Abstract
High dietary intake of saturated fat and cholesterol, and elevated low-density-lipoprotein (LDL) cholesterol levels are some of the modifiable risk factors for cardiovascular disease (CVD). Alpha-cyclodextrin (α-CD) when given orally has been shown in rats to increase fecal saturated fat excretion, and to reduce blood total cholesterol levels in obese hypertriglyceridemic subjects with type 2 diabetes. In this study, the effects of dietary α-CD on lipid metabolism in LDL receptor knock-out (LDLr-KO) mice were investigated. LDLr-KO mice were fed a “western diet” (21% milk fat) with or without 2.1% of α-CD (10% of dietary fat content) for 14 weeks. At sacrifice, there was no difference in body weight, but significant decreases were observed in plasma cholesterol (15.3%), free cholesterol (20%), cholesterol esters (14%) and phospholipid (17.5%) levels in mice treated with α-CD compared to control mice. The decrease in total cholesterol was primarily in the pro-atherogenic apoB-containing lipoprotein fractions, with no significant change in the high density lipoprotein (HDL) fraction. Furthermore, α-CD improved the blood fatty acid profile, reducing the saturated fatty acids (4.5%) and trans-isomers (11%), while increasing (2.5%) unsaturated fatty acids. In summary, the addition of α-CD improved the lipid profile by lowering pro-atherogenic lipoproteins and trans fatty acids, and by decreasing the ratio of saturated and trans fatty acids to polyunsaturated fatty acids (−5.8%), thus suggesting that it may be useful as a dietary supplement for reducing CVD.
Introduction
Cardiovascular disease (CVD) is the number one cause of death in the United States, and life style modifications are recommended for all patients at risk for CVD. The major modifiable risk factors for the CVD include elevated low density lipoprotein cholesterol (LDL-C), decreased high density lipoprotein cholesterol (HDL-C), obesity, diabetes, inactivity, cigarette smoking and a poor diet low in soluble fiber and high in saturated and trans fats, as well as cholesterol (1), (2). In order to reduce the risk of CVD, it is recommended that individuals should reduce their intake of saturated and trans fats, increase dietary soluble fiber intakes, and increase the intake of omega-3 fats or fatty fish (3).
Soluble dietary fibers like psyllium and pectin have been shown to reduce the absorption of dietary fat and cholesterol, thus reducing blood levels of cholesterol and the risk of CVD (4). Alpha-cyclodextrin (α-CD) is a soluble dietary fiber derived from corn starch that is non-absorbable. It is a polymer of 6 glucose units in a cyclic ring structure with the polar hydroxyl groups facing outward (5). The core of the ring is hydrophobic and can bind various hydrophobic compounds, including free fatty acids. The World Health Organization has established an acceptable daily intake of “not specified” and α-CD has been granted the Generally Recognized As Safe (GRAS) status by the United States Department of Agriculture (USDA). α-CD (trade name FBCx®) is currently available as a dietary supplement. It has been shown to reduce weight gain in Wistar rats fed an obesity-promoting high fat diet (6). It also reduced blood triglyceride and leptin levels, and improved the calculated insulin sensitivity in these rats, as well as a tendency to reduce blood total cholesterol and insulin levels (6). In a double blind, placebo controlled clinical trial with obese type 2 diabetic patients, the α-CD treated group were able to maintain their body weight and had increased adiponectin levels, whereas patients in the placebo group gained weight (7). Those patients who began the study with hypertriglyceridemia also had significant reductions in their total cholesterol levels. Hence, α-CD may be considered to have health benefits in obese patients with type 2 diabetes. In the current study, we investigate the impact of α-CD – feeding on the plasma lipid profile of a common mouse model of atherosclerosis (8). Mice deficient of the low density lipoprotein receptor (LDLr-KO mice) develop dyslipidemia due to impaired clearance of chylomycron remnants and hepatic pro-atherogenic lipoproteins, such as very low density lipoproteins (VLDL), low density lipoproteins (LDL) and intermediate density lipoprotein (IDL) (9). LDLr-KO mice were fed a high fat/high cholesterol containing “western” (21% fat, 0.2% cholesterol, w/w) diet with or without 2.1% α-CD for 14 weeks. A significant reduction in the level of pro-atherogenic lipoproteins and an improvement in the fatty acid profile were observed with α-CD treatment, suggesting that supplementation with α-CD may be useful for minimizing the negative impact of high fat diets on serum lipids.
