Summary
Iron acquisition is a vital process for most pathogenic bacteria, as iron is a limiting nutrient during infection. Staphylococcus aureus, an increasingly important pathogen, acquires iron from host heme via elaboration of the iron-regulated surface determinant system (Isd). IsdG and IsdI are heme oxygenases that have been proposed to degrade exogenous heme in the bacterial cytoplasm as a mechanism to liberate free iron for use as a nutrient source. Herein, web report that IsdG and IsdI are both important for S. aureus growth on hemin as a sole iron source and are necessary for full S. aureus pathogenesis. Investigations into the regulation of these enzymes revealed that IsdG and IsdI are differentially regulated by iron and heme through both transcriptional and post-transcriptional mechanisms. Additionally, IsdI was found to be expressed in infected tissues at the sites of abscess formation, suggesting that abscesses are iron-starved microenvironments inside the host. These findings suggest that S. aureus differentially regulates IsdG and IsdI in response to alterations in iron and heme availability during infection.
Keywords: heme oxygenase, iron, post-transcriptional regulation, protein stability, Staphylococcus aureus
Introduction
Iron acquisition is a key strategy for pathogenic bacteria, as free iron in the human host is found at concentrations that are substantially less than is required to sustain bacterial growth (Braun et al., 1991; Chipperfield and Ratledge, 2000). In order to circumvent this impediment to growth in vertebrates, Staphylococcus aureus elaborates the iron-regulated surface determinant system (Isd), which is responsible for capturing nutrient iron from host heme and hemoproteins (Mazmanian et al., 2003). The Isd system is composed of ten proteins that act in concert to bind host hemoproteins, remove the heme cofactor, and passage it through the cell wall and plasma membrane. In accordance with their role in heme-iron acquisition, the cell-wall anchored and membrane transport segments of this system are regulated by the ferric uptake regulator (Fur), such that they are maximally expressed in low iron conditions (Mazmanian et al., 2003). Fur is the canonical iron-dependent regulator that binds DNA at target consensus sequences in order to inhibit transcription when iron is abundant (Escolar et al., 1999). Once heme enters the bacterial cytoplasm it is modeled that IsdG and IsdI, two paralogous heme oxygenases, degrade heme to release free iron for use as a nutrient source (Reniere et al., 2007; Skaar and Schneewind, 2004). In support of this model, IsdG and IsdI have been shown to catalytically degrade hemin in vitro in a reaction that requires oxygen (Lee et al., submitted; Skaar et al., 2004a). Further, S. aureus IsdI expressed in trans is able to complement the heme utilization defect of a Corynebacterium ulcerans heme oxygenase mutant, indicating a role for IsdI in heme-iron utilization in vivo (Skaar et al., 2004a). Orthologues of IsdG and IsdI can be found in a variety of Gram positive pathogens including Bacillus anthracis and Listeria monocytogenes (Skaar et al., 2004a, 2006), suggesting a conserved mechanism by which these important human pathogens catabolize heme during infection.
Despite the accumulating data suggesting a role for IsdG and IsdI in heme degradation during infection, the importance of IsdG and IsdI to staphylococcal biology is not yet known. For instance, the regulatory networks governing the expression of these enzymes have not been explored. Also absent is a mechanistic explanation as to why S. aureus encodes two paralogous enzymes that appear to be functionally redundant. Furthermore, the contribution of bacterial heme degradation to pathogenesis has not been evaluated. This latter point is surprising in light of the fact that heme degrading enzymes have been described in over eight distinct genera of pathogenic bacteria (Ratliff et al., 2001; Ridley et al., 2006; Schmitt, 1997; Skaar et al., 2004a; Suits et al., 2005; Wegele et al., 2004; Wilks and Schmitt, 1998; Wu et al., 2005; Zhu et al., 2000).
The present study addresses each of these missing links by defining the role of heme oxygenases in staphylococcal biology and pathogenesis. Specifically, we demonstrate that IsdG and IsdI are each required for S. aureus heme utilization, as both are necessary for optimal growth on hemin as a sole iron source. Investigation into the regulation of these enzymes reveals that IsdG and IsdI are differentially regulated by local iron and heme environments, providing a functional distinction between these two paralogous enzymes. More specifically, IsdG and IsdI are both transcriptionally regulated by iron in a Fur-dependent manner, while IsdG is also regulated post-transcriptionally by heme. Moreover, in vivo bioluminescence imaging indicates that IsdI is expressed in infected tissues, primarily at the locations of abscess formation. Finally, we demonstrate that IsdG and IsdI are each required for full S. aureus virulence. Taken together, these results establish a role for bacterial heme degradation in pathogenesis and identify a complex regulatory system responsible for ensuring efficient heme catabolism during infection.
Results
S. aureus IsdG and IsdI are required for growth on hemin as a sole iron source
IsdG and IsdI have been shown to catalytically degrade hemin in vitro (Skaar et al., 2004a), however their contribution to staphylococcal heme-iron utilization has not been evaluated. In order to assess the role of IsdG and IsdI in vivo, we inactivated isdG and isdI, individually and in combination, in S. aureus strain Newman. We hypothesized that mutants lacking IsdG and/or IsdI would not be able to grow when hemin is the only available iron source. We sought to test this hypothesis by comparing the growth of S. aureus wild type, ΔisdG, ΔisdI, and ΔisdGI on solid media in which hemin is the sole iron source. This assay has successfully ascribed heme utilization properties to several bacterial proteins involved in the intracellular fate of exogenous hemin (Schmitt, 1997; Skaar et al., 2006; Wyckoff et al., 2005). We observed that S. aureus lacking isdG, isdI or isdGI exhibit significantly impaired growth (p < 0.0001) on hemin as a sole iron source when compared to wild type (Figure 1A). In contrast, all four strains show similar growth kinetics when grown on iron-rich medium and none of the four strains were capable of growth on iron-deplete medium lacking hemin supplementation by the time point tested (data not shown). Interestingly, inactivation of isdG and isdI in combination does not enhance the hemin utilization defect over that of either individual mutant strain. It is possible that this is due to each enzyme having distinct functions in S. aureus. Alternatively, this observation may be the result of the hemin utilization defect of each single mutant reaching the limit of detection of the assay. Importantly, the observation that both enzymes are required for maximal hemin utilization strongly suggests that IsdG and IsdI have distinct contributions to hemin catabolism. Also, the fact that S. aureus ΔisdGI exhibits growth on hemin as a sole iron source suggests that additional heme degrading enzymes may exist in S. aureus.
