Abstract
Palmitoyl-protein thioesterase-1 (PPT1) deficiency causes infantile neuronal ceroid lipofuscinosis (INCL), a devastating childhood neurodegenerative storage disorder. We previously reported that neuronal apoptosis in INCL is mediated by endoplasmic reticulum-stress. ER-stress disrupts Ca++-homeostasis and stimulates the expression of Ca++-binding proteins. We report here that in the PPT1-deficient human and mouse brain the levels of S100B, a Ca++-binding protein, and its receptor, RAGE (receptor for advanced glycation end-products) are elevated. We further demonstrate that activation of RAGE-signaling in astroglial cells mediates pro-inflammatory cytokine production, which is inhibited by SiRNA-mediated suppression of RAGE expression. We propose that RAGE-signaling contributes to neuroinflammation in INCL.
Keywords: Neuroinflammation, Neurodegeneration, Infantile Neuronal Ceroid Lipofuscinosis, Batten disease, Palmitoyl-protein thioesterase-1
1. Introduction
Although the molecular mechanism(s) of pathogenesis in many neurodegenerative diseases remains poorly understood, it is increasingly evident that neuroinflammation contributes to the progression of these diseases [1]. Moreover, it has been reported that neuroinflammation resulting from systemic infection may also hasten the progression of chronic neurodegeneration [reviewed in 2]. In the central nervous system (CNS), inflammatory processes differ from those of the systemic inflammation in that CNS inflammation involves direct cellular effects triggered by multiple causes and pathways [2]. Understanding the molecular mechanism(s) of neuroinflammation in neurodegenerative diseases may advance our knowledge of the disease pathogenesis and facilitate the development of novel therapeutic strategies.
Neuronal ceroid lipofuscinoses (NCLs), commonly known as Batten disease [3, 4], cumulatively represent a group of the most common (1 in 12,500 live births) hereditary lysosomal storage disorders with worldwide distribution. Although the mutations in at least eight different genes (CLN1-CLN8) cause various forms of NCL, their pathological manifestations are remarkably similar [3, 4]. The infantile NCL, or INCL, is a rare (1 in >100,000 births) but the most lethal disease. INCL is caused by mutations in the gene encoding palmitoyl-protein thioesterase-1 (PPT1) [5], a lysosomal enzyme that catalyzes the cleavage of the thioester linkage in polypeptides that are palmitoylated [6]. Palmitoylation (also called S-acylation), is a post-translational lipid-modification of polypeptides by the 16-carbon fatty acid, palmitate [7, 8]. Emerging evidence suggests that this modification plays critical roles in protein-protein interaction, protein-trafficking, stability and membrane anchorage [7, 8]. While palmitoylation is critical for the function of many proteins, the enzymatic removal of the palmitate residues (depalmitoylation) is essential for their degradation and/or recycling. The lack of PPT1 activity causes abnormal intracellular accumulation of S-acylated proteins and peptides [9] that leads to INCL pathogenesis, although the precise molecular mechanism(s) remain poorly understood. The clinical features of INCL include an early loss of vision, rapidly progressive mental deterioration, myoclonus and seizures leading to a complete loss of brain activity around four years of age. At autopsy, characteristic intracellular storage material, known as GRODs (granular osmiophilic deposits), are found in the neurons as well as in other cells and tissues [10].
Recently, using postmortem brain tissues from an INCL patient and those from the PPT1-knockout (PPT1-KO) mice [11] that mimic INCL [12], we have demonstrated that the PPT1-deficient neurons undergo apoptosis triggered by endoplasmic reticulum stress (ER-stress) [13, 14]. To circumvent the ER-stress [15, 16] the cells have evolved a signaling pathway for survival known as the unfolded protein response (UPR) [17]. If the ER-stress is so overwhelming that the UPR cannot resolve, the cysteine proteinases, called caspases, are activated leading to apoptosis. ER-stress also disrupts Ca++-homeostasis [18, 19], causing influx of Ca++. This may stimulate the expression of Ca++–binding proteins, some of which are known to trigger the inflammatory response via receptor-mediated pathway. Consistent with these findings, neuroinflammation-associated interneuron loss and local neuroinflammation have been reported in the brain of the PPT1-KO mice [20-22] although the molecular mechanism(s) of neuroinflammation in these mice remains poorly understood.
Recent reports indicate that with increasing age, the brains of the PPT1-KO mice show widespread astrogliosis [12, 23]. Moreover, S100 family of Ca++-binding proteins via receptor-mediated pathway are capable of triggering the inflammatory response [24, 25]. Indeed, cultured astroglia from the rat [26] has been shown to express S100B, a brain-specific Ca++-binding protein as well as its receptor, RAGE (receptor for advanced glycation) [27]. Further, transgenic mice expressing high levels of S100B in the brain, are highly susceptible to developing ß-amyloid-induced neuroinflammation in an animal model of Alzheimer's disease [28].