Methods and materials
Animals
Twenty female LDLr-KO mice on a C57BL/6 background, 12 weeks old at the beginning of the study, were purchased from Jackson Laboratory (Bar Harbor, ME). They were housed in polycarbonate hanging cages with 5 mice per cage. All mice were fed the rodent diet NIH31 (Zeigler Bros Inc., Gardner, PA) and watered ad-libitum prior to the beginning of the study. All animals were treated according to The Guide for the Care and Use of Experimental Animals and the experimental protocol was approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute, National Institutes of Health.
Diets
The baseline diet (NIH31) had the following composition: 5% fiber, 18% protein and 4% fat with energy content of 3.97 kcal/g. Mice were randomized to either the Control diet (Harlan Teklad “Western Diet” TD.88137) or a modified diet with 21 g/kg of α-CD (10% of dietary fat content, w/w), replacing an equal amount of cellulose. The Control and α-CD diets had an energy content of 4.5 kcal/g, and contained 17% protein, 49% carbohydrates, 0.2% cholesterol and 21% fat (w/w) from dried milk fat (65% saturated fats, 31% monounsaturated fats and 4% polyunsaturated fats). The fatty acid composition of the milk fat as analyzed by Harlan Teklad is shown in Table 1.
Table 1.
Fatty acid composition of the milk fat (% of total fat)
| Fatty acids | % |
|---|---|
| 8:0 | 0.5 |
| 10:0 | 2.0 |
| 12:0 | 2.7 |
| 13:0 | 0.13 |
| 14:0 | 9.8 |
| 14:1 | 0.7 |
| 15:0 | 1.1 |
| 16:0 | 29.9 |
| 16:1 | 1.7 |
| 17:0 | 0.7 |
| 18:0 | 15.1 |
| 18:1 (all cis isomers) | 26.4 |
| 18:1 (trans) | 4.0 |
| 18:2 | 3.7 |
| 18:3 | 0.5 |
| 20:3 | 0.1 |
| 20:4 | 0.2 |
| 22:0 | 0.07 |
| 22:5 | 0.07 |
| Total cholesterol | 0.2 |
Procedures
Fasting blood samples were withdrawn for lipid analysis from retro-orbital sinus of all the mice after anesthesia prior to the beginning of the study. The mice were then randomly assigned to either the Control (n=10) or the α-CD group (n=10) and fed their respective diets ad-libitum for 14 weeks. Body weights were measured weekly. Fasting blood samples were collected after 2, 5, and 10 weeks of feeding. Plasma was separated into EDTA tubes and stored at −80°C until analysis. All mice were sacrificed after 14 weeks of feeding the designated diet by cervical dislocation.
Food intake
The daily food intake was determined by measuring the food consumption for 5 consecutive days during week seven of treatment. All mice were separated into individual cages with 50g of ground diet in feeders within the cage. Data from day four and five were used to calculate the daily food consumption.
Chemical analyses
Plasma lipid and lipoprotein analysis
Plasma total cholesterol (TC), cholesterol ester (CE), free cholesterol (FC), phospholipid (PL) and triglycerides (TG) concentrations were analyzed on a Hitachi 912 Chemistry Analyzer, using commercial enzymatic kits (TC, TG, Roche Molecular Biochemicals, Basel, Switzerland; PL, FC, Wako Pure Chemical Industries Ltd, Richmond, VA). Lipoprotein fractions were analyzed by FPLC separation and subsequent lipid analysis, as described earlier (10, 11).
Plasma fatty acid composition
An aliquot (35–50µl) of thawed plasma from week 10 of the study was directly saponified in 1ml ethanol and 0.1ml 50% KOH at 60°C for 30 min following the addition of an internal standard. Non-saponifiable lipids were extracted with hexane (3ml) and discarded. Glacial acetic acid (0.1ml) was added to acidify the ethanol/KOH mixture and fatty acids were extracted in hexane (3ml). The hexane was evaporated under a stream of nitrogen, while heating the tube at 60°C. Fatty acid moieties were converted to volatile fatty acid methyl esters by the method of Metcalfe et al. (12), and were separated by GC (13).
Statistical analysis
Mean and standard error of means (SEM) were calculated and reported. Analysis of variance with repeated measure was performed. When the treatment effects were significant, Student’s t-tests with Bonferroni adjustment for multiple comparisons were performed. The significance level was set at p<0.05.
Results
Body weight and food intake
There was no difference in body weight between the Control and α-CD groups at anytime during the study. At sacrifice, the Control mice had gained 4.81±1.93 g compared to 4.80±1.13 g in the α-CD group (ns). No difference was observed in food intake between the Control and α-CD groups (45.7±3.7 mg food/d/g of body weight for Control group vs 45.0±2.9 mg food/d/g of body weight, ns).
