Abstract
We report a series of p-hydroxy, p-amino, p-monomethylamino and p-monofluoroethylamino substituted biphenyltrienes (14c, 14e, 14f and 14h), which displayed high binding affinities to β-amyloid (Aβ) plaques. In an in vitro binding assay using postmortem brain homogenates of Alzheimer’s patients and [125I]9, the novel triene compounds showed excellent binding affinities (Ki = 9.0 ± 2.1, 9.0 ± 3.2, 7.5 ± 2.5 and 12 ± 3 nM for 14c, 14e, 14f and 14h, respectively). When labeled with suitable radionuclides they are potentially useful as in vivo imaging agents for detecting Aβ plaques in the brain of patients with Alzheimer’s disease.
Keywords: binding affinity, Alzheimer’s disease, imaging, Aβ peptides
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disease affecting millions in the older population. Common clinical symptoms of AD include cognitive decline, irreversible memory loss, disorientation, language impairment, etc. Major neuropathology observations of postmortem AD brain include the presence of senile plaques, neurofibrillary tangles and neurophil threads containing β-amyloid (Aβ) aggregates and highly phosphorylated tau proteins.1 The exact mechanisms leading to the development of AD are not fully understood; however, formation of Aβ plaques in the brain is a pivotal event in the pathology of Alzheimer’s disease. Significant circumstantial evidence suggests that fibrillary Aβ plaques consisting predominately of aggregates of Aβ40 and Aβ42 peptides play a major role in AD pathogenesis.2 Formation of Aβ aggregates in the brain is now considered as a significant event, which produces various toxic effects in neuronal cells leading to the formation of neuritic plaques.2 In view of the critical roles Aβ plaques play in AD, Aβ-plaque-specific imaging agents may be useful for early detection or monitoring the progression and effectiveness of treatment of AD.3–5
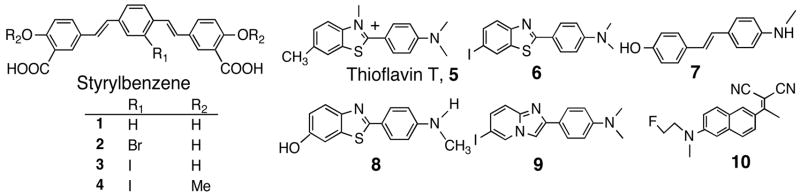
Several research groups have reported Aβ plaque-specific imaging agents based on highly conjugated dyes, such as Congo Red and Chrysamine G.6,7 Thioflavin T, 5, as well as Congo Red have been used in fluorescent staining of plaques and tangles in postmortem AD brain sections.8 More abbreviated forms of Chrysamine G, such as syrylbeneze - including 1–4 (Fig. 1), have been reported as fluorescent dyes for staining amyloid aggregates.9,10 Although these molecules displayed desirable properties: highly conjugated, high binding affinity and moderate lipophilicity, their partial charge prevents them from getting across the intact blood-brain barrier (BBB). A recent report has suggested that it may be possible to prepared near infrared fluorescent imaging agents for imaging the plaques inside the brain.11
Figure 1.
Chemical structures of various probes for Aβ aggregates
To overcome the observed brain penetration deficit associated with the styrylbenzene series (1–4), we and others focused on benzothiazole and other backbone structures. Recently, successful uses of a C-11 labeled benzothiazole derivative, [11C]PIB, 8, and a highly lipophilic F-18 labeled probe, [18F]FDDNP, 10, for plaque and tangle visualization in living AD patients have demonstrated the potential usefulness of in vivo plaque imaging.12,13 Parallel to these efforts, we have similarly prepared [11C]SB-13, 7, a stilbene derivative, for plaque imaging.14 As expected, [11C]SB-13, 7, displayed high accumulation in the affected cortical areas of the brain in mild to moderate AD patients, but not in the age-matched control subjects.15
In order to investigate the significance of distance between the two phenyl groups of the stilbenes, we have added additional double bonds (trienes), instead of one vinyl for the stilbenes. None of the previously reported ligands contains a highly conjugated polyene backbone. To our surprise the addition of a triene bond between the biphenyl groups produce highly selective compounds showing excellent binding affinity towards homogentes of brain tissues from AD patients. Reported herein are syntheses and the structure-activity relationship of a series of derivatives as selective probes potentially useful for detecting amyloid aggregates in the brain.
