Abstract
Enzymes that share the (β/α)8-barrel fold catalyze a diverse range of reactions. Many utilize phosphorylated substrates and share a conserved C-terminal (β/α)2-quarter barrel subdomain that provides a binding motif for the dianionic phosphate group. We recently reported functional and structural studies of D-ribulose 5-phosphate 3-epimerase (RPE) from Streptococcus pyogenes that catalyzes the equilibration of the pentulose 5-phosphates D-ribulose 5-phosphate and D-xylulose 5-phosphate in the pentose phosphate pathway [J. Akana, A. A. Fedorov, E. Fedorov, W. R. P. Novack, P. C. Babbitt, S. C. Almo, and J. A. Gerlt (2006) Biochemistry 45, 2493–2503]. We now report functional and structural studies of D-allulose 6-phosphate 3-epimerase (ALSE) from Escherichia coli K-12 that catalyzes the equilibration of the hexulose 6-phosphates D-allulose 6-phosphate and D-fructose 6-phosphate in a catabolic pathway for D-allose. ALSE and RPE prefer their physiological substrates but are promiscuous for each other’s substrate. The active sites (RPE complexed with D-xylitol 5-phosphate and ALSE complexed with D-glucitol 6-phosphate) are superimposable (as expected from their 39% sequence identity), with the exception of the phosphate binding motif. The loop following the eighth β-strand in ALSE is one residue longer than the homologous loop in RPE, so the binding site for the hexulose 6-phosphate substrate/product in ALSE is elongated relative to that for the pentulose 5-phosphate substrate/product in RPE. We constructed three single-residue deletion mutants of the loop in ALSE, ΔT196, ΔS197 and ΔG198, to investigate the structural bases for the differing substrate specificities; for each, the promiscuity is altered so that D-ribulose 5-phosphate is the preferred substrate. The changes in kcat/Km are dominated by changes in kcat, suggesting that substrate discrimination results from differential transition state stabilization. In both ALSE and RPE, the phosphate group hydrogen bonds not only with the conserved motif but also with an active site loop following the sixth β-strand, providing a potential structural mechanism for coupling substrate binding with catalysis.
The (β/α)8-barrel (or TIM1-barrel) fold is the most common fold in the PDB database (1, 2). The most recent SCOP database (http://scop.mrc-lmb.cam.ac.uk/scop/; Release 1.73, November 2007) lists 33 “superfamilies” that contain a domain with the (β/α)8-barrel fold. Most are enzymes that catalyze a diverse range of reactions. Seven of the “superfamilies” contain structurally conserved motifs for binding the phosphate group of a phosphorylated substrate; almost always, this motif is located at the ends of the seventh and eight β-strands of the domain.
Sterner and Wilmanns and coworkers first provided evidence that the (β/α)8-barrel fold evolved from smaller subdomains (3). Based on both sequence and structures, they noted that two enzymes in the histidine biosynthetic pathway, phosphoribosylformimino-5-aminoimidazole carboxamide ribonucleotide isomerase (HisA) and imidazole glycerolphosphate synthase (HisF), are formed by two tandem copies of a (β/α)4-half barrel that form the intact (β/α)8-barrel. Sterner and coworkers later demonstrated that the N- and C-terminal (β/α)4-half barrels of HisF from Thermotoga maritima could be separately expressed as stable, folded proteins (4). The substrates for both HisA and HisF contain two phosphate groups, with both (β/α)4-half barrels containing a conserved phosphate binding motif (at the ends of the third and fourth and, also, the seventh and eighth β-strands in the complete (β/α)8-barrel) (5).
The recognition of a conserved phosphate binding motif allows the suggestion that (β/α)8-barrels may be assembled by the modular construction from not only (β/α)4-half barrels but also (β/α)2-quarter barrels, thereby allowing the widespread occurrence of the motif in intact β/α)8-barrels domains to be rationalized (3, 4, 6–8).2 In indirect support of this possibility, Drennan and coworkers analyzed the sequences and structures of S-adenosyl-L-methionine-dependent radical enzymes and predicted the occurrence of enzymes containing (β/α)4-half barrels, (β/α)6-three quarter barrels, and the full (β/α)8-barrels, with the number of (β/α)2-quarter barrel modules inversely related to the size of the substrate (9).
The seven “superfamilies” in SCOP that contain the conserved phosphate binding motif are designated the 1) triose phosphate isomerase (TIM), 2) ribulose-phosphate binding barrel, 3) thiamin phosphate synthase, 4) pyridoxine 5’-phosphate synthase, 5) FMN-linked oxidoreductase, 6) inosine monophosphate dehydrogenase, and 7) PLP-binding barrel “superfamilies.” The phosphate-binding motif is located in the C-terminal (β/α)2-quarter barrel and is formed from the backbone amides in glycine-rich loops following the seventh and eighth β-strands and the side chain of at least one residue in the loop following the eighth β-strand.
We are interested in understanding structure-function relationships in several members of the functionally diverse “ribulose-phosphate binding barrel” superfamily (10). The “superfamily” includes HisA and HisF in the histidine biosynthetic pathway, phosphoribosylanthranilate isomerase (TrpF), indole-3-glycerophosphate synthase (TrpC), and the α-subunit of tryptophan synthase in the tryptophan biosynthetic pathway, orotidine 5’-monophosphate decarboxylase (OMPDC) and 3-keto-L-gulonate 6-phosphate decarboxylase (KGPDC) in the OMPDC suprafamily, a putative N-acetylmannosamine 6-phosphate 2-epimerase, and D-ribulose 5-phosphate 3-epimerase (RPE). The diversity of these reactions and the divergent sequences of the members suggest that these are not derived from a common (β/α)8-barrels progenitor by the mutational events associated with divergent evolution. Indeed, we concluded that although OMPDC and KGPDC are derived from a common progenitor, they share sequence and structural homology with only the C-terminal (β/α)2-quarter barrel in RPE that forms the conserved phosphate binding motif (10), consistent with an ancient evolutionary history that involved assembly of these proteins from fractional barrels.
The C-terminal (β/α)2-quarter barrel phosphate binding motif provides the structural elements for recognition of the dianionic phosphate group of the substrate. However, the intrinsic binding energy of the phosphate group is used not only to “grip” the substrate but also for catalysis. In the case of TIM, Amyes and Richard compared the rates of isomerization of D-glyceraldehyde 3-phosphate and D-glyceraldehyde and concluded that 84% of the 4 × 1010 enzymatic rate acceleration with the phosphorylated substrate is provided by the intrinsic binding energy of the phosphate group, although it is structurally remote from the general basic Glu 165 and the electrophilic His 95 (11). In the case of OMPDC, a member of the ribulose-phosphate binding barrel “superfamily,” Amyes and Richard reported that phosphite anion accelerates the decarboxylation of a 5’-truncated analogue of OMP that lacks the 5’-phosphomonoester group by a factor of 80,000-fold (12). Although this factor is a small fraction of the 1017 rate acceleration observed with the OMP substrate, the conclusion is that intrinsic binding energy of the remote phosphate group is important for catalysis.
In both TIM (13) and OMPDC (14), the phosphate group of the substrate not only occupies the conserved phosphate binding motif in the C-terminal (β/α)2-quarter barrel but also forms hydrogen bonding interactions with backbone amides and/or side chains in an active site loop, resulting in closure of the loop, exclusion of solvent from the active site, and reorganization of active site functional groups that are directly involved in catalysis. These interactions likely provide the structural mechanism for utilization of the intrinsic binding energy of the phosphate ester group in catalysis (15).
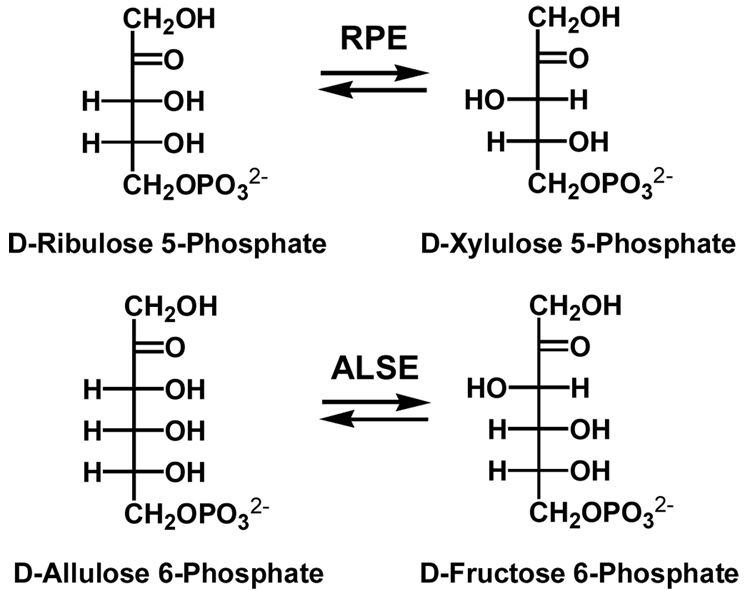
We are interested in the role of the phosphate binding motif in ketose phosphate 3-epimerases that are members of the ribulose-phosphate binding barrel “superfamily.” RPE catalyzes the 1,1-proton transfer reaction that equilibrates the pentulose 5-phosphates D-ribulose 5-phosphate and D-xylulose 5-phosphate in the pentose phosphate pathway (Scheme I).