Total plasma lipids
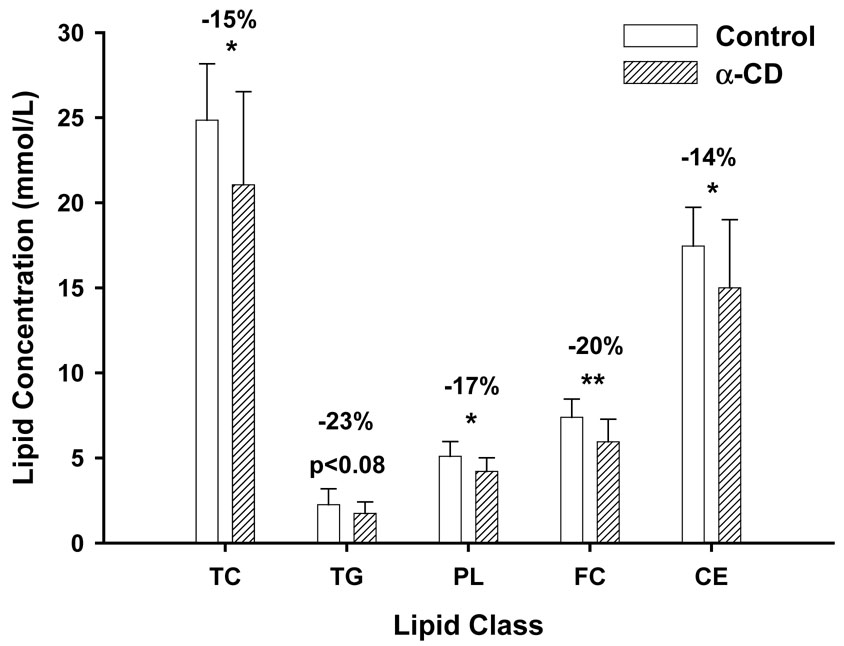
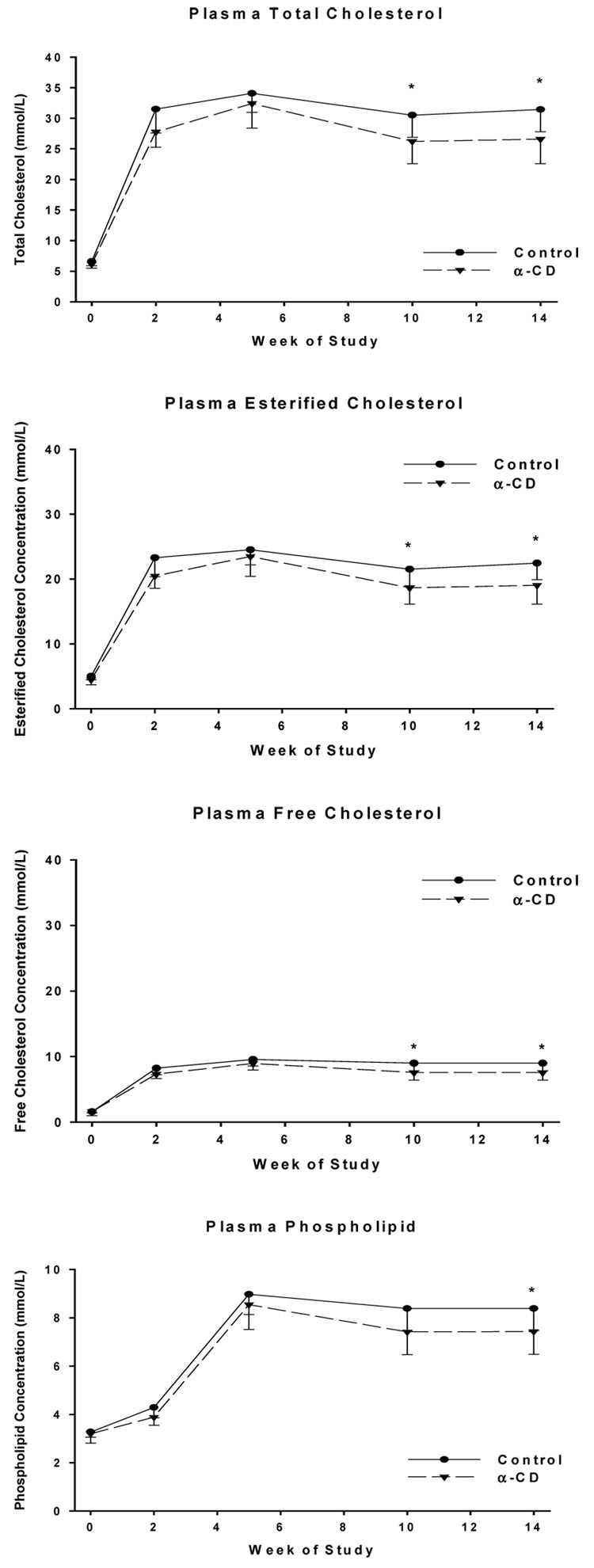
Both the treatment (diet) effect (p<0.05 or 0.01) and the time (weeks) effect (p<0.001) were statistically significant for TC, CE, FC and PL. The diet effect for plasma TG failed to reach significant (p<0.08), although the time effect was significant (p<0.001). As expected, we first observed a significant increase in plasma cholesterol levels after the mice were switched from the baseline diet to a milk-fat based diet, but to a lesser extent for the α-CD treated mice. The increases were significantly lower in the α-CD group as compared to the Control group (TC by 15%, PL by 17%, FC by 20%, and CE by 14%) (Fig. 1). The increase in the TG levels of the α-CD group was also lowered by 23%, but the difference failed to reach statistical significance (p<0.08). The reduction in plasma lipid levels first became apparent two weeks after the start of α-CD feeding. Changes in plasma cholesterol levels throughout the study are depicted in Fig. 2. Student’s t-tests with Bonferroni adjustment for the multiple comparisons revealed that the statistical differences were observed at 10 and 14 weeks between the α-CD and Control groups for TC, CE and FC, and at 14 weeks for PL. The plasma lipid concentrations at sacrifice are presented in Table 2.