RESULTS AND DISCUSSION
Chemistry
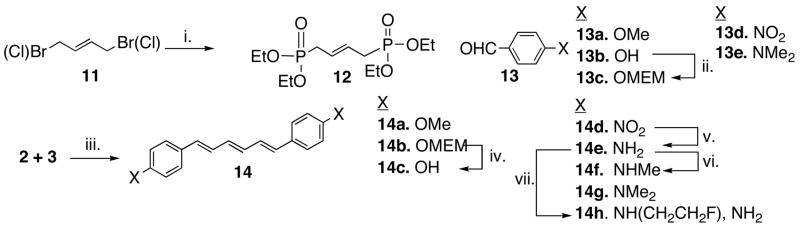
The key step for the synthesis of biphenyltrienes is the Wittig reaction between various aldehydes 13 and bisdiethyl phosphonate, 12, which was readily prepared from 1,4-dibromo (or chloro)-2-butene (Scheme 1). The Wittig reagents, 12 and various aldehydes, 13, were readily reacted in the presence of NaH and THF under a refluxing condition.16,17 The hydroxyl phenyl triene 14c was obtained from hydrolysis of the MEM protected precursor 14b. The bisamino compound 14e and bismonomethylamino compound 14f were synthesized from the correspondent nitro compound 14d through reduction and monomethylation. The bis-p-(N,N-dimethylamino) derivative, 14g, was prepared directly through the Wittig reaction between the p-(N,N-dimethylamino)benzaldehyde 13e and the bisdiethyl phosphonate, 12. One un-symmetrically substituted derivative, N-monofluoroethyl compound, 14h, was prepared by reacting the di-amino derivative, 14e, with 1-bromo-2-fluoroethane in the presence of potassium carbonate in DMF.
Scheme 1.
i. P(OEt)3; ii. MEMCl/DIEA, CH2Cl2; iii. NaH, THF, Reflux; iv. HCl, THF/MeOH(2:1); v. SnCl2/EtOH; vi. NaOMe/(CH2O)n; NaBH4/MeOH; vii. BrCH2CH2F, K2CO3/DMF; Except for 4h, all are symmetrical.
In vitro Binding of Aβ plaques in AD brain tissue homogenates
Using in vitro binding of preformed Aβ peptide aggregates and two different ligands, [125I](E,E)-1-iodo-2,5-bis-(3-hydroxycarbonyl-4-methoxy)styrylbenzene, [125I]4, and [125I] 2-[4′-dimethyl-aminophenyl]-6-iodobenzothiazole, [125I]6, it was demonstrated that there are at least two mutually exclusive binding sites on these aggregates.18 It has been reported recently from our laboratory that [125I] 2-(4′-dimethylaminophenyl)-6-iodo-imidazo[1,2-a]pyridine, [125I]9, binds to the same binding sites as those for 6 in transgenic mice as well as in postmortem homogenates of AD brain tissues, and 9 has a better in vivo biodistribution profile as an imaging agent.18–20 Specific in vitro binding of [125I]9 can be clearly measured in the cortical gray matter, but not in the white matter of AD cases. The location and density of specific signal detected by [125I]9 correlated with the distribution of amyloid plaques in these brain specimens, as confirmed by thioflavin-S staining.
