Abstract
The Ser/Thr phosphatase PP2A is a set of multisubunit enzymes that regulate many cellular processes. In yeast, the PP2A regulatory subunit Tap42 forms part of the Target of Rapamycin (TOR) signaling pathway that links nutrient and energy availability to cell growth. The physiological intersection between the mammalian orthologs of Tap42 and TOR, α4 and mTOR, has not been fully characterized. We used two in vivo models of liver growth in the rat, late gestation fetal development and regeneration after partial hepatectomy, to explore the regulation of the α4-containing form of PP2A. The α4/PP2A catalytic subunit (α4/PP2A-C) complex was present in both fetal and adult liver extracts. There was a trend towards higher levels of α4 protein in fetal liver, but the complex was more abundant in adult liver. Fractionation of extracts by ion exchange chromatography and transient transfection of the AML12 mouse hepatic cell line indicated that α4 associates with PP2A-C but that these complexes have low catalytic activity with both peptide and protein substrates. α4 was able to associate with forms of PP2A-C that were both methylated and non-methylated at the carboxy-terminus. The mTOR inhibitor rapamycin did not block the formation of α4/PP2A-C in liver or hepatic cells, nor did it appear to modulate PP2A activity. Furthermore, sensitivity to the growth inhibitory effects of rapamycin among a panel of hepatic cell lines did not correlate with levels of α4 or α4/PP2A-C. Our results indicate that the yeast Tap42/TOR paradigm is not conserved in hepatic cells.
Keywords: Protein phosphorylation, mTOR, rapamycin, regeneration, cell growth
PP2A is a major Ser/Thr phosphatase that plays a significant role in the regulation of many cellular processes, including metabolism, transcription, translation, cell cycle progression, cell growth and apoptosis [Janssens and Goris, 2001; Mumby and Walter, 1993; Sontag, 2001; Zolnierowicz, 2000]. The activity of such a versatile enzyme must therefore be tightly controlled in vivo. Indeed, the catalytic subunit of PP2A (PP2A-C) is regulated at many levels. The C-terminus of PP2A-C is post-translationally modified by phosphorylation at Y307 and methyl esterification at L309. Phosphorylation results in inhibition of PP2A activity [Chen et al., 1992; Guo and Damuni, 1993] while methylation is thought to play an important function in regulatory subunit binding [Bryant et al., 1999; Tolstykh et al., 2000; Wu et al., 2000; Yu et al., 2001]. More specifically, PP2A-C methylation can influence the association of PP2A-C with specific regulatory subunits, thereby serving to determine the composition, subcellular localization and function of PP2A-C holoenzymes in a variety of cell types [Gentry et al., 2005; Longin et al., 2007; Nunbhakdi-Craig et al., 2007].
The prototypical PP2A enzyme is a heterodimer that consists of the catalytic C subunit and scaffolding A subunit. The substrate specificity and subcellular localization of PP2A are thought to be defined by an array of B-type regulatory subunits that bind to the AC dimer to form a variety of heterotrimeric complexes [Mumby and Walter, 1993]. Among various B-type subunits, B56α/β/ε complexed with the A and C subunits localize to the cytoplasm, whereas heterotrimeric forms of PP2A that include B56δ/γ1/γ3 are concentrated in the nucleus [McCright et al., 1996]. Likewise, the two regulatory subunits Cdc55p (a B-type subunit) and Rts1p (a B′-type subunit) of Saccharomyces cerevisiae display distinct, dynamic localization patterns that support the hypothesis that regulatory subunits determine localization of PP2A [Gentry and Hallberg, 2002]. Recent structural studies further support the role of the B subunit in determining substrate specificity by showing that regulatory B′ subunit interacts with the PP2A-C subunit near the active site and that the B′ subunit is responsible for the ultimate tertiary structure of the catalytic subunit [Cho and Xu, 2007; Xu et al., 2006].
Studies in yeast have established a model in which PP2A, through its interaction with Tap42, functions as a component of the rapamycin-sensitive TOR signaling pathway, a pathway required for initiation of protein synthesis in response to nutrient availability. In this model, TOR phosphorylates Tap42 to promote association with the catalytic subunits of several Ser/Thr phosphatases, including the yeast homologs of PP2A-C [Jiang Y and Broach JR, 1999]. Under nutrient-rich conditions, phosphorylated Tap42 binds to and inhibits PP2A activity towards downstream effectors of TOR, such as the transcriptional regulator GLN3 [Beck and Hall, 1999] and the Ser/Thr kinase NPR1 [Schmidt et al., 1998]. In contrast, nutrient starvation or rapamycin block the phosphorylation of Tap42, inducing the dissociation of Tap42 from PP2A-C [Di Como CJ and Arndt KT, 1996]. Thus, the overall model suggests that TOR regulates protein phosphorylation in yeast by indirectly restraining the activity of PP2A through phosphorylation of Tap42.
In mammalian cells, PP2A-C has been found to associate with the homolog of Tap42, a novel regulatory protein termed α4 [Murata et al., 1997; Prickett and Brautigan, 2004]. α4 binds to PP2A-C independent of the A and B subunits. Thus, α4/PP2A-C is considered to represent a unique, heterodimeric form of the phosphatase [Jiang Y and Broach JR, 1999].
Whether mTOR regulation of α4/PP2A-C plays a role analogous to that in yeast is controversial. Some studies suggest that mTOR can directly phosphorylate its effectors, such as S6K1 and 4E-BP1, while others claim that the mTOR-dependent phosphorylation of S6K1 and 4E-BP1 is an indirect result of the inhibition of PP2A [Gingras AC et al., 2001a; Gingras AC et al., 2001b]. PP2A is able to dephosphorylate S6K1 in vitro [Ferrari et al., 1991], and okadaic acid, a potent PP2A inhibitor, antagonizes dephosphorylation of TOR substrates (i.e. S6K1 and 4E-BP1), promoting S6K1 activation and cell growth in Drosophila [Cygnar et al., 2005]. A role for PP2A is supported by studies showing a direct interaction of PP2A with S6K1 in mammalian cells [Peterson RT et al., 1999; Westphal et al., 1999].
The α4 protein has been found to associate with the catalytic subunits of PP2A, PP4 and PP6, making it a common regulator of multiple PP2A family members [Chen et al., 2004; Kloeker et al., 2003; Murata et al., 1997]. Mutations in PP2A-C that induce the loss of binding to A subunit enhance its binding to α4, whereas mutations that eliminate the α4 association increase AC content [Prickett and Brautigan, 2004]. This suggests a competitive and mutually exclusive association of A subunit and α4 with PP2A-C in cells. Furthermore, previous studies show that single mutations of PP2A-C at the site of phosphorylation (Y307F) or methyl esterification (L309Q) result in recovery of only AC dimers whereas double mutation of both residues favor the formation of α4/PP2A-C [Chung et al., 1999]. This suggests a role for PP2A-C post-translation modification in the association with α4. Recent structural studies reveal that the tetratricopeptide repeat-like fold in Tap42/α4 may have a scaffolding function that promotes the dephosphorylation of substrates recruited to the C-terminal region of PP2A-C [Yang et al., 2007]. It has been proposed that α4, rather than simply inhibiting PP2A activity, may protect mTOR downstream effectors from being dephosphorylated by modulating the activity of other undefined phosphatases [Inui et al., 1998]. It has been shown that α4 interacts with S6K1 through PP2A-C in a rapamycin-sensitive manner [Yamashita et al., 2005]. Recent studies in Drosophila have suggested that PP2A, but not other members of this subfamily, is the major S6K phosphatase [Bielinski and Mumby, 2007]. Studies in Drosophila led to the interesting observation that Tap42 induces nuclear accumulation of PP2A-C but loss of TOR has no effect on PP2A-C localization [Cygnar et al., 2005]. This suggests that Tap42 may act independently of TOR and regulate the localization of PP2A, similar to the B regulatory subunits.
A number of questions regarding the function and regulation of the mTOR/α4/PP2A system remain unanswered. Rapamycin sensitivity of the interaction between α4 and PP2A-C has been observed in some systems [Inui et al., 1998; Kong et al., 2004; Murata et al., 1997], but in others this association is unaffected by rapamycin [Kloeker et al., 2003; Nanahoshi et al., 1998; Nien et al., 2007]. In addition, the issue of whether α4 has a positive or negative effect on PP2A activity has not been definitively determined.
Early studies indicated that yeast strains that overexpressed Tap42 mutants were almost completely resistant to rapamycin [Di Como CJ and Arndt KT, 1996]. α4 transfection in Jurkat cells was shown to result in rapamycin resistance [Inui et al., 1998]. Conditions that promote the association of Tap42 with the yeast homologs of PP2A-C, such as deletion of TPD3 (yeast A subunit) or CDC55 (yeast B subunit), induce partial rapamycin resistance [Jiang Y and Broach JR, 1999]. These relationships between a4 and resistance to the anti-proliferative effects of rapamycin have been relatively unexplored in mammalian models for rapamycin resistance.