Scheme I.
Orthologous homologues of RPE catalyze the D-allulose 6-phosphate 3-epimerase reaction (ALSE), a 1,1-proton transfer reaction that equilibrates the hexulose 6-phosphates D-allulose (D-psicose) 6-phosphate and D-fructose 6-phosphate (Scheme I) in a catabolic pathway for D-allose that is encoded by the genomes of several strains of Escherichia coli (Scheme II) (16). The D-ribulose 5-phosphate substrate for RPE and the D-allulose 6-phosphate substrate for ALSE differ by a hydroxymethylene group.
Scheme II.
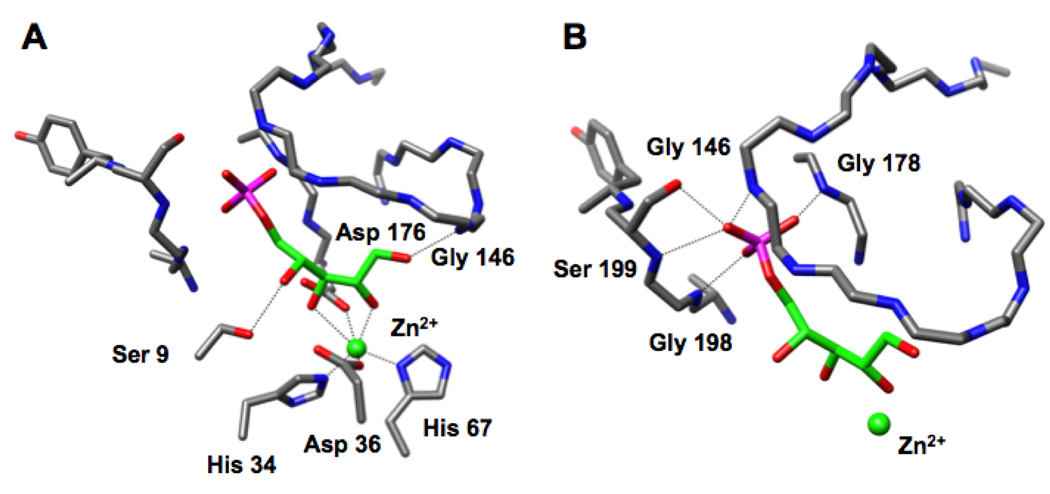
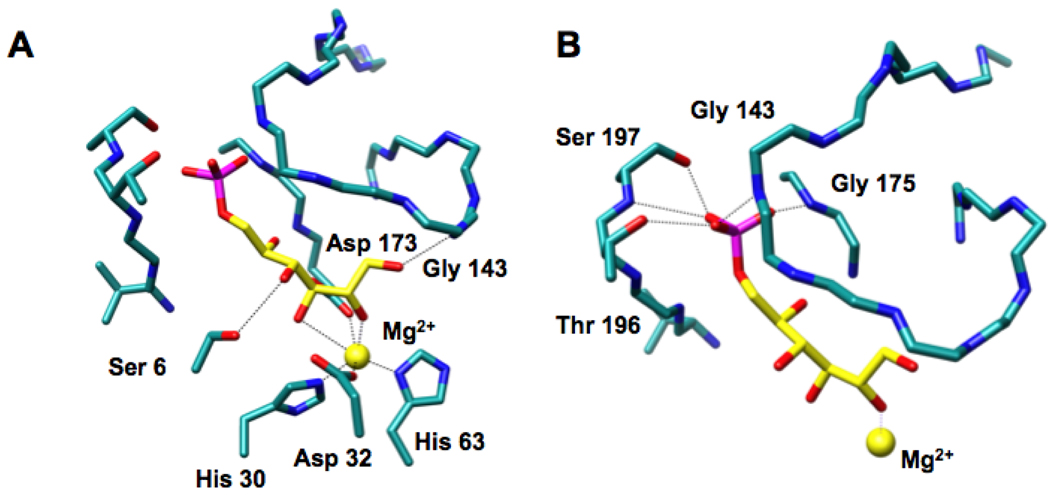
We recently reported the structure of the RPE from Streptococcus pyogenes liganded with a catalytically essential Zn2+ and D-xylitol 5-phosphate, an inert substrate analog (10). In this structure, the 2- and 3-OH groups of the analog are coordinated to a Zn2+, suggesting that the Zn2+ stabilizes the enediolate intermediate generated by abstraction of the 3-proton (Figure 1, panel A). The protein provides four additional ligands for the Zn2+, His 34, His 67, Asp 36, and Asp 176. The substrate analog directly contacts the protein via 1) the 1-OH group that is hydrogen bonded to the amide of Gly 143 in the active site loop following the sixth β-strand, and 2) the 4-OH group that is hydrogen bonded to the OH group of Ser 9 in a loop following the first β-strand. On the basis of this structure, we assigned Asp 36 as the D-ribulose 5-phosphate specific acid/base catalyst and Asp 176 as the D-xylulose 5-phosphate specific acid/base catalyst in the 1,1-proton transfer reaction.
Figure 1.
The active site of the RPE from S. pyogenes (10). Panel A, the interactions of the substrate analog D-xylitol 5-phosphate (green) with the active site. Panel B, the interactions of the phosphate group of D-xylitol 5-phosphate with the phosphate binding motif (Gly 178, Gly 198, and Ser 199) and the active site loop (Gly 146). A 3.2 Å threshold was used for drawing hydrogen bonding and liganding interactions.
In this structure, the phosphate group of the D-xylitol 5-phosphate ligand is located in the conserved binding motif where it forms hydrogen bonds to the amide of Gly 178 at the end of the seventh β-strand, the amide of Gly 198 at the end of the eighth β-strand, and the OH group of Ser 199 also at the end of the eighth β-strand (Figure 1, panel B). The phosphate group also forms a hydrogen bond with the amide of Gly 146 in the active site loop.
We now report the structure of the ALSE from E. coli K-12 in the presence of D-glucitol 6-phosphate, an inert analogue of the D-fructose 6-phosphate product. The phosphate group occupies the phosphate binding motif, which differs from that in RPE by the insertion of a single amino acid residue in the loop following the eighth β-strand. The insertion displaces the position of the binding site for the phosphate group by ~1.5 Å from the binding site for the divalent metal ion relative to the geometry in RPE, thereby forming an elongated site for the phosphorylated hexulose substrate. We kinetically characterized three mutants of ALSE in which a single residue in the loop following the eighth β-strand was deleted, ΔT196, ΔS197 and ΔG198. The values of kcat/Km, the specificity constant, were significantly increased for the RPE reaction and modestly decreased for the ALSE reaction. That these effects result from changes in the values of kcat, not of Km, suggests that the specificity constants for RPE and ALSE reflect the ability of the phosphate groups of their substrates to transmit their intrinsic binding energy to the catalytic groups in the active sites, presumably via its interactions with the active site loops, and not simply the affinities for the substrates.
MATERIALS AND METHODS
1H NMR and 31P NMR spectra were recorded on a Varian Unity 500NB MHz spectrometer. All compounds used were of the highest available commercial grade.
Preparation of D-Allulose 6-Phosphate
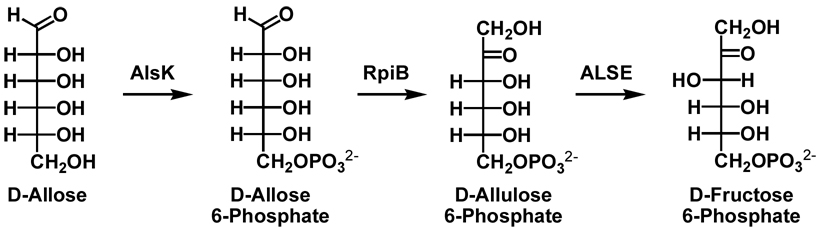
A 50:50 mixture of D-allulose 6-phosphate and D-allose 6-phosphate was prepared from D-allose (Omicron Biochemicals, Inc.) using D-allose kinase (AlsK), a gift from Dr. Brian Miller (17, 18), and D-ribose-5-phosphate isomerase B (RpiB).