In the present study, we sought to determine whether increased astrogliosis in INCL and in the PPT1-KO mice that mimic INCL, trigger S100B-RAGE signaling mediating neuroinflammation. Using postmortem brain tissues from an INCL patient and those of the PPT1-KO mice, we report here that PPT1-deficiency leads to elevated levels of S100B and RAGE. We further demonstrate that RAGE-signaling mediates the activation of Src- as well as MAP-kinases and stimulates the activation of the transcription factor, NF-kB. The activation of NF-kB mediates the production of pro-inflammatory cytokines. Most significantly, the blocking of RAGE expression by SiRNA inhibits proinflammatory cytokine production in the PPT1-deficient cultured astroglial cells. We propose that the activation of the S100B-RAGE signaling pathway in astroglial cells mediate proinflammatory cytokine production, which at least in part contributes to neuroinflammation in INCL.
2. Materials and Methods
2.1 PPT1-KO mice
PPT1-KO mice (a generous gift from Dr. S.L. Hofmann, University of Texas Southwestern Medical Center, Dallas, TX) were generated and characterized as previously described [11]. These mice were backcrossed to obtain the isogenic C57 genetic background in the laboratory of Dr. M. S. Sands (Washington University School of Medicine, St. Louis, MO). All mice were maintained and housed in a germ-free facility and animal procedures were carried out in accordance with institutional guidelines after the NICHD Animal Care and Use Committee approved an animal study protocol.
2.2 Postmortem INCL and normal control brain tissues
Postmortem brain tissues from an INCL patient, with clinical and pathological diagnosis of INCL were obtained from Human Brain and Spinal Fluid Resource Center, Los Angeles, CA. A sister of this patient who also died of INCL was a compound heterozygote for PPT1 mutations [del 398T in exon 4 and C451T in exon 5]; see Table 2 [in ref. 29]. Because INCL is an autosomal recessive disease, we assume that the patient was also a compound heterozygote for the same PPT1 mutations as indicated above. Postmortem brain tissues from age-matched normal control subjects were obtained from NICHD Brain and Tissue Bank for Developmental Disorders, University of Maryland School of Medicine, Baltimore, MD.
2.3 Mouse astroglial cell culture
Astroglial cultures were initiated from El8 (embryo day 18) mouse cortex using a modification of a previously described method [30]. The cells were counted, viability tested by trypan blue dye exclusion test and were then seeded at 5×106 cells/cm2 onto poly-D-lysine-coated plates. The medium was changed on culture day 4 and every 2 days thereafter until confluency was reached.
2.4 Western blot analysis and cytokine ELISA
Western blot analyses of proteins from the cytosolic and nuclear fractions were performed as previously described [23]. Briefly, the brain tissues were homogenized in protein extracting buffer (50 mM Tris–HCI, 150 mM NaCl, 0.25% SDS, 1 mM EDTA and 1% NP-40) containing protease- and/or phosphatase-inhibitor cocktails (Sigma–Aldrich). For the preparation of total lysates from cultured astroglial cells from WT or PPT1-KO mice, cells were homogenized in Phosphosafe extraction reagent (EMD Biosciences) containing protease inhibitors (Sigma–Aldrich). Nuclear extracts from astroglial cells were isolated using the ProteoExtract subcellular proteome extraction kit (Calbiochem) following the manufacturer's instruction. Total proteins (30 μg) from each sample were resolved by SDS–PAGE, electro-transferred and immunodetected. Densitometric measurements of the protein bands were performed using at least three independently derived samples. The error bars indicate standard deviation (SD) of the mean (n=3). The primary antibodies used are: anti-S100B (1:1000, a generous gift from Dr. Alexander Marks, Department of Physiology, University of Toronto, Toronto, Ontario, CA), anti-RAGE (1:750, Abcam) and anti-phospho-ERK1/2 (1:5000), anti-ERK1/2 (1:1000), anti-IkB-α (1:1000), anti-phospho-IκB-α (1:1000), anti-phospho-p38 MAP kinase (1:2000), anti-p38 MAP kinase (1:1000) and anti-p-Src (1:2000) (Cell Signaling), anti-c-Src (1:1000), anti-NF-kB p65 (1:1000) (Santa Cruz Biotechnology) and anti-ß-actin (1:5000, US Biological). The secondary antibodies used are: goat anti-rabbit IgG, donkey anti-goat IgG and goat anti-mouse IgG (Santa Cruz Biotechnology). Amount of proteins from WT and PPT1-KO brain tissue lysates were adjusted to a concentration of 0.5mg/ml and used for ELISA (Assaygate Inc) for quantitating the released IL-1ß, IL-6, MCP-1 and TNFα. Equal number (5×106 cells) of WT and PPT1-KO astroglial cells in 10ml media were seeded in 75cm2 flasks and cultured until the 85−90% confluency was reached. From these confluent astroglial cultures, conditioned media were collected for cytokine ELISA (Assaygate Inc).