In the present studies, we have explored the in vivo relationship between mTOR, α4 and PP2A-C in liver. We used two in vivo models of liver growth, the rapamycin-resistant hepatocyte proliferation that occurs in the late gestation fetal rat, and the rapamycin-sensitive process of liver regeneration induced by 2/3 partial hepatectomy in the adult rat [Curran et al., 1993; Fausto and Webber, 1993; Gruppuso et al., 1997]. We also utilized in vitro hepatic cell models in an effort to facilitate the study of the functional regulation of PP2A. The normal diploid nontumorigenic rat hepatic epithelial line (WB-F344, abbreviated as WB) was originally isolated from an adult Fischer rat [Tsao MS et al., 1984]. We have utilized a spectrum of cell lines derived from the WB line or from other sources [Campbell et al., 2000; Hixson et al., 1985] that we have found exhibit varying degrees of rapamycin sensitivity. Based on the limited transfection efficiency seen with these rat hepatic cell lines, we also utilized a hepatic cell line derived from mice transgenic for human TGFα, AML12 [Wu et al., 1994]. Overall, our studies have found that the regulation of the association between PP2A-C and α4 and the effect of rapamycin on the interaction in hepatic cells do not follow the yeast paradigm.
Materials and Methods
Materials
Antibodies to HA, full-length PP2A-C and the p85 subunit of phosphatidylinositol 3-kinase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). C-terminus PP2A-C antibody and PP2A assay reagents were obtained from Millipore Corporation (Billerica, MA). FLAG antibody was from Sigma-Aldrich (St. Louis, MO). Antibodies directed towards ribosomal protein S6 and phosphorylated S6Ser235/236 were obtained from Cell Signaling Technology, Inc. (Danvers, MA). Mid-portion PP2A-C antibody and α4 antibody were prepared as described previously [Chen et al., 1994; Prickett and Brautigan, 2004]. Myelin basic protein from bovine brain (Sigma-Aldrich Corp., St. Louis, MO) was used as substrate in a PP2A assay. It was first phosphorylated using recombinant, full length, active human MAP Kinase1/Erk1 from Millipore Corporation and [γ-32P]-ATP (3000 Ci/mmol; Perkin Elmer Life and Analytical Sciences, Boston, MA). Lipofectamine and Plus reagents were purchased from Invitrogen Corporation (Carlsbad, CA). The MonoQ HR5/5 column was purchased from GE Healthcare (Piscataway, NJ). Rapamycin was purchased from LC Laboratories (Woburn, MA).
Animals and Preparation of Liver Extracts
Adult Sprague-Dawley male rats and pregnant rats of known gestational age (term being 21 days) were used for all studies (Charles River Laboratories, Wilmington, MA). Adult male rats (125-175 g) were anesthetized with pentobarbital (50 mg/kg body weight, administered by intraperitoneal injection) and exsanguinated prior to removal of the liver. For pregnant rats, cesarean sections were performed under pentobarbital anesthesia and fetal (embryonic day 19) livers were harvested before sacrifice. Two-thirds partial hepatectomy was performed on adult male rats (125-175 g) under isofluorane anesthesia as previously described [Sanders and Gruppuso, 2005]. For rapamycin studies, the animals were injected with DMSO vehicle or 2.5 μg rapamycin/g body weight prior to sacrifice at the times specified. Except where noted, livers were flash-frozen in liquid nitrogen and stored at −70°C until use. Wherever replicate analyses are referred to, samples were prepared from separate adult livers or from separate pools of fetal livers. Fetal livers were taken from multiple dams. All animal studies complied with guidelines set by the Rhode Island Hospital Institutional Animal Care and Use Committee. Rat liver extracts were prepared as previously described [Yoo et al., 2007].
Cell Lines and Preparation of Extracts
AML12 cells were cultured in Dulbecco's modified Eagle's medium/Ham's F-12 (DMEM/F-12) medium containing 10% fetal bovine serum and supplemented with 0.1% gentamicin (Invitrogen Corporation, Carlsbad, California), insulin, transferrin and selenious acid (ITS; Becton Dickinson, Franklin Lakes, NJ) and 0.04 μg/ml dexamethasone. The WB, GN5, WB311 and GP6 cell lines were routinely cultured in DMEM/F-12 containing 10% FBS, 2 mM L-glutamine, gentamicin and ITS. H4-II-E cells were cultured in MEM with 5% FBS. 1682-A, THC 252 and H5D cells were routinely cultured in Weymouth's medium containing 10% FBS.
Where noted, cell lines were treated with rapamycin at various concentrations (0 to 200 nM) and for various durations (10 min to 24 hrs). Analysis for [3H]thymidine incorporation was performed as previously described [Curran et al., 1993]. Cell lysates were prepared using a buffer composed of 20 mM Tris-HCl, pH 7.5, 5 mM EGTA, 2 mM EDTA, 1 mM DTT, 0.1% NP-40 and 10% glycerol plus protease inhibitors (10 μg/ml leupeptin, 10 μg/ml aprotinin and 34.4 μg/ml 4-(2-aminoethyl)-benzenesulfonyl fluoride). Cell lysates were clarified by centrifugation for 10 min at 14,000 rpm. Supernatants were frozen at −70°C until analysis.
Determination of PP2A Activity
PP2A activity in liver extracts, cell lysates and immunoprecipitates was determined using a peptide-based assay. The reaction mixture (50 μl) included 5 μl of 1 mM peptide [KR(pT)IRR] and sample (5 μg liver extract protein, 3 μl column eluate or immunoprecipitate derived from 100 μg lysate protein). Immunoprecipitates were analyzed while bound to antibody beads. In all cases, the reaction was allowed to proceed for 30 min at 30°C. Following centrifugation, 25 μl of the reaction mixture was added to 100 μl of the Malachite Green Phosphate Detection Solution. As per the manufacturer's instructions, the color was allowed to develop for 30 min following which the absorbance was read at 650 nm. Results, representing phosphate released from substrate, were quantified relative to a phosphate standard curve.
PP2A activity was also determined as the release of 32P from radiolabeled MBP. Preparation of the 32P-MBP substrate was carried out as follows. A kinase reaction mixture containing 25 mM Tris/HCl, pH 7.5, 0.02 mM EGTA, 412.5 μg MBP, 1.5 μg recombinant Erk1, 10 mM Mg(CH3COO)2, 100 μM ATP and 50 μCi [γ-32P]-ATP was incubated 30°C for 3 days. The labeling reaction was terminated by adding trichloroacetic acid to a final concentration of 20% and incubating on ice for 90 min. The resulting pellet was collected by centrifugation at 16,100 × g for 5 min and washed with 10% ice-cold trichloroacetic acid. The pellet was diluted in 100 μl of 0.1M NaOH followed by the addition of 150 μl of Substrate Dilution Buffer (20 mM MOPS, pH 7.5, 50 mM NaCl, 1 mM MgCl2, 1 mM dithiothreitol, 0.1% B-mercaptoethanol, 10% glycerol, and 0.1 mg/ml bovine serum albumin). The substrate was further diluted to 2 μM 32P-ATP and stored at -20°C until use. For the phosphatase assay, sample (10 μl) plus substrate (10 μl) were incubated at 30°C for 10 min. The reaction was stopped by adding 100 μl of 25mg/ml BSA and 880 μl of 20% TCA. Following centrifugation at 16,100 × g, 0.9 ml of the supernatant was counted in 5 ml of Optifluor (Perkin Elmer Life and Analytical Sciences) to determine the amount of 32P released from the substrate.
PP2A-C and α4 Transfection
Plasmids encoding wild-type (WT) PP2A-C, L309Q-PP2A-C and α4 were generated as previously described [Chung et al., 1999; Murata et al., 1997]. AML12 cells were transfected with HA-tagged WT-PP2A-C or L309Q-PP2A-C, or with FLAG-tagged α4. For this procedure, 3 × 105 cells per well were seeded onto 6-well plates to achieve ∼60% confluence. 4 μl of Plus reagent was added to 1 μg of DNA in total volume of 100 μl and incubated for 15 min at room temperature. This mixture was then combined with diluted lipofectamine transfection reagent (4 μl/100 μl) in 1:1 ratio and incubated for 15 min at room temperature. The final mixture (200 μl) was applied to cells in 1 ml of their usual media minus serum and incubated for 3 hr. The transfection was terminated by addition of fresh media containing 10% fetal bovine serum.
For immunoblotting, 100 μg of AML12 cell lysate protein, prepared as described above, was immunoprecipitated with 5 μg of FLAG antibody. The resulting immunoprecipitates were run on 10% SDS-polyacrylamide gel and analyzed using MD-PP2A-C, CT-PP2A-C, anti-α4, anti-HA and anti-FLAG antibodies. Lysates prepared from cells exposed to media only, exposed to media with lipofectamine reagents or transfected with empty vector were used as controls.
Western Immunoblotting
For analysis of liver extracts, 80 μg of total protein in Laemmli sample buffer was separated using a 10% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). For analysis of cell lysates, 10-20 μg of extract protein was used. For all immunoblots, detection employed an enhanced chemiluminescence method (GE Healthcare, Piscataway, NJ). Image acquisition employed the ChemiDoc-It Imaging System with Labworks 4.5 Image Acquisition and Analysis Software (UVP, Inc., Upland, CA).