Figure 1. Growth of S. aureus heme oxygenase mutants on hemin as a sole iron source.
(A) The colony diameter of S. aureus wild type, ΔisdG, ΔisdI, and ΔisdGI strains was measured after growth on solid media containing an iron chelator and various concentrations of hemin. Asterisks denote statistical significance between wild type and mutant strains, as measured by Student’s t test (p < 0.0001). (B and C) Strains listed in A were transformed with an empty vector (pOS1) or a vector expressing either isdG or isdI from a constitutive promoter (pisdG, pisdI) and colony diameter was measured as in A. A single time point at 48 hours is shown, however these trends were observed at all time points tested. Asterisks denote statistical significance between mutant and complemented strains, as measured by Student’s t test (p < 0.0001).
To ensure that the absence of isdG and isdI is responsible for the observed defect in hemin utilization, we performed complementation analyses. To this end, we provided a full-length copy of either isdG or isdI in trans in an effort to complement the observed growth defects of the various mutant strains. We found that constitutive expression of isdG or isdI restores the growth of ΔisdG and ΔisdI, respectively, confirming that the observed hemin utilization defect of the mutants is due to the absence of heme oxygenase activity (Figure 1B and C). Surprisingly, over-expression of either isdG or isdI restores the growth of ΔisdGI to wild type levels when hemin is the sole iron source (Figure 1B and C), indicating that over-expression of IsdG or IsdI is able to compensate for the lack of both heme oxygenases. These results suggest that S. aureus may encode for two heme degrading enzymes as a mechanism to fine-tune the abundance of heme oxygenase in the cell.
IsdG and IsdI are differentially regulated by iron and hemin
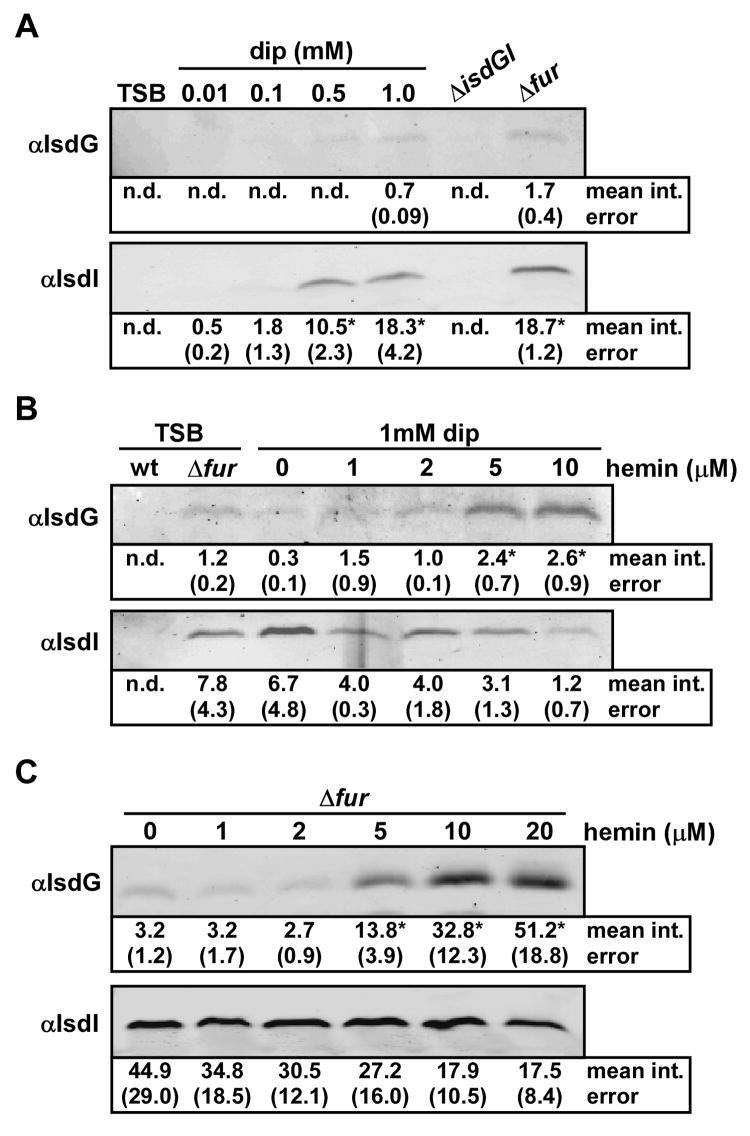
IsdG and IsdI are not functionally redundant given that deletion of either isdG or isdI individually decreases the ability of S. aureus to grow on hemin as a sole iron source. Moreover, over-expression of either IsdG or IsdI complements the loss of both enzymes, suggesting that the efficiency of heme utilization is impacted by the abundance of IsdG and IsdI. Based on these findings, we reasoned that isdG and isdI may be differentially regulated in S. aureus. Due to the location of putative Fur-binding sequences upstream of the transcription start sites for isdG and isdI (Mazmanian et al., 2003; Skaar et al., 2004a), we hypothesized that these enzymes are regulated by iron availability in a Fur-dependent manner. To test this hypothesis we employed quantitative immunoblotting to examine the expression of IsdG and IsdI following growth of S. aureus in medium containing the iron chelator 2,2’dipyridyl. We observed an increase in the abundance of both IsdG and IsdI as the concentration of iron chelator was increased (Figure 2A). This iron-dependent increase was much more pronounced for IsdI as compared to IsdG. As a demonstration that this iron-dependent regulation is mediated by Fur, we found that a S. aureus strain inactivated for fur expresses IsdG and IsdI at levels comparable to those observed in maximally iron-starved S. aureus (Figure 2A). These results demonstrate that IsdG and IsdI are regulated by iron in a Fur-dependent manner.
Figure 2. Regulation of IsdG and IsdI by iron and hemin.
S. aureus strains were grown overnight in the indicated media and protoplast fractions were harvested and normalized by total protein concentration. The mean intensity of each band, as determined by densitometric analyses, is presented under the blots in arbitrary units, with corresponding standard error. Bands that were not detected for quantification are reported as not determined (n.d). Statistical significance was determined using the Student’s t test. Results are representative of at least four independent experiments. (A) The effect of iron chelation by 2,2’dipyridyl (dip) on expression of IsdG and IsdI was analyzed by immunoblot. Asterisks denote statistically significant increases, as compared to 0.01 mM dip (p < 0.0001). (B) The effect of exogenous hemin on expression of IsdG and IsdI in iron-deplete media (dip) was analyzed by immunoblot. Asterisks denote statistically significant increases, as compared to 0 μM hemin (p < 0.03). The slight decrease in IsdI abundance in 10 μM hemin compared to 0 μM hemin is not statistically significant (p > 0.06). (C) The effect of exogenous hemin on expression of IsdG and IsdI in an isogenic Δfur strain was analyzed by immunoblot. Asterisks denote statistically significant increases, as compared to 0 μM hemin (p < 0.0002). The slight decrease in IsdI abundance in 20 μM hemin compared to 0 μM hemin is not statistically significant (p > 0.1).
