Abstract
In vivo transfer and expression of foreign genes allows for the elucidation of functions of genes in living organisms and generation of disease models in animals that more closely resemble the etiology of human diseases. Gene therapy holds promise for the cure of a number of diseases at the fundamental level. Synthetic ‘non-viral’ materials are fast gaining popularity as safe and efficient vectors for delivering genes to target organs. Nanoparticles can not only function as efficient gene carriers, but also simultaneously carry diagnostic probes for direct ‘real-time’ visualization of gene transfer and downstream processes. This review has focused on the central nervous system (CNS) as the target for non-viral gene transfer, with special emphasis on organically modified silica nanoparticles (ORMOSIL) developed in our laboratory. These nanoparticles have shown robust gene transfer efficiency in brain cells in vivo and allowed to investigate mechanisms that control neurogenesis as well as neurodegenerative disorders.
Keywords: Gene therapy, Non-viral vectors, Nanoparticles, Central nervous system, ORMOSIL
Introduction
Gene therapy, the treatment or prevention of disease by gene transfer, is regarded by many as a potential revolution in medicine1, 2. The recently concluded human genome project has substantially increased our knowledge of the molecular mechanisms of cancer and other gene-based diseases3, 4. In addition, there are genes that have been identified whose function in a living organism currently remains unknown. Concurrently, changes in the number of genes were found to be associated with a variety of disorders, some of which have been identified (suspected) as responsible for the onset of several human diseases 5. The application of gene therapy in the suppression or replacement of malfunctioning genes promises progress in understanding physiological roles of genes and in treating diseases at the genetic level. The non-viral gene transfer methods will permit targeting and analyzing biological effects to specific cells/tissues. Ultimately, gene therapy will translate into substantial improvements in therapeutic ratio and cure-rate for a broad-range of diseases, which are presently untreatable or poorly managed.
Though conceptually straightforward, the efficient ectopic expression of foreign genes is the most critical aspect for the success of in vivo gene manipulation experiments or therapy. The first step of gene therapy involves gene delivery, i.e. the presentation of the therapeutic genetic material in the interior of a living cell and its subsequent expression. However, the complexities of the biological system present numerous obstacles to successful gene delivery. The natural immune system of the body will attempt to degrade the foreign, newly introduced gene 6. At the cellular level, the negatively charged cellular membrane will repel the anionic genes. Once within the cell, it must escape intracellular degradation to reach and enter the nucleus to be expressed or inhibit a specific gene function 7, 8. For this, the necessity of a carrier, i.e. a vector, that will protect the genetic material and ferry it to the cellular/nuclear interior of the intended cell type, is paramount. Currently, such vectors of gene transfer are broadly classified into two categories: viral and non-viral 6.
The brain is the most significant challenge to biomedical science in this century. We are living longer and healthier lives due to advances in preventative medicine, medical and surgical breakthroughs. However, with this increased life expectancy, comes the dealing with the diseases that manifest themselves with advancing age. These include higher risks of infection, increase in cancer and increased neurological disorders (e.g. Stroke, Parkinson’s disease, etc.). Because of the complexity of the brain, as well as the lack of effective therapies for these disorders, there is also a need to further understand the pathological mechanisms of these disorders.
In the brain, there are more genes expressed than in any other tissues, reflecting great diversity of the cell types and complexity of the function. With the advent of bioinformatics, the knowledge of the human genome has increased substantially, with a concomitant increase in the understanding of the roles these genes play in normal as well as abnormal brain function. Broadly, in vivo CNS gene transfer can be applied towards understanding the physiological roles of genes in development, homeostasis, and senescence, as well as towards the treatment of (a) inherited (mainly monogenic), and (b) acquired (both monogenic and multigenic) disorders 9, 10. While CNS gene therapy for inherited diseases still remains an extremely difficult goal to achieve, far more promise has been shown towards the reversal of course of acquired CNS diseases such as spinal cord injury, stroke and degenerative (Parkinson’s and Alzheimer’s) diseases. This can be achieved by introduction of transgenes encoding proteins which augment the natural survival and repair systems of the CNS, such as neurotransmitters, neurotrophic factors and their receptors, cytokines, neuronal-survival (anti-apoptotic) agents, etc 9. Such therapeutic molecules, when expressed in sufficient amounts, provide a new lease of life to degenerating neuronal cells. Also, dormant cells like stem and progenitor cells can be genetically induced to differentiate into various neuronal cell types that could replenish areas developmentally impaired or undergoing neurodegeneration.
CNS gene transfer in living animals can be achieved by two different ways, (a) in vivo, where the gene-vector conjugate is directly introduced in the animal, and (b) ex vivo, where neuronal cells in culture are first transfected with the desired gene, followed by re-introduction of the transfected cells in the animal. This review will focus on non-viral methods for the in vivo gene transfer in the CNS.
