and 5′-TCAACTGCTGCAAAATCTGTAGGT-3′ for CSF-1 (accession no. NM_023981); and, 5′-CACGCTTCTGGGCCTGTT-3′ and 5′-TGAGACAGCACGTGGATGCT-3′ for MCP-1 (accession no. NM_031530). The real-time PCR was performed as our previous study (20) with SYBR Green Master mix (Applied Biosystems, Foster City, CA, USA). The relative gene expression (RGE) was calculated with β-actin as an internal control using the formula 2-ΔΔCT. Semi-quantitative PCR also was performed for 26 cycles using the above sequences.
Immunostaining
For detection of EMAP-II expression in vivo, mandibles of postnatal day 5 rats were fixed in formalin, decalcified, dehydrated and sectioned at 5 μm thickness. Endogenous peroxidase activity was quenched by incubating sections in 3% hydrogen peroxide for 10 min. After two washes with Tris-buffered saline (TBS) plus 0.025% Triton X-100, the sections were blocked at room temperature for 1 h with 2% normal rabbit serum (Vector Laboratories, Burlingame, CA, USA) in TBS buffer, and then incubated with goat polyclonal anti-human EMAP-II primary antibody (Pepro Tech, Rock Hill, NJ, USA) at a concentration of 2 μg/ml overnight at 4°C in TBS buffer plus 2% normal rabbit serum. For controls, the primary antibody was replaced by goat IgG (Prepro Tech). After incubation overnight, the sections were washed 3 times and incubated with biotinylated rabbit anti-goat IgG secondary antibody (diluted 1: 100 in TBS buffer plus 1% BSA) for 1 h. Following 3 washes, the sections were incubated with avidin-biotinylated horseradish peroxidase (HRP) (Vector Laboratories) for 30 min and then incubated in 3, 3’-diaminobenzidine (DAB) for 5 min, followed by counterstaining with hematoxylin. The same procedure was applied for the DF cells except that the cells were fixed in ice-cold methanol for 5 min and air-dried.
In vitro transfection of DF cells with siRNA
Dicer substrate siRNA was designed according to the software provided by Integrated DNA Technologies (Coralville, IA, USA) (21). The siRNA duplex, annealed from two RNA strands (5’-rUrGrCrUrUrCrUrCrCrCrArCrArUrCrGrArCrUrUrCrCrUrCrCrArC-3’ and 5’-rGrGrArGrGrArArGrUrCrGrArUrGrUrGrGrGrArGrArArGCA-3’), was synthesized by Integrated DNA Technologies, targeting the rat EMAP-II mRNA at nucleotides 582-606. The DF cells were cultured one day before transfection in T-25 flasks in MEM plus 1 mM sodium pyruvate and 10% heat-inactivated fetal calf serum such that cells were 30-40% confluent at time of transfection. Transfection was carried out using LipofectamineTM RNAiMAX per manufacturer’s instructions (Invitrogen). Briefly, siRNA was diluted to 100 nM and transfection reagent was diluted 50-fold using Opti MEM I Reduced Serum Medium (Invitrogen). After 5 min of incubation at room temperature, an equal amount of diluted siRNA and transfection reagent were combined, and incubated at room temperature for 20 min to form siRNA-LipofectamineT RNAiMAX complex. One ml of the complex was added into 4 ml cell culture to bring the final siRNA concentration to 10 nM. For controls, the cells were transfected without siRNA. The culture was incubated at 37°C with 5% CO2 for 24, 48 and 72 h. After transfection, the cells were collected for determination of gene expression using real-time RT-PCR.
Treatment of DF cells with EMAP-II after EMAP-II knockdown
The DF cells were transfected with 2 nM siRNA targeting EMAP-II as described above. Twenty-four h after transfection, the cells were then treated with human EMAP-II (Pepro Tech, Rock Hill, NJ, USA) at a concentration of 50 ng/ml for 24 h. Two controls, one without siRNA, and another with 2 nM siRNA but without EMAP-II treatments, were included in the experiments. The cells were collected, and real-time RT-PCR was used to determine the expression of CSF-1 and MCP-1 after endogenous EMAP-II expression was confirmed to be knocked down.
Chemotaxis assay
The DF cells at passages 6-9 were cultured in an incubator at 37°C until confluent. Two days after medium change, the conditioned medium (CM) was collected and stored at -20°C for chemotaxis assays. The CM from DF cells with EMAP-II knocked down was collected at 24 and 48 h after transfection, and stored at the same conditions.
Bone marrow mononuclear cells were isolated from six-week old rats, purified using density gradient centrifugation (17), and suspended into α-MEM medium (Invitrogen) at a density of approximately 1 × 106 per ml.
