Abstract
Nucleotidyl-transfer enzymes, which synthesize, degrade and rearrange DNA and RNA, often depend on metal ions for catalysis. All DNA and RNA polymerases, MutH-or RNase H-like nucleases and recombinases, and group I introns appear to require two divalent cations to form a complete active site. The two-metal ion mechanism has been proposed to orient substrate, facilitate acid-base catalysis, and allow catalytic specificity to exceed substrate-binding specificity owing to the stringent metal-ion (Mg2+ in particular) coordination. Not all nucleotidyl-transfer enzymes use two metal ions for catalysis, however. The ββα-Me and HUH nucleases depend on a single metal ion in the active site for the catalysis. All of these one- and two- metal ion dependent enzymes generate 5′-phosphate and 3′-OH products. We compare and contrast their structures and mechanisms and show that they share a functionally equivalent metal ion.
INTRODUCTION
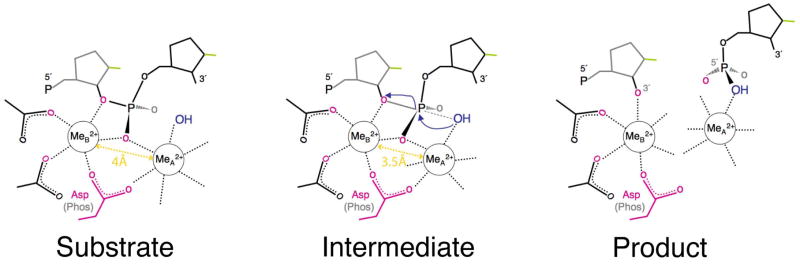
Polymerases 1–3 and nucleases 4–7 using two-metal ion catalysis share a common active site structure. In the enzyme-substrate complexes, the two metal ions are jointly coordinated by the scissile phosphate and an active site Asp (Fig. 1) 8. They are bisected by the scissile phosphate with metal ion A on the nucleophile side and metal ion B on the leaving group side. After the observation of two metal ions in the active site of alkaline phosphatase and 3′-5′ exonuclease 9, 10, Steitz and Steitz proposed that metal ions can substitute protein side chains in catalytic RNA and function as general base and acid 11. It was proposed that metal ion A deprotonates the nucleophilic water and metal ion B stabilizes the pentacovalent phosphate intermediate 4, 11. Recent studies of polymerases and nucleases indicate that metal ion B may destabilize the substrate and promote nucleophilic attack 6, 8. In addition the two metal ions are intimately linked, and the distance between the metal ions is likely shortened from ~4 Å in a substrate complex to ~3.5 Å in the transition state 6, 12. The closely positioned metal ions can effectively neutralize the highly negatively charged pentacovalent phosphate. Both the number and location of metal ions in the active site are sensitive to the environment and can deviate from the norm in the presence of non-cognate substrates, altered active site residues, or metal ion substitution 5, 12–16. This sensitivity to the coordination environment may be the basis for the exceedingly high catalytic specificity of polymerases, restriction endonucleases, and other nucleic acid enzymes that utilize the two-metal ion mechanism8.
Figure 1. A diagram of two-metal ion catalysis by RNase H.
Coordination of metal ions A and B is indicated by the dashed lines. The Asp conserved in all nucleotidyl-transfer enzymes using two-metal ions is highlighted in pink. This Asp is replaced by a backbone phosphate in group I introns. The oxygen atoms from the protein and scissile phosphate that chelate metal ion B are highlighted in pink, and the attacking nucleophile is highlighted in blue.
Many nucleases and recombinases, however, don’t use the two-metal ion mechanism. A large fraction of those that generate 5′-phosphate and 3′-OH products use one metal ion for catalysis according to structural and biochemical analyses. To understand the different metal ion requirements, enzymes known to use one-metal ion catalysis are categorized and compared with the two-metal ion dependent enzymes. Surprisingly, these two seemingly different mechanisms share a conserved metal-ion binding site. The comparative studies and functional implications are summarized here.