D-Allose 6-phosphate was prepared from D-allose using AlsK and an ATP regenerating system. D-Allose (5.5 mmole), ATP (0.1 mmole), MgCl2 (1.8 mmole) and acetyl phosphate (4.4 mmole) were dissolved in 200 mL of 50 mM Tris-HCl, pH. 7.6. AlsK (100 U) and acetate kinase (450 U) were added. After the reaction was complete, the enzymes were removed by ultrafiltration, and the solution was applied to a column (2.5 × 25 cm) of DEAE Sepharose Fast Flow (HCO3− form) and eluted with a 0 to 1 M linear gradient of triethylammonium bicarbonate, pH 8.2. Fractions containing the product were identified by TLC using the Hanes-Isherwood method, pooled, and triethylammonium bicarbonate was removed by rotary evaporation. 1H and 31P NMR spectra were recorded to verify the identity of the product. 1H NMR (D2O, 500 MHz): δ4.72 (d, J = 8.28 Hz, 1H), 4.01 (t, J = 2.98 Hz, 1H), 3.93 (m, 1H), 3.82 (m, 1H), and 3.71 (q, J = 4.77 Hz, 1H) . 31P NMR (D2O, 500 MHz, 1H coupled): 5.66 (t, J = 6.53 Hz).
An equimolar mixture of D-allose 6-phosphate and D-allulose 6-phosphate was prepared from D-allose 6-phosphate using RpiB. RpiB (2 mL of an 80 µM solution) was added to a solution (40 mL) of 1.49 mM D-allose 6-phosphate, 150 mM NaCl, and 50 mM sodium HEPES, pH 7.5. The reaction progress was monitored by removing a 400 µL aliquot, adding 400 µL of D2O, and recording the 31P NMR spectrum. After the reaction came to equilibrium, RpiB was removed by ultrafiltration. The concentration of D-allulose 6-phosphate was determined by measuring the total phosphate concentration by the Ames method (19) and assuming, based on the integration of the 31P NMR spectrum, that 50% of the equilibrated mixture was D-allulose 6-phosphate. 31P NMR (D2O, 500 MHz coupled) 5.63 (t, J = 6.55 Hz), 5.02 (t, J = 5.39 Hz), and 4.94 (t, J = 5.48 Hz).
Preparation of D-Glucitol 6-Phosphate
D-Glucitol-6-phosphate was prepared by reduction of D-glucose-6-phosphate (7 mmoles; Sigma) using NaBH4 (14 mmoles) and purified by passing the resulting solution through a column (2.5 × 25 cm) of DEAE Sepharose Fast Flow (HCO3− form) and eluting with a 0 to 0.6 M linear gradient of triethylammonium bicarbonate (pH 8.2). Fractions containing product (identified by the Hanes-Isherwood method) were pooled, and triethylammonium bicarbonate was removed by rotary evaporation. 1H, 13C, and 31P NMR spectra were recorded to verify the product. 1H NMR (D2O, 500 MHz): δ 3.84 (d, J = 3.55 Hz, 1H), δ 3.76 (m, 2H), δ 3.70 (m, 2H), and δ 3.52 (m, 1H). 31P NMR (D2O, 500 MHz coupled) 3.01 (t, J = 6.45 Hz). 13C NMR (D2O, 500 MHz): δ 73.14, δ 70.36, δ 70.07 (d, J = 7.14 Hz), δ 69.69, δ 65.95 (d, J = 5.23 Hz), and δ 62.49.
Insertional Disruption/Deletions of the Genes Encoding RPE, ALSE, and SGCE
We constructed a mutant strain of E. coli K-12 in which the genes encoding RPE, ALSE, and SGCE, a third homologue of unknown function, were disrupted (rpe, alse, and sgce). The method described by Datsenko and Wanner (20) was used to separately construct disruptions of the three genes in E. coli BW25113 by the insertion of genes encoding antibiotic resistance proteins. An rpe::kanamycin strain was used to produce a phage P1 lysate that was used to transduce an alse::chloramphenicol strain to obtain the double mutant in which the genes encoding both RPE and ALSE had been disrupted. The transduction product was identified by selection on LB plates containing kanamycin and chloramphenicol; the desired junctions were verified by DNA sequence analysis.
The genes encoding the chloramphenicol and kanamycin resistance proteins were eliminated using the pCP20 ampicillin CamR plasmid. After testing for loss of antibiotic resistance, the sgce::cam strain was used to produce a phage P1 lysate that was used to transduce the strain containing the rpe and alse disruptions. The transduction product was identified by selection on LB plates containing chloramphenicol; the desired junctions were verified by DNA sequence analysis. This chloramphenicol-resistant strain contains disruptions of the genes encoding RPE, ALSE, and SGCE and was used for purification of the wild type and mutant proteins described in this manuscript.
Cloning, Expression, and Purification of Wild Type ALSE
The gene encoding ALSE (gi:16131911) was PCR-amplified from E. coli MG 1655 genomic DNA using Platinum Pfx DNA polymerase (Invitrogen). The PCR mixture (100 µL) contained 1 ng of genomic DNA, 10 µL of 10× Pfx amplification buffer, 1 mM MgSO4, 0.4 mM concentrations of each of the four deoxynucleoside triphosphates (dNTPs), 40 pmol of each primer, and 2 U of Platinum Pfx DNA polymerase. The genes were amplified using a PTC-200 gradient thermal cycler (MJ Research), with the following parameters: 94°C for 2 min; followed by 40 cycles of 94°C for 1 min, a gradient annealing temperature range of 45 to 60°C for 1 min 15 s and 68°C for 2 min; and a final extension of 68°C for 10 min. The amplified genes were cloned into the pDMS1a and the pKKHis10 vectors. Both vectors are modifications pKK223-3 vector (Novagen); pDMS1a does not encode an N-terminal His-tag (21), and pKKHis10 encodes an N-terminal His10-tag.
Preparation of Wild-type ALSE with an N-Terminal His10-Tag
ALSE with the N-terminal His10-tag was expressed in the strain lacking RPE, ALSE, and SGCE and grown at 37 °C for 30 hrs in LB containing 100 µg/mL ampicillin and 35 µg/mL chloramphenicol. The cells were collected by centrifugation, resuspended in binding buffer (5 mM imidazole, 0.5 M NaCl, and 20 mM Tris-HCl, pH 7.9), and lysed by sonication. The lysate was applied to a chelating Sepharose Fast Flow column (Pharmacia Biotech) charged with Ni2+. The column was washed with 15% elution buffer (1 M imidazole, 0.5 M NaCl, and 20 mM Tris-HCl, pH 7.9)/85% wash buffer (60 mM imidazole, 0.5 M NaCl, and 20 mM Tris-HCl, pH 7.9), and the protein was eluted with 50% wash buffer/50% strip buffer (100 mM EDTA, 0.5 M NaCl, and 20 mM Tris HCl, pH 7.9). The protein was dialyzed into 10 mM Tris HCl, pH 7.5, containing 150 mM NaCl, concentrated to 30 – 60 mg/mL by ultrafiltration, and stored at −80 °C.
Construction and Purification of Mutants
The ΔT196, ΔS197 and ΔG198 deletion mutants were constructed by the overlap extension method. The resulting PCR products were digested with Nde I and Bam HI and ligated into the pDMS1a vector. The plasmids were transformed into the alse:rpe:sgce::choramphenicol strain of E. coli for expression and purification of the mutant proteins.
Preparation of Wild-type and Mutants of ALSE Reconstituted with Divalent Metal Ions
Wild type and mutants of ALSE without His10-tags were expressed in the strain lacking RPE, ALSE, and SGCE and grown at 20 °C for 30 hours in LB with a metal ion supplement (0.5 mM CoCl2, 1 mM ZnCl2, or 1 mM MnCl2), 100 µg/mL ampicillin, and 35 µg/mL chloramphenicol. The cells were collected by centrifugation, resuspended in binding buffer (5% glycerol, 80 µM divalent metal ion, and 20 mM Tris-HCl, pH 7.9), and lysed by sonication. After centrifugation, the lysate was applied to a DEAE-Sepharose FF column. The column was washed with 2.5 column volumes of binding buffer, and the protein was eluted with a linear gradient (from 0 to 50%) of 1 M NaCl in binding buffer. Fractions containing ALSE or its mutants were pooled, and an equal volume of a solution containing 2 M ammonium sulfate, 5% glycerol, 80 µM of the desired divalent metal ion salt, and 20 mM Tris-HCl, pH 7.9. Pooled fractions were then applied to a Phenyl-Sepharose column equilibrated with 1 M ammonium sulfate, 5% glycerol, 80 µM of the desired divalent metal ion salt, and 20 mM Tris-HCl, pH 7.9, was added. The column was washed with the 1 M ammonium sulfate-containing buffer, and the protein was eluted with a linear gradient (from 100 to 0%) of the 1 M ammonium sulfate-containing buffer. Fractions containing ALSE or mutants were pooled and dialyzed against a solution containing 5% glycerol, 80 µM of the desired divalent metal ion salt, and 20 mM Tris-HCl, pH 7.9. The dialyzed protein was then purified further with a Source 15Q column, eluted with a linear gradient (from 0 to 50%) of 1 M NaCl in 5% glycerol, 80 µM of the desired divalent metal ion salt, and 20 mM Tris-HCl, pH 7.9. Fractions containing pure protein (>95%) were pooled, concentrated, and dialyzed against 150 mM NaCl, 5% glycerol. 80 µM of the desired divalent metal ion salt, and 20 mM Tris-HCl, pH 7.5, concentrated to 20 – 63 mg/mL by ultrafiltration, and stored at −80 °C.