The chemotaxis assays were conducted in Costar Transwell® 24-well plates (Costar Corning, Cambridge, MA, USA), each well of which has lower chamber and upper chamber separated by a polycarbonate membrane with 5 μm pores. The upper chambers were loaded with 1 × 105 bone marrow mononuclear cells in 100 μl, and the lower chambers were filled either with 600 μl MEM medium for chemotactic activity of EMAP-II, or 600 μl CM from the DF cell culture for chemotactic activity of EMAP-II secreted by the DF cells. For chemotaxis assays of EMAP-II, human recombinant EAMP-II (Pepro Tech, Rock Hill, NJ, USA) was added into the lower chamber at concentrations of 0, 0.1, 1 or 10 nM. For chemotaxis assays of the CM of DF cells, two sets of experiments were conducted: A) EMAP-II antibody added to the CM or B) CM from DF cells with EMAP-II knocked down by siRNA. For experiment A, three treatments were included: 1) MEM; 2) CM only; and 3) CM plus 10 μg/ml EMAP-II antibody (R & D Systems, Mineapolis, MN, USA). For experiment B, five treatments were included: 1) MEM; 2) and 3) CM from 0 and 2 nM siRNA transfection for 24 h respectively; 4) and 5) CM from 0 and 2 nM siRNA transfection for 48 h respectively. The plates were incubated for 2 h at 37°C with 5% CO2.
After incubation, the medium in the lower chambers containing cells that had migrated through the membrane from the upper chambers was collected, centrifuged at 12,000 × g for 5 min, and resuspended into 20 μl medium. The cell number of each well was represented by the total of two counts, each with 0.9 μl of the cell suspension counted with a hemocytometer in all of the 9 mm2 scaled area. The chemotactic activity was expressed as a migration index; i.e., the number of cells that had migrated into the lower chamber after treatment divided by the number of cells that had migrated into the lower chamber with MEM medium.
Statistical analysis
Data from DNA microarray (three repeats), gene expression from EMAP-II treatments (three repeats) and chemotaxis assay of human EMAP-II (four repeats) were analyzed using SAS program (version 9). Analysis of variance (ANOVA) was carried out to evaluate the treatment effects, and then the means were separated with the least significant difference (LSD) test at a significant level of P < 0.05. Data from gene expression of CSF-1 and MCP-1 (three repeats) and chemotactic activity of conditioned medium from DF cells (four repeats) were analyzed using paired t-test at P < 0.05.
Results
Expression of EMAP-II mRNA in the DF
The results from RT-PCR showed a strong expression of EMAP-II in the DF cells, as compared to its low expression in spleen and no expression in bone (Fig. 1A).
Fig. 1.
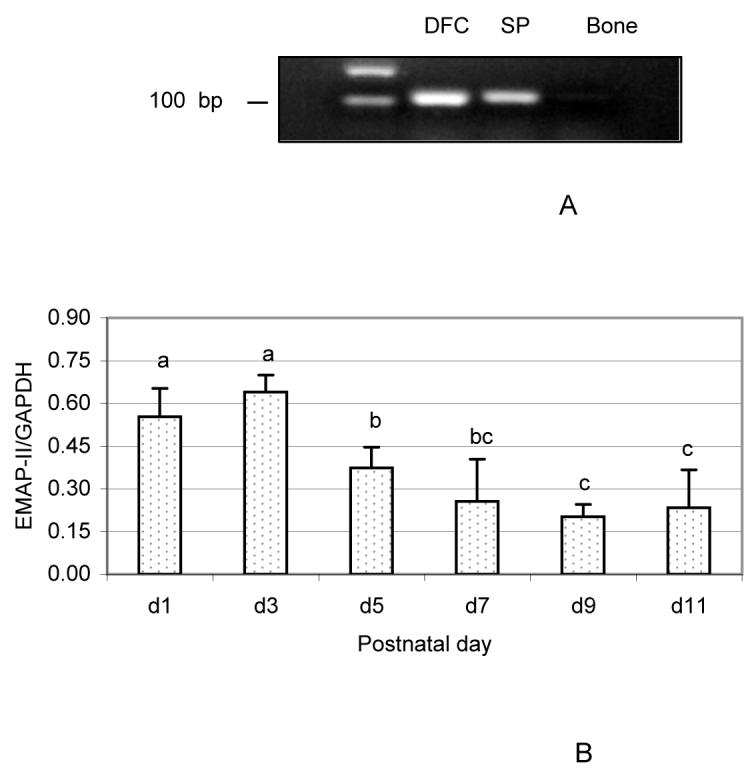
Expression of EMAP-II in the dental follicle cells (DFC) and dental follicle (DF). (A) High expression levels of EMAP-II in the DFC by PCR as compared to weaker expression in spleen (SP) and no expression in bone. (B) Chronological expression of EMAP-II in the DF of postnatal days 1 to 11 rats as determined by DNA microarray. EMAP-II expression was expressed as the ratio of EMAP-II gene to the internal control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. The results were presented as means ± standard deviations of three independent litters of rats. The bars labeled without same letter are statistically different each other. Note that the expression of EMAP-II was higher at days 1 and 3 as compared to other days.