Figure 1.
Changes in plasma lipid at sacrifice (14 weeks) as compared to baseline levels
* α-CD group was significantly different from Control group at p<0.05
* *α-CD group was significantly different from Control group at p<0.01
Figure 2.
Plasma lipid levels over the course of the study
* α-CD group was significantly different from Control group at p<0.05
Table 2.
Plasma lipid levels (mmol/L) at sacrifice (14 weeks) of the 2 groups of mice
| Control | α-CD | p | |
|---|---|---|---|
| Total cholesterol | 31.18±3.62 | 26.36±4.0 | <0.05 |
| Phospholipids | 16.16±1.76 | 14.33±1.81 | <0.05 |
| Free cholesterol | 8.91±1.13 | 7.49±1.14 | <0.05 |
| Esterified cholesterol | 22.27±2.51 | 18.88±2.87 | <0.05 |
| Triglyceride | 8.20±1.81 | 6.97±1.58 | ns |
Lipoprotein fractions
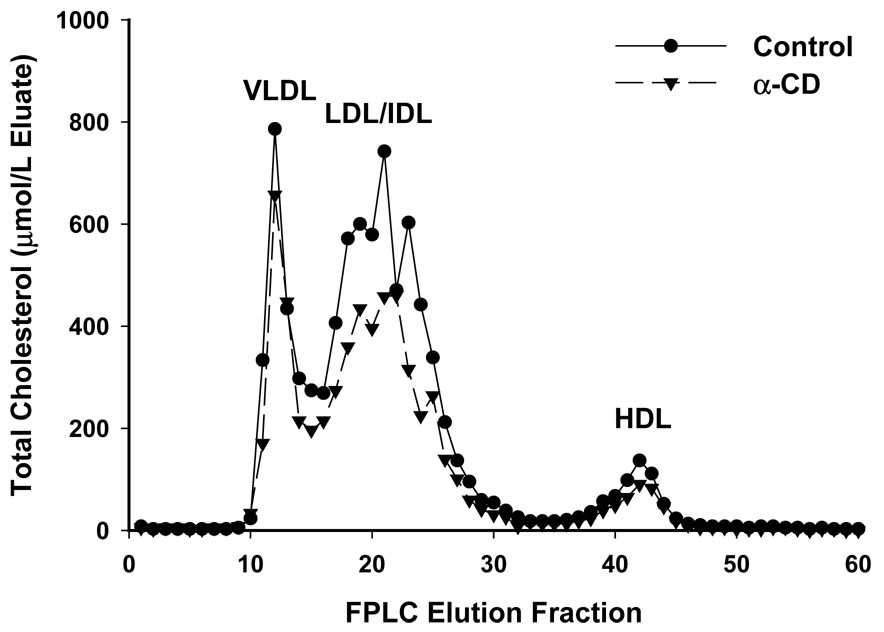
Fig. 3 depicts the lipoprotein fractions of the cholesterol components, as measured by FPLC after 10 weeks of feeding the designated diets. It is apparent that the reduction observed in the α-CD group was mainly due to the reduction in the pro-atherogenic apo-B-containing VLDL and LDL/IDL fractions. No changes in the HDL fractions were detected. As a result, the LDL/IDL to HDL ratio was reduced from 8.3 for the Control group to 7.9 for the α-CD group, a 4.8% reduction.