2.5. Fluorescence microscopy
The method for immunofluorescence microscopy has been described previously [14]. Rabbit NF-kB-anti-p65 (1:50) as the primary antibody and goat anti-rabbit-alexafluor594 (1:200) conjugated secondary antibody (Molecular probes) were used. Nuclei were stained with 4, 6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma).
2.6 RNA isolation and quantitative PCR analysis
Total RNA from the brains of PPT1-KO mice and their WT littermates as well as that from cultured astroglial cells were isolated and quantitated by real-time PCR as previously described [23]. The primers used are presented in Supplemental Table 1. The results were analyzed using ABI Prism Software version 1.01 (Applied Biosystems). The final data were normalized to ß-actin standard and presented as fold change in PPT1-KO mice, compared with those of the WT mice.
2.7 Si-RNA transfection
For blocking of the S100B-RAGE signaling pathway astroglial cells were transfected with a RAGE-specific siRNA (Ambion) for reducing RAGE expression. As a negative control, cells were transfected with a scrambled siRNA (Ambion). SiPORT NeoFX Transfection agent (Ambion) was used and transfection was performed following the manufacturer's protocol. Total proteins were prepared from the cells 72 h after transfection using the PhosphoSafe extraction reagent (EMD Biosciences). Cytokine levels in the culture media were quantitated by ELISA (Asssaygate Inc).
2.8 Statistical analysis
Results are expressed as the mean of at least three experiments ± standard deviation (SD). Statistical analyses were performed by Student's t-test using Microsoft Excel 2002 and p< 0.05 was considered statistically significant.
3. Results
3.1 Expression of S100B in the brain of the PPT1-KO mouse and INCL patient
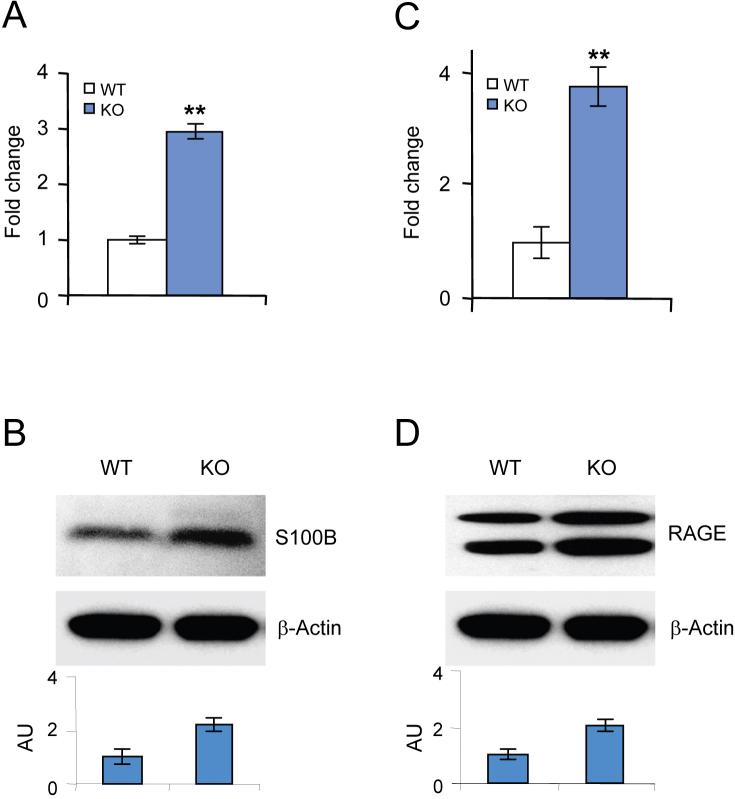
Since ER-stress disrupts Ca++-homeostasis, we sought to determine whether the expression of S100B in the brain of the PPT1-deficient human and mouse is elevated. Accordingly, we first determined the S100B-mRNA levels in the brain tissues from 1, 3 and 6 month-old PPT1-KO mice and in those of their WT littermates by quantitative RTPCR. The results show that compared with the WT mice, S100B-mRNA levels in the brains of the PPT1-KO mice of all three age groups are markedly higher (Fig. 1A). Consistent with these results, the Western blot analyses show that the S100B protein levels in the PPT1-KO brains are also elevated (Fig. 1B). To determine whether these results in mice are reproducible in human INCL brain, we analyzed the S100B protein levels in postmortem brain tissues from an INCL patient and those of an age-matched normal control by Western blot analysis. The results show that compared with the normal control, the brain tissues of the INCL patient contain markedly higher levels of S100B protein (Fig. 1C). Taken together, these results show that PPT1-deficient human and mouse brains express higher levels of S100B.
Fig. 1.

Elevated expression of S100B and RAGE in the brain tissues of PPT1-KO mice and their WT littermates. Expression of S100B- (A) and RAGE- (D) mRNAs in the brain of 1, 3 and 6-month old PPT1-KO mice and in those of their WT littermates. Western blot analyses of brain lysates from PPT1-KO mice and their WT littermates showing elevated expression of S100B- (B) and RAGE (E) in the PPT1-KO mouse brain. Western blot analyses of S100B (C) and RAGE (F) in postmortem brain tissue lysates from a normal control and an INCL patient. The bar graphs below the Western blots represent densitometric quantitation of the protein bands. The error bars indicate the standard deviation of the mean (n=3). Asterisks indicate statistical significance: *, p< 0.010; **, p< 0.005. AU, arbitrary units.