To determine EMAP-II expression levels and chronological changes in vivo, DNA microarrays were performed. As seen in Fig. 1B, EMAP-II was strongly expressed in the DF from day 1 to day 11. Chronologically, EMAP-II showed a strong expression at early days (day 1 and 3), with a significant reduction starting at day 5 and reaching its lowest level at day 9. The expression levels at day 1 and day 3 were 2.7- and 3.2-fold higher, respectively, than those at day 9.
EMAP-II protein expression in DF
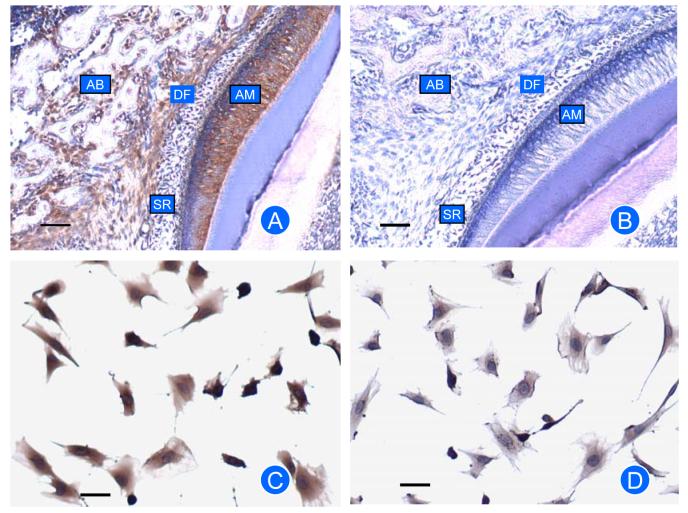
Immunostaining for EMAP-II protein expression showed that DF strongly stained for EMAP-II (Fig. 2A). Some staining in the apex of ameloblasts was observed, which was likely due to a physical entrapment of the antibody. Slight staining of the alveolar bone also was observed. In vitro, the DF cells strongly stained for EMAP-II protein (Fig. 2C). No staining was seen in the control (Fig. 2B,D).
Fig. 2.
Immunostaining for EMAP-II protein in rat mandibles (A, B) and DF cells (C, D). Expression of EMAP-II was seen in dental follicle (DF) and DF cells. There is some expression in ameloblasts (AM) and alveolar bone (AB), but no expression in stellate reticulum (SR). Controls for immunostaining (B and D) with IgG used to replace the primary antibody showed little or no staining. Scale bar: 50 μm.
Reduction of CSF-1 and MCP-1 expression in the EMAP-II-knockdown DF cells
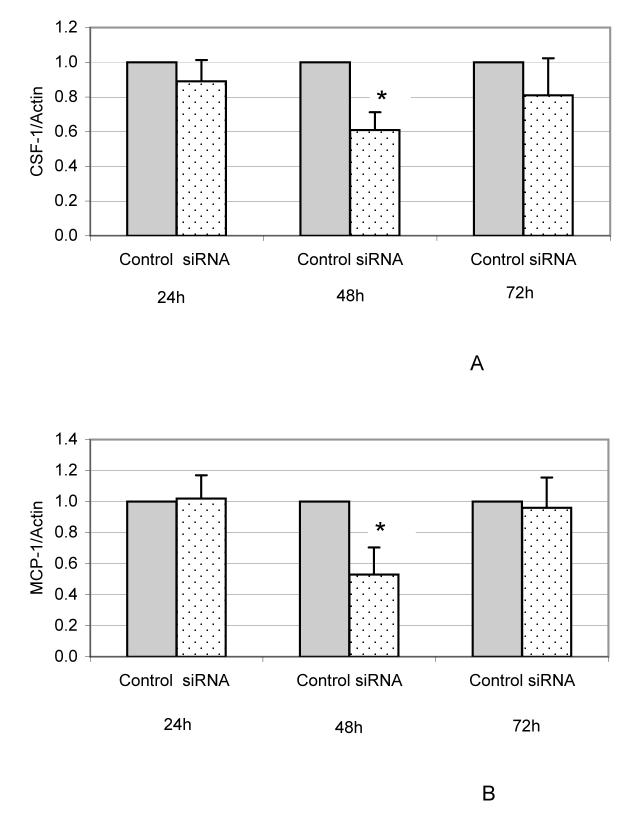
Because the expression of both CSF-1 and MCP-1 are elevated at day 3, experiments were conducted to determine if EMAP-II affects their expression. To that end, expressions of CSF-1 and MCP-1 in the DF cells were examined after EMAP-II expression was knocked down by Dicer siRNA targeting EMAP-II mRNA. Dicer siRNA knocked down EMAP-II expression in DF cells by 96-98% from 24 to 72 h after transfection, as determined by real-time RT-PCR (data not shown). As shown in Fig. 3A, CSF-1 expression was reduced after transfection, with a significant reduction at 48 h. Similarly, MCP-1 expression was also reduced significantly at 48 h after transfection (Fig. 3B).