Preparation of Metal-Free D-Ribulose 5-Phosphate Epimerase and Reconstitution with Divalent Metal Ions
Metal-free RPE and wild type RPE reconstituted with Zn2+ were prepared as previously described (10). Wild type RPE reconstituted with Mn2+ or Cl2+ was similarly prepared except that 20 mM L-glutamate, 20 mM L-arginine, and 20% glycerol were omitted.
Metal Ion Analyses
The procedures used for removing adventitious divalent metal ions from buffers were described previously (10). Free or loosely associated metal ions were removed from protein samples by gel filtration using prepacked PD-10 columns (Amersham) that were washed and equilibrated with metal-free 10 mM sodium HEPES, pH 7.5. Inductively coupled plasma emission spectroscopy (ICP) data were obtained by the Garratt-Callahan Company, Burlingame, CA Laboratory as described previously (10). Some additional ICP analyses for Zn and Co were performed at the University of Illinois Microanalysis Laboratory.
Coupled-Enzyme Assay of ALSE Activity
The production of D-fructose-6-phopshate from D-allulose 6-phosphate catalyzed by ALSE was quantitated with an irreversible, coupled-enzyme, continuous spectrophotometric assay. The assay (0.2 mL) contained D-allulose 6-phosphate (0.02–7 mM), 0.16 mM NADP+, 4 U of glucose-6-phosphate dehydrogenase (Sigma), 4 U of phosphoglucoisomerase (Sigma), and 150 mM NaCl in 50 mM sodium HEPES, pH 7.5. The change in absorbance at 340 nm was measured as a function of time, with the measured value providing the rate of conversion of D-allulose 6-phosphate to D-fructose 6-phosphate.
Coupled-Enzyme Assay of RPE Activity
RPE was assayed as previously described (10).
Polarimetric Assay of RPE activity
A polarimetric assay was used to avoid divalent metal contamination and also served to verify the coupled-enzyme spectrophotometric assay. The assays were performed as previously described for Zn2+-reconstituted RPE using 80 µM MnCl2, 80 µM CoCl2, or zinc (10).
Crystallization and Data Collection
Two different crystal forms (Table 1) were grown by the hanging drop method at room temperature: 1) ALSE, Mg2+, and sulfate, and 2) ALSE, Mg2+, and the substrate analog D-glucitol 6-phosphate.
Table 1.
Data collection and refinement statistics
| ALSE·Mg2+·SO4 | ALSE·Mg2+·D-glucitol 6-phosphate | |
|---|---|---|
| Data Collection | ||
| Wavelength (Å) | 0.979 | 0.979 |
| Space group | P212121 | P212121 |
| Mol. in a.u. | 6 | 6 |
| Unit cell parameters | ||
| a (Å) | 75.46 | 75.71 |
| b (Å) | 129.21 | 129.04 |
| c (Å) | 154.45 | 154.87 |
| Resolution (Å)a | 30-2.50 (2.59-2.50) | 25.0-2.2 (2.28-2.2) |
| Unique reflections | 48811 | 74982 |
| Completeness (%)a | 93.4 (92.1) | 96.5 (92.6) |
| Rmergea | 0.053 (0.246) | 0.087 (0.039) |
| Average I/σa | 40.7 (7.6) | 14.5 (3.1) |
| Refinement | ||
| Resolution (Å) | 25-2.5 | 25-2.2 |
| Rcryst | 0.222 | 0.243 |
| Rfree | 0.249 | 0.265 |
| rmsd, bonds (Å) | 0.007 | 0.007 |
| rmsd, angles (°) | 1.41 | 1.39 |
| no. of atoms | ||
| Protein | 10475 | 10475 |
| Water | 201 | 174 |
| Mg2+ | 6 | 6 |
| SO42−− | 30 | |
| bound ligand | 96 | |
| PDB entry | 3CT7 | 3CTL |
Numbers in parentheses indicate values for the highest resolution shell.
For ALSE, Mg2+, and sulfate, the protein solution contained ALSE (60 mg/mL) in 20 mM Tris-HCl, pH 7.9, containing 100 mM NaCl and 5 mM MgCl2; the precipitant contained 16% pentaerythritol ethoxylate, 0.1 M Bis-Tris, pH 6.5, and 50 mM ammonium sulfate. Crystals appeared in 8–9 days and exhibited diffraction consistent with the space group P212121 with six molecules of ALSE per asymmetric unit.
For ALSE, Mg2+, and the substrate analog D-glucitol 6-phosphate, the protein solution contained ALSE (30 mg/mL) in 20 mM Tris-HCl, pH 7.9, containing 100 mm NaCl, 5 mM MgCl2, and 20 mM D-glucitol 6-phosphte; the precipitant contained 15% PEG 3350, and 100 mM succinic acid, pH 7.0. Crystals also appeared in 8–9 days and exhibited diffraction consistent with the space group P212121 with six molecules of ALSE per asymmetric unit.
Prior to data collection, the crystals were transferred to cryoprotectant solution composed of their mother liquid and 20% glycerol and flash-cooled in a nitrogen stream. X-Ray diffraction data sets for the complexes with Mg2+ and sulfate (Table 1, column 1), and Mg2+ and D-glucitol 6-phosphate (column 2) were collected at the NSLS X4A beamline (Brookhaven National Laboratory) on an ADSC CCD detector to 2.5, and 2.2 Å resolution, respectively. Diffraction intensities were integrated and scaled with programs DENZO and SCALEPACK (22). The data collection statistics are given in Table 1.
Structure Determination and Model Refinement
The structure of the ALSE.Mg2+·sulfate complex was solved by molecular replacement with the program EPMR (23), using the hexamer of RPE from Synechocystis (PDB 1TQJ) as the search model. A single clear solution was found using all data between 15 and 4 Å resolution. Rigid body refinement with CNS (24) yielded an electron density map with clear features for a number of amino acid side chains in the ALSE sequence. The bound sulfate anion and Mg2+ were clearly visible in electron density maps calculated immediately after the first cycle of rigid body refinement of the protein molecule alone. Iterative cycles of manual rebuilding with TOM (25) and refinement with CNS resulted in a model with Rcryst and Rfree 0.222 and 0.249, respectively. The final structure contained 10475 protein atoms, 201 water molecules, 6 Mg2+ atoms and 6 sulfate ions for one hexamer in the asymmetric unit. All nonglycine residues lie in allowed regions of the Ramachandran plot.
The structure of the ALSE crystallized with Mg2+ and D-glucitol 6-phosphate was determined by molecular replacement using the previous structure as the search model. Iterative cycles of manual rebuilding with TOM and refinement with CNS were performed. The model was refined at 2.2 Å with an Rcryst of 0.243 and an Rfree of 0.265. The structure contained a well-defined Mg2+ and D-glucitol 6-phosphate in each polypeptide of the hexamer.
Final crystallographic refinement statistics are provided in Table 1.
