Abstract
Total parenteral nutrition (TPN), with the complete removal of enteral nutrition, results in marked changes in intestinal intraepithelial lymphocyte (IEL) function and phenotype. Previous work shows that TPN results in a loss of intestinal epithelial cell (EC)-derived interleukin-7 (IL-7), and this loss may play an important role in development of such TPN-associated IEL changes. To further understand this relation, we generated a transgenic mouse (IL-7vill), which over-expresses IL-7 specifically in intestinal ECs. We hypothesized that this localized over-expression would attenuate many of the observed TPN-associated IEL changes. Our study showed that TPN administration led to significant changes in IEL phenotype, including a marked decline in the CD8αβ+, CD4+, and αβ-TCR+ populations. IEL basal proliferation decreased 1.7-fold compared to Controls. TPN administration in wild-type mice resulted in several changes in IEL-derived cytokine expression. IL-7vill mice given TPN, however, maintained IEL proliferation, as well as sustaining normal IEL numbers and phenotype. We conclude that specific intestinal IL-7 over-expression significantly attenuated many IEL changes in IEL phenotype and function after TPN administration. These findings suggest a mechanism by which TPN results in observed IEL changes.
Keywords: Transgenic, Intraepithelial lymphocyte, Interleukin-7, Mouse, epithelial cells
INTRODUCTION
Total parenteral nutrition (TPN) is vital for patients unable to take enteral feedings. However, TPN is associated with several major complications, which include the loss of immune reactivity and an increase in infectious complications and generalized septicemia 1–4. Although the underlying mechanism for this is unknown, it may be that a loss of TPN-associated intestinal immune functionality leads to bacterial translocation and subsequent sepsis 5, 6. Our previous studies have also shown that TPN administration resulted in marked changes in intestinal intraepithelial lymphocyte (IEL) phenotype and function; and these changes may appear to have an important role in how administration of TPN is associated with a loss of immune function 5, 7. Although it is unknown what mechanism(s) lead to these TPN-associated IEL changes, deraignment of mucosal immune function has also been observed by other investigators 8, 9. IEL are a distinct population of T lymphocytes, which are located at the basolateral side of EC in the epithelial layer of the intestine. This cell population, although not fully defined, appears to play a critical role in modulation of mucosal immune responses, as well as in the control of epithelial cell growth and function 10, 11. Studies from our laboratory have found that TPN administration results in a marked decline in intestinal epithelial cell (EC)-derived IL-7 expression 7, 12, 13. Recently, our laboratory has shown that systemic IL-7 administration into healthy wild-type mice significantly altered IEL phenotype and function; including an increase in αβ-T-cell receptor (TCR)+ and CD8αβ+ IEL, as well the formation of a mature (CD44+) IEL population 14. Additionally, systemic IL-7 resulted in significant changes in IEL derived cytokine expression. Furthermore, we also showed a close physical cross-communication between EC-derived IL-7 and IEL 15, 16. Finally, our laboratory, and other researchers, have shown that systemic administration of IL-7 may prevent some of the observed TPN-associated changes in the mucosal immune system 16, 17
IL-7 has been found to be essential for homeostatic proliferation of naïve peripheral T cells 18. IL-7 is produced by both thymic and intestinal epithelial cells (EC) 19-21; and in turn, IL-7 receptors (IL-7R) have been detected on the surface of thymocytes and IEL 16, 20. Recently, a study from our group found that IL-7R was identified on CD4+, CD8αα+, as well as the CD8αβ+ IEL subtypes. IL-7R were also identified in both αβ-TCR+ IEL and γδ-TCR+ IEL sub-populations 16. Several studies have indicated that EC may play an important role in mucosal immune responses by helping to regulate neighboring IEL 16, 22. It has been shown that IL-7 knock-out and IL-7 receptor knock-out mice show distinct declines in absolute numbers of thymocytes and IEL; and for the latter, a reduction in αβ-TCR+ IEL and a complete absence of γδ-TCR+ IEL have been noted 23.