3.2 Expression of RAGE in the PPT1-deficient mouse and human brain
S100B is a ligand of RAGE, and since S100B protein levels are markedly elevated in the brain homogenates of the PPT1-KO mice as well as in those of the INCL patient, we determined whether RAGE expression is also elevated. Accordingly, we first determined the RAGE-mRNA levels in the brain tissues of 1, 3 and 6 month-old PPT1-KO mice and in those of their WT littermates. The results show that compared with the WT brains, the RAGE-mRNA levels in those of the PPT1-KO mice are significantly higher (Fig. 1D). Consistent with these results, the Western blot analyses also show markedly elevated levels of RAGE-protein in the brains of PPT1-KO mice of all three age groups studied (Fig. 1E). We then performed Western blot analyses of the postmortem brain tissues from an INCL patient and those of an age-matched normal control. The results show that compared with the normal control, the RAGE protein levels in the INCL brain cortex and hippocampus are significantly higher (Fig. 1F). These results show that in the brains of PPT1-deficient mouse and human there is elevated expression of RAGE.
3.3 Astroglia contribute to the elevated S100B and RAGE levels in the PPT1-KO mouse brain
We previously reported that PPT1-deficient neurons and astroglia undergo ER-stress and it is known that ER stress disrupts Ca++ homeostasis [13, 14]. Thus, we sought to determine whether PPT1-deficient cultured astroglia express elevated levels of S100B and RAGE and accounts for the observed levels of the Ca++-binding protein, S100B, and its receptor, RAGE, mediating the neuroinflammatory response. Accordingly, we determined the S100B- and RAGE-mRNA levels in cultured PPT1-deficient and WT astroglial cells by quantitative RT-PCR. We also determined S100B- and RAGE-protein levels by Western blot analyses in these cells from PPT1-KO mice and from their WT littermates. The results show that compared with the mRNA and protein levels in the WT astroglia those derived from the PPT1-KO mice express markedly higher levels of S100B-mRNA (Fig. 2A) and S100B-protein (Fig. 2B). Moreover, we found that the RAGE-mRNA (Fig. 2C) and RAGE-protein (Fig. 2D) levels are also upregulated in the PPT1-deficient astroglial cells. Taken together, these results show that in the PPT1-deficient astroglia the expression of both S100B as well as RAGE is elevated and may trigger the activation of the RAGE-signaling pathway.
Fig. 2.
Expression of S100B and RAGE in cultured astroglial cells from PPT1-KO mice and WT littermates. Expression of S100B- (A) and RAGE- (C) mRNAs in WT and PPT1-KO astroglial cells. Western blot analyses of S100B (B) and RAGE (D) in WT and PPT1-KO astroglial cells. Quantitation (bar graphs) is provided under each Western blot. Error bars indicate standard deviation of the mean (n=3). Asterisks indicate statistical significance at p<0.005.
3.4 Activation of Src tyrosine kinase in PPT1-deficient cultured astroglia
It has been reported that several receptors that lack intrinsic tyrosine kinase activity utilize Src tyrosine kinases for signaling to induce gene expression, cell migration and proliferation [31]. Recently, it has been shown that Src kinase is essential for S100B mediated RAGE signaling and inflammation [32]. Since we found that in the PPT1-KO brain tissues both S100B and RAGE expressions are elevated, we sought to determine whether the level of phosphorylated Src kinase in cultured PPT-deficient astroglia is also elevated. Accordingly, we determined the levels of phospho-Src (p-Src) in these cells from PPT1-KO and WT mice by Western blot analysis. The results show that compared with the WT, p-Src level is markedly elevated in astroglial cells from the PPT1-KO mice (Fig. 3A).
Fig. 3.
The activation of Src-kinase, MEK1/2, ERK1/2 and p38 MAP kinases in astroglial cells from the PPT1 KO mice. (A) Western blot analyses of WT and PPT1-KO astroglial cell extracts using anti-phospho-Src antibody; anti-c-Src was used as the control. (B) Western blot analyses of WT and PPT1-KO astroglial cell extracts using anti-phospho-MEK1/2 antibody; anti-MEK1/2 was used as the control. (C) Western blot analyses of WT and PPT1-KO astroglial cell extracts using anti-phospho-ERK1/2 antibody; anti- ERK1/2 was used as the control. (D) Western blot analyses of WT and PPT1-KO astroglial cell extracts using anti-phospho-p38 antibody; anti- p38 was used as the control. Note the elevation of Src- and MAPK- phosphorylation in PPT1-KO astroglial cells. The error bars indicate standard deviation of the mean (n=3).