As IsdG and IsdI are heme-degrading enzymes that are required for hemin utilization (Skaar et al., 2004a; Figure 1), we next sought to investigate the effect of hemin exposure on IsdG and IsdI expression. In an attempt to represent the predicted nutrient environment encountered during infection, we performed these experiments on iron-starved S. aureus. These experiments revealed that IsdI expression is not significantly affected by exposing iron-starved S. aureus to hemin (Figure 2B). In contrast, the abundance of IsdG is enhanced up to 8-fold upon exposure of iron-starved S. aureus to exogenous hemin (Figure 2B). This hemin-dependent increase in IsdG only occurs when S. aureus has been starved for iron, as IsdG was not detected when S. aureus was grown in iron-replete medium supplemented with exogenous hemin (data not shown).
To determine if Fur contributes to the hemin-dependent increase in IsdG expression, we monitored IsdG abundance in S. aureus Δfur exposed to hemin. In this strain, a similar increase in the abundance of IsdG upon hemin exposure was observed compared to iron-starved wild type, indicating that Fur is not responsible for the hemin-dependent regulation of IsdG (Figure 2C). Taken together, these experiments show that IsdG and IsdI are differentially regulated by iron and hemin. More specifically, IsdI is robustly expressed upon iron starvation whereas IsdG abundance is maximal in iron-deplete medium containing hemin.
Proteins encoded within the Isd operon are differentially regulated by hemin
isdG is the terminal gene in the isd operon (Mazmanian et al., 2003; Skaar et al., 2004a). In keeping with this genomic assignment, isdG is predicted to be co-transcribed with the upstream genes isdCDEFsrtB (Figure 3A). In order to test this, we analyzed the expression of IsdG in a S. aureus strain in which isdC has been inactivated by the insertion of an antimicrobial resistance cassette (ΔisdC), leading to transcriptional termination of the operon (Mazmanian et al., 2003). IsdG expression was again found to be enhanced in iron-deplete medium supplemented with hemin whereas IsdI expression is increased in iron-deplete medium, independently of hemin (Figure 3B). Expression of IsdG in the ΔisdC mutant was abolished regardless of the growth conditions under study (Figure 3B), supporting the model that isdC and isdG are encoded within the same transcriptional unit.
Figure 3. Hemin-dependent regulation of Isd protein expression.
(A) Schematic representation of the isd locus. The genes are indicated by open arrows and the putative Fur-binding sites are denoted by black boxes upstream of putative transcription start sites (black arrows). orfX is an uncharacterized open reading frame upstream of isdI, and these genes are co-transcribed. (B) Expression of IsdG and IsdI was analyzed by immunoblot. Wild type S. aureus and ΔisdC were grown in iron-replete or iron-deplete (dip) media in the presence or absence of hemin (10 μM) and protoplast fractions were harvested and normalized by total protein concentration. (C) Immunoblot analysis of Isd proteins in S. aureus wild type grown in iron-deplete medium containing hemin (wt dip+hemin) compared to isogenic strains lacking individual genes of the isd locus in identical growth media. The isogenic Δfur strain was grown in TSB. All results are representative of at least three independent experiments.
Based on the assignment of isdG as the terminal gene in the isd operon, we reasoned that all of the proteins encoded within this operon would be up-regulated when iron-starved S. aureus encounters hemin. To test this, a series of strains inactivated for various components of the isd operon were examined for protein expression in iron-deplete media containing hemin. As a negative control in these experiments, we analyzed the abundance of IsdI, as its expression is not affected by hemin. As expected, IsdC, IsdD, IsdE, and SrtB were expressed in S. aureus Δfur. Surprisingly, IsdG is the only protein expressed from the isdCDEFsrtBisdG operon with enhanced abundance in the presence of hemin (Figure 3C, wt dip+hemin vs Δfur). Moreover, transcriptional termination at any gene upstream of isdG in the isdCDEFsrtBisdG operon abolishes expression of all downstream genes, further confirming that these genes are transcribed as a single unit (Figure 3C). Taken together, these data suggest that the hemin-dependent increase in IsdG expression is occurring post-transcriptionally.
IsdG is regulated by hemin via a post-transcriptional mechanism
In order to investigate the mechanism responsible for the observed hemin-dependent regulation of IsdG, we analyzed transcript levels of several isd genes in iron-rich medium, iron-deplete medium, and iron-deplete medium containing hemin. We observed that transcript levels of isdC, isdD, and isdG are increased 40-70-fold upon iron starvation, consistent with the Fur-regulation of this operon (Figure 4A). However, the transcript levels of isdC, isdD, and isdG from iron-starved S. aureus were not affected by addition of exogenous hemin (Figure 4A). This is in stark contrast to results of the immunoblot analyses that showed an increase of IsdG upon addition of hemin to iron-deplete medium (Figures 2 and 3). These experiments confirm that the hemin-dependent up-regulation of IsdG is occurring post-transcriptionally.
Figure 4. Role of transcription in the hemin-dependent regulation of IsdG.
(A) Transcript levels of isdC, isdD, and isdG were analyzed by real time RT-PCR and normalized to 16S rRNA. S. aureus wild type was grown in iron-deplete media (dip, gray bars) and iron-deplete media containing 5 μM hemin (dip + hemin, black bars) and transcript levels were compared to those grown in TSB. Error bars represent the average range of triplicate experiments. (B) IsdG abundance in S. aureus ΔisdG pisdG grown in iron-deplete media and various concentrations of hemin was analyzed by immunoblotting. Results depict normalized IsdG protein levels in arbitrary units (a.u.). Asterisk denotes statistical significance compared to iron-deplete media alone, as measured by Student’s t test (p < 0.015).