Fig. 3.
Reduction of CSF-1 and MCP-1 expression in the DF cells after EMAP-II expression was knocked down by siRNA. DF cells were transfected without siRNA (control) or with 10 nM of siRNA (siRNA) targeting EMAP-II mRNA for 24, 48 and 72 h, and expression of CSF-1 and MCP-1 was determined using real-time RT-PCR. The experiments were repeated three times, and the data were analyzed using paired t-tests. A significant difference between treatment pairs is indicated by an asterisk (*). The expression of CSF-1 (A) and MCP-1 (B) were reduced significantly at 48 h after transfection with siRNA (P < 0.05).
Human EMAP-II was also used to treat DF cells and expression of CSF-1 and MCP-1 were evaluated. However, the results showed that expression of both CSF-1 and MCP-1 remained unchanged (data not shown). This could be attributed to a high endogenous expression of EMAP-II that is maximal, and thus, the cells do not respond. Therefore, EMAP-II expression in the DF cells was knocked down using siRNA, and then the cells were treated with human EMAP-II. As seen in Fig. 4, EMAP-II (50 ng/ml) significantly increased the expression of CSF-1 and MCP-1 after endogenous EMAP-II expression was knocked down. These results suggest that endogenous EMAP-II elevates the expression of CSF-1 and MCP-1.
Fig. 4.
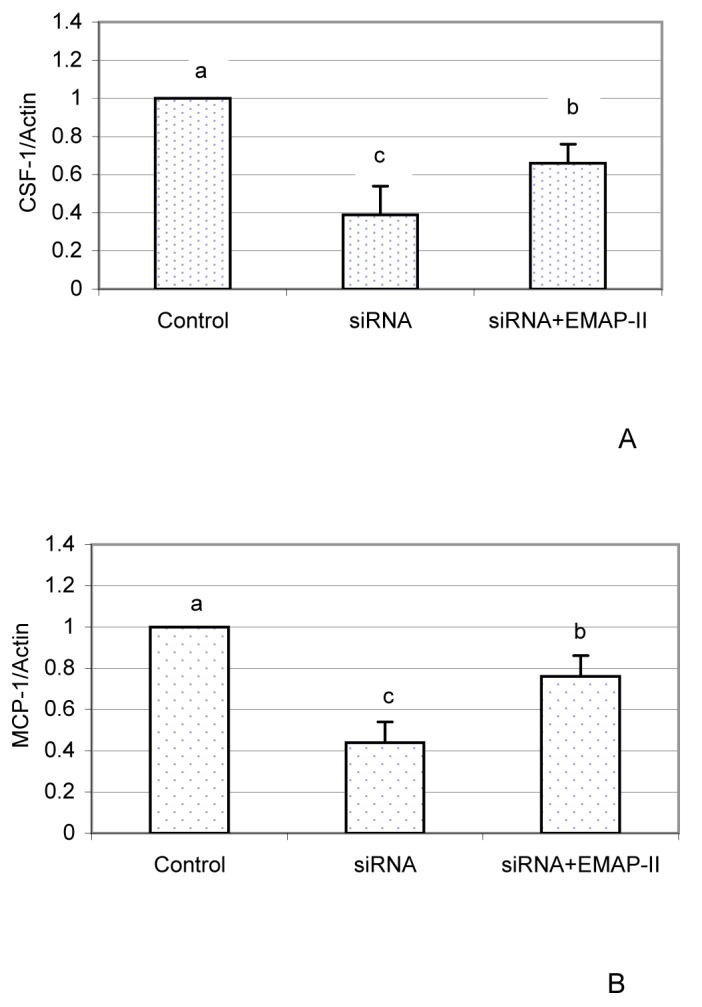
Up-regulation of CSF-1 and MCP-1 expression by EMAP-II in the DF cells after EMAP-II was knocked down by siRNA. DF cells were treated with EMAP-II (50 ng/ml) for 24 h after endogenous EMAP-II expression was knocked down. Expression of CSF-1 (A) and MCP-1 (B) were determined using real-time RT-PCR. Control: transfection without siRNA; siRNA: transfection with 2 nM siRNA; siRNA+EMAP-II: transfection with 2 nM siRNA for 24 h, and then treated with 50 ng/ml EMAP-II for 24 h. The experiments were repeated three times and the results were analyzed using SAS software. Knockdown of EMAP-II resulted in reduction of CSF-1 and MCP-1 expression, and the expression of CSF-1 and MCP-1 were increased significantly by EMAP-II treatments (P < 0.01).
Chemotactic activity of EMAP-II in DF cells
To determine if EMAP-II attracted mononuclear cells from rat bone marrow, human EMAP-II was applied in a chemotaxis assay. As shown in Fig. 5, EMAP-II at a concentration of 0.1 nM facilitated mononuclear cell migration. With an increase of EMAP-II concentrations from 0.1 to 10 nM, the migration index had a 1.8-fold increase (Fig. 5).