Results
The α4/PP2A Complex in Fetal and Adult Liver
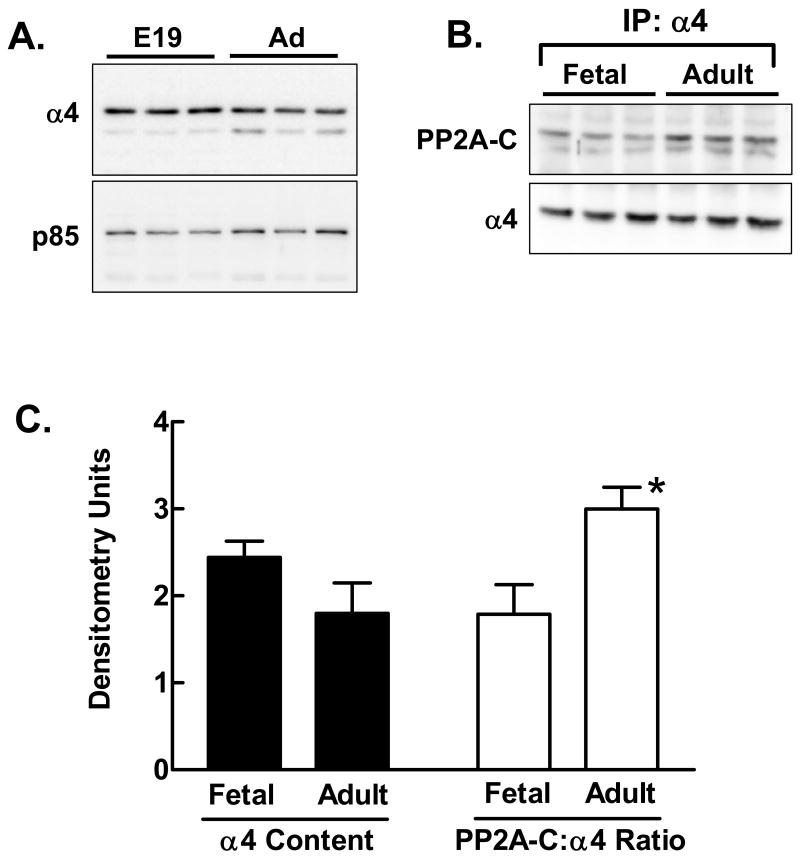
Direct immunoblotting of triplicate samples of unfractionated homogenates showed that α4 content tended to be slightly higher in fetal liver than in adult liver (Fig. 1A). The immunoblot was also probed for the p85 subunit of PI3-kinase in order to demonstrate the consistency of transfer. Immunoprecipitation with α4 antibody followed by analysis of the immunoprecipitates by immunoblotting for α4 and PP2A-C showed that more PP2A-C was bound to α4 in adult compared to fetal liver (Fig. 1B). PP2A-C appeared as a doublet, as previously observed by us [Yoo et al., 2007] and by others [Turowski et al., 1995; Yu et al., 2001]. Quantification of the results of the direct immunoblotting and immunoprecipitation (Fig. 1C) showed that the trend towards higher α4 levels in fetal liver was not significant while the higher content of the α4/PP2A-C complex in adult liver reached significance at the level of P<0.01.
Fig. 1.
Content of α4 and α4/PP2A-C in fetal and adult liver. A: Liver extracts were prepared from triplicate fetal and adult livers. Extracts (80 μg protein per lane) were analyzed for α4 protein content by direct immunoblotting. Blots were stripped and reprobed for the p85 subunit of phosphatidylinositol 3-kinase to demonstrate consistency in the transfer. B: Extracts from triplicate fetal and adult livers were analyzed for content of the α4/PP2A-C complex by immunoprecipitation of α4 followed by immunoblotting for PP2A-C. The blot was stripped and reprobed for α4 to verify equivalent immunoprecipitation in all samples. C: The results of the immunoblots in panels A and B were quantified. Results are shown as the mean + 1 standard deviation. *, P<0.01 versus fetal results.
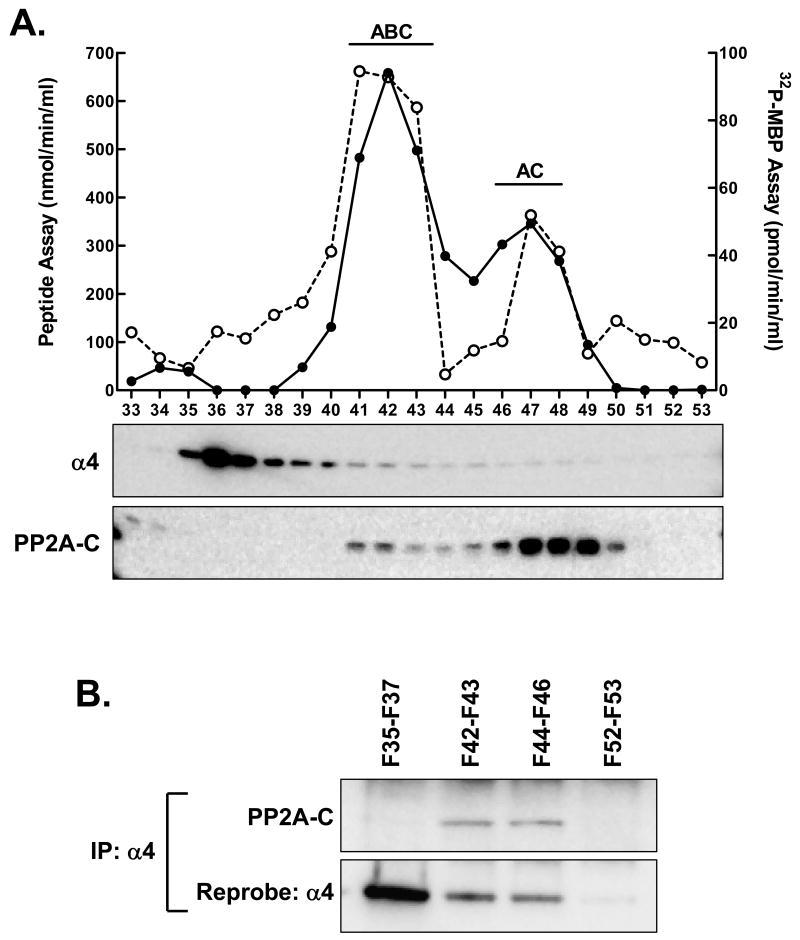
In order to examine the activity of α4/PP2A-C complexes, liver extracts were fractionated by MonoQ chromatography as described previously [Yoo et al., 2007]. Fractions were analyzed for PP2A activity using a peptide substrate and 32P-MBP (Fig. 2A). The activity profiles obtained with both substrates were similar, demonstrating two main peaks of activity. Our analysis of numerous such profiles by immunoblotting for regulatory subunits allowed us to assign the first and second peaks to the ABC and AC forms of PP2A, respectively [Yoo et al., 2007].
Fig. 2.
Chromatographic separation of adult liver extract PP2A complexes. A: Extract protein was applied to a MonoQ ion-exchange column and eluted with a linear salt gradient from 0 to 0.6 M. PP2A activity was determined in individual fractions using peptide substrate (filled circles/solid line) and 32P-MBP (unfilled circles/dashed line). Samples of each fraction were then analyzed by immunoblotting with antibodies directed towards PP2A-C and α4. The previously determined locations of the ABC and AC forms of PP2A are shown above the major activity peaks. B: Fractions were combined to give four pools; fractions 35-37, 42-43, 44-46 and 52-53. An equal volume of each fraction pool (1 ml) was analyzed by α4 immunoprecipitation followed by immunoblotting for PP2A-C. The blot was then stripped and reprobed for α4.
Immunoblotting of the MonoQ chromatography fractions for α4 showed a major peak that eluted early in the gradient (fractions 36 to 40) with continued elution through later fractions (up to fraction 46). The elution of PP2A-C occurred in two peaks that were in near alignment with the activity peaks and that corresponded to the ABC and AC forms of the enzyme. In order to determine the position of the α4/PP2A-C complex, four fraction pools were analyzed by immunoprecipitation for α4 followed by immunoblotting for α4 and PP2A-C (Fig. 2B). Results showed that the first peak of α4 was not associated with PP2A-C. Two fraction pools from the later-eluting α4, one under the major activity peak and the second between the two peaks, showed the presence of α4/PP2A-C. Given the relationship between activity and the position of the α4/PP2A-C complex, it did not appear that this complex made a significant contribution to PP2A activity with either substrate. A pool derived from the end of the gradient served as a negative control, showing neither α4 nor PP2A-C in the immunoprecipitate.
The elution pattern of the α4/PP2A-C complex was replicated in a second experiment (data not shown). Similar results were also observed in analyses of fetal liver extracts (data not shown).