In order to determine if the post-transcriptional regulation of IsdG requires cis-acting sequences in the 5’ untranslated region of isdG, we analyzed the effect of hemin on IsdG expression when isdG transcription is driven by a constitutive promoter. The ΔisdG pisdG strain was utilized for this assay, as the coding sequence of isdG is cloned directly downstream of the constitutive lgt promoter, which is not affected by hemin (Bubeck Wardenburg et al., 2006; Stauff et al., 2007). This vector does not contain any of the sequences typically present in the genomic context surrounding isdG, and hence, expression can be monitored independently of potential regulatory cis-acting sequences. We observed a 2.4-fold increase (p < 0.015) in the abundance of IsdG when ΔisdG pisdG was grown on hemin as the only iron source (Figure 4B), indicating that the isdG coding sequence is sufficient for the hemin-dependent regulation of IsdG.
IsdG protein stability is enhanced by hemin
The hemin-dependent increase in IsdG abundance in iron-deplete medium (Figure 2) is not a result of increased isdG transcription in this environment (Figure 4A). One possible explanation for this is that the presence of hemin stabilizes the IsdG protein. To investigate the iron- and hemin-dependent stability of IsdG we utilized the S. aureus ΔisdG pisdG strain that constitutively expresses isdG. Following a pulse with 35S-methionine, the fate of IsdG was analyzed by immunoprecipitation over time. We observed that IsdG is more stable in conditions containing hemin, independent of the iron status of the culture (Figure 5A and C). In medium lacking hemin, IsdG turnover is more rapid, with a half-life of 66.9 minutes. However, IsdG is comparatively stable in medium containing hemin, with a half-life of 166.3 minutes. Thus, in the presence of hemin, the half-life of IsdG is increased 2.5-fold (p < 5×10-7), independently of the iron status of the medium. To determine if this hemin-dependent stabilization is specific for IsdG, we performed a similar pulse-chase experiment to monitor the iron- and hemin-dependent stability of IsdI. For these experiments, we utilized a S. aureus ΔisdI pisdI strain that constitutively expresses isdI. These experiments revealed that IsdI stability is unaffected by hemin or iron status (Figure 5B and D). The half-life of IsdI in the absence of hemin is 329.0 minutes, while in the presence of hemin it is 325.4 minutes (p = 0.98). Taken together with data obtained from the quantitative RT-PCR experiments described above, these results lead us to conclude that IsdG is most abundant in low iron conditions containing hemin, an environment that is potentially encountered following erythrocyte lysis during infection.
Figure 5. Role of hemin in the stability of IsdG and IsdI.
(A and B) Phosphorimages of radiolabeled IsdG and IsdI immunoprecipitated from cells pulsed with 35S-methionine, followed by a chase with unlabeled methionine for the time indicated. Pulse-chase analyses were carried out in media treated with iron sulfate (+Fe) or 2,2’dipyridyl (-Fe), and 10 μM hemin (+H). Dash indicates control cells in which primary antibody was omitted during immunoprecipitation. (C and D) Quantification of IsdG and IsdI from quadruplicate experiments as described in A and B. Error bars represent standard deviation of data from at least three independent experiments. Asterisks denote statistical significance between conditions with and without hemin irrespective of iron status, as measured by Student’s t test (p < 0.003).
IsdG and IsdI are required for staphylococcal pathogenesis
We have shown that IsdG and IsdI are required for maximal staphylococcal growth when hemin is the sole iron source (Figure 1). An abundance of available heme in an otherwise iron-free host is an environment likely encountered during the course of infection. Additionally, it has been shown that hemoglobin binding and heme acquisition systems are required for staphylococcal pathogenesis (Skaar et al., 2004b; Torres et al., 2006). Therefore, we sought to test the pathological relevance of heme catabolism by investigating the requirement for IsdG and IsdI in S. aureus pathogenesis. To determine the contribution of IsdG- and IsdI-mediated heme-iron utilization to staphylococcal virulence, a mouse model of systemic abscess formation was employed. Mice were infected intravenously with wild type, ΔisdG, ΔisdI, or ΔisdGI S. aureus. Enumeration of bacteria four days after infection revealed a decrease in bacterial load of up to 2 logs (p < 0.04) for each mutant strain as compared to wild type in the hearts of infected animals (Figure 6A & B). Additionally, infection with the ΔisdG and ΔisdGI strains resulted in a 1-log decrease (p < 0.02) in bacterial load compared to wild type in the kidneys (Figure 6C & D). No difference in bacterial load was observed in the livers of infected animals. Taken together, these results demonstrate the importance of IsdG- and IsdI- mediated heme catabolism in staphylococcal virulence. The virulence decrease of ΔisdG and ΔisdGI observed in the kidneys suggests that heme degradation by IsdG is more significant than that mediated by IsdI upon S. aureus colonization of this organ. The reason for this organ-specific requirement for IsdG is not known at this time. Importantly, this is the first demonstration that heme degrading enzymes contribute to the virulence of a bacterial pathogen.
Figure 6. Contribution of IsdG and IsdI to S. aureus pathogenesis.
S. aureus colonization of the hearts and kidneys of female Balb/c mice was analyzed four days post retroorbital infection. (A and C) Colonization was compared between wild type, ΔisdG, and ΔisdI strains. (B and D) In a separate experiment, colonization of wild type S. aureus was compared to the ΔisdGI strain. Each data point represents the colony forming units (CFU) per organ in a single animal. The horizontal lines represent the mean CFU/organ. Asterisks denote statistical significance between wild type and the representative mutant strain as measured by Student’s t test (p < 0.04).
IsdI is expressed in tissue abscesses
The decreased virulence of staphylococcal strains lacking isdG and isdI indicates that IsdG and IsdI are expressed and functional during infection. In order to investigate the expression of these enzymes in the context of infection we employed in vivo bioluminescence imaging (IVIS). This technique requires cloning the promoter of the gene of interest in front of the modified luciferase operon of Photorhabdus luminescens (Francis et al., 2000). When the promoter is activated the luciferase enzyme and its substrate are expressed and luminescence can be detected and quantified. The luminescent signal produced is of sufficient intensity to be detected through the tissues of an animal, thus enabling the detection of promoter activity in vivo. Because this technique employs a transcriptional reporter gene, and our data indicate that IsdG is primarily regulated by a post-transcriptional mechanism, we were not able to investigate the in vivo expression of IsdG using this technology.