A major problem with previous studies examining IL-7 function is that the cytokine has either been delivered by systemic administration16, 17, systemic IL-7 over-expression, or conversely, the systemic blockade of the IL-7/IL-7R pathway (using anti-IL-7R antibody) 15, 24. Such studies may result in a confounding of the systemic versus local actions of IL-7 on the mucosal immune system. Unfortunately, this prevents one from gaining a detailed analysis of how the EC and IEL populations might be modulated by local IL-7 production. In the single study that examined the local expression of IL-7, Laky et al, created a restitution of IL-7 epithelial cell expression in IL-7 knock-out mice, and demonstrated that this was sufficient to regain development of αβ-TCR+ IEL and γδ-TCR+ IEL populations 25. To better understand the role that intestinal EC-derived IL-7 has in directing IEL lineage and function, we established an intestinal EC-specific over-expressing IL-7 transgenic mouse model. We hypothesized the specific over-expression of IL-7 by intestinal epithelial cells would attenuate the effect of TPN on the IEL.
METHODS
Animals
Studies reported here conformed to the guidelines for the care and use of laboratory animals established by the University Committee on Use and Care of Animals at the University of Michigan, and protocols were approved by that committee. Mice were maintained in temperature, humidity and light-controlled conditions. During administration of intravenous solutions, mice were housed in metabolic cages.
Creation of IL-7 Transgenic Mice
1. Development of a P12.4KVill-IL-7 transgene
We utilized a previously characterized 12.4 Kb murine villin promoter/enhancer fragment to drive the expression of an IL-7 cDNA exclusively in the epithelium of the large and small intestine 26. The full-length sequence of mouse IL-7 cDNA (generously donated by Dr. Jay Bream, NIH/NIAMS) was cloned behind the 12.4Kb villin promoter/enhancer. The final clone used for transgenesis (P12.4KVILL-IL-7) was confirmed by restriction digestion and sequence analysis (across the complete IL-7 and into the villin sequences).
2. Generation of IL-7vill Transgenic Mice
The transgene, containing villin regulatory sequences linked to IL-7, was prepared by digestion of P12.4KVILL-IL-7 with PmeI. After purification, transgene DNA was injected into the pronuclei of fertilized ova (C57BL/6J × SJL/J) by the University of Michigan Transgenic Animal Core. Founders were identified by PCR amplification of tail DNA with primers specific for the transgene (Forward primer: ACAGGCACTAAGGGAGCCAATG; Reverse primer: TCCTGTCAT TTTGTC CAATTCA). Founders were then mated to C57BL/6J mice for the creation of F1 hybrids. Transgenic lines were maintained by crossing transgenic mice with C57BL/6J mice; transgenic animals were identified by PCR analysis of their tail DNA.
Total Parenteral Nutrition Model
Administration and composition of TPN was performed as previously described 5. IL-7vill and wild-type (C57BL/6J) were infused with a crystalloid solution (dextrose 5% in 0.45 normal saline with 20 mEq KCl/liter) at 4 ml/day. After 24 hrs, mice were randomized into four groups (n=6 each group). Control Groups: Wildtype and IL-7vill mice received the same intravenous crystalloid solution at 7 ml/24 hrs, in addition to standard laboratory mouse chow and water ad libitum. TPN Groups: Wildtype and IL-7vill TPN mice received an intravenous TPN solution at 7 ml/24 hrs. The TPN solution contained a balanced mixture of amino acids, lipids and dextrose in addition to electrolytes, trace elements and vitamins 5. Caloric delivery was similar in each group, as based on estimates of caloric intake measurements in the control group, and from previous investigators 5. All animals were sacrificed at 7 days using CO2.
IEL Isolation and Purification
IEL isolation from small intestine
Small bowel IEL and ECs were isolated using a Percoll gradient as previously described 5. Following mucosal cell isolation, supernatants contained an enriched IEL population, but also contained ECs. In some cases, the IEL was further purified by filtering through a glass wool column, and by using magnetic beads conjugated with antibody to CD3 (BioMag SelectaPure Anti-Mouse Antibody Particles, Polyscience Inc, Warrington, PA). Cells bound to beads were considered purified IEL; remaining EC were in the supernatant. Flow cytometry confirmed purity of sorted cells, which was greater than 99%.
Reverse Transcriptase Polymerase Chain Reaction
1. Isolation of total RNA
A guanidinium isothiocyanate–chloroform extraction method was used. Total RNA from isolated EC or IEL was extracted using Trizol reagent (Life Technologies, Inc., MD, USA) according to the manufacturer’s directions.