Viral vectors
Viral directed gene delivery/therapy employs the use of engineered, recombinant viruses as gene carriers, by exploiting their ability to deliver genes to the nucleus of a cell and their expression through integration into the host genome 11. Both RNA and DNA viruses have been evaluated as possible gene carriers. These recombinant viruses are genetically modified to eliminate their pathogenicity, while retaining their infectivity. They are, however, difficult to produce and are limited due to immunogenicity and the size of the inserted genetic materials. It has been recognized that there is a potential risk of excessive immune response and insertional mutagenesis, which can activate oncogenes or silence tumor-suppressor genes, leading to cancerous development 12, 13. Additionally, there is the possibility of reversion of an engineered virus to the pathogenic, wild type 14. Human fatality has been reported in clinical trials, leading to a halt in further use of viral vectors for gene therapy 15–17. Still, in recent years, a significant progress has been made in this area as reviewed 18, 19.
Non-viral vectors
In gene therapy, the research focus has shifted towards the development of non-viral gene delivery vectors. In most cases, these vectors are relatively simple to synthesize, and devoid, to a large extent, of the health risks that are associated with their viral counterparts 1. The other major advantage of non-viral vectors is the absence of limitation on the size and number of the genetic insert/s. This is particularly important from the point of view that treatment of several diseases will require the introduction of not one, but multiple genes simultaneously, for effective therapy. The simplest non-viral techniques involve the direct injection of naked DNA through the use of electrical impulses (electroporation), or bombardment with gold particles (gene gun), to force them across cellular membranes 20. However, these methods were very inefficient, localized, and severely limited in terms of diversity of applications. Subsequently, other non-viral carriers were developed using cationic lipids, polymers, ceramic based nanomaterials such as inorganic-phosphate nanoparticles, carbon- nanotubes, metal-nanorods, and silica-based nanoparticles 21–28. These materials can electrostatically complex with anionic genetic materials. These materials are designed as a nanosized assembly with the genetic material, with some residual positive charge. These modifications greatly reduced the chances of enzymatic degradation of the complexed genetic materials and are, in most cases, readily taken up by cells. The cellular uptake mechanism of such a complex is probably mediated by the electrostatic interaction of the nanocomplex with the anionic cellular membrane 29. Once inside the cell, the complex unwinds freeing the genetic material to execute its function. The efficiency of transfection could be further enhanced by the incorporation of targeting ligands like folic acid, peptide hormones, transferrin or cell/tissue- specific monoclonal antibodies 30–33. The applications of these non-viral vectors in CNS gene delivery are summarized below.
Liposomes in CNS gene delivery
Reports of CNS gene transfer using cationic liposomes began to appear in the literature from the early 90’s, when Holt et al. successfully transfected neurons in the embryonic brain of Xenopus by direct injection of the liposome-DNA complexes 34. Subsequently, numerous laboratories reported CNS gene transfer using a variety of formulations of the cationic liposomes (reported as ‘lipofectins’ in the early articles) 35. Probably the first report of successful liposome-mediated CNS gene therapy appeared in 1998 when Imoaka et al. claimed to bring about therapeutic changes in rats carrying experimental Parkinson’s disease (PD) by continuous direct injection of liposomal complex of a plasmid encoding tyrosine hydroxylase (TH) 36. In this study a significant recovery in apomorphine-induced rotational behavior of PD models was obtained by transfection of TH gene and this effect continued for up to 5 weeks after injection. Another application of CNS gene therapy soon appeared when Zou et al. alleviated neuronal injury in rats by transfecting a gene encoding neurotrophic growth factor (NGF) using DC-cholesterol based cationic liposomes 37. Recently, da Cruz et al reported an increase in the efficiency of NGF gene transfer in the CNS by conjugating the protein transferrin with the liposomes 38. Another interesting gene therapy approach against neuronal injury was proposed by Cao et al, where they transfected the anti-apoptotic gene by injecting the lipoplex in the cerebrospinal fluid, as opposed to direct brain injection reported by the other groups 39.
Gene transfer in the CNS following systemic administration of DNA-liposome complex would be much more acceptable clinically. However, this is not feasible using cationic liposomes for two reasons, (a) these liposomes are extremely unstable in biological fluids and are prone to RES capture, and (b) the presence of the blood-brain barrier (BBB) restricts the access to the brain of macromolecular vehicles like the lipoplexes circulating in the blood. To circumvent this problem, Shi et al. has synthesized neutral/anionic liposomes containing the polymer polyethylene glycol (PEG) 40. Using this liposome, it was shown that the genetic material of interest is actually encapsulated within the lipid core (as opposed to surface attachment for cationic liposomes). Furthermore, the presence of PEG prevented the RES capture of the lipoplexes. The diffusional impedance offered by the BBB was overcome by using conjugated antibody fragments to the transferrin receptor present on the BBB and therefore enabling the lipoplex to efficiently transverse the BBB. Using a brain-specific promoter, the authors have also demonstrated normalization of straital TH and reversal of motor impairment in an experimental Parkinsonian rat model, following systemic injection of the lipoplex 41.
