Abstract
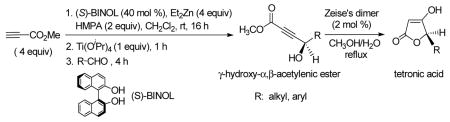
The optically active γ-hydroxy-α,β-acetylenic esters are obtained from the enantioselective reaction of methyl propiolate with both aliphatic and aromatic aldehydes. These compounds can undergo regiospecific hydration in the presence of Zeise’s dimer, [PtCl2(C2H4)]2, to generate the optically active tetronic acids.
Optically active γ-hydroxy-α,β-acetylenic esters are a class of highly functional compounds with versatile synthetic applications.1,2 These compounds are generally prepared by oxidation of the racemic γ-hydroxy-α,β-acetylenic esters to the corresponding γ-oxo-α,β-acetylenic esters followed by asymmetric reduction (Scheme 1).2 The racemic γ-hydroxy-α,β-acetylenic esters are normally prepared from the treatment of a propynoate, e.g., methyl propiolate, with nBuLi at very low temperature, often ≤−78 °C, followed by addition to an aldehyde.1a Although it would be much more efficient to synthesize the optically active γ-hydroxy-α,β-acetylenic esters by the asymmetric addition of methyl propiolate to aldehydes, no such process was developed until recently.3,4 We discovered that 1,1′-bi-2-naphthol (BINOL) in combination with Et2Zn, Ti(OiPr)4 and HMPA can catalyze the highly enantioselective reaction of methyl propiolate with aromatic aldehydes at room temperature (Scheme 2).3 In this paper, we describe a further expansion of the scope of the substrates for this reaction to include various types of aliphatic aldehydes. In addition, we have discovered that the optically active γ-hydroxy-α,β-acetylenic ester products can undergo a regiospecific hydration to generate tetronic acids while maintaining the optical purity. Herein these results are reported.
Scheme 1.

Synthesis of Optically Active γ-Hydroxy-α,β-acetylenic Esters by Asymmetric Reduction.
Scheme 2.

Asymmetric Addition of Methyl Propiolate to Aldehydes.
We first used (S)-BINOL to catalyze the reaction of methyl propiolate with aliphatic aldehydes in the presence of Et2Zn and Ti(OiPr)4 to synthesize the optically active γ-hydroxy-α,β-acetylenic esters (Scheme 2). The results are summarized in Table 1. Good enantioselectivity has been observed for the reaction of methyl propiolate with linear (entries 1 and 2), α-branched (entry 3) and β-branched aliphatic aldehyde (entry 4). High enantioselectivity was also observed for the addition to an α,β-unsaturated aldehyde though the yield is not high (entry 5). The result in entry 6 for the addition to an ortho-substituted aromatic aldehydes was reported3 and it is included here because of the subsequent hydration study (vide infra). On the basis of the previous study,3 the products generated from (S)-BINOL should have a R configuration and those from (R)-BINOL should have a S configuration.
Table 1.
Results for the Enantioselective Addition of Methyl Propiolate to Aldehydes Catalyzed by BINOL-HMPA-Ti
| entry | aldehyde | γ-hydroxy-α,β-acetylenic ester | yield (%) | ee (%) |
|---|---|---|---|---|
| 1 |
|

|
72 | 81a |
| 2 |
|

|
76 | 89a |
| 3 |
|

|
60 | 81a |
| 4 |
|

|
73 | 83a |
| 5 |
|

|
38 | 90a |
| 63 |

|

|
96 | 91b |
Measured by analyzing the 1H NMR spectrum of the mandelate acetate.
Obtained by using HPLC-chiral column
The mechanism for the BINOL-catalyzed alkyne addition to aldehydes is unknown at the current stage. An intermediate shown in Scheme 2 is only a tentative hypothesis provided to help us understand this process. The molecular modeling structure of this intermediate was established with the PC Spartan-Semiempirical PM3 program. In this intermediate, the ethyl group on the zinc center is down in order to avoid the steric interaction between its α-sp3 carbon with the 3-H of the (S)-BINOL ligand. The alkyl group of the aldehyde is up in order to avoid the steric interaction with the ethyl group on the zinc and to reduce the interaction with the isopropoxy groups on the titanium. The alkynyl group on the zinc will then attack the si face of the aldehyde to give the observed R propargylic alcohol product.
We studied the hydration of the optically active γ-hydroxy-α,β-acetylenic esters by using Ziese’s dimer as the catalyst.5,6 In a refluxing CH3OH/H2O (3:1) solution, the hydration of a γ-hydroxy-α,β-acetylenic ester occurred with water attacking the β-position specifically to generate tetronic acids of general formula 1 (Scheme 3). The 300 MHz 1H NMR spectra of these tetronic acids generally showed that both the keto and enol tautomers were present in solution. This compound belongs to the family of the biologically significant tetronic acids.7,8 One of the best known examples of tetronic acids is vitamin C (ascorbic acid).
Scheme 3.

Regiospecific Hydration of γ-Hydroxy-α,β-acetylenic Esters Catalyzed by the Zeise’s Dimer.

