Abstract
Objective
To examine the relationship of male obesity and reproductive function.
Design
Observational study
Setting
Academic medical center
Patients
87 adult males, body mass index (BMI) range from 16.1 to 47.0 kg/m2 (mean = 29.3 kg/m2 ,SD=6.5 kg/m2).
Interventions
None
Main Outcome Measures
Reproductive history, physical examination, inhibin B, FSH, LH, testosterone, unbound testosterone (uT) levels and semen-analysis.
Results
BMI was negatively correlated with testosterone (R = −0.38, P <0.001), FSH (R = −0.22, P = 0.042) and inhibin B levels (R = −0.21, P = 0.048), and was positively correlated with estradiol levels (R = 0.34, P = 0.001). Testosterone also negatively correlated with skin fold thickness (SFT) (R = −0.30, P = 0.005). There was no correlation of BMI or SFT with semen-analysis parameters (sperm density, volume, motility or morphology). Inhibin B level correlated significantly with sperm motility (R = 0.23, P = 0.045). Males with paternity had lower BMIs (28.0 kg/m2 vs. 31.6 kg/m2; P = 0.037) and lower SFT (24.7 mm vs. 34.1 mm; P = 0.016) than males without.
Conclusions
Obesity is an infertility factor in otherwise normal males. Obese males demonstrate a relative hypogonadotropic hypogonadism. Reduced inhibin B levels and diminished paternity suggest compromised reproductive capacity in this population.
Keywords: Male infertility, inhibin B, semen, body fat distribution
INTRODUCTION
Infertility, generally defined as the inability to conceive after one year of unprotected intercourse, affects approximately 15% of couples in Western countries (1, 2). In almost half of these couples, a male fertility factor is involved (2). It has been known for some time that obese males exhibit altered levels of circulating sex steroids; decreased levels of total testosterone (T) and sex hormone binding globulin (SHBG) and increased estrogens levels (3). Most studies have shown an inverse correlation between obesity and testosterone and a direct correlation with estrogen (3–7). Decreased follicle stimulating hormone (FSH) and inhibin B levels are also found in obese male populations (5).
Inhibin B, a gonadal glycoprotein hormone, negatively inhibits FSH production and release from the pituitary gland. It is a novel, sensitive marker of spermatogenesis (8, 9). Subnormal serum levels of inhibin B correlate with abnormal spermatogenesis (9). In cryptorchid men, inhibin B levels and paternity are both significantly reduced, suggesting that inhibin B may be a marker of both testicular malfunction and paternity (10). In obese infertile females, inhibin levels are inversely related to BMI, suggesting a relationship between these parameters (11). Increasing BMI in males is associated with decreased levels of serum T, SHBG and inhibin B and increased free androgen index and estradiol levels (5).
Infertility may be more prevalent among men with elevated BMIs. Forty percent of men presenting to one infertility clinic were overweight (12). However, the relationship between male obesity and other fertility parameters has not been well established. Decreased T, SHBG and testosterone/estrogen ratios have been documented among infertile obese men compared with infertile non-obese men and fertile obese men (13). Additional studies regarding the association of obesity parameters and reproductive hormone levels with semen analyses and paternity are lacking. The aim of the current study was to investigate these relationships in otherwise normal populations of obese and non-obese men.
MATERIALS AND METHODS
Over a three-year period (from July 2000 to August 2003), a prospective sample of adult males (N = 87, age range 19–48 y) were enrolled in a study protocol at the Penn State College of Medicine Hershey Medical Center in Hershey, PA. Inclusion criteria included: males age 18 or older who were able to provide a blood and semen sample, were willing to undergo a physical exam, complete a survey and could give informed consent. Males with a history of varicocele or prior vasectomy were excluded. Institutional review board approval was obtained prior to the start of the study and written informed consent was obtained prior to examination and collection of blood and semen samples. None of the men had any major illnesses or chronic disease.