RESULTS AND DISCUSSION
Nucleases containing one-metal ion in the active site
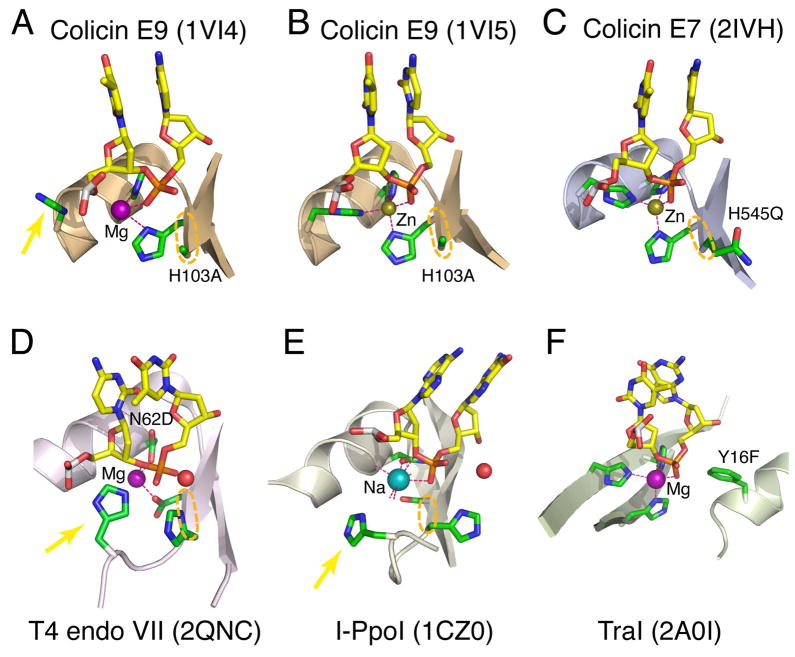
A single metal ion has been observed in the active site of endonucleases with the ββα-Me motif 17, 18, which is characterized by the consecutive β, β, α secondary structure elements arranged in a V shape (Fig. 2A–E). The β strands form one arm of the “V”, and the helix the other. A single metal ion located at the opening of the “V” is coordinated by active site residues and the scissile phosphate including the leaving group (3′-O) (Fig. 2A–E) 19–21. Many of the ββα-Me endonucleases like Caspase-Activated DNase (CAD), Serratia, Vvn and E-group colicins hydrolyze nucleic acids with no sequence or ribose (DNA versus RNA) specificity 19, 22, 23. Other superfamily members like HNH 24 and His-Cys homing endonucleases 20 are highly sequence specific, but in these cases protein domains besides the ββα-Me motif mediate base-specific interactions.
Figure 2. Examples of one-metal ion-dependent nucleases.
(A–E) The ββα-Me family. (F) The flipped ββα-Me family. The active site residues are shown in green(C)/blue(N)/red(O) and the DNA substrate centered on the scissile phosphate in yellow(C)/blue/red/orange(P) stick models. The Na+, Mg2+ and Zn2+ in the active site are shown as color-coded spheres. Coordination of metal ion is represented by pink dashed lines. The mobile metal-ion ligand is indicated by a yellow arrow. When determined, the nucleophile water is shown as a red sphere. The adjoining two active site residues in the ββα-Me nucleases are highlighted by the orange ovals. Mutations of the active site residues, which enable crystallization of enzyme-substrate complexes are labeled.
A conserved single metal-ion binding site is also found in HUH endonucleases required for Y1-type DNA transposition 25, 26 and for initiation of rolling-circle DNA replication 27, a mechanism used in bacterial conjugation 28–30 and propagation of ssDNA viruses (Adeno-associated virus (AAV), parvovirus, etc) 31–33. The name of HUH comes from the sequence motif of two conserved His’s (H) separated by a hydrophobic residue (U) 27. These nucleases catalyze a two-step reaction. In the first step a conserved tyrosine serves as the nucleophile to cleave DNA and generate a tyrosyl-5′-phosphate and 3′-OH. After DNA rearrangement or replication, in the second step the phospho-tyrosyl bond is cleaved by a 3′-OH, so the DNA is re-ligated and the active site Tyr regenerated 26, 27, 34. The first step has been structurally well studied and shown to be very similar to nucleic acid hydrolysis, while the configuration of the second step of the reaction is less known. The active site of these enzymes consists of β-strands and an α-helix, but they are not contiguous in the amino-acid sequence 26, 31, 35, 36 (Fig. 2F). The two conserved His’s in the HUH motif are involved in metal ion binding as proposed27. Interestingly, the metal ions, scissile phosphates and nucleophiles of the HUH and ββα-Me enzymes can be superimposed, although the locations of β-strands and α-helix are approximately switched (Fig. 2).