Polymeric nanoparticles in CNS gene delivery
Polyethyleneimine (PEI) is a cationic polymer which is ideally suited for brain specific gene delivery, owing to its ability to form stable complexes with genetic materials, escape from endosomes due to associated proton-sponge effect, as well as transfect non-dividing, post-mitotic cells (e.g. neurons) 27. Efficient in vivo gene delivery in the brain using PEI was first demonstrated by Abdallah et al., using plasmid DNA complexed with branched PEI (25 kD) at a electrostatic charge close to neutral 42. Transgene expression was found more than 3 months post-injection in cortical neurons. Later, this method was modified by Goula et al., who have used the linear 22 kD polymer to condense DNA 43. The complex was found to be highly stable and diffusible in biological fluids, and intraventricular injection resulted in diffusion of the complex from the injection site to the entire brain ventricular space. Later, Lemkine et al. were able to demonstrate that using this technique it is possible to transfect neuronal stem cells in the subventricular zone (SVZ) of the brain, and also modify the nature of these cells using functional genes 44. It has been was reported that in patients with Parkinson’s disease (PD) have a decrease in Fibroblast Growth Factor-2 (FGF-2) in the dopaminergic neurons of the substantia nigra prior to cell degeneration45. We investigated the effect of PEI-mediated delivery of the mutant plasmid for fibroblast growth factor receptor-1 (FGFR-1) on dopamine-producing cells of the SN. Following PEI-mediated delivery of a dominant negative FGFR1 mutant 46, 47, we observed a decrease in number of TH positive neurons in SN on the FGFR1(TK-) injected side, as opposed to control side 48. This efficiency of PEI has been found to be similar to that of helper-free HSV-1 amplicon as a FGFR1 (TK-) DNA delivery vector 48.
Ceramic nanoparticles in CNS gene delivery
A number of cationic ceramic nanoparticles have been tested as gene delivery vectors. Prominent among them are cationic silica nanoparticles, calcium phosphate nanoparticles and carbon nanotubes 22, 23, 26. While these nanoparticles have shown significant gene transfer efficiency in vitro, no reports exist concerning their use in in vivo CNS gene delivery. Our group have tested calcium phosphate nanoparticles condensed with the reporter plasmid for β-galactosidase by directly injecting them into the substantia nigra (SN) region of the rat brain 49. One month post-injection, moderate gene expression could be observed in the midbrain area, thus highlighting their potential in CNS gene delivery with improved formulations.
ORMOSIL nanoparticles in CNS gene delivery
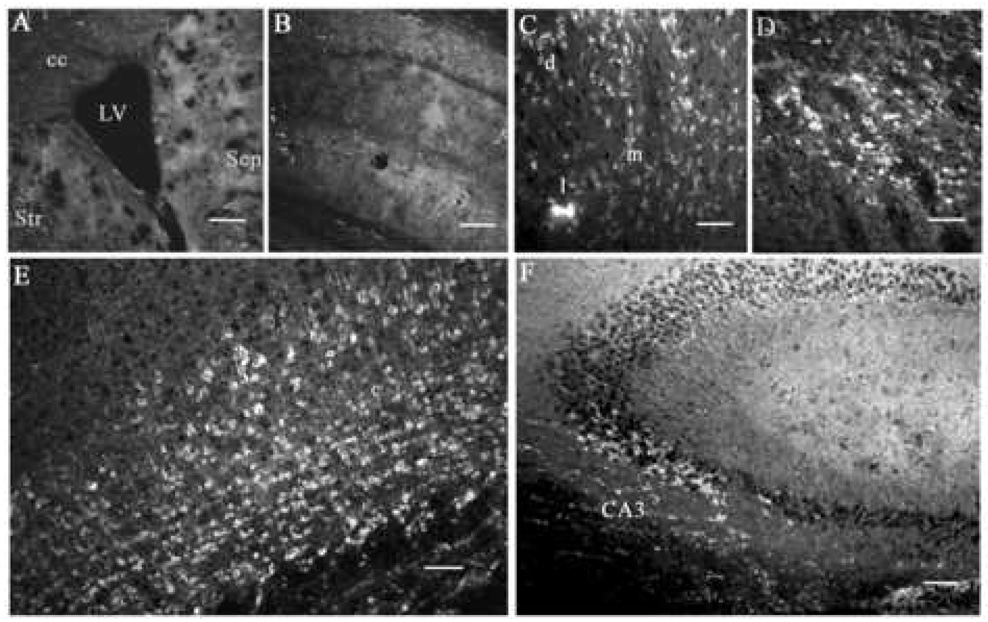
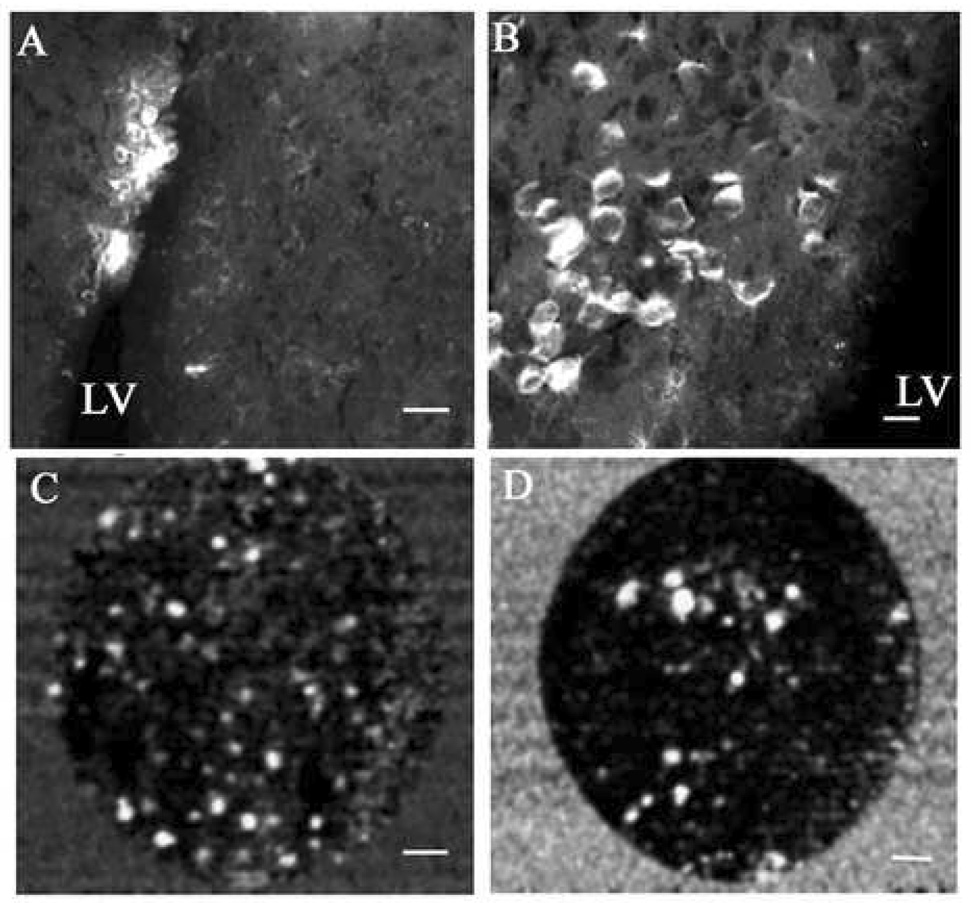