Fig. 5.
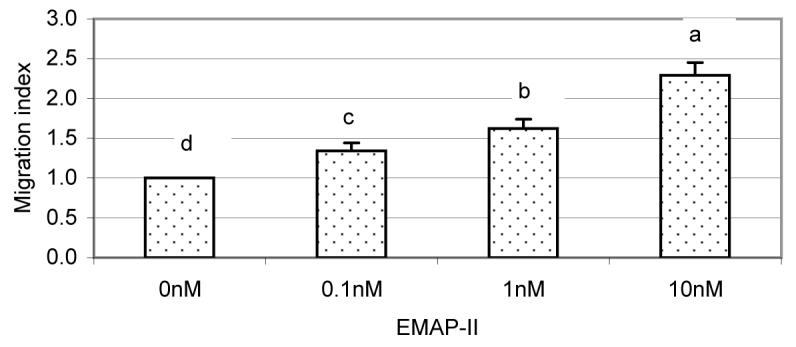
Chemotaxis assay of human EMAP-II on rat bone marrow mononuclear cells. In Transwells, the control was 600 μl MEM medium loaded in the lower chambers (0 nM) and treatments were MEM plus human EMAP-II (0.1-10 nM). In the upper chamber, 100 μl bone marrow mononuclear cells were loaded. After 2 h, the cells that migrated through the membrane into lower chambers were collected and counted. The migration index was expressed as the ratio of the cell number in lower chambers with EMAP-II to that in lower chambers without EMAP-II. The experiments were repeated four times, and the results were analyzed with SAS software. The human EMAP-II attracted a significant number of cells (P < 0.01) at all concentrations tested.
To determine if EMAP-II secreted by the DF has chemotactic activity, a chemotactic assay was performed using the conditioned medium (CM) of the DF cells in the presence of EMAP-II antibody. As seen in Fig. 6A, CM had a strong chemotactic activity with a migration index of 6.1 in the absence of antibody. Addition of 10 μg anti-human EMAP-II resulted in reduction of chemotactic activity with a migration index of 4.4, about a 30% reduction in chemotactic activity.
Fig. 6.
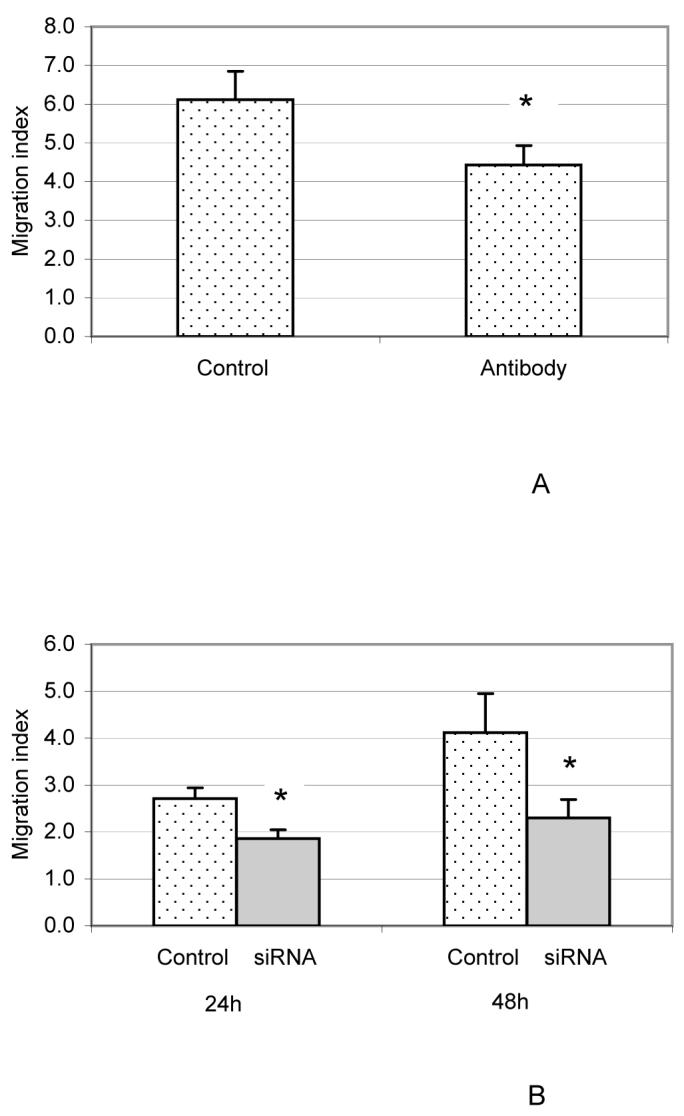
Reduction in chemotactic activity of the conditioned medium (CM) from the DF cells with EMAP-II antibody added (A) and CM from DF cells with EMAP-II knocked down (B). The chemotaxis assay was performed as in Fig. 5 except that 600 μl CM (Control) or CM plus 10 μg anti-EMAP-II antibody (antibody) or CM from DF cells with EMAP-II knocked down by 10 nM siRNA (siRNA) was loaded in the lower chambers of Transwells. The controls in 6B were CM from the DF cells transfected without siRNA. The migration index was calculated as the ratio of cells migrating into conditioned medium to those migrating into MEM medium. The experiments were repeated four times, and the results were analyzed using paired t-tests with an asterisk (*) denoting a significant difference between treatment pairs. Both anti-EMAP-II antibody (6A) and siRNA targeting EMAP-II mRNA expression (6B) reduced the chemotactic activity of the CM significantly (P < 0.01).