Men for this protocol were recruited from two sources. The first group was fathers of babies born at our Ob/Gyn practice. The second group was couples seeking fertility assistance from the Division of Reproductive Endocrinology. Men whose female partners had an identified cause of infertility and who themselves had no other history of infertility were invited to participate.
All subjects were evaluated by one of the primary investigators (PAL). Physical examination included measurements of height, weight and arm skin-fold thickness (SFT). Current BMI was calculated for all subjects according to the formula BMI = weight (kg) / stature (m2). Arm SFT (in mm) was measured using standard calipers. Testicular examination evaluated for previously undiagnosed testicular pathology. Testicular volume (in ml) was determined from measurements of the long and short axis using standard conversion tables.
Subjects also completed a questionnaire including information about demographics, health and paternity status (including a history of infertility in self or partner) and life-style factors (including smoking history).
Semen was analyzed according to the World Health Organization (WHO) guidelines on samples obtained by masturbation after 48–72 hours of abstinence (14). All semen analyses were performed in our andrology laboratory, with observers blinded to the study design. The normal values used for the analysis included a semen volume of ≥2.0 mL and a semen concentration of ≥20 × 106/mL.
Hormonal analysis was performed from a single venipuncture obtained between 08:00 and 10:00 a.m. Blood samples were analyzed for inhibin B, FSH, lutenizing hormone (LH), testosterone, free testosterone and estradiol. As some males in this study had partners undergoing infertility treatment, they too may have had a prior medical evaluation for infertility. Only the blood and semen samples obtained after study enrollment and completion of physical examination and were used for statistical analysis.
Inhibin-B was analyzed by enzyme immuno-assay on a microtiter plate using standard reagents (Oxford Bio-Innovation LTD, Oxfordshire, UK) Interassay precision averaged 12% at an inhibin-B concentration of 327 pg/ml The assay has a functional sensitivity of 15 pg/ml. FSH and LH levels were analyzed by the Delfia® fluoroimmunoassay method. The lower limits of detection were 0.15 U/L for LH and 0.50 U/L for FSH. Testosterone was determined using the solid phase I 125 radioimmunoassay (Diagnostic Products, Los Angeles, CA). Free and weakly bound testosterone was determined using an ammonium sulfate precipitation method. The functional sensitivity of the total testosterone assay is 10 ng/dl. Estradiol levels were determined by radioimmunoassay using I 125 labeled estradiol in an antibody coated tube format (Diagnostic Products). Assay sensitivity is 10 pg/ml with an interassay imprecision of 8% at a mean concentration of 42 pg/ml.
All samples could not be measured in the same assay because of the total number of sample assayed and the duration of time required to collect the samples. Samples from the same subject were batched for assay when possible. The reference ranges for these assays in our laboratory for normal adult males can be found in Table 1. The intra- and interassay coefficients of variation for these assays were < 10%.
Table 1.
Normal adult male hormonal assay reference ranges
| Hormone Assayed | Reference Range |
|---|---|
| FSH | 2.3–13.6 U/L |
| LH | 2.0–12.6 U/L |
| Testosterone | 285–980 ng/dL |
| Free testosterone | 12–40 pg/dL |
| Estradiol | 15–40 pg/ml |
| Inhibin B | 110–365 pg/mL |
Descriptive data are reported as means and standard deviations for continuous measurements and as frequencies and percentages for categorical measurements. Bilateral arm SFT was calculated by summing the right and left arm skin-fold thicknesses. One subject had an inhibin B value below the lowest detectable limit and was assigned a value of 15 pg/mL for statistical purposes Males were classified as having a history of paternity if they had fathered any pregnancies with their current or previous partners, including spontaneous or elective abortions.