3.5 MAPK pathways are activated in cultured PPT1-deficient astroglia
Interaction of RAGE with its ligands mediates increased levels of oxidative stress, cell migration, proliferation as well as the expression of genes that are known to play critical roles in mediating inflammation [33]. Moreover, RAGE-mediated signaling can stimulate multiple pathways, including MAPK [34]. Since we previously reported that elevated levels of oxidative stress is readily detectable in the brain of the PPT1-KO mice, we sought to determine whether elevated expression of S100B and RAGE in the PPT1-KO brain triggers RAGE-signaling pathways leading to MAPK activation. Accordingly, we determined the levels of phosphorylated-MEK1/2 (p-MEK1/2), phosphorylated-ERK1/2 (p-ERK1/2) and phosphorylated-p38 (p-p38) in cultured astroglial cells from the PPT1-KO mice and in their WT littermates. The results show that the levels of p-MEK1/2 (Fig. 3B), p-ERK1/2 (Fig. 3C) and p-p38 (Fig. 3D) are significantly elevated in the PPT1-deficient astroglial cells.
3.6 Activation of NF-kB in the PPT1-deficient cultured astroglial cells
The interaction of RAGE with S100 proteins leads to inflammation and RAGE is a potential drug target for controlling inflammation [35]. Since it has been reported that the activation of nuclear factor-kappa B (NF-kB) plays critical roles in generating the inflammatory response, we investigated whether this pathway might be involved in causing neuro-inflammation in INCL. It has been reported that rapid phosphorylation and degradation of IκBα and subsequent release and translocation of NF-kB (p65) from the cytosol to the nucleus preceed the inflammatory response. Accordingly, we determined the levels of phosphorylated-IkBα (p-IkBα) in the cytosol and NF-kB (p65) in the nuclear fraction of cultured astroglia from the PPT1-KO mice and their WT littermates. The results show that compared with the WT littermates, the levels of p-IkBα in the cytosolic fraction (Fig. 4A) and NF-kB (p65) in the nuclear fraction (Fig. 4B) of the PPT1-deficient astroglia are markedly higher. Further evidence for NF-kB activation was obtained by cytochemical analysis of the cultured astroglial cells from the PPT1-KO mice and their WT littermates. The results show that compared with WT cells, the nuclear translocation of the p65 subunit of NF-kB in the PPT1-deficient cells is appreciably elevated (Fig. 4C).
Fig. 4.
Increased phosphorylation of IkBα and nuclear translocation of NF-kB p65 subunit in PPT1-KO astroglial cells. (A). Western blot analyses of astroglial cell extracts from PPT1-KO and WT littermates using phospho-IkBα antibody showing increased phosphorylation of IkBα in PPT1-KO astroglial cells. (B) Western blot analyses of nuclear extracts from PPT1-KO and WT astroglial cells using NF-kB p65 antibody. Histone H4 was used as the loading standard for the nuclear protein fractions (C) Activation of NF-kB in cultured astroglial cells from the PPT1-KO and WT mice. The cells were processed for immunofluorescence staining detecting sub-cellular localization of NF-kB subunit p65 (red) in the WT (Left panels) and PPT1-KO (Right panels). Nuclei were stained with DAPI (blue; middle panel). Increased immunoreactivity for p65 in the nuclei of KO cells was also observed. Scale bars, 100μm. The error bars indicate standard deviation of the mean (n=3).
3.7 Expression of pro-inflammatory mediators in the brain of the PPT1-deficient mice and in cultured astroglia
Because the activation of NF-kB mediates increased expression of pro-inflammatory cytokines, we analyzed the levels of four key cytokines, IL-1ß, IL-6, MCP-1 and TNF-α, in six-month-old PPT1-KO mice and their WT littermates. Six-month old PPT1-KO mice were chosen because at this age astrogliosis is at a very high level in the PPT1-KO mouse brain. We also analyzed the levels of the cytokines in the conditioned media from WT and PPT1-deficient astroglia cultures by ELISA. Our results show that compared with the levels of these cytokines in the WT mouse brain and in WT astroglial cell culture media (Fig. 5A-D, open bars), those in the PPT1-KO littermates and culture media from the PPT1-deficient astroglia are markedly elevated (Fig. 5A-D, solid bars). These results are consonant with the high levels of RAGE and S100B expression we found in the brain tissues of the PPT1-KO mice as well as in those of the INCL patient. Taken together, these results raise the possibility that RAGE-signaling mediates the production of proinflammatory cytokines in astroglia, which in turn cause neuroinflammation in INCL.
Fig. 5.

Elevated secretion of IL-1ß, IL-6, MCP-1 and TNFα in PPT1-KO mouse brain extract as well as in those of the PPT1-KO conditioned media from astroglial cell cultures. The results are expressed as the mean (n=3) ±SD.