In this study we have extended the in vitro binding assay to using homogenates of AD brain tissue and the two ligands for two different and mutually exclusive binding sites using two different radiolabeled ligands, [125I]4 and [125I]9 (Table 1). It was demonstrated that the new biphenyltrienes showed variable binding affinities depending on the substitution groups at the p-position of the phenyl groups. Generally, the biphenyltrienes preferentially bind to the binding sites for 9. Specifially, compounds 14c, 14e, 14f and 14h containing substitution groups, -OH, -NH2, -NHMe and –NH(CH2CH2F) displayed moderate binding affinities towards [125I]4 giving Ki values of 150, 375, 122 and 217 nM, respectively. However, these four compounds exhibited highly potent binding towards [125I]9 showing Ki values of 9.0, 9.0, 7.5 and 12 nM, respectively. The binding data suggest that the biphenyltrienes are highly competitive to the binding sites of 9 on the Aβ aggregates, while the binding towards the binding sites for 4 are 15–50 fold lower in affinity. It is expected that the compound with a [18F]fluoroethyl group on 14h can be prepared and the labeled [18F]14h may be useful as a PET imaging agent specifically targeting Aβ plaques in the brain.
Table 1.
Inhibition constants (Ki, nM)* of compounds on ligand binding to homogenates of brain tissue
| Compounds | vs[125I]4 | vs[125I]9 | Compounds | vs[125I]4 | vs[125I]9 |
|---|---|---|---|---|---|
| 14a -OMe | >8,000 | >4,000 | 14h- NH(CH CH F) | 217 ± 20 | 12 ± 3 |
| 14c -OH | 150 ± 30 | 9.0 ± 2.1 | 4 | 5.1 ± 1.2 | >1,000 |
| 14d -NO2 | >4,000 | 500 ± 30 | 9 | >5,000 | 5.0 ± 0.4 |
| 14e -NH2 | 375 ± 150 | 9.0 ± 3.2 | 7 | >3,000 | 1.2 ± 0.2 |
| 14f -NHMe | 122 ± 40 | 7.5 ± 2.5 | 8 | >10,000 | 2.8 ± 0.5 |
| 14g -NMe2 | >7,000 | 837 ± 80 | 10 | >8,000 | 152 ± 20 |
Values are the mean ± SEM of three independent experiments, each in duplicate.
There are several unexpected findings for this series of compounds. There is a dramatic difference between –OMe, 14a, and –OH, 14c, in binding affinities (>4,000 vs 9.0 nM using [125I]9 and >8,000 vs 150 nM using [125I]4). Addition of the methyl group significantly reduces the binding affinities to both binding sites. The same dramatic difference is observed between –NHMe, 14f, and –NMe2, 14g, in binding affinities (7.5 vs 837 nM using [125I]9 and 122 vs >7,000 nM using [125I]4). It is a striking difference in binding affinity between two seemingly related compounds; 14f has a mono-N-methylamino and 14g has a N,N-dimethylamino substitution at the p-position of the phenyl rings. In the same assay system, as expected, non-radioactive 9 showed a high binding affinity towards [125I]4 binding (Ki = 5.1 nM), while it displayed a very low binding affinity to the [125I]9 sites suggesting that there are two distinct biding sites. Both 9 and 7 showed strong competitive binding to [125I]9 binding sites (Ki = 5.0 and 1.2 nM, respectively). The results are consistent with previously reported values.18 Recently, 8 has been tested in AD patients as a Aβ plaque-specific imaging agent.13 It has been reported previously that [3H]8 binds to thehomogenates of postmortem AD brain tissue with a high binding affinity (Kd = 1.4 nM).3 In our laboratory competition of 8 to [125I]9 binding sites showed a measured Ki value of 2.8 nM, which is comparable to that reported previously. It was also observed that in our study another PET imaging agent, 10, which binds to plaques as well as tangles, however showed a moderate binding affinity towards the [125I]9 binding site (Ki = 152 nM) 18,19 indicating 10 might not compete with [125I]9 for identical binding pockets on amyloid plaques. There may be a third binding site not related to 4 or 9. This observation has not been reported previously. Significantly, the multiple binding sites on the Aβ plaques in human AD brain may be important and should be carefully considered in the future in developing in vivo imaging agents.