2. Reverse Transcriptase (RT) and Polymerase Chain Reaction (PCR)
poly-A tailed mRNA was reversed transcribed into complementary DNA by adding total cellular RNA to the following reaction mixture: PCR Nucleotide Mix (Invitrogen, CA), M-MLV Reverse Transcriptase (Invitrogen), Oligo(dT)12–18 Primer (Invitrogen), and RNAse Inhibitor (40 U/μl, Roche Diagnostics, Germany). DEPC-treated H2O was added to yield the appropriate final concentration. For quantification of the IL-7 mRNA (including transgenic IL-7 mRNA) expression study, IL-7 primers were designed by using sequence from the encodable sequence of mouse IL-7cDNA. Forward primer: TGCCCGAATAATGAACCAA, Right primer: CAACCTCTCCAAGTTATGAACCA GTAG16. For transgenic IL-7 gene detection, we used a unique primer set which stretched across a portion of the villin promoter and IL-7 sequence; the forward primer was designed from Exon-1: CAACTTCCTAAGATCTCCCAGGTG, the reverse primer was designed from IL-7 cDNA: GTTCCTGTCATTTTGTCCAATTC. The PCR products were run out on a 2% agarose gel. Quantification of cDNA product was completed using a Kodak 1D image quantification software (Kodak Co, NY), and target PCR products were compared by normalizing each sample to the production of β-actin.
Immunoblot analysis for EC-derived IL-7 Expression
Briefly, isolated EC were homogenized on ice in lysis buffer 27. Protein determination was performed by using a Micro BCA™ Protein Assay Kit (PIERCE, Rockford, IL). Approximately 40 μg of total protein in loading buffer was loaded per lane in a SDS-polyacrylamide-gel (13%), and separated using electrophoresis. Proteins were then transferred to a PVDF membrane (Bio-Rad Laboratories, CA). The membrane was blocked with blocking solution (Zymed Laboratories Inc, CA), and probed with goat anti-mouse biotinylated-IL-7 antibody (R&D Systems, Inc. MN) (0.15 μg/ml in blocking solution) for 1 hour. Bound antibodies were exposed to a Strepavidin-HRP conjugate (1:10000, Zymed Laboratories Inc, CA), detected on X-ray film. Blots were then stripped and re-probed with monoclonal mouse anti β-actin antibody (1:8000 in blocking solution; Sigma, St Louis, MO) to confirm equal loading of protein. The peroxidase-conjugated second antibody was goat anti-mouse IgG (1:8000 in blocking solution; Invitrogen). Quantification of results was performed using Kodak 1D image quantification software (Kodak Co, NY). Thus, results of immunoblots are expressed as the relative expression of IL-7 to beta actin expression.
Flow Cytometric Analysis
IEL Phenotype analysis
IEL phenotype was studied using fluorescent-labeled antibody staining detected with flow cytometry. Monoclonal antibodies (BD PharMingen, San Diego, CA) consisted of anti-CD4, CD8α, CD8β, αβ-TCR, γδ-TCR, CD44 and CD69 antibodies were used to examine phenotype expression of IEL subsets. Flow cytometry was performed using standard techniques 28. Acquisition and analysis were performed on a FACSCalibur (Becton-Dickinson, Mountain View, CA) using CellQuest software (Becton-Dickinson). The IEL population was gated based on forward and side scatter characteristics. Quantification of each subpopulation was based on its percentage of the gated IEL population.
IEL cell cycle analysis
The modified protocol of Geiselhart et al 19 was used. Briefly, separated IEL were labeled with FITC-CD3. Surfaced-labeled or unlabeled IEL were permeabilized by resuspension in saponin buffer (0.1% BSA, 0.01 M HEPES, 0.1% saponin in PBS) at a concentration of 0.5 × 106 cells/ml for 10 minutes, followed by centrifugation at 1500 rpm for 5 minutes at 4°C. The supernatant was decanted and the pellet was resuspended in a saponin buffer containing 200 μg/ml of propidium iodide (Sigma Aldrich, St. Louis, MO) and 50 μg/ml of RNase (Roche Diagnostics Corporation, Indianapolis, IN) followed by incubation for 15 min at 4°C. Labeled cells were analyzed by using a FACSCalibur flow cytometer and CellQuest (Becton-Dickinson) software for cell cycle analysis.