Baseline characteristics were compared between males with and without a history of paternity using a two-sample t-test for continuous measurements or a chi-square test for categorical measurements. The association between markers of fertility including serum sex-hormone levels and semen analysis parameters, and indices of body fat including BMI and bilateral arm skin-fold thickness, were quantified using Pearson’s correlation coefficients (R) and 95% confidence intervals. Analyses were not adjusted for multiple hypothesis testing. All statistical comparisons were performed using the SAS software package (SAS v. 9.1, SAS Institute Inc., Cary, NC).
RESULTS
Baseline patient characteristics
Of the males evaluated in this study, fifty-seven (68%) had a history of paternity with either their current partner or a previous partner. Men with or without a history of paternity did not differ significantly with respect to tobacco usage, age or systolic and diastolic blood pressure (Table 2). Males with paternity had a significantly lower BMI (28.0 kg/m2 vs. 31.6 kg/m2; P = 0.037) and bilateral arm skin-fold thickness (24.7 mm vs. 34.1 mm; P = 0.016) compared to males without a history of paternity.
Table 2.
Baseline characteristics of subjects with and without a history of paternity
| Paternity Mean (SD) | No paternity Mean (SD) | Mean Difference (95% CI) | P Value | |
|---|---|---|---|---|
| Age (yrs) | 32.4 (5.8) | 31.3 (5.0) | −1.1 (−3.7,1.6) | 0.430 |
| BMI (kg/m2) | 28.0 (5.0) | 31.6 (8.0) | 3.6 (0.8,6.5) | 0.037 |
| Diastolic Blood pressure (mmHg) | 77.2 (7.8) | 80.1 (7.6) | 2.9 (−0.9, 6.6) | 0.128 |
| Systolic Blood pressure (mmHg) | 130.3 (12.5) | 128.3 (13.7) | −2.0 (−8.2, 4.2) | 0.523 |
| Bilateral Arm Skin-fold Thickness (mm) | 24.7 (11.7) | 34.1 (17.6) | 9.5 (2.9,16.0) | 0.016 |
| Smoking* | 12 (28.6%) | 8 (32.0%) | 0.767 |
N(%) and Chi-Square test are reported for the proportion of men who have smoked.
Body fat indices vs. serum hormone analysis
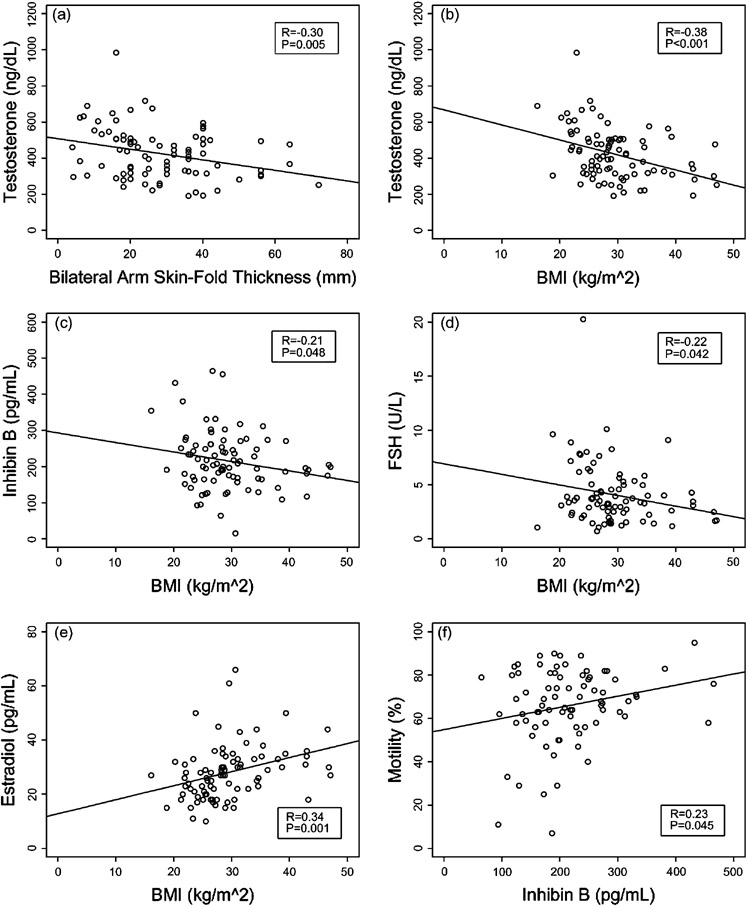
Mean BMI was 29.3 kg/m2 (SD=6.5 kg/m2), (range: 16.1 − 47.0 kg/m2). The strength and type of associations of serum hormone levels and indices of body fat can be found in Table 3. Serum testosterone levels were negatively correlated with BMI (R = −0.38, P < 0.001) and with bilateral arm skin-fold thickness (R = − 0.30, P = 0.005) (Figure 1a and 1b). Although there was no significant