In summary, results of the binding study suggest that this series of novel ligands based on biphenyltrienes showed potent binding towards Aβ plaques in human AD brain tissue homogenates. The competitive binding study showed excellent binding affinities with biphenyltrienes containing -OH, -NH2, –NHMe and –NH(CH2CH2F) groups. When labeled with suitable short-lived radionuclides they may be useful as imaging agents for detecting amyloid aggregates in the living human brain.
EXPERIMENTAL
Reagents used in the synthesis were purchased from Aldrich Co. or Fluka Co., and were used without further purification unless otherwise indicated. Anhydrous Na2SO4 was used as a drying agent. 1H NMR spectra were obtained on a Bruker spectrometer (Bruker AC 200). Chemical shifts are reported as δ values with chloroform as the internal reference unless otherwise mentioned. Coupling constants are reported in Hz. The multiplicity is defined by s (singlet), d (doublet), t (triplet), br (broad) and m (multiplet). PTLC : Preparative Thin Layer Chromatography, silica gel, GF, 2000 microns from Analtech Inc. Elemental Analysis was performed by Atlantic Microlab, Inc.
General procedure for Wittig reaction
1,6-Bis(4′-methoxyphenyl)-hexa-1,3,5-triene (14a)
To a suspension of NaH (60 mg, 60% in mineral oil, 1.5 mmol) in THF (5 mL) was added a solution of tetraethyl-2-butene-1,4-diphosphonate (12) (164 mg, 0.5 mmol) in THF (5 mL) dropwise followed by a solution of 4-methoxybenzaldehyde (136 mg, 1 mmol) in THF (10 mL). The mixture was stirred under reflux overnight. Isopropyl alcohol was added to destroy the excesses of NaH after cooling. Ice-water was added and the precipitate was collected by suction, washed with water and dried. The crude product was recrystallized in benzene to afford 34 mg of product (m.p. 244–245°C, 23%).
1HNMR (200 MHz, CDCl3): δ 3.81 (s, 6H), 6.46 (s, 2H), 6.52 (d, J = 15.6 Hz, 2H), 6.74 (m, 2H), 6.85 (d, J = 8.5 Hz, 4H), 7.34 (d, J = 8.7 Hz, 4H).
Anal(C20H20O2) H; C: calcd, 82.16; found, 81.24.
1,6-Bis[4′-(2″-methox-ethoxy)-methoxyphenyl]-hexa-1,3,5-triene (14b)
Prepared as described above from 4-(2′-methoxyethoxy-)methoxy-benzaldehyde (13c, 2.1g, 10 mmol) and 12 (1.64g, 5 mmol) to afford 1.18 g of product (29%).
1HNMR (200 MHz, CDCl3): δ 3.37 (s, 6H), 3.33 (m, 4H), 3.81 (m, 4H), 5.28 (s, 4H), 6.45 (d,d, J = 6.9, 2.8 Hz, 2H), 6.51 (d, J = 15.5 Hz, 2H), 6.76 (d,d,d, J = 15.5, 7.0, 3.2 Hz, 2H), 7.00 (d, J = 8.7 Hz, 4H), 7.33 (d, J = 8.7 Hz, 4H).
Anal(C26H32O6) C, H.
1,6-Bis(4′-nitrophenyl)-hexa-1,3,5-triene (14d)
Prepared as described above from 4-nitro-benzaldehyde (302 mg, 2 mmol) and 12 (328 mg, 1 mmol) to afford 200 mg of product (62%).
1HNMR (200 MHz, CDCl3): δ 6.64 (d,d, J = 7.0, 3.0 Hz, 2H), 6.70 (d, J = 15.6 Hz, 2H), 7.04 (d,d,d, J = 15.4, 7.0, 2.9 Hz, 2H), 7.54 (d, J = 8.8 Hz, 4H), 8.19 (d, J = 8.8 Hz, 4H).