We first grew wild type S. aureus carrying either the pXen1 vector (no promoter) or the pisdI.Xen1 (isdI promoter) vector in iron-rich medium or iron-deplete medium. As expected from the iron-dependent regulation of IsdI seen by protein analysis (Figure 2A), the luminescent signal from bacteria harboring the pisdI.Xen1 vector is significantly increased in iron-deplete conditions (Figure 7A & B). Importantly, luminescence from up to 108 bacteria harboring the control vector is not above background levels, irrespective of the growth medium. To investigate the expression of IsdI in infected tissues, we infected Balb/c mice with wild type S. aureus harboring either pXen1 or pisdI.Xen1, using the same infection protocol described above. Four days post-infection the organs were harvested and imaged ex vivo via the IVIS imaging system. Luminescence was detected in the kidneys and livers of mice infected with bacteria carrying the pisdI.XenI plasmid (Figure 7C and data not shown). Notably, the luminescence co-localizes with sites of abscess formation in both organs and this trend was reproducible in every mouse analyzed (n=5). Although the number of bacteria and abscesses were similar in the organs from mice infected with bacteria harboring the control vector, no luminescence was detected in any mouse infected with this strain (n=6). These results indicate that isdI is expressed in vivo during infection. Considering that the total number of colony forming units in the kidneys does not typically exceed 107 (Figure 6), the significant luminescence observed in each individual abscess strongly suggests that S. aureus is starved for iron inside tissue abscesses.
Figure 7. In vivo bioluminescence imaging of IsdI expression.
(A) S. aureus harboring pXen1 or pisdI.Xen1 was grown overnight in TSB or iron-deplete media (+dip). Serial dilutions were performed and luminescence was measured as described in the Experimental Procedures. Luminescence scale is in p/s/cm2/sr. (B) Quantification of luminescence depicted in A. Results are shown as fold change in luminescence in iron-deplete medium compared to TSB. Asterisks denote statistical significance between pXen1 (white bars) and pisdI.XenI (black bars), as measured by Student’s t test (p < 0.004). (C) At four days post-infection kidneys were removed from mice imaged ex vivo. Pictured are kidneys from mice infected with S. aureus carrying pXen1 or pisdI.Xen1 (left panels) and bioluminescent images of the same kidneys (right panels). Arrows indicate representative abscesses. Luminescence scale is as in A. Pictures and bioluminescent images are representative of at least five mice in each group. Bacterial load and number of abscesses were consistent between strains.
Discussion
S. aureus heme-iron acquisition is vital for pathogenesis, but the intracellular components of this process have yet to be fully described (Skaar et al., 2004b; Torres et al., 2006). IsdG and IsdI, two cytoplasmic proteins of the Isd heme-uptake machinery, are capable of degrading exogenously acquired heme to release free iron in vitro (Skaar et al., 2004a). Here we have shown that IsdG- and IsdI-catalyzed heme degradation is biologically significant, as IsdG and IsdI are each required for S. aureus growth on hemin as a sole iron source (Figure 1). Additionally, we have provided a mechanistic explanation for the presence of two similar enzymes, in that they are differentially regulated according to the environment encountered by the bacteria. We have also demonstrated the importance of IsdG and IsdI in staphylococcal pathogenesis, as strains lacking these enzymes exhibit decreased virulence (Figure 6). Moreover, we have shown that IsdI is expressed at the site of infection coincident with the location of abscess formation (Figure 7C). These in vivo data suggest that abscesses are iron-starved microenvironments.
Although heme oxygenases have been described in eight genera of pathogenic bacteria, very little is known about the regulatory mechanisms controlling expression of these enzymes. The exception to this is HmuO of the Gram positive bacterium Corynebacterium diphtheriae (Schmitt, 1997). Although HmuO shares no sequence or structural homology to IsdG, HmuO is also maximally expressed in low-iron high-heme (or hemoglobin) conditions (Bibb et al., 2007). However, unlike IsdG, HmuO is regulated at the level of transcription by the combined activity of the diphtheria toxin repressor protein (DtxR) and the response regulators ChrA and HrrA (Bibb et al., 2007).
The observation that IsdG and IsdI are differentially regulated provides insight into the basis for two paralogous enzymes in S. aureus with seemingly identical functions. IsdG and IsdI are both regulated at the transcriptional level by Fur in response to iron availability (Figure 2A). Additionally, IsdG abundance is regulated by hemin availability via a post-transcriptional mechanism controlling protein stability (Figure 4 & 5). Pseudomonas aeruginosa is the only other pathogenic bacteria known to encode for two heme oxygenases (PigA and BphO). These have been shown to differ in their catalytic regiospecificity, in that they each produce a distinct isomer of biliverdin upon heme degradation (Wegele et al., 2004). Although the regiospecificity of IsdG- and IsdI-catalyzed heme degradation is not yet known, it appears that in the case of S. aureus, the significance of encoding for two paralogous heme oxygenases is to fine-tune the expression of these enzymes according to the microenvironment encountered by the bacteria.
S. aureus is able to colonize and infect virtually every tissue of the vertebrate host. This diverse ecological range invariably requires significant flexibility in nutrient acquisition systems. It is tempting to speculate that, in the case of heme degradation, this flexibility is mediated by alterations in the abundance of IsdG and IsdI in response to heme and iron. The human body is virtually devoid of free iron due to the fact that the vast majority of iron is complexed as a component of heme (Bullen, 1999). In healthy individuals, heme is bound to hemoglobin within erythrocytes and myoglobin within myocytes. During systemic infections, it is modeled that S. aureus can access this heme through the secretion of a number of potent hemolysins. Hemolysin expression is regulated in response to bacterial density by global virulence factors, which allow for controllable erythrocyte lysis at a time when colonization has been established and nutrient-iron needs are high (Dunman et al., 2001; Ji et al., 1995). In keeping with this model, it is possible that IsdI is expressed upon entry into the host to ensure that the low level of heme entering the staphylococcal cytoplasm is degraded and the iron is released. Following bacterial seeding of host organs, significant erythrocyte lysis is likely. Based on the efficiency of the Isd system for hemoglobin recognition and heme transport (Mazmanian et al., 2003), this lysis would lead to a commensurate increase in heme transport into the staphylococcal cytoplasm. The intracellular amassing of heme would then stabilize IsdG, hence increasing its abundance. In effect, the simultaneous expression of both IsdG and IsdI would increase the capacity of S. aureus to deal with a heme surplus. We predict that IsdG and IsdI abundance remains high until the nutrient iron needs of the organism have been satisfied. Based on the fact that IsdI remains expressed inside abscesses four days following infection, it appears that S. aureus remains iron-starved during the course of infection in the animal model used in this work.