correlation with unbound testosterone, percent free testosterone was positively correlated with BMI (R = 0.23, P = 0.035). BMI was also negatively correlated with inhibin B levels (R= −0.21, P = 0.048 and with FSH (R = −0.22, P = 0.042), and was positively correlated with estradiol (R = 0.34, P = 0.001) (Figure 1c, 1d, and 1e respectively). The association of BMI and LH approached significance (R = − 0.20, P = 0.068) for a negative correlation.
Table 3.
Associations of serum hormone levels and indices of body fat
| BMI (kg/m2) | Bilateral Arm Skin-Fold Thickness (mm) | |||
|---|---|---|---|---|
| Pearson Correlation Coefficient (95% CI) | P Value | Pearson Correlation Coefficient (95% CI) | P Value | |
| FSH (U/L) | −0.22 (−0.41, −0.01) | 0.042 | −0.05 (−0.26, 0.17) | 0.645 |
| LH (U/L) | −0.20 (−0.39, 0.01) | 0.068 | −0.09 (−0.30, 0.12) | 0.395 |
| Testosterone (ng/dL) | −0.38 (−0.55, −0.18) | < 0.001 | −0.30 (−0.48, −0.09) | 0.005 |
| Unbound Testosterone (ng/dL) | −0.18 (−0.38, 0.03) | 0.091 | −0.13 (−0.34, 0.08) | 0.225 |
| % Free Testosterone | 0.23 (0.02, 0.42) | 0.035 | 0.18 (−0.03, 0.38) | 0.093 |
| Inhibin B (pg/mL) | −0.21 (−0.41, −0.002) | 0.048 | −0.10 (−0.31, 0.12) | 0.365 |
| Estradiol (pg/mL) | 0.34 (0.13, 0.51) | 0.001 | 0.21 (−0.002, 0.41) | 0.052 |
Conversion to SI units: Testosterone × 3.467= nmol/L, Estradiol × 3.671 = pmol/L, Inhibin × 1.0 = pmol/L
Figure 1.
Correlations between hormones and morphometric parameters (Conversion to SI units: Testosterone × 3.467= nmol/L, Inhibin × 1.0 = pmol/L).
Body fat indices and inhibin B levels vs. semen analysis
There was no correlation of BMI or bilateral arm skin-fold thickness with any of the semen analysis parameters, including sperm density, semen volume, total motile sperm (including both rapid progressive and slow progressive sperm) and motility percentage as determined by Kruger criteria. Inhibin B was not correlated with any semen analysis parameters except for an association with motility (R = 0.23, P = 0.045) (Figure 1f).
Body fat indices and inhibin B levels vs. physical examination
There was no statistically significant relationship between BMI and right (R = 0.05, P = 0.692), left (R = 0.10, P = 0.454), or combined (R = 0.06, P = 0.627) testicular volume or between inhibin B and right (R = 0.15, P = 0.256), left (R = 0.05, P = 0.714) or combined (R = 0.17, P = 0.170) testicular volume.