Anal(C18H14N2O4) C, H, N.
1,6-Bis(4′-dimethylaminophenyl)-hexa-1,3,5-triene (14g)
Prepared as described above from 4-dimethyaminobenzaldehyde (149 mg, 1 mmol) and 12 (164 mg, 0.5 mmol) to afford 60 mg of product (38%).
1HNMR (200 MHz, CDCl3): δ 2.97 (s, 12H), 6.42 (d,d, J = 6.9, 2.9 Hz, 2H), 6.47 (d, J = 13.8 Hz, 2H), 6.68 (d, J = 8.8 Hz, 4H), 6.70 (m, 2H), 7.30 (d, J = 8.9 Hz, 4H).
Anal(C22H26N2) C: calcd, 82.97; found, 76.55; H: calcd, 8.23; found, 8.65; N: calcd, 8.8; found, 7.89.
4-(2′-methoxyethoxy-)methoxybenzaldehyde (13c)
To a solution of 4-hydroxybenzaldehyde (3.7 g, 30 mmol) and diisopropylethylamine (7.6 mL) in CH2Cl2 (60 mL) was added MEMCl (5.0 mL) in CH2Cl2 (17 mL) dropwise at 0°C. The mixture was stirred at RT for 3 h, quenched with HCl (60 mL, 0.5 N). The mixture was extracted with CH2Cl2. The organic phase was washed with NaOH solution (1 N) and water. The separated organic phase was dried under Na2SO4, filtered and concentrated to give 6.2 g of product (97%), which was pure enough to be used to the next reaction without further purification.
1HNMR (200 MHz, CDCl3): δ 3.36 (s, 3H), 3.54 (m, 2H), 3.83 (m, 2H), 5.41 (s, 2H), 7.16 (d, J = 8.7 Hz, 2H), 7.83 (d, J = 8.7 Hz, 2H), 9.90 (s,1H).
1,6-Bis(4′-hydroxyphenyl)-hexa-1,3,5-triene (14c)
To a solution of compound 14b (237 mg, 0.54 mmol) in a mixed solvent (15 mL, THF:MeOH = 2:1) was added HCl ( 2 mL, conc.). The mixture was stirred at RT overnight. Water was added and the mixture was extracted with ethyl acetate. Usually work up gave crude product which was purified by PTLC (Hex:EA = 2:1 as developing solvent) to give 44 mg of product (31%).
1HNMR (200 MHz, CDCl3): δ 6.44 (d,d, J = 6.7, 2.7 Hz, 2H), 6.49 (d, J = 14.5 Hz, 2H), 6.70 (m, 2H), 6.75 (d, J = 8.6 Hz, 4H), 7.27 (d, J = 8.6 Hz, 4H), 7.55 (s, 2H).
Anal(C18H16O2 ·1/2 H2O) C, H.
1,6-Bis(4′-aminophenyl)-hexa-1,3,5-triene (14e)
A mixture of compound 14d (180 mg, 0.56 mmol) and SnCl2 (1.05 g) in EtOH (20 mL) was refluxed overnight. Water was added and the mixture was made basic with NaOH solution (40%). The mixture was extracted with EA. The separated organic phase was dried under Na2SO4, filtered and concentrated to give crude product which was purified by PTLC (Hexane:Ethylacetate = 1:1) to give 50 mg of product (34%).
1HNMR (200 MHz, CDCl3): δ 6.39 (d,d, J = 7.0, 3.0 Hz, 2H), 6.44 ( d, J = 15.2 Hz, 2H), 6.59 (d, J = 8.5 Hz, 4H), 6.70 (d,d,d, J = 15.5, 7.0, 3.0 Hz, 2H), 7.18 (d, J = 8.5 Hz, 4H).
Anal(C18H18N2) H, N; C: calcd, 82.41; found, 80.09.