An equivalent metal ion in RNase H and TraI
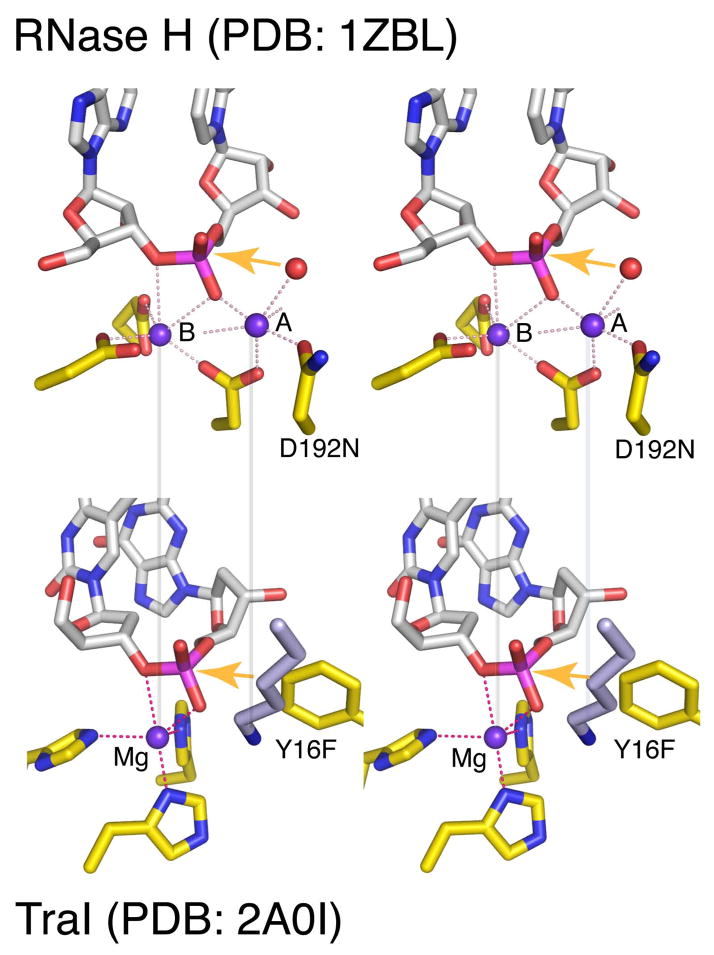
RNase H, for which several crystal structures along the catalytic pathway are available 6, 37, provides an example of two-metal ion catalysis (Fig. 1). The HUH nuclease TraI, crystallized with a ssDNA substrate, Mg2+ ion and the nucleophile Tyr mutated to Phe 35, reveals the most complete active site that utilizes the one-metal ion mechanism (Fig. 2F). If the Tyr were present, its hydroxyl group would be 2.6 Å from the scissile phosphorus and perfectly inline for nucleophilic attack. Similar arrangement of the metal ion and catalytic residues are observed for other HUH members 26, 33, 34. When the active sites of TraI and RNase H are superimposed, metal ion A in the RNase H-substrate complex coincides with the amine group of a highly conserved Lys in TraI (Fig. 3). This Lys, however, is not strictly required for DNA cleavage by TraI 38, nor is it conserved among enzymes utilizing one-metal ion catalysis. Nevertheless and perhaps most astonishingly, metal ion B of RNase H coincides with the single Mg2+ of TraI (Fig. 3).
Figure 3. Comparison of the active site of RNase H and TraI in stereoview.
The nucleic acid is shown in light grey, and the protein in yellow with the N (blue), O (red), P (meganta) highlighted. The Mg2+ ions are shown as purple spheres, and their coordination by macromolecules is indicated by dashed pink lines. The active site mutations (D192N and Y16F) that prevent the chemistry are labeled. Alignments of the metal ions (or Lys) between the two enzymes are shown by the semi-transparent grey lines. The orange arrows represent the nucleophilic attack.