RESULTS AND DISCUSSION
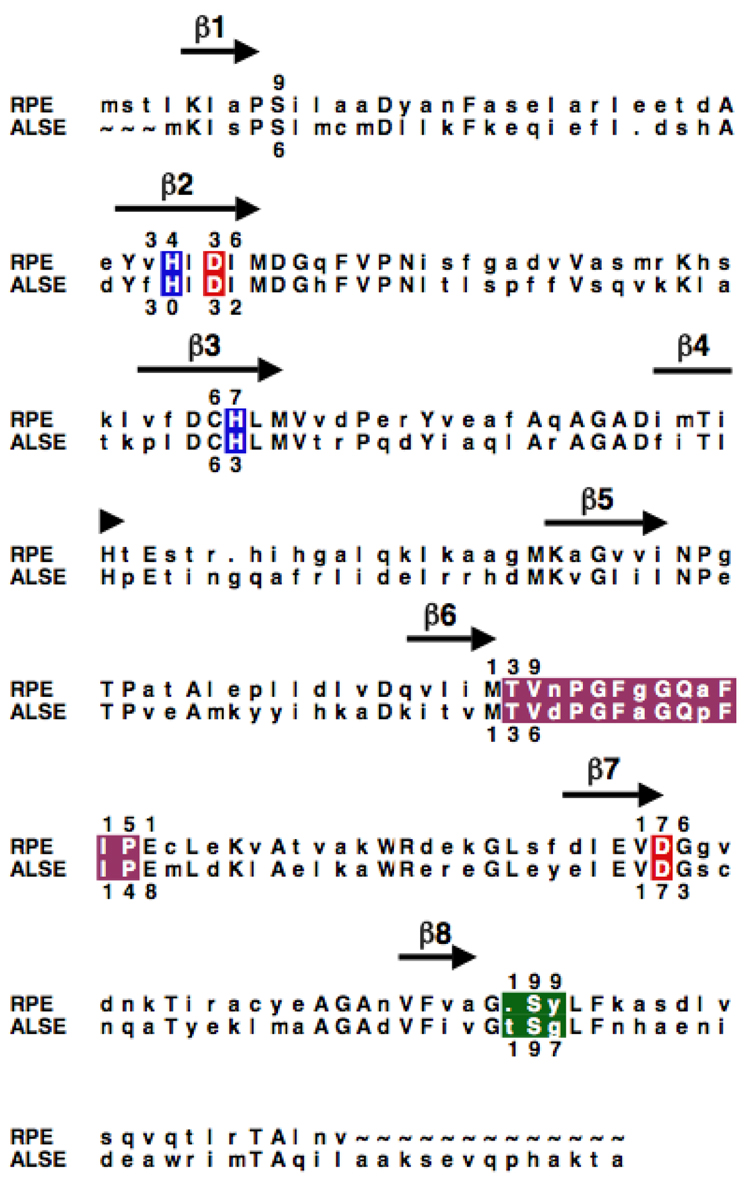
As described in the Introduction, the genomes of species of E. coli encode a catabolic pathway for the rare hexose D-allose that includes ALSE, a homologue of RPE (Scheme II) (16). The structurally characterized RPE from S. pyogenes and the ALSE from E. coli K-12 share 39% sequence identity. On the basis of the sequence alignment (Figure 2), the active site of ALSE is predicted to contain homologues of the ligands for an essential divalent metal ion that are found in RPE, His 30, His 63, Asp 32, and Asp 173, thereby allowing a conserved mechanism for the 1,1-proton transfer reaction.
Figure 2.
Sequence alignment of the RPE from S. pyogenes and the ALSE from E. coli K-12. The positions of the β-strands in the (β/α)8-barrel are marked with arrows; conserved residues are designated with capital letters. The His residues that coordinate the essential divalent metal ion are highlighted in blue, and the Asp residues that are the acid/base catalysts in the 1,1-proton transfer reactions are highlighted in red. The residues in the phosphate binding motifs are highlighted in green, and active site loops are highlighted in magenta.
However, the structural bases for differences in substrate specificity are not as readily predicted from the sequence alignment. Three amino acid substitutions appear to distinguish the active site loops in ALSE and RPE that sequester the bound substrate from the solvent (Asn to Asp at residue 138, Gly to Ala at residue 142, and Ala to Pro at residue 145 in ALSE). But, the side chains of these residues are solvent-exposed in RPE and, therefore, cannot be responsible for differences in substrate specificity. However, the loop that follows the eighth β-strand in the phosphate binding motif is predicted to contain an additional residue. Thus, we were intrigued by structural basis for the presumed different substrate specificities of RPE and ALSE and sought a structural explanation for this difference.
Initial Functional Characterization of ALSE
In the pathway for D-allose utilization, the binding/transport protein for D-allose has been structurally characterized (26, 27) and D-allose kinase (AlsK, Scheme II) has been kinetically characterized (17, 18). But, ALSE had not been isolated and characterized. We cloned the gene encoding the ALSE from E. coli K-12 with an N-terminal His10-tag and initially purified the protein using a Ni2+-chelating column without regard to its metal ion content (determined by ICP as variable, substoichiometric amounts of Mg2+ and trace amounts of Fe2+, Mn2+, Ni2+, and Zn2+). Structures of this protein were determined in the presence of Mg2+ as well as either sulfate anion or D-glucitol 6-phosphate, an inert analogue of D-fructose 6-phosphate (vide infra). However, minimal ALSE activity was detected in the coupled-enzyme spectrophotometric assay (kcat, 0.14 sec−1; kcat/Km, 8 M−1 sec−1). Given our experiences with RPE, we suspected that the low activity could be explained by the requirement for a “missing” transition metal ion: when the His-tagged RPE from S. pyogenes was isolated using a Ni2+-chelating column, the enzyme displayed reduced catalytic activity until adventitious metal ions were removed by incubation with EDTA and Zn2+ was added (10).
Divalent Metal Ion Requirements for ALSE and RPE
We also were concerned that the low activity could result from the N-terminal His10-tag competing for metal ions. Because we could not cleave the tag with thrombin, we expressed and purified ALSE without the affinity tag. But, when this protein was isolated by successive DEAE-anion exchange, phenyl-Sepharose reverse-phase, and Resource-Q anion exchange chromatographies, it also had low ALSE activity.
If the growth medium and buffers for purification were supplemented with ZnCl2, the protein without the tag displayed greater levels of ALSE activity; ICP analysis revealed the presence of 0.66 equivalents of Zn (Table 2). Although Zn was substoichiometric, we hypothesized that other transition metal ions might provide greater activity. Therefore, we analogously expressed and purified ALSE in the presence of either MnCl2 or CoCl2. These proteins also displayed enhanced values for their kinetic constants, with Co2+ providing the greatest level of activity; both proteins contained near stoichiometric amounts of the added metal (Table 2). We used Co2+-containing wild type and mutant enzymes for the remainder of our studies.
Table 2.
Kinetic Parameters, Substrate Specificities, and Metal Contents for Wild Type RPE and ALSE
| Co-ALSE | Mn-ALSE | Zn-ALSE | Co-RPE | Mn-RPE | Zn-RPE | |
|---|---|---|---|---|---|---|
| RPEa | ||||||
| kcat(s−1) | 2 ± 0.8 | -b | -b | 3,800 ± 160 | 1,600 ± 70 | 500 ± 20 |
| Km (mM) | 3.0 ± 0.3 | -b | -b | 1.2 ± 0.1 | 2.2 ± 0.3 | 3.2 ± 0.2 |
| kcat/Km(M−1 s−1) | 670 | 190 | 390 | 3,200,000 | 7,100 | 1,500 |
| ALSEa | ||||||
| kcat (s−1) | 46 ± 4 | 7.3 ± 0.5 | 2.2 ± 0.2 | 0.016 ± 0.002 | 0.012 ± 0.001 | 0.011 ± 0.001 |
| Km (mM) | 1.6 ± 0.4 | 1.1 ± 0.2 | 3.3 ± 0.4 | 1.1 ± 0.3 | 1.1 ± 0.06 | 1.8 ± 0.4 |
| kcat/Km (M−1 s−1) | 29,000 | 6,600 | 700 | 14 | 11 | 6 |
| Substrate Specificity (RPE/ALSE)c | ||||||
| RPE/ALSE | 0.025 | 0.029 | 0.6 | 230,000 | 64,000 | 25,000 |
| Metal Contentd | ||||||
| Co | 0.99 | 0 | 0 | 1.46 | 0 | 0 |
| Zn | 0 | 0.15 | 0.66 | 0.11 | 0.17 | 1.15 |
| Mn | 0 | 0.94 | 0 | 0 | 0.98 | 0 |
Using the spectrophotometric assay described in Materials and Methods.
Could not saturated with substrate; kcat/Km was determined by the dependence of velocity on substrate concentration.
(kcat/Km)RPE/(kcat/Km)ALSE.
Stoichometry of metal to protein; metal content determined by ICP as described in Materials and Methods.
Given the preference of ALSE for Co2+, we re-examined the metal ion requirement for the RPE from S. pyogenes. We previously reported that the RPE was activated by Zn2+ but did not examine whether it also could be activated by either Mn2+ or Co2+. We followed our published procedure for preparing metal free-RPE and reconstituted it with Mn2+ or Co2+. The RPE also is maximally activated by Co2+, with Mn2+ also providing more activity than previously reported for Zn2+ (Table 2).
Promiscuity of ALSE for the RPE Reaction and Vice-Versa
We assayed the various transition metal ion-reconstituted samples of ALSE for RPE activity and analogous samples of RPE for ALSE activity. ALSE was promiscuous for the RPE reaction, and vice versa (Table 2). On the basis of the values of kcat/Km (the specificity constant), ALSE is more promiscuous than RPE. Focusing on the most active Co-containing epimerases, the ratio of the values for kcat/Km (RPE:ALSE) is 0.023 for ALSE and 2.3 × 105 for RPE.