1,6-Bis(4′-methylaminophenyl)-hexa-1,3,5-triene (14f)
To a suspension of amine 14e (15 mg, 0.06 mmol) in MeOH (3 mL) was added NaOMe (30 mg) in solid form followed by (CH2O)n (18 mg, 0.6 mmol) in solid form. The mixture was stirred under reflux for 2h. NaBH4 (44mg, 1.2 mmol) was added in portions after the reaction mixture was cooled down. The resulting mixture was stirred under reflux for 1 h. Ice water was added and the mixture was extracted with CH2Cl2. The organic phase was dried under Na2SO4, filtered, concentrated and purified by PTLC (Hexane:Ethylacetate = 2:1) to give 15 mg of product (90%).
1HNMR (200 MHz, CDCl3): δ 2.85 (s, 6H), 6.40 (d,d, J = 6.9, 2.9 Hz, 2H), 6.46 (s, J = 15.6 Hz, 2H), 6.55 (d, J = 8.6 Hz, 4H), 6.68 (m, 2H), 7.26 (d, J = 8.9 Hz, 4H).
Anal(C20H22N2 ·H2O) C, H, N.
1-(4′-aminophenyl)-6-[4′-(2′-fluoroethylamino)phenyl]-hexa-1,3,5-triene (14h)
To a solution of compound 14e (30 mg, 0.11 mmol) and 1-bromo-2-fluoroethane (100 mg, 0.78 mmol) in DMF (3 mL) was added K2CO3 (160 mg, 5 eq) and KI (5 mg). The mixture was stirred at 90°C overnight. Water was added and the mixture was extracted with CH2Cl2. The solvent was removed and the residue was purified by PTLC (Hexane:Ethylacetate = 2:1) to give 7 mg of product (20 %).
1HNMR (200 MHz, CDCl3): δ 3.45 (t,d, J =26.6, 4.5 Hz 2H), 4.62 (t,d, J = 47.3, 4.5 Hz, 2H), 6.27–6.67 (m, 10 H), 7.20–7.34 (m, 4H).
Anal(C20H21FN2) H; C: calcd, 77.89; found, 74.04; N: calcd, 9.08; found, 8.41.
Preparation of radioiodinated ligands
The desired 125I labeled ligands, 9 and 4, were prepared using iododestannylation reactions. Hydrogen peroxide (50 μL, 3% w/v) was added to a mixture of 50 μL of the correspondent tributyltin precursor (1μg/μL EtOH), 50 μL of 1N HCl and [125I]NaI (1–5 mCi) in a closed vial. The reaction was allowed to proceed for 10 min at room temperature and terminated by addition of 100 μL of sat. NaHSO3. The reaction mixture was either directly extracted (styrylbenzenes) with ethyl acetate (3 × 1 mL) or extracted after neutralization with saturated sodium bicarbonate solution (thioflavins). The combined extracts were evaporated to dryness. For styrylbenzenes the residues were dissolved in 100 μL of EtOH and purified by HPLC using a reversed phase column (Waters μbondpad, 3.9 × 300 mm) with an isocratic solvent of 65 % acetonitrile-35 % trifluoroacetic acid (0.1%) in a flow rate of 0.8 mL/min. [125I]9 was purified on a PRP-1 column (Hamilton, 4.1×250 mm) eluted with an isocratic solvent of 90 % acetonitrile-10 % 3,3-dimethyl-glutaric acid (5 mM, pH 7.0) and a flow rate of 1.0 mL/min. The desired fractions containing the product were collected, condensed and re-extracted with ethyl acetate. The no-carrier-added products were evaporated to dryness and re-dissolved in 100% EtOH (1μCi/μL). The final 125I probes, with a specific activity of 2,200Ci/mmole and a greater than 95% radiochemical purity, were stored at −20 °C up to 6 weeks for in vitro binding studies.