The Effect of Rapamycin on α4/PP2A-C
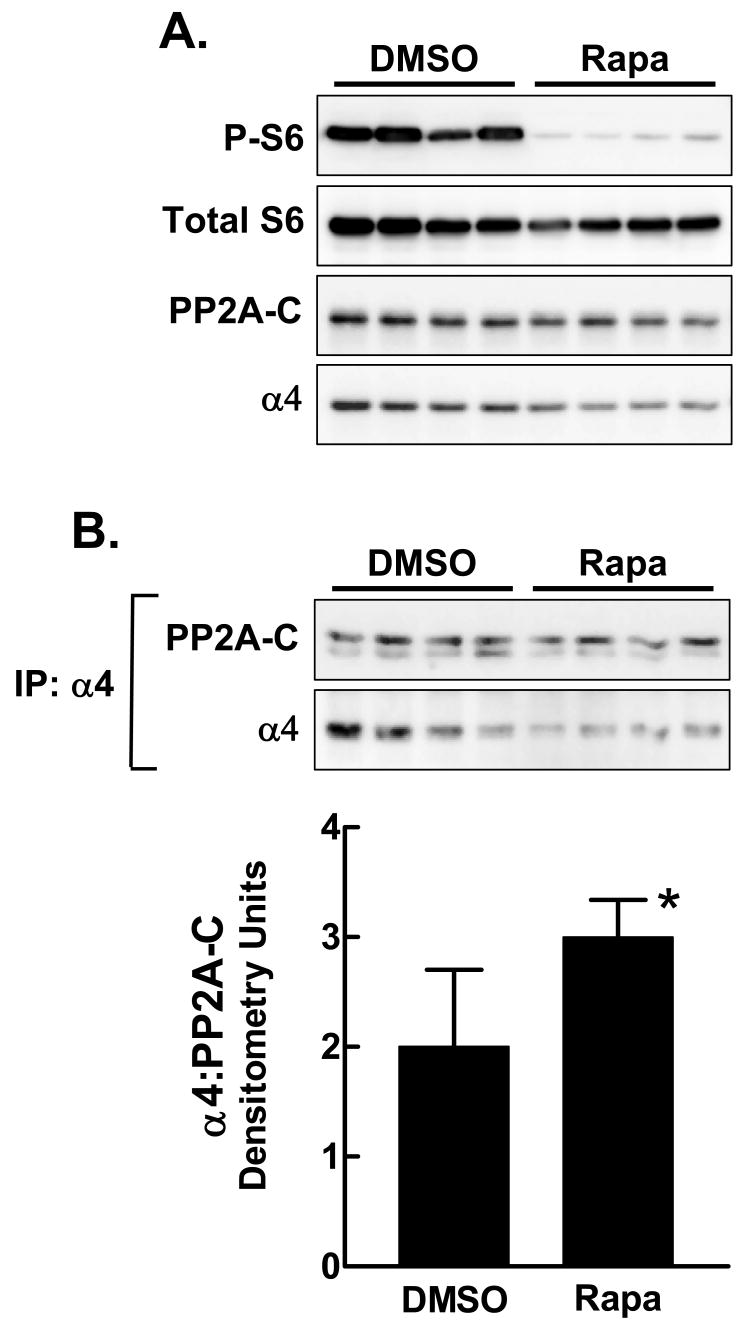
Previous studies in yeast and in mammalian cells have shown that α4 and PP2A-C dissociate in response to rapamycin [Gingras AC et al., 2001a; Inui et al., 1998; Murata et al., 1997]. We sought to determine if the same effect occurs in hepatic cells in vivo. Liver extracts were prepared from rats sacrificed 24 hr after intraperitoneal administration of rapamycin or DMSO vehicle as control (Fig. 3). Reduction of phosphorylated ribosomal protein S6 confirmed that rapamycin administration produced a marked inhibition of mTOR signaling. Liver PP2A-C content was unaffected, though the level of α4 was slightly reduced by rapamycin administration. Immunoprecipitation with α4 antibody followed by immunoblotting showed a modest increase in the content of the α4/PP2A-C complex in animals that received rapamycin relative to those that were given vehicle alone. There was most certainly no evidence for a rapamycin-induced reduction in the content of the α4/PP2A-C complex.
Fig. 3.
The effect of rapamycin on formation of the α4/PP2A-C complex in adult liver. Extract protein was prepared from livers taken from adult rats injected 24 hr previously with DMSO or rapamycin. A: The extracts (80 μg protein per lane) were analyzed by direct immunoblotting for phosphorylated ribosomal protein S6 (P-S6), total S6, PP2A-C and α4. B: Extracts (1 mg protein) were analyzed by immunoprecipitation with α4 antibody, followed by immunoblotting for PP2A-C and α4. The ratio of PP2A-C to α4 in the immunoprecipitates is shown in the bar graph as the mean + 1 standard deviation. *, P<0.05 versus DMSO control.
In order to repeat these analyses under conditions of maximal mTOR activation [Anand and Gruppuso, 2006], animals were fasted for 48 hr then injected with DMSO or rapamycin 15 min prior to a 1 hr refeeding period. The effectiveness of the rapamycin injection was again confirmed using immunoblotting for phosphorylated S6 as an indicator of mTOR signaling. Under these conditions, rapamycin administration did not change the amount of α4 associated with hepatic PP2A-C in vivo (data not shown).
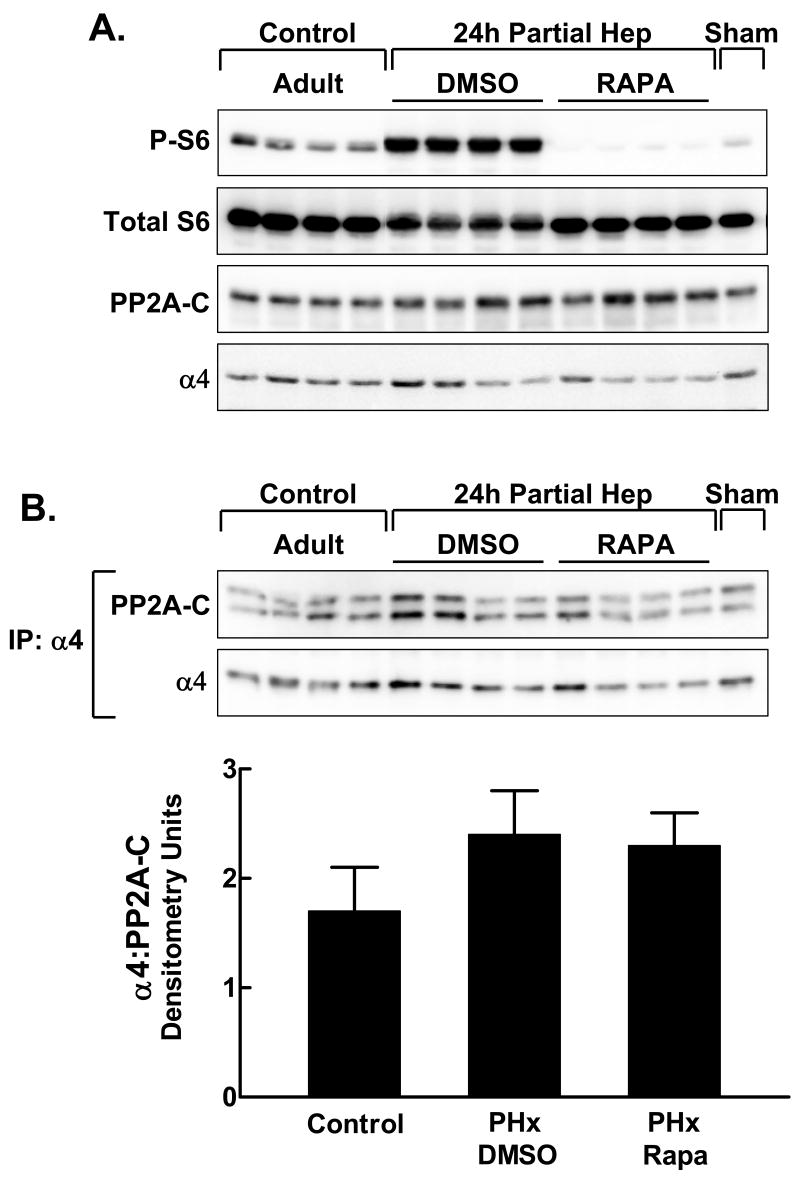
We went on to examine α4/PP2A-C in animals that underwent partial hepatectomy with or without prior administration of rapamycin. Animals were sacrificed 24 hr after partial hepatectomy. As expected, S6 phosphorylation was induced by partial hepatectomy and fully inhibited by the prior administration of rapamycin (Fig. 4A). Partial hepatectomy did not induce changes in the content of either PP2A-C or α4 proteins (Fig. 4A). The content of the α4/PP2A complex recovered by α4 immunoprecipitation also was unaffected by partial hepatectomy or by rapamycin (Fig. 4B). Similar results were obtained in animals sacrificed 6 hr and 48 hr after partial hepatectomy (data not shown).
Fig. 4.
The effect of rapamycin on α4/PP2A-C in regenerating liver. Adult rats were injected with DMSO vehicle or rapamycin 1 hr before partial hepatectomy. Control animals were not subjected to surgery. One animal underwent sham hepatectomy in which the liver was exteriorized but not ligated or excised. Animals were sacrificed and livers obtained 24 hr after surgery. A: The extracts (80 μg protein per lane) were analyzed by direct immunoblotting for phosphorylated ribosomal protein S6 (P-S6), total S6, PP2A-C and α4. B: Extracts (1 mg protein) were analyzed by immunoprecipitation with α4 antibody followed by immunoblotting for PP2A-C and α4. The ratio of PP2A-C to α4 in the immunoprecipitates is shown for the control, partial hepatectomy/DMSO and partial hepatectomy/rapamycin groups in the bar graph as the mean + 1 standard deviation.
In Vitro Studies on the Regulation of α4/PP2A-C in Hepatic Cells
Most hepatic cell lines show poor transfection efficiency. Among those that we tested, only AML12 cells showed adequate transfection efficiency to acquire interpretable data. Preliminary studies (data not shown) revealed that the AML12 cells are highly rapamycin-sensitive and that the transfection conditions used were associated with excellent cell viability and a transfection efficiency of approximately 10%.
AML12 cells were transfected with HA-tagged PP2A-C constructs that encoded the wild-type enzyme (WT) or one mutated at leucine 309 to an uncharged polar residue, glutamine (L309Q). The latter cannot be methylated. These plasmids were transfected alone or in combination with FLAG-tagged α4. Transfection with FLAG-α4 alone was also performed. Direct immunoblotting was carried out using antibodies directed towards HA, FLAG, PP2A-C and α4 (Fig. 5A). Immunoblotting for HA showed that WT-PP2A-C migrated at 45 kDa while L309Q-PP2A-C migrated at 43 kDa. Both antibodies detected a minor form at 41 kDa. Levels of both the WT-PP2A-C and L309Q-PP2A-C were enhanced by co-transfection with FLAG-α4. This suggested interaction between the proteins that resulted in stabilization of the PP2A-C. Immunoblotting with the antibody directed towards the mid-portion of PP2A-C detected both WT-PP2A-C and L309Q-PP2A-C as well as endogenous PP2A-C at 39 kDa. Levels of the endogenous protein were not affected by transfection.