Not only are the Mg2+ ions of TraI and RNase H (B) coincident relative to the scissile phosphate, they also share similar coordination geometry. Both are devoid of inner-sphere water ligands, and both are coordinated by three active site residues plus two oxygen atoms from the scissile phosphate (Fig. 3). The deviation from the normal octahedral Mg2+ coordination geometry and the absence of water ligands 39 indicate that the metal ion and its ligands are likely strained. This conserved metal-ion binding site in RNase H and TraI, which share no structural or functional similarity and use different amino acids (Asp and Glu vs His) for metal ion coordination, suggests an essential and convergent role of the metal ion in degradation of nucleic acid. It is worth noting that metal ion B in polymerases, which catalyze nucleic acid synthesis instead of degradation, has six ligands but is often devoid of water ligand 3. As proposed for metal ion B in the two-metal ion mechanism 8, the single metal ion in TraI may also destabilize the substrate and weaken the scissile bond for breakage.
Similarity between the ββα-Me and HUH superfamilies
Coordination of the metal ion in the ββα-Me nucleases colicin E7 and E9 resembles that of TraI (Fig. 2). To crystallize colicin E7 and E9 with DNA substrate, the general base His, which activates the nucleophilic water molecule, was mutated to Ala, Glu or Gln (Fig. 2A–C) 40–42. In the presence of Zn2+, the single metal ion in the active site of colicin E7 and E9 is coordinated by three His’s and the scissile phosphate as the Mg2+ in TraI (Fig. 2B–C). The distance between the Zn2+ and the 3′-O leaving group varies from 2.8 to 3.2 Å, which leaves the Zn 2+ with only 4 ligands in a more or less tetrahedral geometry. When Zn2+ in colicin E9 is substituted by Mg2+, which supports the catalysis, one His coordinating the metal ion moves away, and so does the 3′-O leaving group (Fig. 2A) 40. The active site mutation and consequent changes of the scissile-phosphate orientation and coordination environment may prevent Zn2+ and Mg2+ from adopting the strained five-ligand coordination and engaging the 3′ leaving group.
A similar alignment of the metal ion, scissile phosphate and nucleophile is also observed in the substrate complexes of other ββα-Me enzymes, I-PpoI, I-HmuI, Vvn and T4 endo VII 19–21 (Fig. 2D–E), although a mixture of His, Asn, Asp, Glu, Ser, etc instead of three His’s are used as the metal ion ligands. One of the three protein residues participating in the metal ion coordination is difficult to identify due to the lack of conservation in amino acid type and location in the tertiary structure 19, 20, 24 (Fig. 2A–E). This elusive metal-ion ligand is also mobile like the flexible His in the colicin E’s and may be located > 4Å from the metal ion in crystal structures due to non-functional metal ion substitution (Fig. 2E) 43 or active site mutations (Fig. 2A, D) 21, 40, 44, 45. However, there is a notably conserved feature among ββα-Me nucleases. Two catalytically essential residues are adjacent in the sequence and structure (at the end of the first β strand): the invariable His, which serves as the general base, and its N-terminal neighbor, which coordinates the metal ion (circled in Fig. 2A–E). The metal ion ligand varies from His in colicins, Glu in Vvn, Ser in I-PpoI, to Asp in T4 endo VII and I-HmuI (Fig. 2A–E), but the peptide bond between the general base activating the nucleophile and the metal-ion ligand that orients the scissile phosphate is conserved and likely essential for the catalysis. This is reminiscent of the coupling of nucleophile and scissile phosphate by the A and B metal ions jointly coordinated by the conserved Asp in the two-metal ion mechanism (Fig. 1, 2A–E). The coupling may be such that the nucleophile doesn’t form unless the scissile bond is aligned and strained.