Structure of ALSE
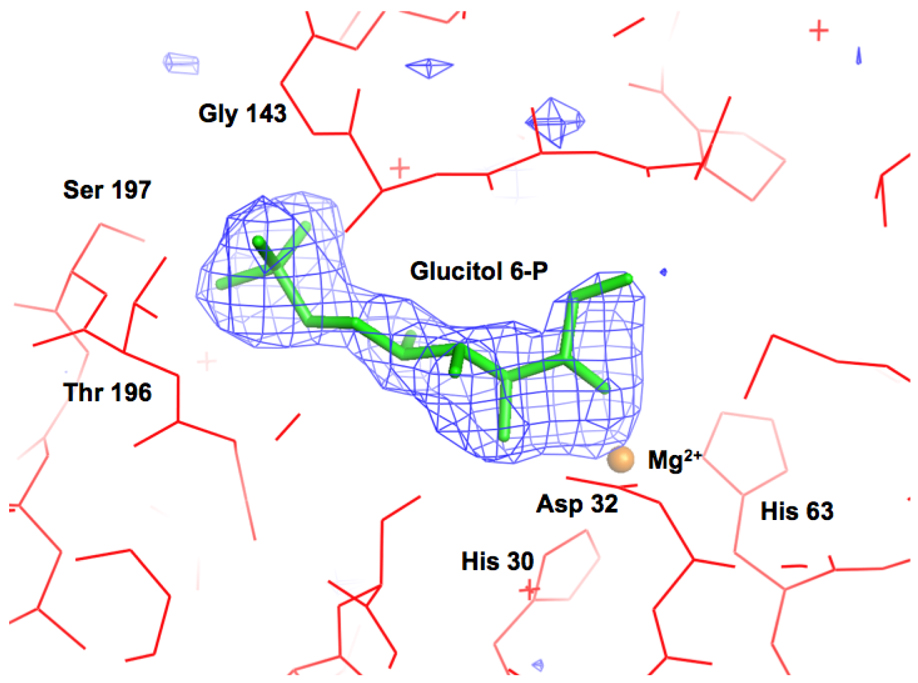
We determined structures of the low activity, N-terminal His-tagged ALSE in the presence of 5 mM Mg2+ and either 1) sulfate anion at 2.5 Å resolution or 2) D-glucitol 6-phosphate, an inert structural analog of the D-fructose 6-phosphate product, at 2.2 Å resolution. Unfortunately, we were unable to obtain any crystals of ALSE without the His-tag that had been reconstituted with Zn2+, Mn2+, or Co2+. Because the sample of ALSE used to obtain these crystals contained some Mg2+ but essentially no transition metal ions as assessed by ICP analyses (vide supra), we assign Mg2+ to the electron density to which O2 and O3 of the analog is coordinated. Although the protein used to obtain crystals had low levels of ALSE activity, we believe that the structures of the complexes can be used to infer the structural bases for substrate specificity, the focus of this study, given 1) the excellent superposition of the sulfate/phosphate binding motifs at the ends of the seventh and eighth β-strands in the two complexes; and 2) the global similarity of the structures of these complexes to the structure of RPE liganded with Zn2+ and D-xylitol 5-phosphate (10).
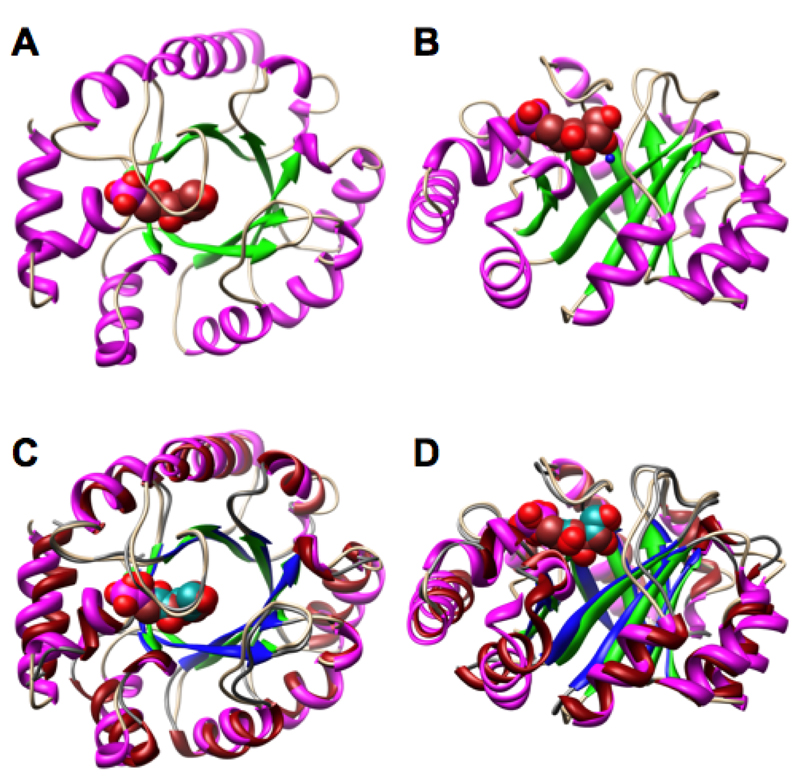
In each structure, the asymmetric unit contains six polypeptides. These are arranged as trimers of dimers to form hexamers; we reported a similar hexameric structure for the homologous RPE from S. pyogenes (10). Like RPE, ALSE is a (β/α)8-barrel, with the active site located at the C-terminal ends of the β-strands.
The structure of a polypeptide of ALSE liganded with D-glucitol 6-phosphate as well as its superposition with a polypeptide of RPE liganded with D-xylitol 5-phosphate are shown in Figure 4. Like RPE, a loop that follows the sixth β-strand sequesters the active site of ALSE from the solvent. As expected based on the sequence alignment (Figure 2), the polypeptides are well-superimposed (rmsd 1.05 Å for 168 pairs of α-carbons).
Figure 4.
Structures of the polypeptides of ALSE and RPE. Panels A and B, the D-glucitol 6-phosphate-liganded complex of ALSE: panel A, view from the C-terminal ends of the β-strands of the (β/α)8-barrel domains; panel B, view from the sides of the (β/α)8-barrel domains. Panels C and D, the D-glucitol 6-phosphate-liganded complex of ALSE superimposed on the D-xylitol 5-phosphate-liganded complex of RPE (10): panel C, view from the C-terminal ends of the β-strands of the (β/α)8-barrel domains; panel D, view from the sides of the (β/α)8-barrel domains. In ALSE, the backbone is shown in tan, the β-strands are shown in green, the α-helices are shown in magenta, and the D-glucitol 6-phosphate ligand is shown in brown. In RPE, the backbone is shown in gray, the β-strands are shown in blue, and α-helices are shown in red, and the D-xylitol 5-phosphate ligand is shown in dark cyan.
Active Site of ALSE
The structure of the active site of the D-glucitol 6-phosphate-liganded ALSE (Figure 5) is similar to that of the D-xylitol 5-phosphate-liganded RPE (Figure 1): the positions of the metal ions, their protein-derived ligands, and the 2- and 3-OH groups of D-glucitol 6-phosphate in ALSE and of D-xylitol 5-phosphate in RPE superimpose closely, as would be expected from mechanistically conserved geometric requirements for the “same” 1,1-proton transfer reactions involving ketose substrates. Therefore, in analogy to the structure-function relationships we determined for RPE (10), we propose that Asp 32 is the D-allulose 6-phosphate specific acid/base catalyst and Asp 173 is the D-fructose 6-phosphate specific acid/base catalyst in the ALSE-catalyzed 1,1-proton transfer reaction.
Figure 5.
The active site of ALSE. Panel A, the interactions of the substrate analog D-glucitol 6-phosphate (yellow) with the active site. Panel B, the interactions of the phosphate group of D-glucitol 5-phosphate with the phosphate binding motif (Thr 196 and Ser 197) and the active site loop (Gly 143). A 3.2 Å threshold was used for drawing hydrogen bonding and liganding interactions.