Data analysis
Data are expressed as mean ± standard deviation. Results were analyzed using ANOVA, and a Bonferroni post hoc analysis was used to detect differences between groups. Statistical significance was defined as P<0.05.
RESULTS
General descriptions of specific intestinal EC over-expression of IL-7 in IL-7vill mice
It has been shown that IL-7 is produced by intestinal EC and plays an important role in mucosal immune responses by regulation of growth and function of IEL 20. More recently, a study from our laboratory showed that TPN results in a loss of intestinal epithelial cell (EC)-derived IL-7, and this may play an important role in TPN-associated IEL changes 16. To further address this, we generated a transgenic mouse (IL-7vill), using a 12.4kb villin promoter fragment drives IL-7 cDNA specific expression in intestinal ECs from the pylorus, throughout the small bowel, and colon 26. Six initial founders were identified by Genotype PCR analysis, and bred on a background of C57Bl/6 mice.
Intestinal EC-derived IL-7 mRNA expression was significantly higher in transgenic mice than wild-type mice (3-fold increase compared to wild-type by PCR). Western blot analysis showed significantly increased EC derived IL-7 expression when compared with wild-type mice (P<0.05; Figure 1). Thus, EC-derived IL-7 expression increased nearly 3-fold when compared with wild-type mice. This correlated with the significant increase in IEL numbers, and significantly altered phenotype, maturation as well as function of the IEL.
Figure 1.
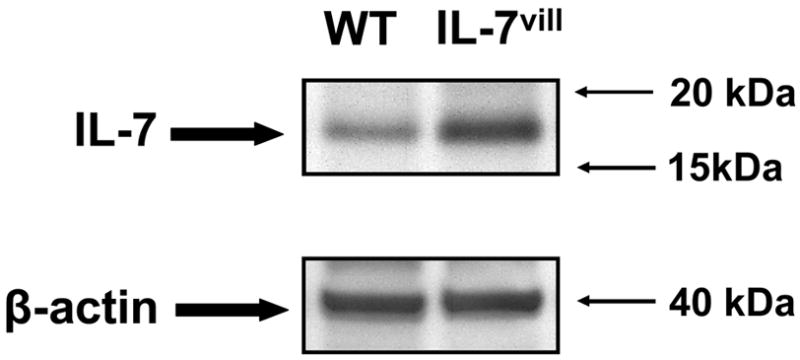
Western immunoblot analysis of intestinal EC derived IL-7 expression. Composite representative immunoblot of EC-derived IL-7 expression in wild-type and IL-7vill mouse, β-actin was used to normalize loading. EC-derived IL-7 expression was significantly higher in transgenic mice than wild-type mice.
Changes in IEL number
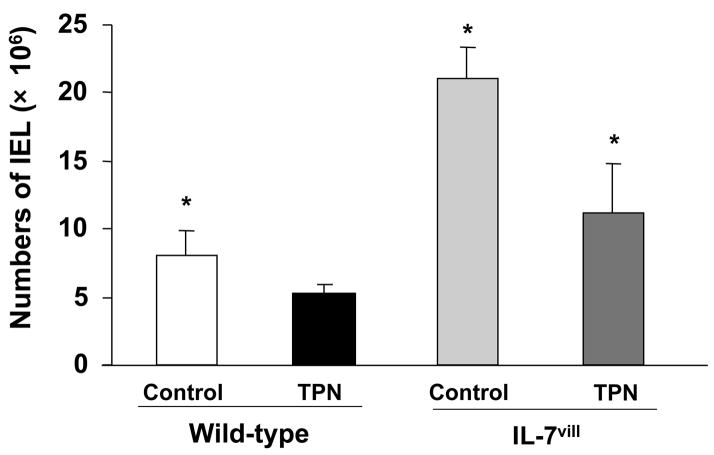
TPN administration led to a significant decrease in IEL numbers when compared with controls. Numbers of IEL significantly decreased (by 35%) after TPN administration. IEL numbers in IL-7vill mice, however, were significantly higher compared to wild-type mice. IEL numbers in transgenic mice increased 3-fold when compared with wild-type mice. When IL-7vill mice were given TPN, IEL numbers were much higher that wild-type mice who were receiving TPN, and numbers of IEL were similar to enterally fed mice (P<0.05; Figure 2). This represents a 2-fold increase in the number of harvested IEL in IL-7vill TPN mice compared with wild-type TPN mice.