3.8 Suppression of RAGE-expression inhibits proinflammatory mediator production
To determine whether the production of proinflammatory cytokines is mediated via RAGE signaling, we suppressed RAGE expression in cultured astroglial cells derived from PPT1-KO mice and measured the cytokine levels before and after the suppression of RAGE. We used RAGE-specific siRNA to suppress RAGE expression and transfection of the cells with a scrambled siRNA served as controls. We measured the levels of RAGE, phosphorylated as well as non-phosphorylated Src, ERK1/2 and p38 MAPK by Western blot analysis. Our results show that treatment of the PPT1-deficient cells with RAGE-specific siRNA not only reduced the level of RAGE but also the levels of p-ERK1/2, p-Src and p-p38 (Fig. 6). We then sought to determine the effects of suppressing RAGE expression on the production of proinflammatory mediators. Accordingly, we quantitated the levels of IL-1ß, IL-6, MCP-1 and TNF-α in the culture media of astroglial cells that are either mock transfected, scrambled Si-RNA transfected or transfected with RAGE-specific SiRNA. The results show that suppression of RAGE expression occurred only when the cells were transfected with RAGE-specific SiRNA, which also inhibited the production of IL-1 ß, IL-6, MCP-1 and TNF-α (Fig. 7). Taken together, our results suggest that proinflammatory cytokine production by PPT1-deficient astroglial cells is mediated by RAGE- signaling and most likely a contributing factor in mediating neuroinflammation in INCL.
Fig. 6.

Effect of a RAGE-specific siRNA transfection of PPT1-deficient astroglial cells to suppress RAGE expression. Both WT and PPT1-KO astroglial cells were transfected with siRNAs or with a scrambled siRNA (Ambion, USA) as control. Western blot analyses were performed using anti-RAGE , -ß-Actin, -pSrc, c-Src, -pERK1/2, -ERK1/2, -pP38 or –P38 antibodies.
Fig. 7.

Effect of RAGE-SiRNA on the expression of pro-inflammatory cytokines. Note that suppression of RAGE-expression by SiRNA markedly decreased the cytokine production.
4. Discussion
In this study, we sought to determine the molecular mechanism(s) of neuroinflammation in INCL, a devastating neurodegenerative storage disorder in children that has no effective treatment. We have demonstrated that in the brain tissues of both the INCL patient and in those of the PPT1-KO mice that mimic INCL, the expression of S100B, a brain-specific Ca++-binding protein, and its receptor, RAGE, are appreciably higher than that if normal controls. We further demonstrated that in the brain of the PPT1-KO mice significantly higher levels of the following cytokines, IL-1ß, IL-6, MCP-1 and TNF-α, are readily detectable. These cytokines are well-known mediators of inflammation. Moreover, our results show that the production of these mediators requires RAGE-signaling as the inhibition of RAGE expression markedly inhibited the production of the proinflammatory cytokines. Thus, our results show that increased S100B in conjunction with elevated levels of RAGE expression may trigger neuroinflammation in INCL.
In several brain disorders including traumatic brain injury, the concentrations of S100B in the serum and cerebrospinal fluid tend to increase [36]. We previously reported that in INCL and in the PPT1-KO mice neuronal apoptosis, at least in part, is mediated by ER-[13] and oxidative [37]-stresses. ER stress disrupts Ca++-homeostasis [18] and excessive Ca++ release in the cytosol due to ER stress may stimulate the production of S100B, which also augments the expression of its receptor, RAGE. The interaction of S100B with RAGE activates signaling pathways leading to NF-kB activation, which in turn induces the expression of the genes that encode proinflammatory cytokines [38]. Consistent with the results of our present study, it has been reported that NF-kB may be a sensor of oxidative stress [reviewed in 39]. Indeed, increased production of reactive oxygen species (ROS), as observed in the brain of PPT1-KO mice [14, 37] has been reported and it has been shown that ROS production is associated with up regulation of activated NF-kB [40]. Moreover, it has been demonstrated that the activation of NF-kB in certain circumstances is coupled with the activation of the Src- [32] and MAP-kinases [41, 42]. Thus, the results of our present investigation are consistent with our previous demonstration that PPT1-deficiency leads to ER- and oxidative-stresses [13, 37]. To our knowledge, this is the first report demonstrating that S100B-RAGE signaling is instrumental in the elevated production of pro-inflammatory cytokines, which most likely contributes to neuroinflammation in INCL.
It is increasingly evident that neuroinflammation contributes to the progression of neurodegeneration. Recently, it has been proposed that RAGE may be a potential target for amyloid beta-mediated cellular perturbation in Alzheimer's disease [43]. One of the major complications in neurodegenerative diseases is the activation and brain infiltration of astroglia. The activation of astroglia has been reported in neurodegenerative diseases such as Sandhoff's disease [44], in animal models of lysosomal storage disorders such as mucopolysaccharidoses I and IIIB [45] and in INCL [12, 23]. In the central nervous system, astroglial cells represent the immune cells that play critical roles in mediating neuroinflammation [43]. The results of our present investigation demonstrate that these immune cells in the brain of the PPT1-KO mice, which mimic INCL, are capable of secreting proinflammatory cytokines via RAGE signaling pathway and most likely stimulate the neuroinflammatory response. This raises the possibility that suppressing RAGE expression, signaling or blocking the interaction of S100B with RAGE may have beneficial effects in ameliorating neuroinflammation in INCL.