Binding assays using homogenates of AD brain tissue
Postmortem brain tissues were obtained from AD patients at autopsy, and neuropathological diagnosis was confirmed by current criteria (NIA-Reagan Institute Consensus Group, 1997). Homogenates were then prepared from dissected gray matters from AD patients in phosphate buffered saline (PBS, pH 7.4) at the concentration of approximately 100 mg wet tissue/ml (motor-driven glass homogenizer with setting of 6 for 30 sec). The homogenates were aliquoted into 1 ml-portions and stored at −70°C for 3–6 month without loss of binding signal.
Binding assays were carried out in 12 × 75 mm borosilicate glass tubes. For competition studies, the reaction mixture contained 50 μl of AD brain tissue homogenates (containing 20–50 μg protein), 50 μl of [125I]9 (diluted in PBS, 0.02–0.04 nM for [125I]9) and 50 μl of inhibitiors (10−7−10−10 M diluted serially in PBS containing 0.1 % bovine serum albumin) in a final volume of 1 ml. Similarly, [125I]4 (diluted in PBS, 0.02–0.04 nM) was used for the binding assay. Nonspecific binding was defined in the presence of 600 nM 9, or 4, in the same assay tubes. The mixture was incubated at 37°C for 2 hr and the bound and the free radioactivity were separated by vacuum filtration through Whatman GF/B filters using a Brandel M-24R cell harvester followed by 2 × 3 mL washes of PBS at room temperature. Filters containing the bound I-125 ligand were counted in a gamma counter (Packard 5000) with 70% counting efficiency. The results of inhibition experiments were based on the assumption that the compounds under the evaluation competed for the same binding site with the hot ligand and the data were subjected to nonlinear regression analysis with the EBDA and Ligand programs 21 by which Ki values were calculated.
Supplementary Material
Acknowledgments
This work was supported by grants awarded from the National Institutes of Health (AG022559 H.F.K and AG-21868 M-P. K.), Institute for the Study of Aging (M-P. K.) We thank Dr. George Barrio for kindly providing cold FDDNP for competition study.
References
- 1.Selkoe DJ. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140:627–38. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Mathis CA, Wang Y, Klunk WE. Imaging b-amyloid plaques and neurofibrillary tangles in the aging human brain. Curr Pharm Des. 2004;10:1469–1492. doi: 10.2174/1381612043384772. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Imaging Alzheimer’s amyloid. Nat Biotechnol. 2000;18:823–824. doi: 10.1038/78422. [DOI] [PubMed] [Google Scholar]
- 5.Mathis CA, Klunk WE, Price JC, DeKosky ST. Imaging technology for neurodegenerative diseases: progress toward detection of specific pathologies. Arch Neurol. 2005;62:196–200. doi: 10.1001/archneur.62.2.196. [DOI] [PubMed] [Google Scholar]
- 6.Klunk WE, Debnath ML, Pettegrew JW. Small-molecule beta-amyloid probes which distinguish homogenates of Alzheimer’s and control brains. Biol Psychiatry. 1994;35:627. [Google Scholar]
- 7.Klunk WE, Debnath ML, Koros AM, Pettegrew JW. Chrysamine-G, a lipophilic analogue of Congo red, inhibits Aβ-induced toxicity in PC12 cells. Life Sci. 1998;63:1807–1814. doi: 10.1016/s0024-3205(98)00454-8. [DOI] [PubMed] [Google Scholar]
- 8.Elhaddaoui A, Pigorsch E, Delacourte A, Turrell S. Competition of congo red and thioflavin S binding to amyloid sites in Alzheimer’s diseased tissue. Biospectroscopy. 1995;1:351–356. [Google Scholar]