Figure 2.
Changes in IEL numbers. TPN administration led to a significant decrease in IEL numbers when compared with control. The number of IEL significantly increased in IL-7vill mice when compared with wild-type control mice. IL-7vill TPN mice had much higher IEL number than wild-type TPN mice (n =6 each group), *P<0.05 compared to TPN group.
Changes in IEL phenotype
Several changes were identified in IEL surface phenotypic markers with TPN administration. Both the CD4+,CD8− and CD4+,CD8+ IEL sub-populations decreased significantly compared with controls; CD4+,CD8− IEL decreased 87% and CD4+,CD8+ IEL decreased 80%, respectively. In contrast, IL-7vill mice have much larger CD4+,CD8− and CD4+,CD8+ IEL subtype populations; CD4+,CD8− IEL were increased 5-fold, and CD4+,CD8+ IEL increased 3.1-fold, respectively compared with wild-type mice. IL-7vill mice given TPN had higher levels of CD4+,CD8− and CD4+,CD8+ IEL sub-populations compared with wild-type TPN mice (P<0.05; Figure 3). The levels of these populations were not significantly different than wild-type control mice.
Figure 3.
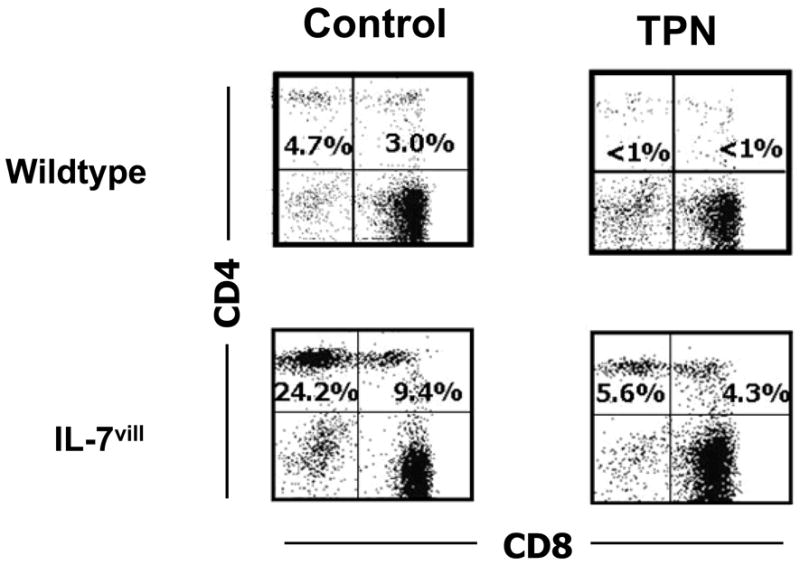
Representative flow cytometry results of gated IEL populations. Cell populations are expressed as the percentage of gated cells with CD4 and CD8 markers based on isotype-matched control antibodies. Data are expressed as the percent of total gated population of lymphocytes. TPN resulted in the loss of single CD4+CD8− and double CD4+CD8+ IEL. In contrast, IL-7vill mice receiving TPN had higher levels of CD4+CD8− and CD4+CD8+ IEL than wild type TPN mice (n =6 each group); *P<0.05 compared to TPN group.
Loss of the CD8αβ+ IEL was also observed after TPN administration. The percentage of CD8αβ+IEL decreased nearly 6-fold when compared with wild-type control mice (P<0.05). In contrast, IL-7vill mice have a much larger population of CD8αβ+IEL. The CD8αβ+ IEL population increased 5.6-fold when compared with wild-type mice. Additionally, IL-7 transgenic mice receiving TPN have a much higher CD8αβ+ population compared to wild-type TPN mice (P<0.05; Figure 4).
Figure 4.