Supplementary Material
Acknowledgements
We thank Drs. J.Y. Chou and I. Owens for critical review of the manuscript and helpful suggestions.
Abbreviations
- INCL
infantile neuronal ceroid lipofuscinosis
- PPT1
palmitoyl-protein thioesterase-1
- RAGE
receptor for advanced glycation end products
- ER
endoplasmic reticulum
- CNS
central nervous system
- GROD
granular osmiophilic deposit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Consilvio C, Vincent AM, Feldman EL. Neuroinflammation, COX-2, and ALS--a dual role? Exp. Neurol. 2004;187:1–10. doi: 10.1016/j.expneurol.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 3.Goebel HH, Wisniewski KE. Current state of clinical and morphological features in human NCL. Brain Pathol. 2004;14:61–69. doi: 10.1111/j.1750-3639.2004.tb00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchison HM, Mole SE. Neurodegenerative disease: the neuronal ceroid lipofuscinosis (Batten disease). Curr. Opin. Neurol. 2001;14:795–803. doi: 10.1097/00019052-200112000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Vesa J, Hellsten E, Verkruyse LA, Camp LA, Rapola J, Santavuori P, Hofmann SL, Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376:584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- 6.Camp LA, Hofmann SL. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J. Biol. Chem. 1993;268:22566–22574. [PubMed] [Google Scholar]
- 7.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006:re14. doi: 10.1126/stke.3592006re14. 2006. [DOI] [PubMed] [Google Scholar]
- 8.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell. Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 9.Lu JY, Verkruyse LA, Hofmann SL. Lipid thioesters derived from acylated proteins accumulate in infantile neuronal ceroid lipofuscinosis: correction of the defect in lymphoblasts by recombinant palmitoyl-protein thioesterase. Proc. Natl. Acad. Sci. U S A. 1996;93:10046–10050. doi: 10.1073/pnas.93.19.10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapola J, Haltia M. Cytoplasmic inclusions in the vermiform appendix and skeletal muscle in two types of so-called neuronal ceroid-lipofuscinosis. Brain. 1973;96:833–840. doi: 10.1093/brain/96.4.833. [DOI] [PubMed] [Google Scholar]
- 11.Gupta P, Soyombo AA, Atashband A, Wisniewski KE, Shelton JM, Richardson JA, Hammer RE, Hofmann SL. Disruption of PPT1 or PPT2 causes neuronal ceroid lipofuscinosis in knockout mice. Proc. Natl. Acad. Sci. U S A. 2001;98:13566–13571. doi: 10.1073/pnas.251485198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bible E, Gupta P, Hofmann SL, Cooper JD. Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol. Dis. 2004;16:346–359. doi: 10.1016/j.nbd.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Lee YC, Kim SJ, Tsai PC, Saha A, Wei H, Xu Y, Xiao YJ, Zhang P, Heffer A, Mukherjee AB. Palmitoyl-protein thioesterase-1 deficiency mediates the activation of the unfolded protein response and neuronal apoptosis in INCL. Hum. Mol. Genet. 2006;15:337–346. doi: 10.1093/hmg/ddi451. [DOI] [PubMed] [Google Scholar]
- 14.Kim SJ, Zhang Z, Hitomi E, Lee YC, Mukherjee AB. Endoplasmic reticulum stress-induced caspase-4 activation mediates apoptosis and neurodegeneration in INCL. Hum. Mol. Genet. 2006;15:1826–1834. doi: 10.1093/hmg/ddl105. [DOI] [PubMed] [Google Scholar]
- 15.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 16.Hetz CA, Soto C. Stressing out the ER: a role of the unfolded protein response in prion-related disorders. Curr. Mol. Med. 2006;6:37–43. doi: 10.2174/156652406775574578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 18.Paschen W. Dependence of vital cell function on endoplasmic reticulum calcium levels: implications for the mechanisms underlying neuronal cell injury in different pathological states. Cell Calcium. 2001;29:1–11. doi: 10.1054/ceca.2000.0162. [DOI] [PubMed] [Google Scholar]
- 19.Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol. Sci. 1998;19:131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- 20.Jalanko A, Tyynela J, Peltonen L. From genes to systems: new global strategies for the characterization of NCL biology. Biochim. Biophys. Acta. 2006;1762:934–944. doi: 10.1016/j.bbadis.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Woloszynek JC, Coleman T, Semenkovich CF, Sands MS. Lysosomal dysfunction results in altered energy balance. J. Biol. Chem. 2007;282:35765–35771. doi: 10.1074/jbc.M705124200. [DOI] [PubMed] [Google Scholar]