Other known HUH enzymes share a similar active site configuration with TraI, although the third metal ion ligand from protein varies in residue type (His, Gln or Glu) and location 26, 28, 31, 33, 34. Coupling between the attacking nucleophile and leaving group is not evident in the HUH family. This may be due to the two-step reaction that HUH enzymes catalyze, during which the nucleophile Tyr in the first step would become the leaving group in the second step 26, 27. In addition, DNA exchange or replication primed by the cleavage product 3′-OH takes place in between the two steps 26, 27, 34. It is not clear whether the substrate is reoriented so that the phospho-tyrosyl bond replaces the phosphodiester bond of the first cleavage step or the phospho-tyrosine remains more or less in the same place, but the leaving group 3′-OH in the first step becomes the attacking nucleophile. Either way, some rearrangement in the active site is needed 37.
Differences between one- and two-metal ion catalysis
The hallmark of two-metal ion dependent nucleic acid enzymes is an absolutely conserved Asp (Fig. 1), which coordinates both metal ions and is substituted by a backbone phosphate in ribozymes 8. Among the one-metal ion dependent enzymes, there is no conserved Asp. The ββα-Me superfamily is marked by a conserved general base His, and the HUH enzymes by two histidines required for metal ion coordination (Fig. 2). Another major difference is the degree of difficulty in capturing catalytically essential metal ions in crystal structures. The two metal ions are elusive even in enzyme-substrate complexes 8, but one-metal ion dependent enzymes can bind the metal ion in the absence of substrate 19, 20, 29, 31, 46.
In general, enzymes using one-metal ion catalysis are less stringent in metal ion selection and substrate specificity than two-metal ion-dependent enzymes. Mg2+, Ca2+, Mn2+, Zn2+, Cu2+, Cd2+, Co2+ and Ni2+ can support one-metal ion catalysis to varying degrees in many enzymes 19, 20, 38, while Mg2+ is typically required for two-metal ion catalysis 8. The preference for Mg2+ may result from the chemical environment and the stringency imposed by coordination of two metal ions within 3–4 Å. The stringent requirement for two-Mg2+ coordination is likely the basis for catalytic specificity by the two-metal ion mechanism 8. In contrast, catalytic specificity of one-metal ion dependent homing and HUH nucleases appears to derive from substrate binding 20, 21, 26, 28, 35.
Catalytic role of the metal ion
We propose that the ββα-Me and HUH nucleotidyl-transfer enzymes use a common one-metal ion mechanism for catalysis. The single metal ion appears to be spatially and functionally equivalent to metal ion B of two-metal ion catalysis (Fig. 3). The metal ion probably plays a catalytic role in phosphoryl transfer reactions. Firstly, the high charge density of divalent cations obviously can stabilize the electron-rich pentacovalent phosphate intermediate more efficiently than protein sidechains. Secondly, the conserved metal ion is likely coordinated by three protein ligands and two oxygen atoms of the scissile phosphate as observed for TraI and RNase H (Fig. 3). Such coordination deviates from the usual octahedral or tetrahedral geometry preferred by divalent cations 47 and leads to a strained metal ion.. By engaging two oxygen atoms from the scissile phosphate as ligands, which necessarily results in an unfavorably coordination angle (O-Me-O) smaller than 90° (Fig. 3), the metal ion can directly destabilize the scissile bond and facilitate the nucleophilic attack. Finally, the conserved metal ion in RNase H 37 and TraI 35 as well as metal ion B in many DNA and RNA polymerases 3, 8 is also devoid of water ligand and is thus at a high-energy state 39, 48. The metal ion may accelerate product release and turnover rate by regaining water ligands.
The catalytic role of the conserved metal ion in nucleotidyl transfer reactions rationalizes why ligands of metal ion B in two-metal ion catalysis are less mutable than those of metal ion A 37, 49. The absence of a second metal ion in the one-metal ion mechanism may be compensated by the presence of a good general base in the ββα-Me family (His) or a better nucleophile in the HUH family (Tyr), which is easier to deprotonate than a water molecule.
METHODS
Structural superimposition of the actives site between ββα-Me and HUH enzymes and between TraI and RNase H were carried out manually using ONO 50. These enzymes share no sequence or structural conservation. But they all generate 5′ phosphate and 3′-OH. In theory the orientation of nucleophile and scissile phosphate relative to the active site should be conserved, and indeed they were superimposable. Moreover, the metal ions in ββα-Me and HUH were found to have similar locations and thus were included for superposition. Superposition of RNase H and TraI was also achieved by optimizing the alignment of scissile phosphates, nucleophiles and metal ions (B in RNase H).