In our laboratory, we have designed amino terminated organically modified silica (ORMOSIL) nanoparticles that are able to condense, protect and deliver plasmid DNA within cells. The anionic plasmid DNA is electrostatically complexed with the cationic amino groups on the ORMOSIL nanoparticles, and the average diameter of the complexes is 30 nm. Once inside the cells, the plasmid is thought to be released following the destabilization of the plasmid-nanoparticle complex in the acidic environment of intracellular compartments such as endosomes and lysosomes. In addition, this nanoparticle also can be fluorescently labeled in order to optically track their path outside and inside cells 24. After successfully confirming their gene transfer ability in vitro, we explored the possibility of in vivo gene transfer in the CNS, using direct, stereotaxic injection of the ORMOSIL-DNA nanoparticle complexes (nanoplexes) into the mouse brain. Initially, a simple reporter gene expression using the plasmid encoding the enhanced green fluorescent protein (pEGFP) was tested in the substantia nigra per compacta (SNc) region of the brain, an area richly populated with neuronal cells and of considerable interest for its role in Parkinson’s disease and other psychiatric disorders 50, 51. Our initial optical detection of the expressed enhanced green fluorescent protein (EGFP) was disappointing as a result of the obscuring of the fluorescent signal against a backdrop of strong tissue autofluorescence, particularly due to the use of tissue-fixatives. However, subsequent immunofluorescent detection using specific monoclonal antibodies to EGFP resulted in observation of robust EGFP expression of neuron-shaped cells in the SNc 52. This strong in vivo transfection using the nanoplexes was reconfirmed by the successful double-immunostaining using anti-EGFP and anti-TH antibodies, given the fact that most of the SNc neurons are dopaminergic and can be specifically detected using antibodies to TH 53. The immunofluorescent detection of this in vivo transfection is depicted in Figure 1. The efficiency of this nanoplex mediated in vivo transfection was found to be equal to or greater than that obtained using herpes simplex virus (HSV) reported earlier in our laboratory 48, 54. Furthermore, this topical gene expression was observed in multiple areas of the brain (septum, cortex, hippocampus, etc.) surrounding the lateral ventricle (LV) following intraventricular injection of the nanoplex, as shown in Figure 2. It may be noted that no marked toxic effects of the nanoparticles could be observed in the mouse even after two months of stereotaxic injection.
Figure 1.
ORMOSIL nanoparticle mediated EGFP transfection in the SNc in which brain sections were immunostained to detect expression of EGFP. (A) DNA-free ORMOSIL injection showing no substantial immunostaining for EGFP. (B–E) Injection of ORMOSIL-pEGFP-N2 complex into SNc. (B) Multiple cells with typical dopaminergic neuron morphology are immunostained positive for EGFP. (C) No immunostaining is observed without primary anti-EGFP Ab. (D) EGFP immunostaining of neuron-shaped cells. (E) Transfected EGFP (green) is expressed in TH-immunopositive (red) dopaminergic neuron. Scale bar (A–C) = 75 µm, (D) = 25 µm, (C) = 10 µm.
Figure 2.