The presence of hemin delays or prevents IsdG degradation, increasing the half-life of the protein (Figure 5). This regulatory strategy may be mediated by enzyme stabilization upon substrate binding. In keeping with this, it is possible that IsdG undergoes a conformational change or is partially unfolded in the absence of heme and is therefore targeted by proteases for degradation. Alternatively, heme could be stabilizing IsdG by binding outside the active site in a manner that conceals or distorts possible protease target sites. Heme regulatory motifs (HRMs), which have been described in eukaryotes and bacteria, are involved in heme-dependent transcription, translation, protein translocation, and protein stability (Mense and Zhang, 2006; Qi et al., 1999). HRMs are short sequence motifs containing an invariant cys-pro sequence. When heme binds an HRM it induces a conformational change, altering the function of the protein (Mense and Zhang, 2006). This type of mechanism is unlikely in the case of IsdG, due to the lack of any cysteine or proline residues in the protein. Additionally, the recent co-crystal structure of heme-bound IsdG reveals heme bound only in the active site (Lee et al., submitted). However, it is formally possible that heme can bind elsewhere on the protein under in vivo conditions, resulting in enhanced stabilization of IsdG.
In the present study, we demonstrate the role of IsdG and IsdI in staphylococcal heme-iron utilization and pathogenesis. Additionally, we reveal the mode of regulation to be a functional difference between these paralogous enzymes. Interestingly, IsdG and IsdI are 78% similar at the amino acid level, but only IsdG is post-transcriptionally regulated by hemin. Future experiments will seek to provide a mechanistic explanation for the hemin-dependent IsdG stabilization. The finding that IsdG and IsdI are required for full staphylococcal virulence establishes these enzymes as viable targets for the generation of novel antimicrobials to treat S. aureus infections. The potential to design small molecule inhibitors that specifically target bacterial heme oxygenases is further supported by the significant structural differences between this class of enzymes and the human heme oxygenases (Schuller et al., 1999; Wu et al., 2005). Considering the conservation of this enzyme family across B. anthracis, L. monocytogenes, and the pathogenic Staphylococci, antimicrobials targeting the IsdG-family of heme oxygenases have the potential to be broadly applicable across a variety of infectious diseases.
Experimental procedures
Bacterial strains and growth conditions
S. aureus clinical isolate Newman was used in all experiments (Duthie and Lorenz, 1952). Isogenic variants lacking fur, isdC, isdD, isdE, isdF, srtB, isdG have been previously described (Mazmanian et al., 2003). Mutations in isdI were made by allelic replacement of the coding sequence with tetM, according to previously described methods (Bae and Schneewind, 2006). The double heme oxygenase knockout strain (ΔisdGI) was made by transducing the ΔisdI∷tetM allele into ΔisdG using the transducing phage phi-85 as has been described previously (Skaar et al., 2004a). Bacteria were grown overnight in tryptic soy broth (TSB) at 37°C with shaking at 180 rpm unless otherwise stated.
For complementation studies, S. aureus ΔisdG, ΔisdI, and ΔisdGI were transformed with a plasmid encoding a full length copy of isdG or isdI under control of the S. aureus lipoprotein diacylglycerol transferase (lgt) constitutive promoter in the pOS1-derived vector (Bubeck Wardenburg et al., 2006; Schneewind et al., 1992). isdG and isdI coding sequences were PCR amplified (see Table 1 for primers) from Newman genomic DNA and cloned into pCR2.1 (Invitrogen). Successful transformants were screened and those with the correct insert were subcloned into pOS1plgt, making pisdG and pisdI.
Table 1.
Primers used in this study
| Primer | Oligonucleotide sequence (5’ → 3’) |
|---|---|
| isdG into pOS1-F | CTCGAGATGAAATTTATGGCAGAAAATAG |
| isdG into pOS1-R | GGATCCAATTATTTCATGTAACTATAGCC |
| isdI into pOS1-F | CCGGCTCGAGATGTTTATGGCAGAAAATAG |
| isdI into pOS1-R | CCGGGGATCCGTTTTTAATATATTTATTTTTGATAG |
| Real time RT-PCR | |
| isdC-F | GCGGTACTTTGAATTATGAGG |
| isdC-R | GGTTGACAGTTATTTGAACATAC |
| isdD-F | CCGAAACATAAAGATGAAAAATC |
| isdD-R | GGTTCATCAAGTTCTTTAATATC |
| isdG-F | CTTTGATGGCATGTTTGTTAC |
| isdG-R | CGCTGCTTTAAAGACATCAG |
| 16S rRNA-F | GCTGCAGCTAACGCATTAAGCACT |
| 16S rRNA-R | TTAAACCACATGCTCCACCGCTTG |
| Bioluminescence imaging | |
| isdI-F | GAATTCTAATCAGCTCACAGAAGTCTC |
| isdI-R | GGATCCTACTAATGCGCATGTAATATTG |
Hemin nutrition plate assay
S. aureus strains were passaged for 2 days in iron-deplete medium (TSB + 1 mM 2,2’dipyridyl) followed by a 4 hour subculture (1:100) in iron-deplete medium. The cultures were then normalized by optical density at 600 nm and sedimented by centrifugation at 6,000 × g for 10 minutes. The pellets were resuspended in TSB + 6 mM 2,2’dipyridyl and 60 μL was spotted in triplicate on TSA plates containing 6 mM 2,2‘dipyridyl and various concentrations of hemin (Sigma). Chloramphenicol (10 μg/mL) was included in the medium for growth of all strains harboring pOS-1-derived vectors. The iron-depleted, hemin-containing plates were incubated at 37°C in the dark for up to 5 days and the diameter of each colony was measured after 48 hours using the AlphaImager (Alpha Innotech) software. Over 100 colonies were counted per strain for each experiment and the experiment was performed at least three times. Similar trends were conserved across all replicates.
Fractionation of staphylococci for immunoblotting
Overnight cultures of S. aureus (50 ml) were normalized by optical density at 600 nm and sedimented by centrifugation at 6,000 × g for 10 minutes. The bacteria were washed with Tris buffered saline (TBS, 50 mM Tris pH 7.5, 150 mM NaCl), resuspended in TSM buffer (100 mM Tris pH 7.0, 500 mM sucrose, 10 mM MgCl2) and incubated in the presence of lysostaphin (30 μg) at 37°C for 30 minutes. The samples were then pelleted and the supernatants (cell wall fraction) were collected and analyzed by 12% SDS-PAGE followed by immunoblotting. For analysis of membrane and cytoplasmic proteins, the protoplasts were resuspended in BugBuster Protein Extraction Reagent (Novagen) with proteinase inhibitor and sonicated for 10 seconds. The whole protoplast fractions were normalized by total protein concentration, as assessed by BCA analysis, separated by 15% SDS-PAGE, and analyzed by immunoblotting. Antisera was created by injection of purified His-tagged IsdG or IsdI emulsified in complete Freund’s adjuvant (day 7 injection) or incomplete Freund’s adjuvant (two subsequent injections) into female New Zealand rabbits
Immunoblot Protein Quantification
Immunoblotting was performed in quadruplicate as described above. The raw intensities of the protein bands were quantified using the Odyssey System software by LI-COR, Inc, and expressed in arbitrary units.