Figure 3.
The lipoprotein fractions of the cholesterol components as measured by FPLC after 10 weeks of feeding the designated diets. Results were obtained by pooling plasma from 5 mice in each group.
Fatty acid profile
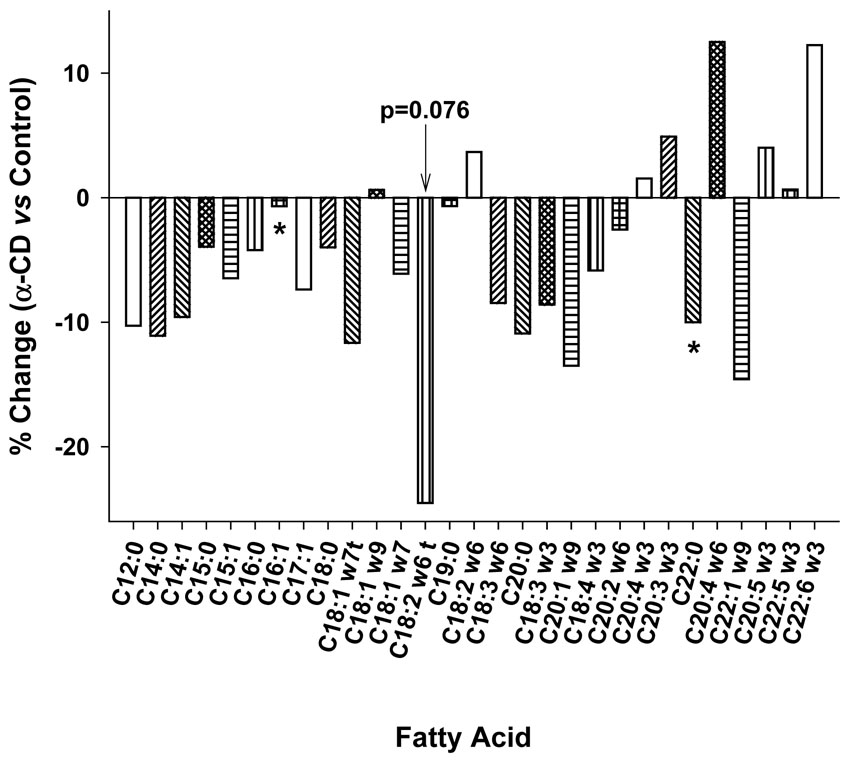
Consistent with the difference in the total triglyceride and phospholipid levels, the plasma total fatty acid levels from the α-CD fed mice were also lower (by 17%) than those of the Control mice (α-CD group: 1137.2±125.6 µg/mL; Control group: 1370.2±164 µg/mL, p<0.005). After calculating each fatty acid as a percent of total lipid in each group, significant reductions in C17:1 and C22:0 were observed in the α-CD group compared to the Control group (Fig 4). The reduction in C16:0 (p<0.055) just missed to be significant. A reduction in the concentration of C18:2w6 trans isomer was also observed, but the difference failed to reach significance (p=0.076). When the percent of saturated and trans fatty acids were added together, the α-CD group tended to have a decreased level (−4.8%, p=0.057) relative to the Control group. The ratio of trans plus saturated FA to unsaturated FA also showed a tendency of reduction (−5.8%, p=0.055).
Figure 4.
Changes (%) in plasma fatty acid profile of α-CD group as compared to the Control group.