Expression of EGFP in multiple brain areas after injection of ORMOSIL-pEGFP-N2 into the brain LV. (A) EGFP expression in the region surrounding the LV. Str, striatum; Sep, septum; cc, corpus callosum. (B) The hippocampal region adjacent to the ventricle. No cellular staining is detected in either region by using anti-EGFP immunocytochemistry. (C–F) ORMOSIL-pEGFP-N2 particles. EGFP expression in neuron-shaped cells in dorsal lateral (d), lateral (l), and medial (m) septal nuclei (C); in the adjacent striatal region of the brain (D); cingulate and motor cortex (E); and pyramidal neurons of the cornu Ammonis 3 (CA3) hippocampal region (F). Scale bar (A, B) = 200 µm, (C–E) = 30 µm, (F) = 60 µm.
One major bottleneck of non-viral gene transfer is that often the efficiency of the in vivo gene expression falls short of the ‘threshold’ efficiency required in order to cause functional or therapeutic changes. Therefore, after confirming the robust expression of reporter genes using our nanoplexes in the CNS, we went on to investigate whether by transfecting functional genes using the nanoparticles we can alter the biology of brain cells. The SVZ of the LV is home to a number of neural stem/progenitor cells (NSPCs), which are capable of differentiating to various cell types like neurons, astrocytes and oligodendrocytes, and therefore is an exciting target from the point of view of CNS gene therapy 55, 56. Using the pEGFP, we found that not only the nanoplexes could successfully transfect in vivo the NSPCs within the SVZ, but also the transfected cells retained their viability, as visualized by a novel fiber-optic based in vivo live animal imaging system and depicted in Figure 3. In addition, we used the nanoplex mediated transfection of a functional gene, nucleus-targeted fibroblast growth factor receptor type-1 [FGFR-1 (SP-/NLS)] 57. Stereotaxic injection of these nanoplexes into the SVZ resulted in significant modulation of the biology of the NSPCs, as evidenced by the marked reduction in the incorporation of bromodeoxiuridine (BrdU), as shown in Figure 4. This demonstration of in vivo manipulation of the NSPCs can potentially open up multiple CNS gene therapeutic approaches including stimulation of neurogenesis, neutralization of growth inhibitory molecules, repair of neuronal injury, etc.
Figure 3.
Transfection of ORMOSIL-pEGFP-N2 complex into the LV cells of the SVZ. (A and B) Seven days postmortem EGFP immunostaining is shown at low magnification (A) and at higher magnification (B) of the positive region to visualize transfected cells. (C and D) In vivo imaging of EGFP fluorescence in cells in the LV. Ten days after transfection, mice were subjected to the second stereotaxic surgery, and a miniature fiber-optic Cell-viZio probe was inserted into the anterior dorsal region (C) or the posterior region (D) of the LV 15 µm from the medial ventricular wall. Dynamic sequences were recorded, and selected frames are shown. Scale bar (A) = 20 µm, (B) = 10 µm, (C,D) = µ22 m.
Figure 4.
Modulation of cell proliferation by using ORMOSIL transfection of nonmembrane nucleus-targeted FGFR1(SP-NLS). ORMOSIL (A, C, and E) or ORMOSIL-pEGFR1(SP-NLS) (B, D, and F) were injected into the anterior region of the brain LV followed seven days later with injection with BrdU. Sagittal brain sections were immunostained for FGFR1 or DNA that had incorporated BrdU. (A, B) Immunostaining of SVZ using antibodies to FGFR1. (C, D) BrdU immunostaining of cell nuclei in SVZ and adjacent tissue. (E, F) BrdU immunostaining of cells in the rostral migratory stream close to SVZ. Scale bar (A–F) = 22 µm.
More recently, we have further extended the application of these nanoparticles in CNS gene delivery by creating a mouse model of Huntington’s disease. After receiving stereotaxic injection of the plasmid encoding hemagglutinin-tagged polypeptides with extended (127)-glutamine repeats (Q127), complexed with the nanoparticles, mice have displayed behavioral abnormalities akin to Huntington’s disease58. Immunocytochemistry revealed the presence of the characteristic nuclear and cytoplasmic Q127 aggregates in numerous striatal, septal and neocortical cells. These mice showed a marked increase in the reactive glial fibrillary acidic protein + astrocytes in striatum, septum and brain cortex, indicative of neurodegenerative changes and accompanied by motor impairments. None of the above characteristics were observed in mice receiving injection of the nanoparticles, complexed with the corresponding plasmid with normal (20)-glutamine repeats (Q20). This demonstrates a direct application of these nanoparticles in gene transfer, leading to the modulation of a gene-based disease.
Conclusions
A number of novel nanosized inorganic-based materials (spheres, rods, tubes etc) are being investigated as non-viral carriers for gene therapy. Nanoparticle-mediated gene delivery will allow the combination of gene therapy with traditional approaches, like chemotherapy. Testing of potential therapeutic genes by generating transgenic mice that carry a disease-causing gene and cross-breeding those mice with mice that carry the corrective gene is a slow procedure, and may be complicated if the genetic changes cause developmental and/or reproductive abnormalities. The ORMOSIL nanoparticle-mediated transfection can be used to model of genetic brain diseases in mice as well as in other species, and provide an effective research tool for in vivo testing of single as well as multi-gene therapies.