Similarly, CM from DF cells with EMAP-II expression knocked down by siRNA showed that knock-down of EMAP-II resulted in reduction of chemotactic activity by the CM (Fig. 6B). Using either 24 h or 48 h CM from siRNA transfection resulted in the migration index being reduced by more than 31% at 24 h as compared to controls, and by more than 43% at 48 h(Fig. 6B).
Discussion
EMAP-II is an inflammatory cytokine originally discovered in the supernatant of murine fibrosarcoma cells (13). Using DNA microarrays, our previous study showed that EMAP-II was highly expressed in DF cells (17). This study further demonstrated that EMAP-II was abundantly expressed in DF both at mRNA and protein levels, and its expression was characterized by maximal expression early (days 1 and 3) but reduced to base level at later days (days 7, 9 and 11). The timing of maximal EMAP-II expression correlates with the influx of mononuclear cells into the DF and the major burst of osteoclastogenesis seen at day 3 (1, 2). In conjunction with the reports that EMAP-II has chemotactic activity for monocytes and macrophages after injection of EMAP-II into mouse footpad (13), and that lungs from rats treated with EMAP-II showed an increase in the number of monocytes/macrophages (18), we hypothesize that EMAP-II in the DF likely acts as a chemotactic agent to attract mononuclear cells that fuse to form osteoclasts for alveolar bone resorption.
As seen from the chemotaxis assay, the CM of DF cells is chemotactic for mononuclear cells as seen by their migration through the membrane into the lower chamber of transwells. Adding EMAP-II antibody into the CM of the DF cell culture resulted in a decrease in the migration index for bone marrow mononuclear cells. This indicated that EMAP-II expressed in the DF cells was secreted into the medium, and contributed to the chemotactic activity of the CM. Our previous studies have shown that MCP-1 and CSF-1 have chemotactic activity for mononuclear cells (4), and their expression was up-regulated at day 3, the time of maximal influx of mononuclear cells (osteoclast precursors) into the DF. Thus, this study shows that in addition to CSF-1 and MCP-1, EMAP-II also likely plays a role in chemotactic attraction of mononuclear cells into the DF.
The chemotactic activity of EMAP-II was also confirmed in CM obtained from the DF cell cultures with EMAP-II expression knocked down. Using siRNA targeting EMAP-II mRNA expression in the DF cells, EMAP-II expression was knocked down by 96% after transfection. In turn, the chemotactic activity of the conditioned medium obtained at 24 h after transfection was reduced by 31%, but MCP-1 expression remained unchanged and CSF-1 had only a slight decrease as determined by real-time RT-PCR. This suggested that reduction in chemotactic activity at this time was mainly due to EMAP-II reduction. Greater reduction in chemotaxis at 48 h (43%) is likely due not only to EMAP-II knockdown, but to the significant reduction in expression of MCP-1 and CSF-1 at this time. On the other hand, knockdown of EMAP-II caused significant reduction in expression of CSF-1 and MCP-I, and addition of EMAP-II partially restored their expression (Figs. 3 and 4). This strongly suggests that endogenous EMAP-II up-regulates the expression of CSF-1 and MCP-1. Therefore, EMAP-II probably plays dual roles in recruitment of mononuclear cells into DF as follows: 1) directly, by attracting mononuclear cells into dental follicle and 2) indirectly, by up-regulating the expression of both MCP-1 and CSF-1, molecules that also attract mononuclear cells into the DF, as previously shown (4).
In addition to recruiting mononuclear cells for osteoclastogenesis, EMAP-II appears to promote acute tissue inflammation. Treatment of rats with LPS resulted in acute inflammation in the lungs and rapid induction of EMAP-II expression, which, in turn, promoted recruitment of monocytes/macrophages into the lung tissue (18). Similarly, injection of EMAP-II into the mouse footpad caused acute inflammation, along with increased numbers of monocytes/macrophages (13). Other studies have also linked chemotactic activity of EMAP-II for monocytes/macrophages to acute inflammation in injured tissues (22, 23, 24). These studies suggest that injury in spinal cord and brain caused EMAP-II expression, followed by accumulation of monocytes/macrophages into the sites of injury, resulting in acute inflammation. The use of Rapamycin to reduce neointima formation induced by vascular injury appears to be due to Rapamycin inhibiting EMAP-II expression and thus reducing the recruitment of inflammatory cells to the adventitia of the vessel (24). Thus, the inflammatory effect of EMAP-II is attributed to its chemotactic activity for recruitment of inflammatory cells.