Structure rendering and distance measurement were made using PyMol (www.pymol.com).
Acknowledgments
I thank Drs. C. Biertümpfel, C. Larkin, M. Nowotny for stimulating discussion, Drs. B. Craigie, F. Dyda, D. Leahy and H. Yuan for critical reading of the manuscript. This research was supported by the Intramural Research Program of the NIDDK, NIH.
References
- 1.Pelletier H, Sawaya MR, Kumar A, Wilson SH, Kraut J. Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science. 1994;264:1891–903. [PubMed] [Google Scholar]
- 2.Doublie S, Tabor S, Long AM, Richardson CC, Ellenberger T. Crystal structure of a bacteriophage T7 DNA replication complex at 2.2 A resolution. Nature. 1998;391:251–8. doi: 10.1038/34593. [DOI] [PubMed] [Google Scholar]
- 3.Steitz TA. A mechanism for all polymerases. Nature. 1998;391:231–2. doi: 10.1038/34542. [DOI] [PubMed] [Google Scholar]
- 4.Beese LS, Steitz TA. Structural basis for the 3′-5′ exonuclease activity of Escherichia coli DNA polymerase I: a two metal ion mechanism. Embo J. 1991;10:25–33. doi: 10.1002/j.1460-2075.1991.tb07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JY, et al. MutH complexed with Hemi- and unmethylated DNAs: coupling base recognition and DNA cleavage. Mol Cell. 2005;20:155–66. doi: 10.1016/j.molcel.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Nowotny M, Yang W. Stepwise analyses of metal ions in RNase H catalysis from substrate destabilization to product release. Embo J. 2006;25:1924–33. doi: 10.1038/sj.emboj.7601076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stahley MR, Strobel SA. RNA splicing: group I intron crystal structures reveal the basis of splice site selection and metal ion catalysis. Curr Opin Struct Biol. 2006;16:319–26. doi: 10.1016/j.sbi.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Yang W, Lee JY, Nowotny M. Making and breaking nucleic acids: two-Mg2+-ion catalysis and substrate specificity. Mol Cell. 2006;22:5–13. doi: 10.1016/j.molcel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Sowadski JM, Handschumacher MD, Murthy HM, Foster BA, Wyckoff HW. Refined structure of alkaline phosphatase from Escherichia coli at 2.8 A resolution. J Mol Biol. 1985;186:417–33. doi: 10.1016/0022-2836(85)90115-9. [DOI] [PubMed] [Google Scholar]
- 10.Freemont PS, Friedman JM, Beese LS, Sanderson MR, Steitz TA. Cocrystal structure of an editing complex of Klenow fragment with DNA. Proc Natl Acad Sci U S A. 1988;85:8924–8. doi: 10.1073/pnas.85.23.8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steitz TA, Steitz JA. A general two-metal-ion mechanism for catalytic RNA. Proc Natl Acad Sci U S A. 1993;90:6498–502. doi: 10.1073/pnas.90.14.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batra VK, et al. Magnesium-induced assembly of a complete DNA polymerase catalytic complex. Structure. 2006;14:757–66. doi: 10.1016/j.str.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brautigam CA, Sun S, Piccirilli JA, Steitz TA. Structures of normal single-stranded DNA and deoxyribo-3′-S-phosphorothiolates bound to the 3′-5′ exonucleolytic active site of DNA polymerase I from Escherichia coli. Biochemistry. 1999;38:696–704. doi: 10.1021/bi981537g. [DOI] [PubMed] [Google Scholar]
- 14.Viadiu H, Aggarwal AK. Structure of BamHI bound to nonspecific DNA: a model for DNA sliding. Mol Cell. 2000;5:889–95. doi: 10.1016/s1097-2765(00)80329-9. [DOI] [PubMed] [Google Scholar]
- 15.Horton NC, Perona JJ. DNA cleavage by EcoRV endonuclease: two metal ions in three metal ion binding sites. Biochemistry. 2004;43:6841–57. doi: 10.1021/bi0499056. [DOI] [PubMed] [Google Scholar]