Acknowledgements
This study was supported by grants from the NCI CA119397, NIH CA104492, the John R. Oishei Foundation, the Chemistry and Life Sciences Division of the Air Force Office of Scientific Research and the University at Buffalo Interdisciplinary Research and Creative Activities Fund. Support from the Center of Excellence in Bioinformatics and Life Sciences is also acknowledged.
Abbreviation List
- Q127
(127)-glutamine repeats
- Q20
(20)-glutamine repeats
- BBB
blood-brain barrier
- BrdU
bromodeoxiuridine
- CNS
central nervous system
- CA3
cornu Ammonis 3
- EGFP
enhanced green fluorescent protein
- HSV
herpes-simplex virus
- LV
lateral ventricle
- LV
lateral ventricle
- NSPCs
neural stem/progenitor cells
- NGF
neurotrophic growth factor
- FGFR-1 (SP-/NLS)
nucleus-targeted fibroblast growth factor receptor type-1
- ORMOSIL
organically modified silica nanoparticles
- pEGFP-N2
plasmid encoding the enhanced green fluorescent protein
- PEG
polyethylene glycol
- PEI
polyethyleneimine
- SNc
substantia nigra per compacta
- SVZ
subventricular zone
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None of the authors has any conflict of interest with information presented in this review.
References
- 1.Verma IM, Somia N. Gene therapy -- promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 2.Prasad PN. Introduction to Biophotonics. New York: Wiley-Interscience; 2004. [Google Scholar]
- 3.Austin CP. The impact of the completed human genome sequence on the development of novel therapeutics for human disease. Annu Rev Med. 2004;55:1–13. doi: 10.1146/annurev.med.55.091902.104426. [DOI] [PubMed] [Google Scholar]
- 4.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10(8):789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 5.Cullen R, Marshall S. Genetic research and genetic information: a health information professional's perspective on the benefits and risks. Health Info Libr J. 2006;23(4):275–282. doi: 10.1111/j.1471-1842.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- 6.Davis SS. Biomedical applications of nanotechnology--implications for drug targeting and gene therapy. Trends Biotechnol. 1997;15(6):217–224. doi: 10.1016/S0167-7799(97)01036-6. [DOI] [PubMed] [Google Scholar]
- 7.Medina-Kauwe LK, Xie J, Hamm-Alvarez S. Intracellular trafficking of nonviral vectors. Gene Ther. 2005;12(24):1734–1751. doi: 10.1038/sj.gt.3302592. [DOI] [PubMed] [Google Scholar]
- 8.Rolland A. Nuclear gene delivery: the Trojan horse approach. Expert Opin Drug Deliv. 2006;3(1):1–10. doi: 10.1517/17425247.3.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Bowers W, Federoff H. Gene therapy for neurological diseases. In: Templeton NS, editor. Gene and Cell therapy: Therapeutic mechanisms and strategies. 2nd ed. 2004. pp. 601–627. [Google Scholar]
- 10.Karpati G, Lochmuller H, Nalbantoglu J, Durham H. The principles of gene therapy for the nervous system. Trends Neurosci. 1996;19(2):49–54. doi: 10.1016/0166-2236(96)89620-2. [DOI] [PubMed] [Google Scholar]
- 11.Anderson WF. Human gene therapy. Nature. 1998;392(6679 Suppl):25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- 12.Uren AG, Kool J, Berns A, van Lohuizen M. Retroviral insertional mutagenesis: past, present and future. Oncogene. 2005;24(52):7656–7672. doi: 10.1038/sj.onc.1209043. [DOI] [PubMed] [Google Scholar]
- 13.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 14.Davis S. Biomedical applications of nanotechnology. Trends Biotechnol. 1997;15:217–224. doi: 10.1016/S0167-7799(97)01036-6. [DOI] [PubMed] [Google Scholar]
- 15.Check E. Cancer risk prompts US to curb gene therapy. Nature. 2003;422:6927. doi: 10.1038/422007a. [DOI] [PubMed] [Google Scholar]
- 16.Marwick C. FDA halts gene therapy trials after leukaemia case in France. British Medical Journal. 2003;326(7382):181–181. doi: 10.1136/bmj.326.7382.181/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999:517–518. doi: 10.1038/43977. [DOI] [PubMed] [Google Scholar]
- 18.Deglo N, Hantraye P. Viral vectors as tools to model and treat neurodegenerative disorders. J Gene Med. 2005;7(5):530–539. doi: 10.1002/jgm.707. [DOI] [PubMed] [Google Scholar]
- 19.Mancheno-Corvo P, Martin-Duque P. Viral gene therapy. Clin Transl Oncol. 2006;8(12):858–867. doi: 10.1007/s12094-006-0149-y. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa M, Huang L. Nonviral vectors in the new millennium: delivery barriers in gene transfer. Hum Gene Ther. 2001;12(8):861–870. doi: 10.1089/104303401750195836. [DOI] [PubMed] [Google Scholar]