Fig. 5.
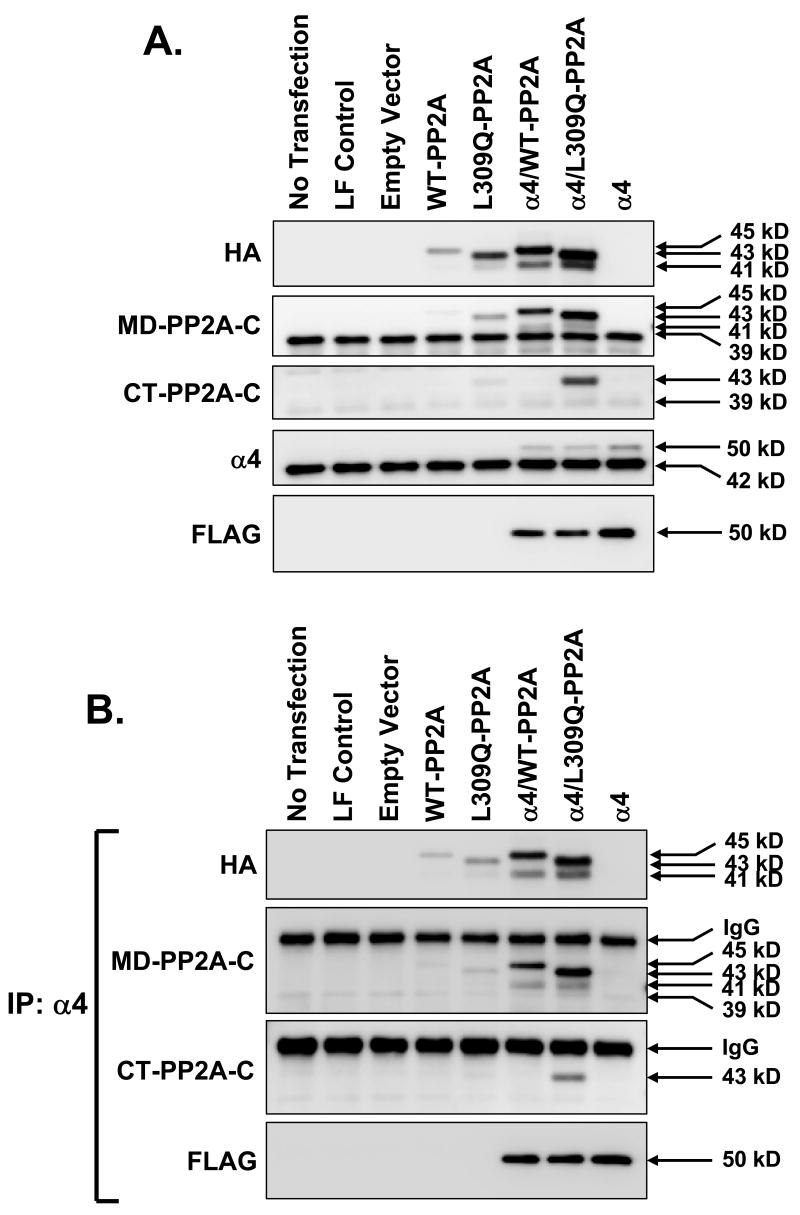
Formation of α4/PP2A-C in AML12 cells. AML12 cells were transfected with 1 μg of DNA encoding HA-tagged wild-type (WT)-PP2A-C, L309Q-PP2A-C and FLAG-tagged α4 alone or in combination. The lysates from untransfected cells, cells exposed to Lipofectamine/Plus transfection reagent (LF control) or cells transfected using empty vector were used as negative controls. A: Lysate protein (10 μg) was analyzed by direct immunoblotting using antibodies against HA, MD-PP2A, CT-PP2A, α4, and FLAG. B: Lysate protein (200 μg) was immunoprecipitated with α4 antibody followed by immunoblotting with HA, MD-PP2A, CT-PP2A, and FLAG antibodies.
We showed previously [Yoo et al., 2007] that immunoblotting with CT-PP2A-C antibody only detects unmethylated PP2A-C. Results of immunoblotting with this antibody indicated that the endogenous PP2A-C was predominantly in the methylated form. In addition, ectopic WT-PP2A-C did not react with CT-PP2A-C antibody, indicating that this form also was methylated. As expected, L309Q-PP2A-C reacted with CT-PP2A-C antibody, consistent with its non-methylated status. Immunoblotting for α4 showed similar levels of the endogenous 42 kDa protein under all conditions.
In order to assess the interactions between transfected PP2A-C and α4, cell lysates were immunoprecipitated with α4 antibody. The immunoprecipitates were analyzed by immunoblotting. Results (Fig. 5B) showed that immunoprecipitation with α4 antibody yielded positive immunoblot results with both the MD-PP2A-C and CT-PP2A-C antibodies. This was interpreted as indicating that the transfected α4 was capable of binding to both the methylated and unmethylated forms of PP2A-C. We did not observe interactions between the transfected α4 and endogenous PP2A-C. This is likely attributable to the relatively low transfection efficiency that we achieved in these cells. While the levels of the transfected PP2A-C and endogenous enzyme appear similar in the immunoblots, the transfected cells are expressing high levels of the transfected protein relative to the endogenous protein. Therefore, the complexes that form with the transfected α4 predominantly include the more abundant co-transfected PP2A-C.
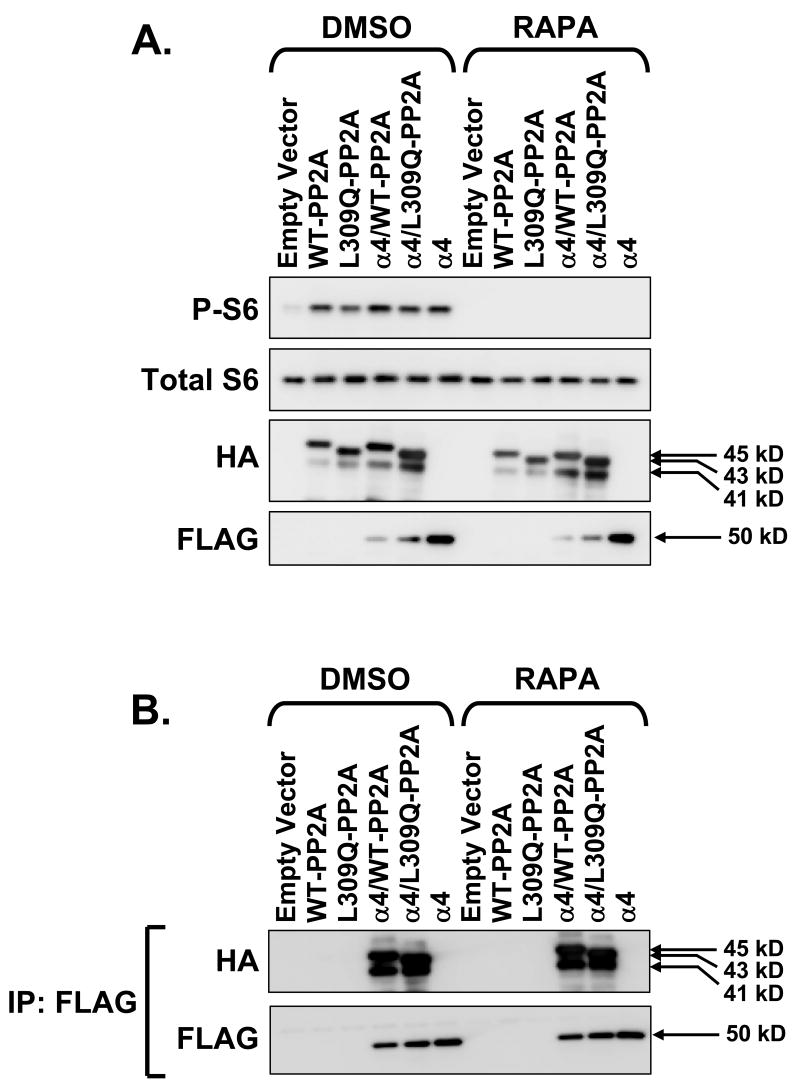
Transfected AML12 cells were treated with either DMSO vehicle or 20 nM rapamycin for 1 hr. Immunoblotting for phospho-S6 confirmed the efficacy of rapamycin in inhibiting mTOR signaling (Fig. 6A). Rapamycin did not affect the levels of ectopic PP2A-C or α4 (Fig. 7A). Most importantly, immunoprecipitation of α4 using anti-FLAG followed by immunoblotting for HA and FLAG showed that rapamycin had no effect on PP2A-C association with α4 (Fig. 6B).
Fig. 6.
In vitro effect of rapamycin on α4/PP2A. AML12 cells were transfected in the same manner as for Figure 5 but were exposed to DMSO vehicle or 20 nM rapamycin for 1 hr prior to preparing lysates. A: Lysate protein (10 μg) was analyzed by direct immunoblotting using anti-HA and anti-FLAG antibodies. Phospho-S6 and total S6 immunoblots were done to verify rapamycin effect. B: Lysate protein (200 μg) was analyzed by immunoprecipitation with anti-FLAG antibody followed by immunoblotting for HA and FLAG.
Fig. 7.