- 16.Nowotny M, et al. Structure of human RNase H1 complexed with an RNA/DNA hybrid: insight into HIV reverse transcription. Mol Cell. 2007;28:264–76. doi: 10.1016/j.molcel.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Friedhoff P, et al. A similar active site for non-specific and specific endonucleases. Nat Struct Biol. 1999;6:112–3. doi: 10.1038/5796. [DOI] [PubMed] [Google Scholar]
- 18.Kuhlmann UC, Moore GR, James R, Kleanthous C, Hemmings AM. Structural parsimony in endonuclease active sites: should the number of homing endonuclease families be redefined? FEBS Lett. 1999;463:1–2. doi: 10.1016/s0014-5793(99)01499-4. [DOI] [PubMed] [Google Scholar]
- 19.Hsia KC, Li CL, Yuan HS. Structural and functional insight into sugar-nonspecific nucleases in host defense. Curr Opin Struct Biol. 2005;15:126–34. doi: 10.1016/j.sbi.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Stoddard BL. Homing endonuclease structure and function. Q Rev Biophys. 2005;38:49–95. doi: 10.1017/S0033583505004063. [DOI] [PubMed] [Google Scholar]
- 21.Biertümpfel C, Yang W, Suck D. Crystal structure of T4 endonuclease VII resolving a Holliday junction. Nature. 2007;449:616–20. doi: 10.1038/nature06152. [DOI] [PubMed] [Google Scholar]
- 22.Meiss G, Gimadutdinow O, Friedhoff P, Pingoud AM. Microtiter-plate assay and related assays for nonspecific endonucleases. Methods Mol Biol. 2001;160:37–48. doi: 10.1385/1-59259-233-3:037. [DOI] [PubMed] [Google Scholar]
- 23.Woo EJ, et al. Structural mechanism for inactivation and activation of CAD/DFF40 in the apoptotic pathway. Mol Cell. 2004;14:531–9. doi: 10.1016/s1097-2765(04)00258-8. [DOI] [PubMed] [Google Scholar]
- 24.Mehta P, Katta K, Krishnaswamy S. HNH family subclassification leads to identification of commonality in the His-Me endonuclease superfamily. Protein Sci. 2004;13:295–300. doi: 10.1110/ps.03115604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ton-Hoang B, et al. Transposition of ISHp608, member of an unusual family of bacterial insertion sequences. Embo J. 2005;24:3325–38. doi: 10.1038/sj.emboj.7600787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barabas O, et al. Mechanism of IS200/IS605 family DNA transposases: activation and transposon-directed target site selection. Cell. 2008;132:208–20. doi: 10.1016/j.cell.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koonin EV, Ilyina TV. Computer-assisted dissection of rolling circle DNA replication. Biosystems. 1993;30:241–68. doi: 10.1016/0303-2647(93)90074-m. [DOI] [PubMed] [Google Scholar]
- 28.Guasch A, et al. Recognition and processing of the origin of transfer DNA by conjugative relaxase TrwC. Nat Struct Biol. 2003;10:1002–10. doi: 10.1038/nsb1017. [DOI] [PubMed] [Google Scholar]
- 29.Datta S, Larkin C, Schildbach JF. Structural insights into single-stranded DNA binding and cleavage by F factor TraI. Structure. 2003;11:1369–79. doi: 10.1016/j.str.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Monzingo AF, Ozburn A, Xia S, Meyer RJ, Robertus JD. The structure of the minimal relaxase domain of MobA at 2.1 A resolution. J Mol Biol. 2007;366:165–78. doi: 10.1016/j.jmb.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hickman AB, Ronning DR, Kotin RM, Dyda F. Structural unity among viral origin binding proteins: crystal structure of the nuclease domain of adeno-associated virus Rep. Mol Cell. 2002;10:327–37. doi: 10.1016/s1097-2765(02)00592-0. [DOI] [PubMed] [Google Scholar]