- 21.Bhakta G, Mitra S, Maitra A. DNA encapsulated magnesium and manganous phosphate nanoparticles: potential non-viral vectors for gene delivery. Biomaterials. 2005;26(14):2157–2163. doi: 10.1016/j.biomaterials.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 22.Kam NW, Liu Z, Dai H. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J Am Chem Soc. 2005;127(36):12492–12493. doi: 10.1021/ja053962k. [DOI] [PubMed] [Google Scholar]
- 23.Roy I, Mitra S, Maitra A, Mozumdar S. Calcium phosphate nanoparticles as novel non-viral vectors for targeted gene delivery. Int J Pharm. 2003;250(1):25–33. doi: 10.1016/s0378-5173(02)00452-0. [DOI] [PubMed] [Google Scholar]
- 24.Roy I, Ohulchanskyy TY, Bharali DJ, Pudavar HE, Mistretta RA, Kaur N, Prasad PN. Optical tracking of organically modified silica nanoparticles as DNA carriers: a nonviral, nanomedicine approach for gene delivery. Proc Natl Acad Sci U S A. 2005;102(2):279–284. doi: 10.1073/pnas.0408039101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salem AK, Searson PC, Leong KW. Multifunctional nanorods for gene delivery. Nat Mater. 2003;2(10):668–671. doi: 10.1038/nmat974. [DOI] [PubMed] [Google Scholar]
- 26.Kneuer C, Sameti M, Bakowsky U, Schiestel T, Schirra H, Schmidt H, Lehr CM. A nonviral DNA delivery system based on surface modified silica-nanoparticles can efficiently transfect cells in vitro. Bioconjug Chem. 2000;11(6):926–932. doi: 10.1021/bc0000637. [DOI] [PubMed] [Google Scholar]
- 27.Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mounkes LC, Zhong W, Cipres-Palacin G, Heath TD, Debs RJ. Proteoglycans mediate cationic liposome-DNA complex-based gene delivery in vitro and in vivo. J Biol Chem. 1998;273(40):26164–26170. doi: 10.1074/jbc.273.40.26164. [DOI] [PubMed] [Google Scholar]
- 30.Foster BJ, Kern JA. HER2-targeted gene transfer. Hum Gene Ther. 1997;8(6):719–727. doi: 10.1089/hum.1997.8.6-719. [DOI] [PubMed] [Google Scholar]
- 31.Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, Cheresh DA. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296(5577):2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- 32.Lee RJ, Huang L. Folate-targeted, anionic liposome-entrapped polylysine-condensed DNA for tumor cell-specific gene transfer. J Biol Chem. 1996;271(14):8481–8487. doi: 10.1074/jbc.271.14.8481. [DOI] [PubMed] [Google Scholar]
- 33.Pirollo KF, Zon G, Rait A, Zhou Q, Yu W, Hogrefe R, Chang EH. Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery. Hum Gene Ther. 2006;17(1):117–124. doi: 10.1089/hum.2006.17.117. [DOI] [PubMed] [Google Scholar]
- 34.Holt CE, Garlick N, Cornel E. Lipofection of cDNAs in the embryonic vertebrate central nervous system. Neuron. 1990;4(2):203–214. doi: 10.1016/0896-6273(90)90095-w. [DOI] [PubMed] [Google Scholar]
- 35.Ono T, Fujino Y, Tsuchiya T, Tsuda M. Plasmid DNAs directly injected into mouse brain with lipofectin can be incorporated and expressed by brain cells. Neurosci Lett. 1990;117(3):259–263. doi: 10.1016/0304-3940(90)90673-w. [DOI] [PubMed] [Google Scholar]
- 36.Imaoka T, Date I, Ohmoto T, Nagatsu T. Significant behavioral recovery in Parkinson's disease model by direct intracerebral gene transfer using continuous injection of a plasmid DNA-liposome complex. Hum Gene Ther. 1998;9(7):1093–1102. doi: 10.1089/hum.1998.9.7-1093. [DOI] [PubMed] [Google Scholar]
- 37.Zou LL, Huang L, Hayes RL, Black C, Qiu YH, Perez-Polo JR, Le W, Clifton GL, Yang K. Liposome-mediated NGF gene transfection following neuronal injury: potential therapeutic applications. Gene Ther. 1999;6(6):994–1005. doi: 10.1038/sj.gt.3300936. [DOI] [PubMed] [Google Scholar]
- 38.da Cruz MT, Cardoso AL, Almeida LP, Simoes S, de Lima MC. Tf-lipoplex-mediated NGF gene transfer to the CNS: neuronal protection and recovery in an excitotoxic model of brain injury. Gene Ther. 2005;12(16):1242–1252. doi: 10.1038/sj.gt.3302516. [DOI] [PubMed] [Google Scholar]
- 39.Cao YJ, Shibata T, Rainov NG. Liposome-mediated transfer of the bcl-2 gene results in neuroprotection after in vivo transient focal cerebral ischemia in an animal model. Gene Ther. 2002;9(6):415–419. doi: 10.1038/sj.gt.3301676. [DOI] [PubMed] [Google Scholar]
- 40.Shi N, Pardridge WM. Noninvasive gene targeting to the brain. Proc Natl Acad Sci U S A. 2000;97(13):7567–7572. doi: 10.1073/pnas.130187497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Schlachetzki F, Zhang YF, Boado RJ, Pardridge WM. Normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism with intravenous nonviral gene therapy and a brain-specific promoter. Hum Gene Ther. 2004;15(4):339–350. doi: 10.1089/104303404322959498. [DOI] [PubMed] [Google Scholar]