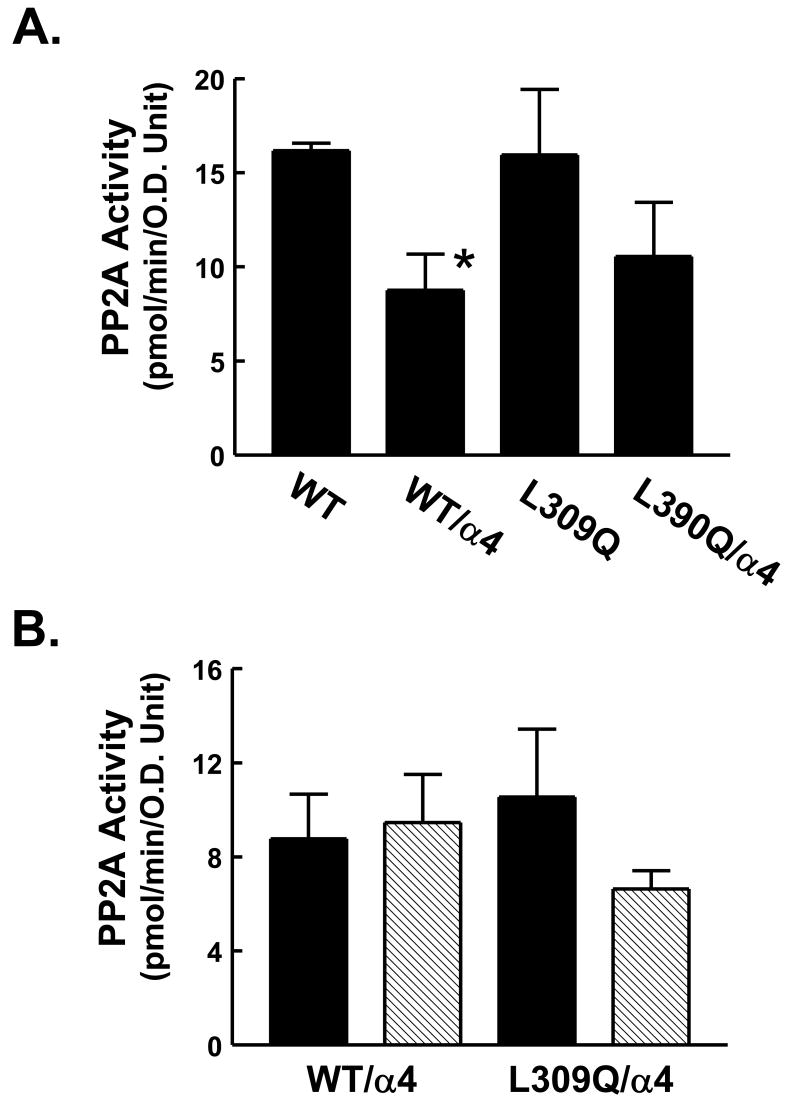
In vitro regulation of PP2A activity by α4 and rapamycin. A: AML12 cells were transfected with HA-tagged WT-PP2A or L309Q-PP2A alone or with FLAG-tagged α4. Cell lysates were immunoprecipitated with anti-HA antibody. PP2A activity was measured in each immunoprecipitate. The activity in pmol/min was normalized to the protein content of PP2A using densitometry values derived from a PP2A immunoblot (not shown). Analysis of triplicate lysates is shown as the mean + 1 standard deviation. *, P<0.01. B: AML12 cells were transfected with HA-tagged WT-PP2A or L309Q-PP2A along with FLAG-tagged α4 as for Panel A. Prior to preparing lysates, cells were exposed to 20 nM rapamycin or DMSO vehicle for 1 hr. The lysates were then analyzed by immunoprecipitation with anti-HA antibody followed by measurement of PP2A activity in the immunoprecipitates. Results are shown as mean + 1 standard deviation (DMSO: solid bars; rapamycin: hatched bars). Differences between groups were not significant as determined by ANOVA.
In order to confirm our in vivo findings indicating the inhibitory effect of α4 on PP2A activity, we performed a phosphatase activity assay with immunoprecipitates from transfected AML12 cells. Immunoprecipitates were analyzed by immunoblotting (not shown) in order to correct activities for the recovery of the ectopic phosphatase. Results (Fig. 7A) showed that α4 co-transfection reduced the cell lysate specific activity of the WT-PP2A-C. Results with L309Q-PP2A-C showed a similar trend, though the effect did not achieve statistical significance. We extended our observations to the effect of rapamycin. Results (Fig. 7B) indicated that rapamycin did not induce a significant change in PP2A activity in cells co-transfected with either WT-PP2A-C or L309Q-PP2A-C and α4. This experiment was repeated in its entirety with similar results.
Cell lysates derived from the above experiment were analyzed by immunoprecipitation with α4 antibody followed by the determination of phosphatase activity. In all cases, negligible activity was detected in these immunoprecipitates.
The Relationship Between the Rapamycin Sensitivity of Hepatic Cells and α4/PP2A-C Content
We examined the α4/PP2A-C complex in rapamycin-sensitive and resistant rat hepatic cell lines. These cell lines were characterized for rapamycin sensitivity by exposing them to various concentrations of rapamycin for 24 hr and measuring [3H]thymidine incorporation during the last 6 hr of the incubation period. The IC50 for rapamycin was determined for each cell line. The nontumorigenic H4-II-E and WB cells were highly sensitive to rapamycin, with IC50 values in the range of 2 to 10 nM. The tumorigenic GN5 and 1682-A cells were also rapamycin-sensitive, whereas four other cell lines, WB311, GP6, H5D and THC-252 were highly resistant to rapamycin, showing an IC50 of above 200 nM.
Among the eight cell lines, there was a greater than 5-fold difference in the level α4 and a 2 to 3-fold difference in levels of PP2A-C (Fig. 8A). The abundance of neither protein corresponded to sensitivity of the cell lines to the anti-proliferative effects of rapamycin. Furthermore, rapamycin sensitivity was not associated with PP2A-C methylation, as indicated by reactivity with the MD-PP2A-C and CT-PP2A-C antibodies (Fig. 8A). PP2A-C association with α4 was examined in these cell lines by co-immunoprecipitation and immunoblotting. Results showed that the amount of the α4/PP2A-C complex followed the level of α4 protein with no apparent correlation with rapamycin sensitivity or tumorigenicity (data not shown).
Fig. 8.
α4/PP2A-C and rapamycin resistance in rat hepatic cell lines. A panel of rapamycin-sensitive (H4-II-E, GN5, 1682-A, WB) and rapamycin-resistant (WB311, GP6, H5D, THC-252) hepatic cells were studied. A: Lysate protein (20 μg) from cells was analyzed by direct immunoblotting for content of α4 and PP2A-C. The latter employed antibodies directed toward the mid-portion (MD-PP2A) and carboxy-terminus (CT-PP2A) of PP2A-C. B: WB and WB311 cells were exposed to rapamycin for times varying from 10 min to 6 hr. Cell lysates were analyzed by immunoprecipitation with α4 antibody followed by immunoblotting for PP2A-C and α4.
The effect of rapamycin on formation of the α4/PP2A-C complex was studied in two of the cell lines, the rapamycin-sensitive WB cell line and a rapamycin-resistant counterpart, the WB311 cell line. These two particular lines were chosen because the WB311 cells were spontaneously derived from the WB cells. There was no change in the content of α4 or PP2A-C (not shown) or of the α4/PP2A-C complex (Fig. 8B) for periods of rapamycin exposure ranging from 10 min to 6 hr.
Discussion
Although previous studies have identified the presence of α4/PP2A complexes in yeast, plants and mammalian cells [Chen et al., 1998; Di Como CJ and Arndt KT, 1996; Harris et al., 1999; Inui et al., 1998; Kloeker et al., 2003; Kong et al., 2004; Murata et al., 1997; Nanahoshi et al., 1998; Nien et al., 2007; Prickett and Brautigan, 2004; Yamashita et al., 2005], information on the physiological control of this distinct form of PP2A in liver and hepatic cells is quite limited. Given the purported role of PP2A in cell growth, the possible link to mTOR and the limitations in the available data on the α4/PP2A complex, we carried out studies in a well defined in vivo system, liver growth in the laboratory rat. The hypothesis was that the abundance of PP2A, in particular the α4/PP2A-C complex, might be associated with differences in the rapamycin sensitivity and proliferative activity of fetal versus adult hepatocytes in vivo. We showed previously that PP2A activity and abundance were slightly higher in unfractionated E19 fetal compared to adult liver extracts [Yoo et al., 2007]. This was unexpected based on the reported inverse correlation between PP2A activity and cell growth and proliferation [Janssens et al., 2005; Janssens and Goris, 2001].
We detected the α4/PP2A-C complex in unfractionated liver extracts and in α4-containing fractions obtained by MonoQ chromatography. MonoQ fractionation also provided evidence that liver extracts contain α4 not bound to PP2A-C. This observation may be a function of the ability of α4 to associate with other PP2A-related phosphatases [Chen et al., 1998; Nanahoshi et al., 1999], or it may indicate the presence of a pool of free α4 in hepatocytes.