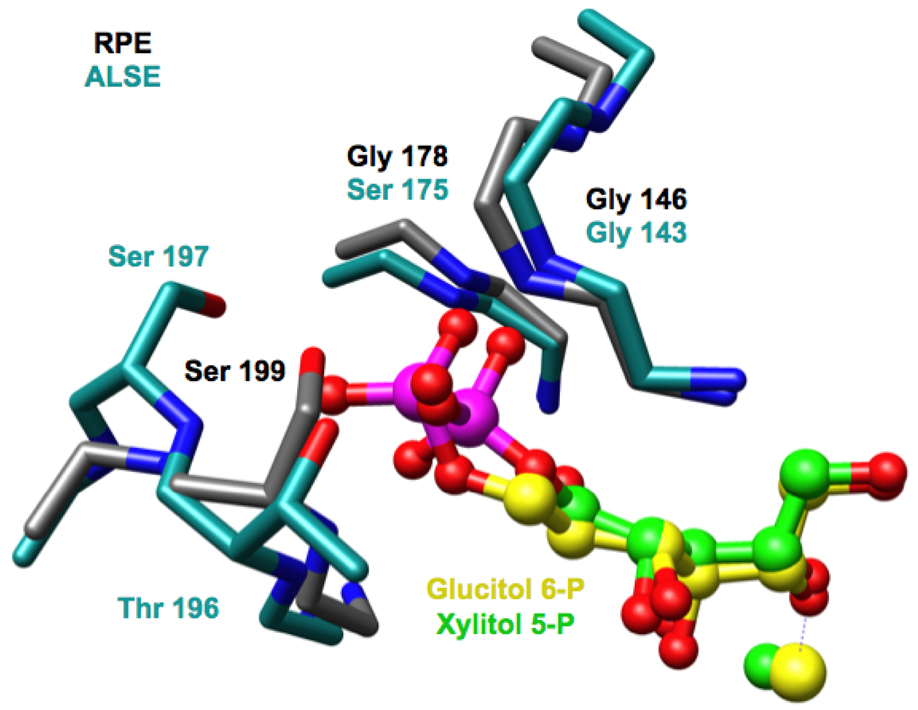
With these conserved geometric features, the phosphate groups must occupy different positions as a consequence of the differing lengths of the substrates. As suggested by the sequence alignment (Figure 2), the loop following the eighth β-strand in ALSE has an additional residue so that it can accommodate the longer hexulose 6-phosphate substrates for the ALSE-catalyzed reaction (Figure 5). The phosphate group of D-glucitol 6-phosphate makes several hydrogen-bonding contacts with the expanded phosphate binding motif in the C-terminal (β/α)2-quarter barrel: the amide of Ser 175 at the end of seventh β-strand, the amide of Thr 196 at the end of the eighth β-strand, and the OH groups of both Thr 196 and Ser 197. In addition, the phosphate group also forms a hydrogen bond with the amide of Gly 143 in the closed active site loop.
The locations of the backbone and side chain hydrogen bond donors in the loop following the eighth β-strand of ALSE are displaced from those in RPE as permitted by the insertion of a single amino acid residue at this position in the sequence of ALSE (Figure 6). As a result, the active site of RPE sterically should be unable to accommodate the longer substrate for the ALSE reaction, but the active site of ALSE should be able to accommodate the shorter substrate for the RPE reaction. These predictions are in accord with the experimentally measured substrate promiscuities (Table 2): the ratio of the values for kcat/Km (RPE:ALSE) is 0.023 for ALSE and 2.3 × 105 for RPE.
Figure 6.
A superposition of the phosphate binding motifs in RPE (gray) and ALSE (cyan). In RPE the D-xylitol 5-phosphate ligand is shown in green; in ALSE, the D-glucitol 6-phosphate ligand is shown in yellow.
Deletion Mutants of the Phosphate Binding Motif of ALSE
Given the differences in both the substrate specificities and structures of the phosphate binding motifs in ALSE and RPE, we examined the consequences of contracting the phosphate binding motif in ALSE and, thereby, allowing it to assume the conformation of the motif in RPE. We constructed three mutants in which one of the residues in the loop following the eighth β-strand is deleted, ΔT196, ΔS197 and ΔG198, thereby reducing the length to that found in RPE (Figure 2). In each mutant, the position of the backbone amide that hydrogen bonds to the phosphate group of the substrate (residue 196, Thr in wild type) should be altered; in addition, in the ΔT196 and ΔS197 mutants, one of the side chain OH groups that hydrogen bonds to the phosphate group is missing.
The mutants were expressed and purified so that Co2+ would be present in the active site; when assayed for ALSE activity, the values of kcat/Km were modestly reduced for all three mutants (Table 3). In contrast, when assayed for RPE activity, the values of kcat/Km were significantly increased. As a result, the promiscuity for the RPE reaction was increased for the all three mutants, such that D-ribulose 5-phosphate was the preferred substrate (RPE:ALSE = 5.2, 7.8, and 4.3 for the ΔT196, ΔS197 and ΔG198 mutants, respectively). However, for all three mutants the values for kcat and kcat/Km for the RPE reaction remained less than those for the RPE from S. pyogenes.
Table 3.
Kinetic Parameters, Substrate Specificities, and Metal Contents for Co-Reconstituted Wild Type ALSE, ALSE Mutants, and Wild Type RPE
| Co-ALSE | Co-ΔT196 | Co-ΔS197 | Co-ΔG198 | Co-RPE | ||
|---|---|---|---|---|---|---|
| RPEa | ||||||
| kcat (s−1) | 2 ± 0.8 | 130 ± 7 | 240 ± 14 | 440 ± 20 | 3800 ± 160 | |
| Km (mM) | 3.0 ± 0.3 | 3.9 ± 0.5 | 2.8 ± 0.6 | 5.1 ± 0.5 | 1.2 ± 0.1 | |
| kcat/Km (M−1 s−1) | 670 | 33,000 | 85,000 | 86,000 | 3,200,000 | |
| ALSEa | ||||||
| kcat (s−1) | 46 ± 4 | 7.5 ± 0.3 | 12 ± 0.8 | 34 ± 2 | 0.016 ± 0.002 | |
| Km (mM) | 1.6 ± 0.4 | 1.2 ± 0.1 | 1.1 ± 0.2 | 1.7 ± 0.2 | 1.1 ± 0.3 | |
| kcat/Km (M−1 s−1) | 29,000 | 6,300 | 11,000 | 20,000 | 14 | |
| Substrate Specificity (RPE/ALSE)b | ||||||
| RPE/ALSE | 0.023 | 5.2 | 7.8 | 4.3 | 230,000 | |
| Metal Contentc | ||||||
| Co | 0.99 | 1.00 | 0.95 | 1.11 | 1.46 | |
| Zn | 0 | 0 | 0 | 0 | 0.11 | |
Using the spectrophotometric assay described in Materials and Methods.
(kcat/Km)RPE/(kcat/Km)ALSE.
Stoichometry of metal to protein; metal content determined by ICP as described in Materials and Methods.
For both activities, the changes in specificity are dominated by changes in the values of kcat (Table 3). We were not able to determine the structures of any of the mutants, so a structure-based explanation for the changes in their substrate specificities is not possible. However, the structures of RPE liganded with D-xylitol 5-phosphate and ALSE liganded with D-glucitol 6-phosphate reveal hydrogen bonding interactions not only between the phosphate monoester dianions and the binding motif in the C-terminal (β/α)8-quarter barrel but also with the active site loops (the amides of Gly 146 and Gly 143, respectively). This is reminiscent of the interactions of the phosphate groups of the substrates in the active sites of TIM (13) and OMPDC (14) with their active site loops. For both TIM (11) and OMPDC (12), Amyes and Richard proposed that the active site loops transmit the energy obtained by binding the phosphate group to catalysis, given that phosphite “allosterically” activates both enzymes for utilization of desphospho-truncated substrates.
Thus, the significant increases in the values of kcat for the RPE reaction (> 100-fold) that dominate the change in specificity from D-allulose 6-phosphate to D-ribulose 5-phosphate in the mutants likely reflects the ability of the altered binding site to better couple phosphate binding with catalysis via ligand-induced changes in the structure of the active site, including the loop at the end of the sixth β-strand (15). Similarly, the small decreases in the values of kcat for the ALSE reaction (< 6-fold) likely result from less efficient utilization of the intrinsic binding energy of the phosphate group. Indeed, the values of both kcat and kcat/Km for the RPE reaction catalyzed by the RPE from S. pyogenes are larger than those for the ALSE reaction catalyzed by ALSE, suggesting that the mechanism for coupling the phosphate binding energy to catalysis has not been optimized in the ALSE-catalyzed reaction.
That this mechanism for determining substrate specificity is more “sophisticated” than “simple” discrimination of the substrates based on their affinities supports the hypothesis that the evolution of function in (β/α)8-barrels that contain the conserved phosphate binding motif has been perfected to optimally utilize the intrinsic binding energy to assist catalysis. Presumably, as in TIM and OMPDC, this coupling involves the active site loop that sequesters the substrate from solvent and productively orients the substrate vis a vis the active site functional groups.