- 42.Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum Gene Ther. 1996;7(16):1947–1954. doi: 10.1089/hum.1996.7.16-1947. [DOI] [PubMed] [Google Scholar]
- 43.Goula D, Remy JS, Erbacher P, Wasowicz M, Levi G, Abdallah B, Demeneix BA. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. Gene Ther. 1998;5(5):712–717. doi: 10.1038/sj.gt.3300635. [DOI] [PubMed] [Google Scholar]
- 44.Lemkine GF, Mantero S, Migne C, Raji A, Goula D, Normandie P, Levi G, Demeneix BA. Preferential transfection of adult mouse neural stem cells and their immediate progeny in vivo with polyethylenimine. Mol Cell Neurosci. 2002;19(2):165–174. doi: 10.1006/mcne.2001.1084. [DOI] [PubMed] [Google Scholar]
- 45.Tooyama I, Kawamata T, Walker D, Yamada T, Hanai K, Kimura H, Iwane M, Igarashi K, McGeer EG, McGeer PL. Loss of basic fibroblast growth factor in substantia nigra neurons in Parkinson's disease. Neurology. 1993;43(2):372–376. doi: 10.1212/wnl.43.2.372. [DOI] [PubMed] [Google Scholar]
- 46.Peng H, Moffett J, Myers J, Fang X, Stachowiak EK, Maher P, Kratz E, et al. Novel nuclear signaling pathway mediates activation of fibroblast growth factor-2 gene by type 1 and type 2 angiotensin II receptors. Mol Biol Cell. 2001;12(2):449–462. doi: 10.1091/mbc.12.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng H, Myers J, Fang X, Stachowiak EK, Maher PA, Martins GG, Popescu G, et al. Integrative nuclear FGFR1 signaling (INFS) pathway mediates activation of the tyrosine hydroxylase gene by angiotensin II, depolarization and protein kinase C. J Neurochem. 2002;81(3):506–524. doi: 10.1046/j.1471-4159.2002.00833.x. [DOI] [PubMed] [Google Scholar]
- 48.Corso TD, Torres G, Goulah C, Roy I, Gambino AS, Nayda J, Buckley T, et al. Transfection of tyrosine kinase deleted FGF receptor-1 into rat brain substantia nigra reduces the number of tyrosine hydroxylase expressing neurons and decreases concentration levels of striatal dopamine. Brain Res Mol Brain Res. 2005;139(2):361–366. doi: 10.1016/j.molbrainres.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 49.Corso TD, Torres G, Goulah C, Roy I, Gambino AS, Nayda J, Buckley T, et al. Assessment of Viral and Non-Viral Gene Transfer into Adult Rat Brains Using HSV-1, Calcium Phosphate, and PEI-Based Methods. Folia Morphol (Warsz) 2005;64(3):130–144. [PubMed] [Google Scholar]
- 50.Fisher LJ, Gage FH. Radical directions in Parkinson's disease. Nat Med. 1995;1(3):201–203. doi: 10.1038/nm0395-201. [DOI] [PubMed] [Google Scholar]
- 51.Wong AH, Van Tol HH. Schizophrenia: from phenomenology to neurobiology. Neurosci Biobehav Rev. 2003;27(3):269–306. doi: 10.1016/s0149-7634(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 52.Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, Bergey EJ, et al. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci U S A. 2005;102(32):11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frielingsdorf H, Schwarz K, Brundin P, Mohapel P. No evidence for new dopaminergic neurons in the adult mammalian substantia nigra. Proc Natl Acad Sci U S A. 2004;101(27):10177–10182. doi: 10.1073/pnas.0401229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tabbaa S, Goulah C, Tran RK, Lis A, Korody R, Stachowski B, Horowitz JM, et al. Gene transfer into the central nervous system using herpes simplex virus-1 vectors. Folia Morphol (Warsz) 2000;59(4):221–232. [PubMed] [Google Scholar]
- 55.Goldman J. Peripheral blood stem cells for allografting. Blood. 1995;85(6):1413–1415. [PubMed] [Google Scholar]
- 56.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90(5):2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stachowiak MK, Fang X, Myers JM, Dunham SM, Berezney R, Maher PA, Stachowiak EK. Integrative nuclear FGFR1 signaling (INFS) as a part of a universal 'feed-forward-and-gate' signaling module that controls cell growth and differentiation. J Cell Biochem. 2003;90(4):662–691. doi: 10.1002/jcb.10606. [DOI] [PubMed] [Google Scholar]
- 58.Klejbor I, Stachowiak EK, Bharali DJ, Roy I, Spodnik I, Morys J, Bergey FJ, et al. ORMOSIL nanoparticles as a non-viral gene delivery vector for modeling polyglutamine induced brain pathology. Journal of Neuroscience Methods. 2007;165(2):230–243. doi: 10.1016/j.jneumeth.2007.06.011. [DOI] [PubMed] [Google Scholar]