Previous studies have been interpreted as indicating that the A regulatory subunit is displaced by anti-HA antibody [Prickett and Brautigan, 2004]. This was demonstrated by showing that ectopically expressed HA-PP2A-C could associate with the endogenous A subunit in column chromatography fractions but that HA immunoprecipitates yielded only low co-precipitations with endogenous A subunit. In the present studies, the association between HA-PP2A-C and FLAG-α4 was maintained, thus allowing us to tentatively conclude that α4 exerts an inhibitory effect on the activity of the catalytic subunit. Overall, our findings are consistent with published studies showing that α4 has an inhibitory effect on PP2A activity towards 32P-phosphorylated 4E-BP1 and p-nitrophenyl phosphate (pNPP) in vitro [Nanahoshi et al., 1998; Nanahoshi et al., 1999]. While our observations do not extensively address the issue of substrate specificity, analysis of liver extracts showed similar activity profiles using peptide and 32P-MBP as substrate. It has been demonstrated that α4 enhances PP2A activity toward 32P-MBP [Murata et al., 1997; Prickett and Brautigan, 2006]. In fact, α4 has been shown to have opposing effects on PP2A and PP6 activity [Prickett and Brautigan, 2006]. Our own data do not indicate that 32P-MBP is a preferred substrate for the hepatic α4/PP2A-C complex.
We showed previously [Yoo et al., 2007] that a high proportion of PP2A-C in adult liver is unmethylated while fetal liver PP2A-C is predominantly methylated. Given the higher abundance of the α4/PP2A-C complex in adult liver relative to fetal liver, we hypothesized that α4 preferentially binds to unmethylated PP2A-C. Instead, our studies on AML12 cells co-transfected with α4 and WT-PP2A-C and L309Q-PP2A-C indicated that α4 associates with both the methylated and unmethylated forms of PP2A-C. Previous studies using MonoQ chromatographic fractionation of COS-7 cell extracts [Chung et al., 1999] have shown that a mutation of Leu309 in PP2A-C results in the recovery of only AC and α4/PP2A-C dimers and that a double mutation of Tyr307 and Leu309 favors the formation of α4/PP2A-C. Our prior studies indicated an absence of PP2A-C phosphorylated at Tyr307 in liver homogenates [Yoo et al., 2007]. The present results indicate that the interaction of PP2A-C with α4 in hepatic cells is determined by factors other than the tyrosine phosphorylation or methylation state of the PP2A-C carboxy terminus.
Prior studies provided ample reason to anticipate that changes in hepatic mTOR signaling, whether induced by refeeding or partial hepatectomy or inhibited by rapamycin, might be associated with changes in the abundance of α4/PP2A-C. While we found that the administration of rapamycin to fed rats induced a modest increase in the content of the α4/PP2A-C complex, our studies provided no evidence that rapamycin could induce the reduction in α4/PP2A-C formation that would be predicted by studies in yeast. In fact, we found that the in vivo administration of rapamycin could induce the dephosphorylation of S6 without changing the amount of α4/PP2A-C. This makes it less likely that α4 mediates mTOR signaling in liver.
The absence of a direct effect of rapamycin on α4/PP2A-C was corroborated by the transfection studies in AML12 cells. Of the studies examining the regulation of this complex in mammalian systems, several have shown that the interaction of PP2A-C with α4 is unaffected by rapamycin [Kloeker et al., 2003; Nanahoshi et al., 1998; Nien et al., 2007]. We also found that while co-transfection of α4 and PP2A-C resulted in decreased specific activity of the transfected PP2A-C, there was no apparent effect of rapamycin on activity of the complex.
Studies in yeast [Di Como CJ and Arndt KT, 1996; Jiang Y and Broach JR, 1999] have indicated that the conditions that promote formation of the α4/PP2A-C complex induce partial rapamycin resistance. Our intent was to test the hypothesis that high levels of α4/PP2A-C in fetal liver contribute to the resistance of fetal hepatocytes in vivo to the cell cycle inhibitory effects of rapamycin. We found that fetal hepatocytes in vivo have lower levels of α4/PP2A-C than rapamycin-sensitive adult hepatocytes. We extended our observations to a panel of well characterized hepatic cell lines that in preliminary studies were found to have highly variable sensitivity to rapamycin. Similar to the in vivo model, we found no correlation between α4 content or α4/PP2A-C content and sensitivity to the anti-proliferative effects of rapamycin.
Our studies have a number of limitations that should be taken into consideration. We did not directly examine the effect of α4/PP2A-C overexpression on the ability of rapamycin to inhibit DNA synthesis in cultured hepatic cells. These studies were precluded by the effects of transfection protocols on rapamycin sensitivity. Our results do not rule out the possibility that the effect of rapamycin on α4/PP2A-C is mediated by changes in the phosphorylation state of α4, PP2A-C or both. In the absence of data on the phosphorylation state of proteins that are endogenous substrates of α4/PP2A-C, we cannot conclude the absence of an effect of rapamycin on substrate-specific activity. Nonetheless, our results do lead to several conclusions. The methylation status of hepatic PP2A-C does not appear to be a primary determinant of the formation of α4/PP2A-C in liver. The yeast paradigm in which modulation of TOR activity affects the association of α4 and PP2A-C does not appear to pertain to liver. Finally, the abundance of α4 or the α4/PP2A-C complex is not a primary determinant of the rapamycin sensitivity of hepatic cells in vivo or in vitro.
Acknowledgments
We thank Dr. Nancy Thompson (Department of Medicine, Brown University) for providing rat hepatic cell lines. We also thank Shu-Whei Tsai for her assistance in the performance of animal studies.
Grant sponsor: National Institutes of Health; Grant numbers: R01HD24455, R01HD35831 and R01CA77584. RHJ is supported by a NIH minority predoctoral fellowship grant, F31HD041893.
References
- 1.Anand P, Gruppuso PA. Rapamycin inhibits liver growth during refeeding in rats via control of ribosomal protein translation but not cap-dependent translation initiation. J Nutr. 2006;136:27–33. doi: 10.1093/jn/136.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck T, Hall MN. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- 3.Bielinski VA, Mumby MC. Functional analysis of the PP2A subfamily of protein phosphatases in regulating Drosophila S6 kinase. Exp Cell Res. 2007;313:3117–3126. doi: 10.1016/j.yexcr.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant JC, Westphal RS, Wadzinski BE. Methylated C-terminal leucine residue of PP2A catalytic subunit is important for binding of regulatory Balpha subunit. Biochem J. 1999;339(Pt 2):241–246. [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell WA, Sah DE, Medina MM, Albina JE, Coleman WB, Thompson NL. TA1/LAT1/CD98 light chain and system L activity, but not 4F2/CD98 heavy chain, respond to arginine availability in rat hepatic cells. J Biol Chem. 2000;275:5347–5354. doi: 10.1074/jbc.275.8.5347. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Parsons S, Brautigan DL. Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J Biol Chem. 1994;269:7957–7962. [PubMed] [Google Scholar]
- 8.Chen J, Peterson RT, Schreiber SL. Alpha 4 associates with protein phosphatases 2A, 4, and 6. Biochem Biophys Res Commun. 1998;247:827–832. doi: 10.1006/bbrc.1998.8792. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Possemato R, Campbell KT, Plattner CA, Pallas DC, Hahn WC. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell. 2004;5:127–136. doi: 10.1016/s1535-6108(04)00026-1. [DOI] [PubMed] [Google Scholar]
- 10.Cho US, Xu W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature. 2007;445:53–57. doi: 10.1038/nature05351. [DOI] [PubMed] [Google Scholar]
- 11.Chung H, Nairn AC, Murata K, Brautigan DL. Mutation of Tyr307 and Leu309 in the protein phosphatase 2A catalytic subunit favors association with the alpha 4 subunit which promotes dephosphorylation of elongation factor-2. Biochemistry. 1999;38:10371–10376. doi: 10.1021/bi990902g. [DOI] [PubMed] [Google Scholar]
- 12.Curran TRJ, Bahner RIJ, Oh W, Gruppuso PA. Mitogen-independent DNA synthesis by fetal rat hepatocytes in primary culture. Experimental Cell Research. 1993;209:53–57. doi: 10.1006/excr.1993.1284. [DOI] [PubMed] [Google Scholar]
- 13.Cygnar KD, Gao X, Pan D, Neufeld T. The phosphatase subunit Tap42 functions independently of TOR to regulate cell division and survival in Drosophila. Genetics. 2005;130:733–740. doi: 10.1534/genetics.104.039909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Como CJ, Arndt KT. Nutrients, via the Tor proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes Dev. 1996;10:1904–1916. doi: 10.1101/gad.10.15.1904. [DOI] [PubMed] [Google Scholar]
- 15.Fausto N, Webber EM. Control of liver growth. Crit Rev Eukaryot Gene Expr. 1993;3:117–135. [PubMed] [Google Scholar]
- 16.Ferrari S, Bandi HR, Hofsteenge J, Bussian BM, Thomas G. Mitogen-activated 70K S6 kinase. Identification of in vitro 40 S ribosomal S6 phosphorylation sites. J Biol Chem. 1991;266:22770–22775. [PubMed] [Google Scholar]