* α-CD group was significantly different from Control group at p<0.05
Discussion
In this study, we have observed that α-CD, when given in the diet at a rate of 10% of the fat (w/w), significantly reduces plasma TC, PL, FC, and CE, primarily in the plasma LDL fraction, while maintaining blood HDL-cholesterol levels in LDLr-KO mice as compared to that of the controls. Milk fat, high in saturated fatty acids and cholesterol content, was used as a fat source in order to induce elevated plasma lipid levels. This goal was achieved as demonstrated by the early onset of significant increases in plasma TC, EC, FC and PL levels between baseline and 2 weeks of feeding (Fig. 2). α-CD also showed its lipid lowering effects as early as 2 weeks of feeding, even though the differences just missed the significance levels (TC, p=0.053, PL, p=0.08). It should be noted that both groups of mice had the same amount of fiber in the diet. The only difference was that 21 g (10% of diet fat, w/w) of α-CD was used to replace 21 g of cellulose in the α-CD group. The difference in the lipid profile therefore couldn’t be attributed to the quantity of the fiber. These results demonstrated that α-CD is efficacious in lowering blood lipid levels not only in normal rats fed a high fat diet (6), but also in LDL-r KO mice fed a moderate fat diet.
It should be noted that α-CD has been reported to prevent weight gain in high-fat (40% w/w) fed Wistar rats (6). No difference, however, in body weight and food intake was identified in this 14 week study. In the present study, all mice were fed a milk fat-based diet with 21% fat (w/w). Mice in the α-CD group also ingested 2.1% of α-CD (w/w) in their diet. It is possible that a higher dietary fat content, as was used in the previous rat study (40%, w/w) (6), is needed to show the weight loss property of α-CD. One may also speculate that mice lacking the LDL receptor may not be an ideal animal model to investigate the effect of a high fat diet on body weight regulation, because of reduced uptake of apoB containing lipoproteins in peripheral cells. However, LDLr-KO mice have the advantage of being susceptible to insulin resistance-linked to diet-induced obesity, an effect that could not be observed in apolipoprotein E-deficient (apoE-KO) mice, another common murine model of atherosclerosis (14). Further research to investigate the insulin resistance status of these LDLr-KO mice fed the α-CD-containing diet is warranted.
Soluble dietary fibers are known to reduce blood cholesterol levels; however, a meta analysis concluded that soluble fibers reduce total cholesterol by relatively small amounts, approximately 0.045 mmol/L per gram of soluble fiber (4). For a normal weight subject following the recommended diet of 2000 kcal and 30% fat per day, this amounts to about 4.6% reduction in total cholesterol levels. Another meta analysis of 8 controlled intervention trials reported a 4% reduction in total cholesterol levels after consuming 10.2 g psyllium per day in hypercholesterolemic subjects (15). In contrast, we have reported that in hypertriglyceridemic obese subjects with type 2 diabetes, 6 g of α-CD (2 g/meal) per day significantly reduced serum total cholesterol by 8% (7). In a previous rat study, α-CD added to food in the amount of 10% of the fat content reduced total cholesterol by 13.2% in low fat fed rats, whereas a reduction of 8.5% was observed in rats fed the high fat-containing diet. In the present study of LDL-r KO mice, the reduction in the plasma concentration of total cholesterol by α-CD was about 15%, more than 3 times the reduction in TC observed in humans treated with psyllium fibers (15). Overall, these results consistently demonstrate that α-CD reduces total cholesterol levels by at least 8% when taken in an amount of 6 g/day (humans) or 10% of fat content of animal foods.
It has also been reported that α-CD preferentially binds and reduces the absorption of saturated fatty acids from the diet. Its ability to reduce saturated fatty acid absorption was found to be significantly higher than that of chitosan (16). Because saturated fatty acids promote the hepatic synthesis of cholesterol and reduce its clearance (17), and reduction of saturated fat intake lowers blood cholesterol levels (18), (19), the ability of α-CD to lower plasma cholesterol may be related to its effect on saturated fatty acid absorption. This is supported by the data shown in Figure 4, showing that α-CD lowers the total level of saturated fatty acids in the plasma. FPLC analysis of the plasma (Fig. 2) demonstrated that feeding of the α-CD diet preferentially lowers the pro-atherogenic apoB lipid fractions, leading to a major 29 % decrease in LDL, IDL and VLDL. There was no observed change, however, in the athero-protective HDL cholesterol fractions in the plasma of the studied mice, which is in agreement with the results of previous studies on the effect of fibers on plasma cholesterol levels (4). The ratio of LDL/HDL was lower (−4.8%) in the α-CD treated mice, suggesting that α-CD treatment may lower the risk for atherosclerosis. In addition, it is now understood that the anti-atherogenic potential of HDL can vary significantly between individuals (20, 21). HDL from different subjects can vary in its anti-oxidant and anti-inflammatory ability (21), although the reason for this is still not fully understood. There is evidence that the consumption of saturated fat impairs the anti-inflammatory potential of HDL, while this beneficial property improves after consuming a PUFA-rich diet (22) Hence, besides its ability to improve the LDL-C/HDL ratio, α-CD may have other potential beneficial effects on lipoprotein metabolism through its ability to modulate the plasma fatty acid profile (Fig. 4).