The chemotactic activity of EMAP-II also plays a role in tissue remodeling and development. In mouse embryos, EMAP-II is most abundant at sites of tissue remodeling, where many apoptotic cells are present (15, 25). It is believed that apoptosis results in release of mature EMAP-II, and it recruits macrophages to remove the dead cells for tissue remodeling or development. In that vein, given that the DF develops into the periodontal ligament (PDL), it would be of interest to determine if apoptosis and EMAP-II regulate PDL formation.
EMAP-II also has anti-angiogenic activity, which makes EMAP-II a potential cytokine to suppress primary and metastatic tumor growth (26, 27).
The molecule(s) that contribute to the up-regulation of EMAP-II at early days postnatally in the DF is unknown. Although LPS, apoptosis and injury can induce EMAP-II expression (15, 18, 22, 23, 25), these factors are unlikely responsible for the tooth eruption process. The LPS released during periodontitis might affect EMAP-II and inflammation in the PDL, but the factors that up-regulate EMAP-II in the DF for eruption need to be determined in future studies.
Acknowledgments
This work was supported by NIH grant DE008911-16 to G.E.W.
References
- 1.Wise GE, Fan W. Changes in the tartrate-resistant acid phosphatase cell population in dental follicles and bony crypts of rat molars during tooth eruption. J Dent Res. 1989;68:150–156. doi: 10.1177/00220345890680021001. [DOI] [PubMed] [Google Scholar]
- 2.Cielinski MJ, Jolie M, Wise GE, Ando DG, Marks SC., JR . Colony-stimulating factor-1 (CSF-1) is a potent stimulator of tooth eruption in the rat. In: Davidovitch Z, editor. The biological mechanisms of tooth eruption, resorption and replacement by implants. EBSCO Media; Birmingham, AL: 1994. pp. 429–436. [Google Scholar]
- 3.Wise GE, Lin F. Regulation and localization of colony-stimulating factor-1 mRNA in cultured rat dental follicle cells. Arch Oral Biol. 1994;39:621–627. doi: 10.1016/0003-9969(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 4.Que BG, Wise GE. Colony-stimulating factor-1 and monocyte chemotactic protein-1 chemotaxis for monocytes in the rat dental follicle. Arch Oral Biol. 1997;42:855–860. doi: 10.1016/s0003-9969(97)00072-1. [DOI] [PubMed] [Google Scholar]
- 5.Wise GE, Que BG, Huang H. Synthesis and secretion of MCP-1 by dental follicle cells—implications for tooth eruption. J Dent Res. 1999;78:1667–1681. doi: 10.1177/00220345990780110301. [DOI] [PubMed] [Google Scholar]
- 6.Wise GE, Lumpkin SJ, Huang H, Zhang Q. Osteoprotegerin and osteoclast differentiation factor in tooth eruption. J Dent Res. 2000;79:1937–1942. doi: 10.1177/00220345000790120301. [DOI] [PubMed] [Google Scholar]
- 7.Wise GE, Yao S, Odgren PR, Pan F. CSF-1 regulation of osteoclastogenesis for tooth eruption. J Dent Res. 2005;84:837–841. doi: 10.1177/154405910508400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu D, Yao S, Pan F, Wise GE. Chronology and regulation of gene expression of RANKL in the rat dental follicle. Eur J Oral Sci. 2005;113:404–409. doi: 10.1111/j.1600-0722.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 9.Weber C, Schober A, Zernecke A. Chemokines: key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1997–2008. doi: 10.1161/01.ATV.0000142812.03840.6f. [DOI] [PubMed] [Google Scholar]
- 10.Ono SJ, Nakamura T, Miyazaki D, Ohbayashi M, Dawson M, Toda M. Chemokines: roles in leukocyte development, trafficking, and effector function. J Allergy Clin Immunol. 2003;111:1185–1199. doi: 10.1067/mai.2003.1594. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y, Shin J, Li R, Cheong C, Kim K, Kim S. A novel anti-tumor cytokine contains an RNA binding motif present in aminoacyl-tRNA synthetases. J Biol Chem. 2000;275:27062–27068. doi: 10.1074/jbc.C000216200. [DOI] [PubMed] [Google Scholar]
- 12.Hou Y, Plett PA, Ingram DA, Rajashekhar G, Orschell CM, Yoder MC, March KL, Clauss M. Endothelial-monocyte-activating polypeptide II induces migration of endothelial progenitor cells via the chemokine receptor CXCR3. Exp Hematol. 2006;34:1125–1132. doi: 10.1016/j.exphem.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Kao J, Ryan J, Brett G, Chen J, Shen H, Fan YG, Godman G, Familletti PC, Wang F, Pan YC, Stern D, Clauss M. Endothelial monocyte-activating polypeptide II. A novel tumor-derived polypeptide that activates host-response mechanisms. J Biol Chem. 1992;267:20239–20247. [PubMed] [Google Scholar]