- 9.Styren SD, Hamilton RL, Styren GC, Klunk WE. X-34, a fluorescent derivative of Congo Red: a novel histochemical stain for Alzheimer’s disease pathology. J Histochem Cytochem. 2000;48:1223–1232. doi: 10.1177/002215540004800906. [DOI] [PubMed] [Google Scholar]
- 10.Link CD, Johnson CJ, Fonte V, Paupard MC, Hall DH, Styren S, Mathis CA, Klunk WE. Visualization of fibrillar amyloid deposits in living, transgenic Caenorhabditis elegans animals using the sensitive amyloid dye, X-34. Neurobiol Aging. 2001;22:217–226. doi: 10.1016/s0197-4580(00)00237-2. [DOI] [PubMed] [Google Scholar]
- 11.Hintersteiner M, Enz A, Frey P, Jaton AL, Kinzy W, Kneuer R, Neumann U, Rudin M, Staufenbiel M, Stoeckli M, Wiederhold KH, Gremlich HU. In vivo detection of amyloid-beta deposits by near-infrared imaging using an oxazine-derivative probe. Nat Biotechnol. 2005;23:577–83. doi: 10.1038/nbt1085. [DOI] [PubMed] [Google Scholar]
- 12.Agdeppa ED, Kepe V, Liu J, Flores-Torres S, Satyamurthy N, Petric A, Cole GM, Small GW, Huang SC, Barrio JR. Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for β-amyloid plaques in Alzheimer’s disease. J Neurosci. 2001;21:RC189. doi: 10.1523/JNEUROSCI.21-24-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang G-f, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging Brain Amyloid in Alzheimer’s Disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 14.Ono M, Wilson A, Nobrega J, Westaway D, Verhoeff P, Zhuang ZP, Kung MP, Kung HF. 11C-Labeled Stilbene Derivatives as Aβ-aggregate-specific PET Imaging Agents for Alzheimer’s Disease. Nucl Med Biol. 2003;30:565–571. doi: 10.1016/s0969-8051(03)00049-0. [DOI] [PubMed] [Google Scholar]
- 15.Verhoeff NP, Wilson AA, Takeshita S, Trop L, Hussey D, Singh K, Kung HF, Kung MP, Houle S. In vivo imaging of Alzheimer disease beta-amyloid with [11C]SB-13 PET. Am J Geriatr Psychiatry. 2004;12:584–595. doi: 10.1176/appi.ajgp.12.6.584. [DOI] [PubMed] [Google Scholar]
- 16.Kauffman JM, Moyna G. Diarylamino groups as photostable auxofluors in 2-benzoxazolylfluorene, 2,5-diphenyloxazoles, 1,3,5-hexatrienes, 1,4-distyrylbenzenes, and 2,7-distyrylfluorenes. J Org Chem. 2003;68:839–53. doi: 10.1021/jo020333+. [DOI] [PubMed] [Google Scholar]
- 17.Chen CH, Doney JJ, Reynolds GA, Saeva FD. New donors with two-electron oxidation. Synthesis and electrochemical properties of highly conjugated bis(4H-thiopyrans) and bis(flavenes) J Org Chem. 1983;48:2757–61. [Google Scholar]
- 18.Kung MP, Hou C, Zhuang ZP, Skovronsky D, Kung HF. Binding of two potential imaging agents targeting amyloid plaques in postmortem brain tissues of patients with Alzheimer’s disease. Brain Res. 2004;1025:89–105. doi: 10.1016/j.brainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Kung MP, Hou C, Zhuang ZP, Cross AJ, Maier DL, Kung HF. Characterization of IMPY as a potential imaging agent for β-amyloid plaques in double transgenic PSAPP mice. Eur J Nucl Med Mol Imaging. 2004;31:1136–1145. doi: 10.1007/s00259-004-1487-z. [DOI] [PubMed] [Google Scholar]
- 20.Zhuang ZP, Kung MP, Wilson A, Lee CW, Plossl K, Hou C, Holtzman DM, Kung HF. Structure-activity relationship of imidazo[1,2-a]pyridines as ligands for detecting beta-amyloid plaques in the brain. J Med Chem. 2003;46:237–243. doi: 10.1021/jm020351j. [DOI] [PubMed] [Google Scholar]
- 21.Munson PJ, Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.