Analysis of the plasma fatty acid profile revealed that all fatty acid concentrations were reduced in the α-CD treated group relative to the Control group. Considering the reductions in plasma triglyceride, as well as significant reductions in cholesterol ester and phospholipid levels, this finding is not unexpected. However, when the fatty acids were expressed as percent of the total lipids and the difference between the 2 groups were compared, an additional effect of α-CD was observed. α-CD reduced saturated (C16:0, C18:0, and C22:0) and trans fatty acids (C18:1 trans, and C18:2 trans), while polyunsaturated fatty acids such as arachidonic acid (C20:4 ω-6), docosahexanoic acid (C22:6 ω-3) were increased. Gallaher et al. have reported that α-CD preferentially bound with saturated fats and promoted their excretion into the feces (16). Our current finding thus is consistent with the fecal fat findings of Gallaher et al. (16). The mechanism for this alteration would appear to be related to the higher affinity of α-CD for saturated and trans dietary fat over unsaturated fats.
Trans fatty acids are known to increase the risk of cardiovascular disease and type 2 diabetes (23), (24). They also cause systemic inflammation and endothelial dysfunctions, and may alter the plasma lipoprotein fractions towards a more pro-atherogenic profile (24). In addition, a study with monkeys has shown that trans fats increased abdominal fat deposition and impaired glucose tolerance (25). Abdominal obesity and reduced insulin sensitivity in turn increase the risk of developing type 2 diabetes. Not all trans fatty acids, however, are equally pro-atherogenic. Trans isomers of C18:1 and C18:2 have more detrimental effects than other trans fatty acids, such as C16:1 isomers (26, 27). A recent finding from The Cardiovascular Health Study showed that elevated plasma phospholipid trans 18:2 was associated with higher risk for fatal ischemic heart disease after adjusting for other risk factors (28). Although it did not reach statistical significance, perhaps because of the relatively low content of trans fat in the milk fat diet used in this study, the α-CD treated group had a 25% reduction in trans C18:2 compared to the control group. This amount of reduction may have significant clinical importance. In summary, this study demonstrated that in LDLr-KO mice, feeding α-CD as 10% (w/w) of the dietary fat not only improved blood lipid levels, but also improved the fatty acid profile. This improvement was shown in reduced saturated and trans fatty acid levels with concomitant increase in polyunsaturated fatty acid levels. Considering the relative safety and tolerability of α-CD (7), and the fact that both saturated fats and trans fats are associated with increased risk of CVD, type 2 diabetes, abdominal obesity and inflammation, future clinical studies assessing the benefits of the addition of α-CD to foods or as a food supplement should be performed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Smith SJ. Multiple risk factors for cardiovascular disease and diabetes mellitus. Am J Med. 2007;120 Suppl 1:S3–S11. doi: 10.1016/j.amjmed.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Lefevre M, Kris-Etherton P, Zhao G, Tracy R. Dietary fatty acids, hemostasis, and cardiovascular disease risk. J Am Diet Assoc. 2004;104:410–419. doi: 10.1016/j.jada.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein A, Appel L, Brands M, et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 4.Brown L, Rosner B, Willett W, Sacks F. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69:30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- 5.Allegre M, Deratani A. Cyclodextrinu use: from concept to industrial reality. Agro Food Industry Hi-Tech. 1994;5:9–17. [Google Scholar]
- 6.Artiss J, Brogan K, Brucal M, Moghaddam M, Jen K-L. The effects of a new soluble dietary fiber on weight gain and selected blood parameters in rats. Metabolism. 2006;55:195–202. doi: 10.1016/j.metabol.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Grunberger G, Artiss J, Jen K-LC. The benefits of early intervention in obese diabetic patients with FBCx™ - a new dietary fibre. Diabetes Metab Res Rev. 2005;23:56–62. doi: 10.1002/dmrr.687. [DOI] [PubMed] [Google Scholar]