- 22.Cooper JD, Russell C, Mitchison HM. Progress towards understanding disease mechanisms in small vertebrate models of neuronal ceroid lipofuscinosis. Biochim. Biophys. Acta. 2006;1762:873–889. doi: 10.1016/j.bbadis.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Lee YC, Kim SJ, Choi MS, Tsai PC, Xu Y, Xiao YJ, Zhang P, Heffer A, Mukherjee AB. Production of lysophosphatidylcholine by cPLA2 in the brain of mice lacking PPT1 is a signal for phagocyte infiltration. Hum. Mol. Genet. 2007;16:837–847. doi: 10.1093/hmg/ddm029. [DOI] [PubMed] [Google Scholar]
- 24.Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends. Biochem. Sci. 1996;21:134–140. doi: 10.1016/s0968-0004(96)80167-8. [DOI] [PubMed] [Google Scholar]
- 25.Gebhardt C, Riehl A, Durchdewald M, Németh J, Fürstenberger G, Müller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP, Hess J, Angel P. Division of Signal Transduction and Growth Control, RAGE signaling sustains inflammation and promotes tumor development. J. Exp. Med. 2008;205:275–285. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin J, Goswami R, Dawson S, Dawson G. Expression of the receptor for advanced glycation end products in oligodendrocytes in response to oxidative stress. J. Neurosci. Res. 2008;86:2414–2422. doi: 10.1002/jnr.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J. Biochem. Cell Biol. 2001;33:637–668. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 28.Craft JM, Watterson DM, Marks A, Van Eldik LJ. Enhanced susceptibility of S-100B transgenic mice to neuroinflammation and neuronal dysfunction induced by intracerebroventricular infusion of human beta-amyloid. Glia. 2005;51:209–216. doi: 10.1002/glia.20194. [DOI] [PubMed] [Google Scholar]
- 29.Das AK, Becerra CH, Yi W, Lu JY, Siakotos AN, Wisniewski KE, Hofmann SL. Molecular genetics of palmitoyl-protein thioesterase deficiency in the U.S. J. Clin. Invest. 1998;102:361–370. doi: 10.1172/JCI3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Booher J, Sensenbrenner M. Growth and cultivation of dissociated neurons and glial cells from embryonic chick, rat and human brain in flask cultures. Neurobiology. 1972;2:97–105. [PubMed] [Google Scholar]
- 31.Parsons JT, Parsons SJ. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr. Opin. Cell Biol. 1997;9:187–192. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- 32.Reddy MA, Li SL, Sahar S, Kim YS, Xu ZG, Lanting L, Natarajan R. Key role of Src kinase in S100B-induced activation of the receptor for advanced glycation end products in vascular smooth muscle cells. J Biol Chem. 2006;281:13685–93. doi: 10.1074/jbc.M511425200. 2006. [DOI] [PubMed] [Google Scholar]
- 33.Clynes R, Moser B, Yan SF, Ramasamy R, Herold K, Schmidt AM. Receptor for AGE (RAGE): weaving tangled webs within the inflammatory response. Curr. Mol. Med. 2007;7:743–751. doi: 10.2174/156652407783220714. [DOI] [PubMed] [Google Scholar]
- 34.Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J. Biol. Chem. 1997;272:17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- 35.Bierhaus A, Stern DM, Nawroth PP. RAGE in inflammation: a new therapeutic target? Curr. Opin. Investig. Drugs. 2006;7:985–991. [PubMed] [Google Scholar]
- 36.Petzold A, Jenkins R, Watt HC, Green AJ, Thompson EJ, Keir G, Fox NC, Rossor MN. Cerebrospinal fluid S100B correlates with brain atrophy in Alzheimer's disease. Neurosci. Lett. 2003;336:167–170. doi: 10.1016/s0304-3940(02)01257-0. [DOI] [PubMed] [Google Scholar]
- 37.Wei H, Kim SJ, Zhang Z, Tsai PC, Wisniewski KE, Mukherjee AB. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum. Mol. Genet. 2008;17:469–477. doi: 10.1093/hmg/ddm324. [DOI] [PubMed] [Google Scholar]
- 38.Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 39.Li N, Karin M. Is NF-kappaB the sensor of oxidative stress? FASEB J. 1999;13:1137–1143. [PubMed] [Google Scholar]
- 40.Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid. Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- 41.Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J. Biol. Chem. 1999;274:30858–30863. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- 42.Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, Haegeman G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Walker DG, Schmidt AM, Arancio O, Lue LF, Yan SD. RAGE: a potential target for Abeta-mediated cellular perturbation in Alzheimer's disease. Curr. Mol. Med. 2007;7:735–742. doi: 10.2174/156652407783220741. [DOI] [PubMed] [Google Scholar]
- 44.Wada R, Tifft CJ, Proia RL. Microglial cellsactivation precedes acute neurodegeneration in Sandhoff disease and is suppressed by bone marrow transplantation. Proc. Natl. Acad. Sci. U S A. 2000;97:10954–10959. doi: 10.1073/pnas.97.20.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc. Natl. Acad. Sci. U S A. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.