DISCUSSION
Obese men in this study were less likely to have a history of paternity compared to non-obese men and they had circulating reproductive hormone profiles consistent with diminished reproductive capacity. Serum testosterone and FSH levels were lower and estradiol levels higher with increasing obesity, findings consistent with those previously described in otherwise healthy obese males (4, 16). These data suggest a partial hypogonadotropic hypogonadism based upon decreased FSH and low normal LH levels. Such reduced pituitary FSH stimulation could adversely affect Sertoli cell function, inhibin B and sperm production(5), as well as Leydig cell testosterone production.
A hypothesized mechanism for these changes involves the aromatase enzyme, capable of converting C19 steroid precursors into C18 estrogens, and present in adipose tissue. Increased amounts of adipose tissue would lead to increased conversion of testosterone to estrogen (4, 7); consistent with higher estrogen levels reported to be associated with obesity (3, 4, 13). Such peripheral steroid conversion would result in decreased testosterone levels and increased estrogen including estradiol levels noted elsewhere (4, 7). Suppressed levels of SHBG found in obese men could also lead to greater bioavailability of androgen than estrogen given the preferential binding and sequestration of androgen over estrogen by SHBG (17)
Such altered levels of sex steroids could feed back at the hypothalamic axis to suppress gonadotropins, especially FSH. The inhibin B data reported here are consistent with inhibition at the pituitary level. Although the decreased inhibin B levels seen with obesity should result in increased FSH production, the decreased FSH levels reported here and elsewhere (16, 18) suggest that the pituitary gonadotropin output, particularly FSH, is suppressed. A recent study found a similar inverse relationship between obesity and inhibin-B levels in healthy young adult males, but no effect of obesity on FSH levels (19).
That LH levels were not significantly lower in this and previous studies (13, 16) suggests normal Leydig cell stimulus for testosterone production and an essentially normal level of feedback inhibition by unbound testosterone (which was not correlated with BMI). Decreased production of SHBG would be necessary to have normal unbound testosterone in the face of lower than normal total testosterone levels. While not investigated in this study, such a decrease in SHBG has been found in obese males in prior studies (3, 4, 7, 17) and would account for the seemingly normal bioavailability of testosterone.
Because there were no statistically significant changes in semen analysis parameters, it can be argued that the described hypogonadotropic-hypogonadism is mild. Strain and colleagues reached this conclusion, noting that their study population showed no changes in semen volume, sperm count and sperm motility, libido and potency with increasing obesity (16). Until recently, there has been little evidence that the hormonal changes associated with obesity result in changes in semen analysis parameters or in an overall decreased paternity in an obese population. However, a recent study of a large cohort of Danish men showed that both high and low BMI was associated with reduced semen quality (5). In overweight men (BMI > 25 kg/m2) there was a reduction in sperm concentration and total sperm count (5).
Serum inhibin B levels have been demonstrated to be a sensitive marker of spermatogenesis, indicating quantity and functionality of seminiferous tubules (10, 20). Cryptorchid males, for example, have significantly smaller testicular volumes, lower inhibin B levels and decreased sperm counts (10, 21). Because there was no significant association of testicular volume (the surrogate marker for seminiferous tubule mass) with BMI, the lower concentrations of inhibin B in obese males seems to indicate decreased tubule function not yet resulting in decreased tubular volume.
Our study involved only a single blood sample for hormone determinations. Since gonadotropin secretion displays a pulsatile pattern, and other studies have noted decreases in the mean levels of LH and LH pulse amplitude in obese adult men (22), more frequent sampling may have documented lower levels. Inhibin-B levels are less pulsatile and tend to exhibit a diurnal variation similar to circulating testosterone levels (23). A larger sample size as indicated by the Danish study may be able to detect subtle defects (5) Further, a larger sample size may show stronger associations because many of the correlations from our study, although statistically significant, were relatively weak (R < 0.30).