- 8.Daugherty A, Rateri D. Development of experimental designs for atherosclerosis studies in mice. Methods. 2005;36:129–138. doi: 10.1016/j.ymeth.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Ishibashi S, Brown M, Goldstein J, Gerard R, Hammer R, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joyce C, Amar M, Lambert G, et al. The ATP binding cassette transporter A1(ABCA1) modulates the development of aortic atherosclerosis in C57BL/6 and apoE-knockout mice. Proc Natl Acad Sci. 2002;99:407–412. doi: 10.1073/pnas.012587699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaisman B, Lambert G, Amar M, et al. ABCA1 overexpression leads to hyperalphalipoproteinemia and increased biliary cholesterol excretion in transgenic mice. J Clin Invest. 2001;108:303–309. doi: 10.1172/JCI12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metcalfe L, Schmitz A, Peika J. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38:514–515. [Google Scholar]
- 13.Rudel L, Kelley K, Sawyer J, Shah R, Wilson M. Dietary monounsaturated fatty acids promote aortic atherosclerosis in LDL receptor-null, human ApoB100-overexpressing transgenic mice. Artherioscler Thromb Vasc Biol. 1998;18:1818–1827. doi: 10.1161/01.atv.18.11.1818. [DOI] [PubMed] [Google Scholar]
- 14.Schreyer S, Vick C, Lystig T, Mystkowski P, LeBoeuf R. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Metab. 2002;282:E207–E214. doi: 10.1152/ajpendo.2002.282.1.E207. [DOI] [PubMed] [Google Scholar]
- 15.Anderson J, Allgood L, Lawrence A, et al. Cholesterol-lowering effects of psyllium intake adjunctive to diet therapy in men and women with hypercholesterolemia: meta-analysis of 8 controlled trials. Am J Clin Nutr. 2000;71:472–479. doi: 10.1093/ajcn/71.2.472. [DOI] [PubMed] [Google Scholar]
- 16.Gallaher D, Gallaher C, Plank D. Alpha-cyclodextrin selectively increases fecal excretion of saturated fats. FASEB J. 2007;21:A730. [Google Scholar]
- 17.Spady D, Woollett L, Dietschy J. Regulation of plasma LDL-cholesterol levels by dietary cholesterol and fatty acids. Annu Rev Nutr. 1993;13:355–381. doi: 10.1146/annurev.nu.13.070193.002035. [DOI] [PubMed] [Google Scholar]
- 18.Barr S, Ramakrishnan R, Johnson C, Holleran S, Dell R, Ginsberg H. Reducing total dietary fat without reducing saturated fatty acids does not significantly lower total plasma cholesterol concentrations in normal males. Am J Clin Nutr. 1992;55:675–681. doi: 10.1093/ajcn/55.3.675. [DOI] [PubMed] [Google Scholar]
- 19.Ginsberg H, Kris-Etherton P, Dennis B, et al. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: the DELTA Study, Protocol 1. Arterioscler Thromb Vasc Biol. 1998;18:441–449. doi: 10.1161/01.atv.18.3.441. [DOI] [PubMed] [Google Scholar]
- 20.Ueda M, Sethi A, Freeman L, et al. High density lipoproteins: overview and update on new findings from diagnostics to therapeutics. J. Clin Ligand Assay. 2005;28:216–232. [Google Scholar]
- 21.McMahon M, Grossman J, FitzGerald J, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54:2541–2549. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- 22.Nicholls S, Lundman P, Harmer J, et al. Consumption of saturated fat impairs the anti-inflammatory properties of high-density lipoproteins and endothelial function. J Am Coll Cardiol. 2006;49:1825–1826. doi: 10.1016/j.jacc.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 23.Mozaffarian D, Katan M, Ascherio A, Stamler J, Willett W. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 24.Mozaffarian D. Trans fatty acids - Effects on systemic inflammation and endothelial function. Atherosclerosis Suppl. 2006;7:29–32. doi: 10.1016/j.atherosclerosissup.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Kavanagh K, Jones K, Sawyer J, et al. Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys. Obesity. 2007;15:1675–1684. doi: 10.1038/oby.2007.200. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Garcia E, Schulze M, Meigs J, et al. Consumption f trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005;135:562–566. doi: 10.1093/jn/135.3.562. [DOI] [PubMed] [Google Scholar]
- 27.Lemaitre R, King I, Raghunathan T, et al. Cell membrance trans-fatty acids and risk of primary cardiac arrest. Circulation. 2002;105:697–701. doi: 10.1161/hc0602.103583. [DOI] [PubMed] [Google Scholar]
- 28.Lemaitre R, King I, Mozaffarian D, et al. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults. The Cardiovascular Health Study. Circulation. 2006;114:209–215. doi: 10.1161/CIRCULATIONAHA.106.620336. [DOI] [PubMed] [Google Scholar]