- 14.Murray JC, Heng YM, Symonds P, Rice K, Ward W, Huggins M, Todd I, Robins RA. Endothelial monocyte-activating poplypeptide-II (EMAP-II): a novel inducer of lymphocyte apoptosis. J Leukoc Biol. 2004;75:772–776. doi: 10.1189/jlb.1003487. [DOI] [PubMed] [Google Scholar]
- 15.Knies UE, Behrensdorf HA, Mitchell CA, Deutsch U, Risau W, Drexler HC, Clauss M. Regulation of endothelial monocyte-activating polypeptide II release by apoptosis. Proc Natl Acad Sci USA. 1998;95:12322–12327. doi: 10.1073/pnas.95.21.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emingil G, Atilla G, Baskesen A, Berdeli A. Gingival crevicular fluid EMAP-II, MIP-1α and MIP-1 β levels of patients with periodontal disease. J Clin Periodontol. 2005;32:880–885. doi: 10.1111/j.1600-051X.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu D, Wise GE. A DNA microarray analysis of chemokine and receptor genes in the rat dental follicle—role of secreted frizzled-related protein-1 in osteoclastogenesis. Bone. 2007;41:266–272. doi: 10.1016/j.bone.2007.04.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Journeay WS, Janardhan KS, Singh B. Expression and function of endothelial monocyte-activating polypeptide-II in acute lung inflammation. Inflamm Res. 2007;56:175–181. doi: 10.1007/s00011-006-6162-3. [DOI] [PubMed] [Google Scholar]
- 19.Wise GE, Lin F, Fan W. Culture and characterization of dental follicle cells from rat molars. Cell Tissue Res. 1992;267:483–492. doi: 10.1007/BF00319370. [DOI] [PubMed] [Google Scholar]
- 20.Liu D, Yao S, Wise GE. Effect of interleukin-10 on gene expression of osteoclastogenic regulatory molecules in the rat dental follicle. Eur J Oral Sci. 2006;114:42–49. doi: 10.1111/j.1600-0722.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim DH, Behlke MA, Rose SD, Chang MS, Choi S, Rossi JJ. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat Biotechnol. 2005;23:222–226. doi: 10.1038/nbt1051. [DOI] [PubMed] [Google Scholar]
- 22.Mueller CA, Schluesener HJ, Conrad S, Meyermann R, Schwab JM. Spinal cord injury induces lesional expression of the proinflammatory and antiangiogenic cytokine EMAP-II. J Neurotrauma. 2003;20:1007–1015. doi: 10.1089/089771503770195858. [DOI] [PubMed] [Google Scholar]
- 23.Mueller CA, Schluesener HJ, Conrad S, Meyermann R, Schwab JM. Lesional expression of a proinflammatory and antiangiogenic cytokine EMAP-II confined to endothelium and microglia/macrophages during secondary damage following experimental traumatic brain injury. J Neuroimmunol. 2003;135:1–9. doi: 10.1016/s0165-5728(02)00427-7. [DOI] [PubMed] [Google Scholar]
- 24.Nührenberg TG, Langwieser N, Schwarz JB, Hou Y, Frank P, Sorge F, Matschurat S, Seidl S, Kastrati A, Schömig A, Clauss MA, Zohlnhöfer D. EMAP-II downregulation contributes to the beneficial effects of rapamycin after vascular injury. Cardiovasc Res. 2008;77:580–589. doi: 10.1093/cvr/cvm076. [DOI] [PubMed] [Google Scholar]
- 25.Knies UE, Kröger S, Clauss M. Expression of EMAP-II in the developing and adult mouse. Apoptosis. 2000;5:141–151. doi: 10.1023/a:1009632712876. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz MA, Kandel J, Brett J, Li J, Hayward J, Schwarz RE, Chappey O, Wautier JL, Chabot J, Lo Gerfo P, Stern D. Endothelial-monocyte activating polypeptide II, a novel antitumor cytokine that suppresses primary and metastatic tumor growth and induces apoptosis in growing endothelial cells. J Exp Med. 1999;190:341–354. doi: 10.1084/jem.190.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger AC, Alexander HR, Tang G, Wu PS, Hewitt SM, Turner E, Kruger E, Figg WD, Grove A, Kohn E, Stern D, Libutti SK. Endothelial monocyte activating polypeptide II induces endothelial cell apoptosis and may inhibit tumor angiogenesis. Microvasc Res. 2000;60:70–80. doi: 10.1006/mvre.2000.2249. [DOI] [PubMed] [Google Scholar]