While our data support the assertion that the hormone changes found with obesity are mild and not associated with significant differences in semen analysis parameters, they seem to indicate clinically discernable alterations in fecundity; measurable decreases in inhibin B levels, indicative of seminiferous tubule dysfunction, and decreased paternity. This supports the hypothesis that male obesity is an infertility risk factor, which together with other subtle risk factors may decrease fertility. Obesity may affect fertility by diminishing libido or by affecting sexual performance. Obesity has been associated with erectile dysfunction (24).
In conclusion, obesity is an infertility factor in otherwise normal males. Obese males demonstrate a relative hypogonadal hypogonadism. Reduced inhibin B levels and diminished paternity suggest compromised reproductive capacity in this population. Serum inhibin B levels may therefore be a more sensitive means of detecting compromised fertility than semen analysis characteristics. There may be a clinical role for the measurement of inhibin B as a marker of compromised fertility in obese males.
Acknowledgments
This work was supported by NIH grants K24 HD01476 (RSL), RO1-26477 (PAL), and a PA Tobacco Settlement Fund grant (RSL).
Footnotes
CAPSULE: Increasing BMI among men is associated with lower testosterone, FSH and inhibin B levels, higher estradiol levels and reduced fertility as expressed by paternity.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thonneau P, Spira A. Prevalence of infertility: international data and problems of measurement. Eur J Obstet Gynecol Reprod Biol. 1991;38:43–52. doi: 10.1016/0028-2243(91)90206-z. [DOI] [PubMed] [Google Scholar]
- 2.Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod. 1998;13 Suppl 1:33–44. doi: 10.1093/humrep/13.suppl_1.33. [DOI] [PubMed] [Google Scholar]
- 3.Glass AR, Swerdloff RS, Bray GA, Dahms WT, Atkinson RL. Low serum testosterone and sex-hormone-binding-globulin in massively obese men. J Clin Endocrinol Metab. 1977;45:1211–1219. doi: 10.1210/jcem-45-6-1211. [DOI] [PubMed] [Google Scholar]
- 4.Kley HK, Edelmann P, Kruskemper HL. Relationship of plasma sex hormones to different parameters of obesity in male subjects. Metabolism. 1980;29:1041–1045. doi: 10.1016/0026-0495(80)90214-0. [DOI] [PubMed] [Google Scholar]
- 5.Jensen TK, Andersson AM, Jorgensen N, Andersen AG, Carlsen E, Petersen JH, et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril. 2004;82:863–870. doi: 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 6.Cameron ST, Critchley HO, Thong KJ, Buckley CH, Williams AR, Baird DT. Effects of daily low dose mifepristone on endometrial maturation and proliferation. Hum Reprod. 1996;11:2518–2526. doi: 10.1093/oxfordjournals.humrep.a019151. [DOI] [PubMed] [Google Scholar]
- 7.Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48:633–638. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- 8.Meachem SJ, Nieschlag E, Simoni M. Inhibin B in male reproduction: pathophysiology and clinical relevance. Eur J Endocrinol. 2001;145:561–571. doi: 10.1530/eje.0.1450561. [DOI] [PubMed] [Google Scholar]
- 9.Pierik FH, Burdorf A, de Jong FH, Weber RF. Inhibin B: a novel marker of spermatogenesis. Ann Med. 2003;35:12–20. doi: 10.1080/07853890310004084. [DOI] [PubMed] [Google Scholar]
- 10.Lee PA, Coughlin MT, Bellinger MF. Paternity and hormone levels after unilateral cryptorchidism: association with pretreatment testicular location. J Urol. 2000;164:1697–1701. [PubMed] [Google Scholar]