- 32.Campos-Olivas R, Louis JM, Clerot D, Gronenborn B, Gronenborn AM. The structure of a replication initiator unites diverse aspects of nucleic acid metabolism. Proc Natl Acad Sci U S A. 2002;99:10310–5. doi: 10.1073/pnas.152342699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dyda F, Hickman AB. A mob of reps. Structure. 2003;11:1310–1311. doi: 10.1016/j.str.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Gomis-Ruth FX, Coll M. Cut and move: protein machinery for DNA processing in bacterial conjugation. Curr Opin Struct Biol. 2006;16:744–52. doi: 10.1016/j.sbi.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Larkin C, et al. Inter- and intramolecular determinants of the specificity of single-stranded DNA binding and cleavage by the F factor relaxase. Structure. 2005;13:1533–44. doi: 10.1016/j.str.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Boer R, et al. Unveiling the molecular mechanism of a conjugative relaxase: The structure of TrwC complexed with a 27-mer DNA comprising the recognition hairpin and the cleavage site. J Mol Biol. 2006;358:857–69. doi: 10.1016/j.jmb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Nowotny M, Gaidamakov SA, Crouch RJ, Yang W. Crystal structures of RNase H bound to an RNA/DNA hybrid: substrate specificity and metal-dependent catalysis. Cell. 2005;121:1005–16. doi: 10.1016/j.cell.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 38.Larkin C, Haft RJ, Harley MJ, Traxler B, Schildbach JF. Roles of active site residues and the HUH motif of the F plasmid TraI relaxase. J Biol Chem. 2007;282:33707–13. doi: 10.1074/jbc.M703210200. [DOI] [PubMed] [Google Scholar]
- 39.Maguire ME, Cowan JA. Magnesium chemistry and biochemistry. Biometals. 2002;15:203–10. doi: 10.1023/a:1016058229972. [DOI] [PubMed] [Google Scholar]
- 40.Mate MJ, Kleanthous C. Structure-based analysis of the metal-dependent mechanism of H-N-H endonucleases. J Biol Chem. 2004;279:34763–9. doi: 10.1074/jbc.M403719200. [DOI] [PubMed] [Google Scholar]
- 41.Hsia KC, et al. DNA binding and degradation by the HNH protein ColE7. Structure. 2004;12:205–14. doi: 10.1016/j.str.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Doudeva LG, et al. Crystal structural analysis and metal-dependent stability and activity studies of the ColE7 endonuclease domain in complex with DNA/Zn2+ or inhibitor/Ni2+ Protein Sci. 2006;15:269–80. doi: 10.1110/ps.051903406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galburt ECH, Tang W, Jurica MS, Flick KE, Monnat RJ, Stoddard BL. A novel endonuclease mechanism directly visualized for I-PpoI. Nat Struct Biol. 1999;6:1096–9. doi: 10.1038/70027. [DOI] [PubMed] [Google Scholar]
- 44.Li CL, et al. DNA binding and cleavage by the periplasmic nuclease Vvn: a novel structure with a known active site. Embo J. 2003;22:4014–25. doi: 10.1093/emboj/cdg377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen BWL, Markus Shub, David A, Stoddard Barry L. DNA binding and cleavage by the HNH homing endonuclease I-HmuI. J Mol Biol. 2004;342:43–56. doi: 10.1016/j.jmb.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 46.Raaijmakers H, et al. X-ray structure of T4 endonuclease VII: a DNA junction resolvase with a novel fold and unusual domain-swapped dimer architecture. EMBO J. 1999;18:1447–58. doi: 10.1093/emboj/18.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harding MM. The geometry of metal-ligand interactions relevant to proteins. Acta Crystallogr D Biol Crystallogr. 1999;55(Pt 8):1432–43. doi: 10.1107/s0907444999007374. [DOI] [PubMed] [Google Scholar]
- 48.Harding MM. The architecture of metal coordination groups in proteins. Acta Crystallogr D Biol Crystallogr. 2004;60:849–59. doi: 10.1107/S0907444904004081. [DOI] [PubMed] [Google Scholar]
- 49.Derbyshire V, Grindley ND, Joyce CM. The 3′-5′ exonuclease of DNA polymerase I of Escherichia coli: contribution of each amino acid at the active site to the reaction. Embo J. 1991;10:17–24. doi: 10.1002/j.1460-2075.1991.tb07916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones TA, Zou J-Y, Cowan SW. Improved methods for building models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]