Conclusions
The members of the ribulose-phosphate binding (β/α)8-barrel “superfamily” share a conserved phosphate binding motif formed by the C-terminal (β/α)2-quarter barrel (10). The differing substrate specificities for the 1,1-proton transfer reactions catalyzed by the homologous RPE (pentulose 5-phosphate substrates) and ALSE (hexulose 6-phosphate substrates) are associated with the differing structures of their phosphate binding motifs; in ALSE with the longer substrate, the loop following the eighth β-strand contains an additional residue that increases the distance of the binding site for the phosphate moiety from the active site residues. ALSE is naturally promiscuous for the RPE reaction. That the promiscuity is enhanced, i.e., the substrate specificity is reversed, by deletions of residues in the phosphate binding motif in ALSE, suggests that the phosphate binding motif not only participates in “gripping” the substrate but also plays an essential role in transmitting the energy derived from occupancy of the motif to catalysis via its interactions with the conformationally flexible active site loop that sequesters the substrate from solvent.
Figure 3.
Omit electron density map (Fo–Fc) of the D-glucitol 6-phosphate ligand contoured at 3σ. D-Glucitol 6-phosphte was omitted from the model, and the remainder of the units cell was subjected to a cycle of simulated annealing with CNS at 3000 °C.
Footnotes
This research was supported by Grant GM-65155 from the National Institutes of Health. The X-ray coordinates and structure factors for ALSE complexed with 1) sulfate anion and Mg2+ and 2) D-glucitol 6-phosphate and Mg2+ have been deposited in the Protein Data Bank (PDB accession codes 3CT7 and 3CTL, respectively).
Abbreviations: HisA, phosphoribosylformimino-5-aminoimidazole carboxamide ribonucleotide isomerase; HisF, imidazole glycerolphosphate synthase; ICP, inductively coupled plasma emission spectroscopy; KGPDC, 3-keto-L-gulonate 6-phosphate decarboxylase; OMPDC, orotidine 5’-monophosphate decarboxylase; RPE, D-ribulose 5-phosphate 3-epimerase; TIM, triose phosphate isomerase; TrpC, indole-3-glycerophosphate synthase; TprF, phosphoribosylanthranilate isomerase.
Modular construction from a (β/β)2-quarter barrel that provides the phosphate binding motif does not explain the exclusive occurrence of the motif as the C-terminal (β/α)2-quarter barrel of the (β/α)4-half barrels repeated in HisA and HisF and, also, of the large number of complete (β/α)8-barrels that contain a single copy of the motif.
REFERENCES
- 1.Nagano N, Orengo CA, Thornton JM. One fold with many functions: the evolutionary relationships between TIM barrel families based on their sequences, structures and functions. J. Mol. Biol. 2002;321:741–765. doi: 10.1016/s0022-2836(02)00649-6. [DOI] [PubMed] [Google Scholar]
- 2.Sterner R, Hocker B. Catalytic versatility, stability, and evolution of the (β/α)8-barrel enzyme fold. Chem Rev. 2005;105:4038–4055. doi: 10.1021/cr030191z. [DOI] [PubMed] [Google Scholar]
- 3.Lang D, Thoma R, Henn-Sax M, Sterner R, Wilmanns M. Structural evidence for evolution of the β/α barrel scaffold by gene duplication and fusion. Science. 2000;289:1546–1550. doi: 10.1126/science.289.5484.1546. [DOI] [PubMed] [Google Scholar]
- 4.Hocker B, Beismann-Driemeyer S, Hettwer S, Lustig A, Sterner R. Dissection of a (β/α)8-barrel enzyme into two folded halves. Nat Struct Biol. 2001;8:32–36. doi: 10.1038/83021. [DOI] [PubMed] [Google Scholar]
- 5.Bork P, Gellerich J, Groth H, Hooft R, Martin F. Divergent evolution of a beta/alpha-barrel subclass: detection of numerous phosphate-binding sites by motif search. Protein Sci. 1995;4:268–274. doi: 10.1002/pro.5560040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hocker B, Schmidt S, Sterner R. A common evolutionary origin of two elementary enzyme folds. FEBS Lett. 2002;510:133–135. doi: 10.1016/s0014-5793(01)03232-x. [DOI] [PubMed] [Google Scholar]
- 7.Henn-Sax M, Hocker B, Wilmanns M, Sterner R. Divergent evolution of (β/α)8-barrel enzymes. Biol Chem. 2001;382:1315–1320. doi: 10.1515/BC.2001.163. [DOI] [PubMed] [Google Scholar]
- 8.Gerlt JA. New wine from old barrels [news] Nat Struct Biol. 2000;7:171–173. doi: 10.1038/73249. [DOI] [PubMed] [Google Scholar]
- 9.Nicolet Y, Drennan CL. AdoMet radical proteins--from structure to evolution--alignment of divergent protein sequences reveals strong secondary structure element conservation. Nucleic Acids Res. 2004;32:4015–4025. doi: 10.1093/nar/gkh728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akana J, Fedorov AA, Fedorov E, Novak WR, Babbitt PC, Almo SC, Gerlt JA. D-Ribulose 5-phosphate 3-epimerase: functional and structural relationships to members of the ribulose-phosphate binding (beta/alpha)8-barrel superfamily. Biochemistry. 2006;45:2493–2503. doi: 10.1021/bi052474m. [DOI] [PubMed] [Google Scholar]
- 11.Amyes TL, O'Donoghue AC, Richard JP. Contribution of phosphate intrinsic binding energy to the enzymatic rate acceleration for triosephosphate isomerase. J Am Chem Soc. 2001;123:11325–11326. doi: 10.1021/ja016754a. [DOI] [PubMed] [Google Scholar]
- 12.Amyes TL, Richard JP, Tait JJ. Activation of orotidine 5′-monophosphate decarboxylase by phosphite dianion: the whole substrate is the sum of two parts. J Am Chem Soc. 2005;127:15708–15709. doi: 10.1021/ja055493s. [DOI] [PubMed] [Google Scholar]
- 13.Davenport RC, Bash PA, Seaton BA, Karplus M, Petsko GA, Ringe D. Structure of the triosephosphate isomerase-phosphoglycolohydroxamate complex: an analogue of the intermediate on the reaction pathway. Biochemistry. 1991;30:5821–5826. doi: 10.1021/bi00238a002. [DOI] [PubMed] [Google Scholar]
- 14.Applepy TC, Kinsland C, Belegy TP, Ealick SE. The crystal structure and mechanism of orotidine 5′-monophoste decarboxylate. Proc Natl Acad sci U S A. 2000;97:2005–2010. doi: 10.1073/pnas.259441296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrow JR, Amyes TL, Richard JP. Phosphate Binding Energy and Catalysis by Small and Large Molecules. Acc Chem Res. 2008;41:539–548. doi: 10.1021/ar7002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim C, Song S, Park C. The D-allose operon of Escherichia coli K-12. J Bacteriol. 1997;179:7631–7637. doi: 10.1128/jb.179.24.7631-7637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larion M, Moore LB, Thompson SM, Miller BG. Divergent evolution of function in the ROK sugar kinase superfamily: role of enzyme loops in substrate specificity. Biochemistry. 2007;46:13564–13572. doi: 10.1021/bi700924d. [DOI] [PubMed] [Google Scholar]
- 18.Miller BG, Raines RT. Reconstitution of a defunct glycolytic pathway via recruitment of ambiguous sugar kinases. Biochemistry. 2005;44:10776–10783. doi: 10.1021/bi0506268. [DOI] [PubMed] [Google Scholar]
- 19.Ames BN. Assay of Inorganic Phosphate, Total Phosphate and Phosphatases. 1966;Vol. 8 [Google Scholar]
- 20.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt DM, Hubbard BK, Gerlt JA. Evolution of Enzymatic Activities in the Enolase Superfamily: Functional Assignment of Unknown Proteins in Bacillus subtilis and Escherichia coli as L-Ala-D/L-Glu Epimerases. Biochemistry. 2001;40:15707–15715. doi: 10.1021/bi011640x. [DOI] [PubMed] [Google Scholar]
- 22.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. In: Carter CWJ, Sweet RM, Abelson JN, Simon MI, editors. Methods in Enzymology. New York: Academic Pres; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- 23.Kissinger CR, Gehlhaar DK, Fogel DB. Rapid automated molecular replacement by evolutionary search. Acta Crystallogr D Biol Crystallogr. 1999;55:484–491. doi: 10.1107/s0907444998012517. [DOI] [PubMed] [Google Scholar]
- 24.Brunger AT, Adams PD, Clore GM, DeLAno WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. 1998;D54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 25.Jones AT. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- 26.Magnusson U, Chaudhuri BN, Ko J, Park C, Jones TA, Mowbray SL. Hinge-bending motion of D-allose-binding protein from Escherichia coli: three open conformations. J Biol Chem. 2002;277:14077–14084. doi: 10.1074/jbc.M200514200. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhuri BN, Ko J, Park C, Jones TA, Mowbray SL. Structure of D-allose binding protein from Escherichia coli bound to D-allose at 1.8 A resolution. J Mol Biol. 1999;286:1519–1531. doi: 10.1006/jmbi.1999.2571. [DOI] [PubMed] [Google Scholar]