- 11.Cortet-Rudelli C, Pigny P, Decanter C, Leroy M, Maunoury-Lefebvre C, Thomas-Desrousseaux P, et al. Obesity and serum luteinizing hormone level have an independent and opposite effect on the serum inhibin B level in patients with polycystic ovary syndrome. Fertil Steril. 2002;77:281–287. doi: 10.1016/s0015-0282(01)02968-5. [DOI] [PubMed] [Google Scholar]
- 12.Oliva A, Spira A, Multigner L. Contribution of environmental factors to the risk of male infertility. Hum Reprod. 2001;16:1768–1776. doi: 10.1093/humrep/16.8.1768. [DOI] [PubMed] [Google Scholar]
- 13.Jarow JP, Kirkland J, Koritnik DR, Cefalu WT. Effect of obesity and fertility status on sex steroid levels in men. Urology. 1993;42:171–174. doi: 10.1016/0090-4295(93)90641-m. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Cambridge, UK: Cambridge University Press; Laboratory manual for the examination of human sperm and the sperm-cervical mucus interaction. 1999
- 15.Pigny P, Cortet-Rudelli C, Decanter C, Deroubaix D, Soudan B, Duhamel A, et al. Serum levels of inhibins are differentially altered in patients with polycystic ovary syndrome: effects of being overweight and relevance to hyperandrogenism. Fertil Steril. 2000;73:972–977. doi: 10.1016/s0015-0282(00)00421-0. [DOI] [PubMed] [Google Scholar]
- 16.Strain GW, Zumoff B, Kream J, Strain JJ, Deucher R, Rosenfeld RS, et al. Mild Hypogonadotropic hypogonadism in obese men. Metabolism. 1982;31:871–875. doi: 10.1016/0026-0495(82)90175-5. [DOI] [PubMed] [Google Scholar]
- 17.Kley HK, Deselaers T, Peerenboom H. Evidence for hypogonadism in massively obese males due to decreased free testosterone. Horm Metab Res. 1981;13:639–641. doi: 10.1055/s-2007-1019359. [DOI] [PubMed] [Google Scholar]
- 18.Amatruda JM, Hochstein M, Hsu TH, Lockwood DH. Hypothalamic and pituitary dysfunction in obese males. Int J Obes. 1982;6:183–189. [PubMed] [Google Scholar]
- 19.Winters SJ, Wang C, Abdelrahaman E, Hadeed V, Dyky MA, Brufsky A. Inhibin-B levels in healthy young adult men and prepubertal boys: Is obesity the cause for the contemporary decline in sperm count because of fewer Sertoli cells? J Androl. 2006;27:560–564. doi: 10.2164/jandrol.05193. Epub 2006 Apr 1. [DOI] [PubMed] [Google Scholar]
- 20.Pierik FH, Vreeburg JT, Stijnen T, De Jong FH, Weber RF. Serum inhibin B as a marker of spermatogenesis. J Clin Endocrinol Metab. 1988;83:3110–3114. doi: 10.1210/jcem.83.9.5121. [DOI] [PubMed] [Google Scholar]
- 21.de Gouveia Brazao CA, Pierik FH, Erenpreiss Y, de Jong FH, Dohle GR, Weber RF. The effect of cryptorchidism on inhibin B in a subfertile population. Clin Endocrinol (Oxf) 2003;59:136–141. doi: 10.1046/j.1365-2265.2003.01813.x. [DOI] [PubMed] [Google Scholar]
- 22.Strain GW, Zumoff B, Miller LK, Rosner W. Sex difference in the effect of obesity on 24-hour mean serum gonadotropin levels. Horm Metab Res. 2003;35:362–366. doi: 10.1055/s-2003-41358. [DOI] [PubMed] [Google Scholar]
- 23.Carlsen E, Olsson C, Petersen JH, Andersson AM, Skakkebaek NE. Diurnal rhythm in serum levels of inhibin B in normal men: relation to testicular steroids and gonadotropins. J Clin Endocrinol Metab. 1999;84:1664–1669. doi: 10.1210/jcem.84.5.5708. [DOI] [PubMed] [Google Scholar]
- 24.Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D'Andrea F, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291:2978–2984. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]