RNA Isolation
Overnight cultures of wild type S. aureus were subcultured (1:100) in triplicate into TSB alone, TSB supplemented with 2,2’dipyridyl (1 mM), or TSB supplemented with 2,2’dipyridyl and hemin (5 μM). Mid-log phase cultures (OD600 = 0.25) were mixed with an equal volume of ice-cold ethanol-acetone (1:1) and stored at -80°C. For RNA isolation, samples were thawed on ice and centrifuged at 5,000 × g for 10 minutes. Pellets were resuspended in RLT buffer and transferred to Lysing Matrix B tubes (MP Bio). Cells were lysed by processing in a FastPrep Instrument (MP Bio) two times for 20 sec each, followed by centrifugation. The supernatant was used for RNA isolation using RNeasy Mini kit according to manufacturer’s recommendations (QIAGEN). Contaminating DNA was removed by adding 10 units DNase I (Amersham Biosciences) to RNA samples and incubating in RQ1 buffer (Promega) at 37°C for 30 min. Following DNase treatment the samples were repurified using the RNeasy Mini kit according to manufacturer’s protocol for RNA clean-up (QIAGEN). RNA concentration and purity were measured by optical density at 260nm and 280nm, respectively.
Real time RT-PCR
Real time PCR primers are listed in Table 1. For cDNA synthesis, 2 μg total cellular RNA was reverse transcribed with M-MLV reverse transcriptase according to manufacturer’s recommendations (Promega). 10 ng cDNA was amplified using Platinum Quantitative PCR SuperMix-UDG and SYBR Green I Nucleic Acid Stain (Invitrogen) in an iCycler iQ (Bio-Rad). The quantification of each sample was carried out relative to 16S rRNA and each PCR was measured in quadruplicate. Relative quantification was performed using the comparative CT method according to User Bulletin #2: ABI Prism 7700 Sequence Detection System, Applied Biosystems, 2001.
Pulse-Chase and Immunoprecipitation
Mid-log cultures of S. aureus ΔisdG pisdG or ΔisdI pisdI were pelleted and washed three times in Dulbecco’s Modified Eagle’s Medium (DMEM) lacking methionine (Cellgro). Bacteria were then resuspended in DMEM lacking methionine and supplemented with iron sulfate (10 μM) or 2,2’dipyridyl (1 mM), with or without hemin (10 μM). Cells were then pulsed with 35S-methionine (110 μCi) for two minutes at 37°C. Time zero samples were taken and added to an equal volume of 10% trichloroacetic acid (TCA) on ice. Subsequently, the chase solution (casamino acids, methionine, and cysteine) was added and samples were taken at the indicated time points and mixed with an equal volume of 10% TCA on ice. Labeled bacteria were then pelleted and washed with acetone. To remove the cell wall, bacteria were treated with lysostaphin (40 μg) in TSM buffer for 30 minutes at 37°C and then pelleted and washed with acetone. Protoplasts were lysed by boiling in 0.5 M Tris, pH 7.5, 4% SDS. After pelleting, supernatants were removed and mixed with either IsdG or IsdI antisera diluted 1:500 in RIPA buffer at room temperature for 1.5 hours. Subsequently 50 μL of protein A-sepharose slurry was added and incubated for 1 hour. Protein A beads were then washed extensively and the bound antibody-protein complexes were removed by boiling in reducing SDS-PAGE sample buffer. Samples were separated by electrophoresis, dried, and analyzed using a PhosphorImager.
Murine model of infection
Six to eight week old BALB/c mice (Jackson Laboratories) were infected with 4 × 106 CFU of wild-type S. aureus wild type, ΔisdG, ΔisdI, or ΔisdGI mutant strains suspended in PBS by retroorbital injection. Four days post-infection mice were euthanized with CO2 and hearts, kidneys, and livers were removed and homogenized in sterile PBS. Colonization of each organ was determined by enumerating colony forming units on tryptic soy agar.
Bioluminescence imaging
The promoterless vector pXen1 coding for the luxABCDE operon of Photorhabdus luminescens, and modified for Gram positive bacteria, was obtained from Xenogen (Francis et al., 2000). The promoter region of DNA for isdI was cloned into pXen1 to make pisdI.Xen1 using the primers listed in Table 1. The promoterless vector was used as a control for background luminescence in all experiments. Luminescence in vitro and in vivo was measured using the Xenogen IVIS 200 and LiveImage 2.0 software.
For in vivo bioluminescence imaging, six to eight week old BALB/c mice (Jackson Laboratories) were infected with 3 × 107 CFU of wild type Newman carrying either pXen1 or pisdI.Xen suspended in PBS by retroorbital injection. Beginning 24 hours prior to infection, and continuing throughout the infection, mice were given chloramphenicol (0.5 mg/mL) in their drinking water in order to select for bacteria carrying the plasmid. Four days post-infection the mice were euthanized with CO2 and organs were removed and imaged ex vivo. After imaging, the organs were homogenized in PBS and colonization was determined by enumerating colony forming units on tryptic soy agar in order to ensure the bacterial load in each organ was consistent between strains.
Acknowledgments
We would like to thank the members of the Skaar laboratory for critical reading of the manuscript and Susan Opalenik of the Vanderbilt Molecular Genetics core for technical assistance. We would also like to thank Xenogen for the pXen-1 plasmid. This research was supported by the Searle Scholars Program, and United States Public Health Service Grant AI69233 from the National Institute of Allergy and Infectious Diseases. Eric Skaar, Ph.D. holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Welcome Fund. Michelle Reniere was funded by NIH Training Grant in Mechanisms of Vascular Disease, 5 T32 HL07751.