- 17.Gentry MS, Hallberg RL. Localization of Saccharomyces cerevisiae protein phosphatase 2A subunits throughout mitotic cell cycle. Mol Biol Cell. 2002;13:3477–3492. doi: 10.1091/mbc.02-05-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentry MS, Li Y, Wei H, Syed FF, Patel SH, Hallberg RL, Pallas DC. A novel assay for protein phosphatase 2A (PP2A) complexes in vivo reveals differential effects of covalent modifications on different Saccharomyces cerevisiae PP2A heterotrimers. Eukaryot Cell. 2005;4:1029–1040. doi: 10.1128/EC.4.6.1029-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gingras AC, Raught B, Sonenberg N. Control of translation by the target of rapamycin proteins. Prog Mol Subcell Biol. 2001a;27:143–174. doi: 10.1007/978-3-662-09889-9_6. [DOI] [PubMed] [Google Scholar]
- 20.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001b;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 21.Gruppuso PA, Awad M, Bienieki TC, Boylan JM, Fernando S, Faris RA. Modulation of mitogen-independent hepatocyte proliferation during the perinatal period in the rat. In Vitro Cell Dev Biol Animal. 1997;33:562–568. doi: 10.1007/s11626-997-0099-x. [DOI] [PubMed] [Google Scholar]
- 22.Guo H, Damuni Z. Autophosphorylation-activated protein kinase phosphorylates and inactivates protein phosphatase 2A. Proc Natl Acad Sci U S A. 1993;90:2500–2504. doi: 10.1073/pnas.90.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris DM, Myrick TL, Rundle SJ. The Arabidopsis homolog of yeast TAP42 and mammalian alpha4 binds to the catalytic subunit of protein phosphatase 2A and is induced by chilling. Plant Physiol. 1999;121:609–617. doi: 10.1104/pp.121.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hixson DC, McEntire KD, Obrink B. Alterations in the expression of a hepatocyte cell adhesion molecule by transplantable rat hepatocellular carcinomas. Cancer Res. 1985;45:3742–3749. [PubMed] [Google Scholar]
- 25.Inui S, Sanjo H, Maeda K, Yamamoto H, Miyamoto E, Sakaguchi N. Ig receptor binding protein 1 (alpha4) is associated with a rapamycin-sensitive signal transduction in lymphocytes through direct binding to the catalytic subunit of protein phosphatase 2A. Blood. 1998;92:539–546. [PubMed] [Google Scholar]
- 26.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssens V, Goris J, Van Hoof C. PP2A: the expected tumor suppressor. Curr Opin Genet Dev. 2005;15:34–41. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y, Broach JR. Tor proteins and protein phosphatase 2A reciprocally regulate Tap42 in controlling cell growth in yeast. EMBO J. 1999;18:2782–2792. doi: 10.1093/emboj/18.10.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kloeker S, Reed R, McConnell JL, Chang D, Tran K, Westphal RS, Law BK, Colbran RJ, Kamoun M, Campbell KS, Wadzinski BE. Parallel purification of three catalytic subunits of the protein serine/threonine phosphatase 2A family (PP2A(C), PP4(C), and PP6(C)) and analysis of the interaction of PP2A(C) with alpha4 protein. Protein Expr Purif. 2003;31:19–33. doi: 10.1016/s1046-5928(03)00141-4. [DOI] [PubMed] [Google Scholar]
- 30.Kong M, Fox CJ, Mu J, Solt L, Xu A, Cinalli RM, Birnbaum MJ, Lindsten T, Thompson CB. The PP2A-associated protein alpha4 is an essential inhibitor of apoptosis. Science. 2004;306:695–698. doi: 10.1126/science.1100537. [DOI] [PubMed] [Google Scholar]
- 31.Longin S, Zwaenepoel K, Louis JV, Dilworth S, Goris J, Janssens V. Selection of protein phosphatase 2A regulatory subunits is mediated by the C terminus of the catalytic Subunit. J Biol Chem. 2007;282:26971–26980. doi: 10.1074/jbc.M704059200. [DOI] [PubMed] [Google Scholar]
- 32.McCright B, Rivers AM, Audlin S, Virshup DM. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J Biol Chem. 1996;271:22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- 33.Mumby MC, Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol Rev. 1993;73:673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- 34.Murata K, Wu J, Brautigan DL. B cell receptor-associated protein alpha4 displays rapamycin-sensitive binding directly to the catalytic subunit of protein phosphatase 2A. Proc Natl Acad Sci U S A. 1997;94:10624–10629. doi: 10.1073/pnas.94.20.10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nanahoshi M, Nishiuma T, Tsujishita Y, Hara K, Inui S, Sakaguchi N, Yonezawa K. Regulation of protein phosphatase 2A catalytic activity by alpha4 protein and its yeast homolog Tap42. Biochem Biophys Res Commun. 1998;251:520–526. doi: 10.1006/bbrc.1998.9493. [DOI] [PubMed] [Google Scholar]
- 36.Nanahoshi M, Tsujishita Y, Tokunaga C, Inui S, Sakaguchi N, Hara K, Yonezawa K. Alpha4 protein as a common regulator of type 2A-related serine/threonine protein phosphatases. FEBS Lett. 1999;446:108–112. doi: 10.1016/s0014-5793(99)00189-1. [DOI] [PubMed] [Google Scholar]
- 37.Nien WL, Dauphinee SM, Moffat LD, Too CK. Overexpression of the mTOR alpha4 phosphoprotein activates protein phosphatase 2A and increases Stat1alpha binding to PIAS1. Mol Cell Endocrinol. 2007;263:10–17. doi: 10.1016/j.mce.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Nunbhakdi-Craig V, Schuechner S, Sontag JM, Montgomery L, Pallas DC, Juno C, Mudrak I, Ogris E, Sontag E. Expression of protein phosphatase 2A mutants and silencing of the regulatory B alpha subunit induce a selective loss of acetylated and detyrosinated microtubules. J Neurochem. 2007;101:959–971. doi: 10.1111/j.1471-4159.2007.04503.x. [DOI] [PubMed] [Google Scholar]
- 39.Peterson RT, Desai BN, Hardwick JS, Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycin associated protein. Proc Natl Acad Sci U S A. 1999;96:4438–4442. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prickett TD, Brautigan DL. Overlapping binding sites in protein phosphatase 2A for association with regulatory A and alpha-4 (mTap42) subunits. J Biol Chem. 2004;279:38912–38920. doi: 10.1074/jbc.M401444200. [DOI] [PubMed] [Google Scholar]
- 41.Prickett TD, Brautigan DL. The alpha4 regulatory subunit exerts opposing allosteric effects on protein phosphatases PP6 and PP2A. J Biol Chem. 2006;281:30503–30511. doi: 10.1074/jbc.M601054200. [DOI] [PubMed] [Google Scholar]
- 42.Sanders JA, Gruppuso PA. Nucleolar localization of hepatic c-Myc: a potential mechanism for c-Myc regulation. Biochim Biophys Acta. 2005;1743:141–150. doi: 10.1016/j.bbamcr.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt A, Beck T, Koller A, Kunz J, Hall MN. The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J. 1998;17:6924–6931. doi: 10.1093/emboj/17.23.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sontag E. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal. 2001;13:7–16. doi: 10.1016/s0898-6568(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 45.Tolstykh T, Lee J, Vafai S, Stock JB. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. EMBO J. 2000;19:5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsao MS, Smith JD, Nelson KG, Grisham JW. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of ‘oval’ cells. Exp Cell Res. 1984;154:38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]
- 47.Turowski P, Fernandez A, Favre B, Lamb NJ, Hemmings BA. Differential methylation and altered conformation of cytoplasmic and nuclear forms of protein phosphatase 2A during cell cycle progression. J Cell Biol. 1995;129:397–410. doi: 10.1083/jcb.129.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westphal RS, Coffee RL, Jr, Marotta A, Pelech SL, Wadzinski BE. Identification of kinase-phosphatase signaling modules composed of p70 S6 kinase-protein phosphatase 2A (PP2A) and p21-activated kinase-PP2A. J Biol Chem. 1999;274:687–692. doi: 10.1074/jbc.274.2.687. [DOI] [PubMed] [Google Scholar]
- 49.Wu J, Tolstykh T, Lee J, Boyd K, Stock JB, Broach JR. Carboxyl methylation of the phosphoprotein phosphatase 2A catalytic subunit promotes its functional association with regulatory subunits in vivo. EMBO J. 2000;19:5672–5681. doi: 10.1093/emboj/19.21.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu JC, Merlino G, Fausto N. Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc Natl Acad Sci U S A. 1994;91:674–678. doi: 10.1073/pnas.91.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Y, Xing Y, Chen Y, Chao Y, Lin Z, Fan E, Yu JW, Strack S, Jeffrey PD, Shi Y. Structure of the protein phosphatase 2A holoenzyme. Cell. 2006;127:1239–1251. doi: 10.1016/j.cell.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 52.Yamashita T, Inui S, Maeda K, Hua DR, Takagi K, Sakaguchi N. The heterodimer of alpha4 and PP2Ac is associated with S6 kinase1 in B cells. Biochem Biophys Res Commun. 2005;330:439–445. doi: 10.1016/j.bbrc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Yang J, Roe SM, Prickett TD, Brautigan DL, Barford D. The structure of Tap42/alpha4 reveals a tetratricopeptide repeat-like fold and provides insights into PP2A regulation. Biochemistry. 2007;46:8807–8815. doi: 10.1021/bi7007118. [DOI] [PubMed] [Google Scholar]
- 54.Yoo SJ, Boylan JM, Brautigan DL, Gruppuso PA. Subunit composition and developmental regulation of hepatic protein phosphatase 2A (PP2A) Arch Biochem Biophys. 2007;461:186–193. doi: 10.1016/j.abb.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu XX, Du X, Moreno CS, Green RE, Ogris E, Feng Q, Chou L, McQuoid MJ, Pallas DC. Methylation of the protein phosphatase 2A catalytic subunit is essential for association of Balpha regulatory subunit but not SG2NA, striatin, or polyomavirus middle tumor antigen. Mol Biol Cell. 2001;12:185–199. doi: 10.1091/mbc.12.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zolnierowicz S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem Pharmacol. 2000;60:1225–1235. doi: 10.1016/s0006-2952(00)00424-x. [DOI] [PubMed] [Google Scholar]