In the catalytic hydration of the optically active γ-hydroxy-α,β-acetylenic esters, 2 mol % of the Zeise’s dimer was used. The resulting product 1 was then treated with acetic anhydride and pyridine which quantitatively converted both the enol and keto tautomers of 1 to the acetate 2 (Scheme 3). The ee of the acetate was determined by using GC-β-cyclodextrin column. As the results summarized in Table 2 show, the enantiomeric purities of the tetronic acid products are all very similar to those of the starting aliphatic γ-hydroxy-α,β-acetylenic esters (entries 1–5). The γ-hydroxy-α,β-acetylenic ester derived from ortho-methylbenzaldehyde also gave the hydration product with high enantiomeric purity (entry 6). We tested the hydration of other aromatic γ-hydroxy-α,β-acetylenic esters but significantly reduced enantiomeric purity was observed. It appears that the product derived from the ortho-substituted benzaldehyde has a stable chiral configuration under the reaction conditions, whereas the chiral configurations of the products derived from para- and meta-substituted benzaldehydes are not especially stable.
Table 2.
Results for the Conversion of the γ-Hydroxy-α,β-Acetylenic Esters to 2.
| entry | γ-hydroxy-α,β-acetylenic ester | acetate of tetronic acid (2) | yield (%) | ee (%) |
|---|---|---|---|---|
| 1 |
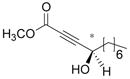
|

|
55 | 80 |
| 2 |

|
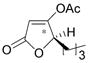
|
66 | 83 |
| 3 |

|

|
61 | 74 |
| 4 |

|

|
78 | 82 |
| 5 |

|

|
46 | 90 |
| 6 |

|

|
41 | 92b |
Obtained by using GC-β-cyclodextrin column.
Estimated (>95% reliable) since baseline resolution was not achieved.
The intermediate 3 is proposed for the Pt(II)-catalyzed hydration of the γ-hydroxy-α,β-acetylenic esters.5,6 In 3, the electron-withdrawing effect of the ester group, the Lewis acidity of the Pt(II) center and the chelate effect in

the coordination of the acetyleneic ester to the Pt(II) center might have all contributed to the observed regiospecific hydration.
In summary, we have demonstrated that the optically active γ-hydroxy-α,β-acetylenic esters can be obtained from the enantioselective reaction of methyl propiolate with both aliphatic and aromatic aldehydes. These compounds can undergo regiospecific hydration in the presence of Zeise’s dimer, a Pt(II) complex, to generate the optically active tetronic acids. This work provides a new and efficient asymmetric synthesis of the biologically significant tetronic acids.
Supplementary Material
Synthesis and characterization of the γ-hydroxy-α,β-acetylenic esters and tetronic acids.
Acknowledgments
Support of this work from the National Institute of Health (R01GM58454/R01EB002037) is gratefully acknowledged.
References
- 1.(a) Midland MM, Tramontano A, Cable JR. J Org Chem. 1980;45:28–29. [Google Scholar]; (b) Molander GA, StJean DJ., Jr J Org Chem. 2002;67:3861–3865. doi: 10.1021/jo0255936. [DOI] [PubMed] [Google Scholar]; (c) Trost BM, Ball ZT. J Am Chem Soc. 2004;126:13942–13944. doi: 10.1021/ja045971j. [DOI] [PubMed] [Google Scholar]; (e) Tejedor D, Garcia-Tellado F, Marrero-Tellado JJ, de Armas P. Chem Eur J. 2003;9:3122–3131. doi: 10.1002/chem.200204579. [DOI] [PubMed] [Google Scholar]; (f) Meta CT, Koide K. Org Lett. 2004;6:1785–1787. doi: 10.1021/ol0495366. [DOI] [PubMed] [Google Scholar]
- 2.(a) Midland MM, McDowell DC, Hatch RL, Tramontano A. J Am Chem Soc. 1980;102:867–869. [Google Scholar]; (b) Noyori R, Yamada TM, Nishizawa M. J Am Chem Soc. 1984;106:6717–6725. [Google Scholar]; (c) Duvold T, Rohmer M. Tetrahedron Lett. 2000;41:3875–3878. [Google Scholar]; (d) Mulzer J, Csybowski M, Bats JW. Tetrahedron Lett. 2001;42:2961–2964. [Google Scholar]; (e) Johansson M, BKöpcke B, Anke H, Sterner O. Tetrahedron. 2002;58:2523–2528. [Google Scholar]
- 3.Gao G, Wang Q, Yu X–Q, Xie R–G, Pu L. Angew Chem Int Ed. 2005;45:122–125. doi: 10.1002/anie.200500469. [DOI] [PubMed] [Google Scholar]
- 4.Trost BM, Weiss AH, von Wangelin AJ. J Am Chem Soc. 2006;128:8–9. doi: 10.1021/ja054871q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiscox W, Jennings PW. Organometallics. 1990;9:1997–1999. [Google Scholar]
- 6.Jenning PW, Hartman JW, Hiscox WC. Inorg Chim Act. 1994;222:317–322. [Google Scholar]
- 7.(a) Tejedor D, Garcia-Tellado F. Organic Preparations and Procedures International. 2004;36:33–59. [Google Scholar]; (b) Roggo BE, Petersen F, Delmendo R, Jenny HB, Peter HH, Roesel JJ. Antibiotics. 1994;47:136–142. doi: 10.7164/antibiotics.47.136. [DOI] [PubMed] [Google Scholar]; (c) Effenberger F, Syed J. Tetrahedron: Asymmetry. 1998;9:817–825. and references cited. [Google Scholar]
- 8.(a) Boll PM, Sorensen E, Balieu E. Acta Chem Scand. 1968;22:3251–3255. [Google Scholar]; (b) Witiak DT, Tehim AK. J Org Chem. 1990;55:1112–1114. [Google Scholar]; (c) Datta A, Schmidt RR. Synlett. 1992:429–430. [Google Scholar]; (d) Datta A, Datta D, Schmidt RR. Tetrahedron Lett. 1992;33:8035–8038. [Google Scholar]; (e) Desmaële D. Tetrahedron. 1992;48:2925–2934. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthesis and characterization of the γ-hydroxy-α,β-acetylenic esters and tetronic acids.