References
- Bae T, Schneewind O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid. 2006;55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Bibb LA, Kunkle CA, Schmitt MP. The ChrA-ChrS and HrrA-HrrS Signal Transduction Systems Are Required for Activation of the hmuO Promoter and Repression of the hemA Promoter in Corynebacterium diphtheriae. Infect Immun. 2007;75:2421–2431. doi: 10.1128/IAI.01821-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V, Gunter K, Hantke K. Transport of iron across the outer membrane. Biol Met. 1991;4:14–22. doi: 10.1007/BF01135552. [DOI] [PubMed] [Google Scholar]
- Bubeck Wardenburg J, Williams WA, Missiakas D. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc Natl Acad Sci U S A. 2006;103:13831–13836. doi: 10.1073/pnas.0603072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen JJ, Griffiths E. Iron and Infection: Molecular, Physiological and Clinical Aspects. New York: John Wiley and Sons; 1999. [Google Scholar]
- Chipperfield JR, Ratledge C. Salicylic acid is not a bacterial siderophore: a theoretical study. Biometals. 2000;13:165–168. doi: 10.1023/a:1009227206890. [DOI] [PubMed] [Google Scholar]
- Dunman PM, Murphy E, Haney S, Palacios D, Tucker-Kellogg G, Wu S, Brown EL, Zagursky RJ, Shlaes D, Projan SJ. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J Bacteriol. 2001;183:7341–7353. doi: 10.1128/JB.183.24.7341-7353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie ES, Lorenz LL. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- Escolar L, Perez-Martin J, de Lorenzo V. Opening the iron box: transcriptional metalloregulation by the Fur protein. J Bacteriol. 1999;181:6223–6229. doi: 10.1128/jb.181.20.6223-6229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis KP, Joh D, Bellinger-Kawahara C, Hawkinson MJ, Purchio TF, Contag PR. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect Immun. 2000;68:3594–3600. doi: 10.1128/iai.68.6.3594-3600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Beavis RC, Novick RP. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci U S A. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O. Passage of heme-iron across the envelope of Staphylococcus aureus. Science. 2003;299:906–909. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- Mense SM, Zhang L. Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res. 2006;16:681–692. doi: 10.1038/sj.cr.7310086. [DOI] [PubMed] [Google Scholar]
- Qi Z, Hamza I, O’Brian MR. Heme is an effector molecule for iron-dependent degradation of the bacterial iron response regulator (Irr) protein. Proc Natl Acad Sci U S A. 1999;96:13056–13061. doi: 10.1073/pnas.96.23.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff M, Zhu W, Deshmukh R, Wilks A, Stojiljkovic I. Homologues of neisserial heme oxygenase in gram-negative bacteria: degradation of heme by the product of the pigA gene of Pseudomonas aeruginosa. J Bacteriol. 2001;183:6394–6403. doi: 10.1128/JB.183.21.6394-6403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reniere ML, Torres VJ, Skaar EP. Intracellular metalloporphyrin metabolism in Staphylococcus aureus. Biometals. 2007;20:333–345. doi: 10.1007/s10534-006-9032-0. [DOI] [PubMed] [Google Scholar]
- Ridley KA, Rock JD, Li Y, Ketley JM. Heme utilization in Campylobacter jejuni. J Bacteriol. 2006;188:7862–7875. doi: 10.1128/JB.00994-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt MP. Utilization of host iron sources by Corynebacterium diphtheriae: identification of a gene whose product is homologous to eukaryotic heme oxygenases and is required for acquisition of iron from heme and hemoglobin. J Bacteriol. 1997;179:838–845. doi: 10.1128/jb.179.3.838-845.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneewind O, Model P, Fischetti VA. Sorting of protein A to the staphylococcal cell wall. Cell. 1992;70:267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- Schuller DJ, Wilks A, Ortiz de Montellano PR, Poulos TL. Crystal structure of human heme oxygenase-1. Nat Struct Biol. 1999;6:860–867. doi: 10.1038/12319. [DOI] [PubMed] [Google Scholar]
- Skaar EP, Gaspar AH, Schneewind O. IsdG and IsdI, heme-degrading enzymes in the cytoplasm of Staphylococcus aureus. J Biol Chem. 2004a;279:436–443. doi: 10.1074/jbc.M307952200. [DOI] [PubMed] [Google Scholar]
- Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. Iron-source preference of Staphylococcus aureus infections. Science. 2004b;305:1626–1628. doi: 10.1126/science.1099930. [DOI] [PubMed] [Google Scholar]
- Skaar EP, Schneewind O. Iron-regulated surface determinants (Isd) of Staphylococcus aureus: stealing iron from heme. Microbes Infect. 2004;6:390–397. doi: 10.1016/j.micinf.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Skaar EP, Gaspar AH, Schneewind O. Bacillus anthracis IsdG, a heme-degrading monooxygenase. J Bacteriol. 2006;188:1071–1080. doi: 10.1128/JB.188.3.1071-1080.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauff DL, Torres VJ, Skaar EP. Signaling and DNA-binding Activities of the Staphylococcus aureus HssR-HssS Two-component System Required for Heme Sensing. J Biol Chem. 2007;282:26111–26121. doi: 10.1074/jbc.M703797200. [DOI] [PubMed] [Google Scholar]
- Suits MD, Pal GP, Nakatsu K, Matte A, Cygler M, Jia Z. Identification of an Escherichia coli O157:H7 heme oxygenase with tandem functional repeats. Proc Natl Acad Sci U S A. 2005;102:16955–16960. doi: 10.1073/pnas.0504289102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol. 2006;188:8421–8429. doi: 10.1128/JB.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegele R, Tasler R, Zeng Y, Rivera M, Frankenberg-Dinkel N. The heme oxygenase(s)-phytochrome system of Pseudomonas aeruginosa. J Biol Chem. 2004;279:45791–45802. doi: 10.1074/jbc.M408303200. [DOI] [PubMed] [Google Scholar]
- Wilks A, Schmitt MP. Expression and characterization of a heme oxygenase (Hmu O) from Corynebacterium diphtheriae. Iron acquisition requires oxidative cleavage of the heme macrocycle. J Biol Chem. 1998;273:837–841. doi: 10.1074/jbc.273.2.837. [DOI] [PubMed] [Google Scholar]
- Wu R, Skaar EP, Zhang R, Joachimiak G, Gornicki P, Schneewind O, Joachimiak A. Staphylococcus aureus IsdG and IsdI, heme-degrading enzymes with structural similarity to monooxygenases. J Biol Chem. 2005;280:2840–2846. doi: 10.1074/jbc.M409526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff EE, Lopreato GF, Tipton KA, Payne SM. Shigella dysenteriae ShuS promotes utilization of heme as an iron source and protects against heme toxicity. J Bacteriol. 2005;187:5658–5664. doi: 10.1128/JB.187.16.5658-5664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Wilks A, Stojiljkovic I. Degradation of heme in gram-negative bacteria: the product of the hemO gene of Neisseriae is a heme oxygenase. J Bacteriol. 2000;182:6783–6790. doi: 10.1128/jb.182.23.6783-6790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]