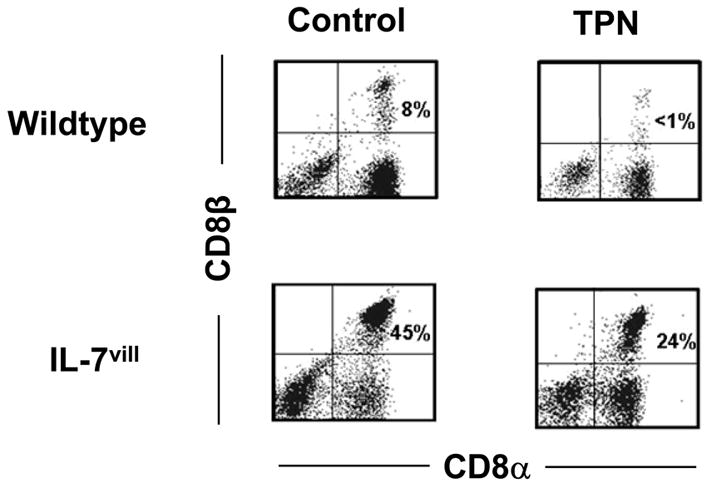
Representative flow cytometry results. Cell populations are expressed as the percentage of gated cells with CD8α and CD8β markers based on isotype-matched control antibodies. Data are expressed as the percent of total gated population of lymphocytes. TPN administration led to a loss of the CD8αβ+IEL subset. IL-7vill mice have a much higher CD8αβ+ population with TPN administration than wild-type TPN mice (n =6 each group); P<0.05 compared to TPN group.
T-cell receptor (TCR) sub-populations were also studied. The αβ-TCR+ IEL decreased after TPN administration; this represented a declined from 33% of all gated IEL to 22% after TPN administration. The relative percentage of αβ-TCR+ IEL was found to be significantly higher in IL-7vill mice when compared with wild-type mice (Figure 5); representing a 2.4–fold increase compared to wild-type mice. Further, IL-7 transgenic mice showed a much higher percent of αβ-TCR+ IEL subtype with TPN administration compared to wild-type TPN mice (P<0.05; Figure 5).
Figure 5.
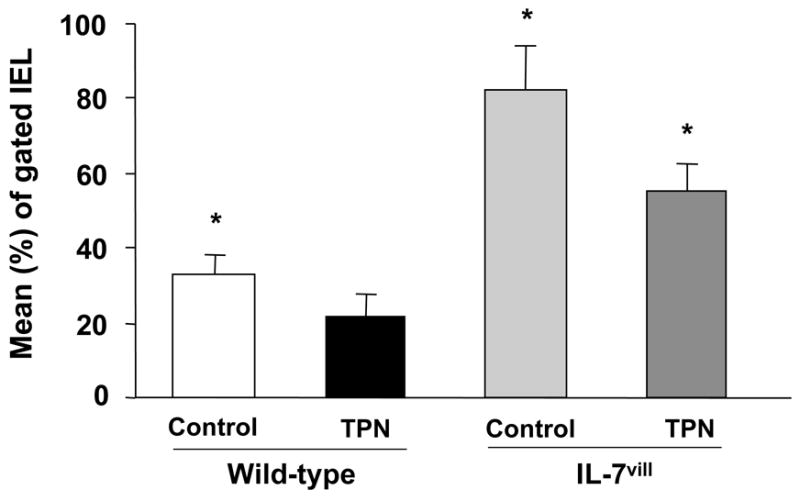
Graphic distribution of phenotypes of IEL data. Staining was performed with FITC-conjugated αβ-TCR antibody. Results from flow cytometric analyses are expressed as the mean (±SD) percent of gated IEL. TPN administration led to a significant decrease in the αβ-TCR+ IEL subset. IL-7vill mice have a higher percentage of αβ-TCR+ IEL withTPN administration compared to wild-type TPN mice (n =6 each group); *P<0.05 compared to TPN group.
Changes in IEL maturation and activation
CD44 was used as a marker of lymphocyte maturation, whereby maturity is manifested by increased expression. The CD44+ IEL subset declined in the TPN group compared to control animals (Figure 6). The CD8+,CD44+ subset in the TPN group showed a 65% decline (P <0.05), and the CD4+,CD44+ subset showed a 55% decline with TPN administration (Figure 6; P <0.05). In contrast, IL-7vill mice have larger CD4+,CD44+ and CD8+,CD44+ IEL populations compared to wild-type control mice, with an increase by 4.7-fold and 1.4 fold over wild-type mice for the CD4+,CD44+ and CD8+,CD44+ IEL subtypes, respectively. IL-7 transgenic mice with TPN administration have much higher CD4+,CD44+ and CD8+,CD44+ sub-populations compared to wild-type TPN mice (P<0.05; Figure 6); such that there was no significant difference between this group and the control, enterally fed wild-type group.
Figure 6.
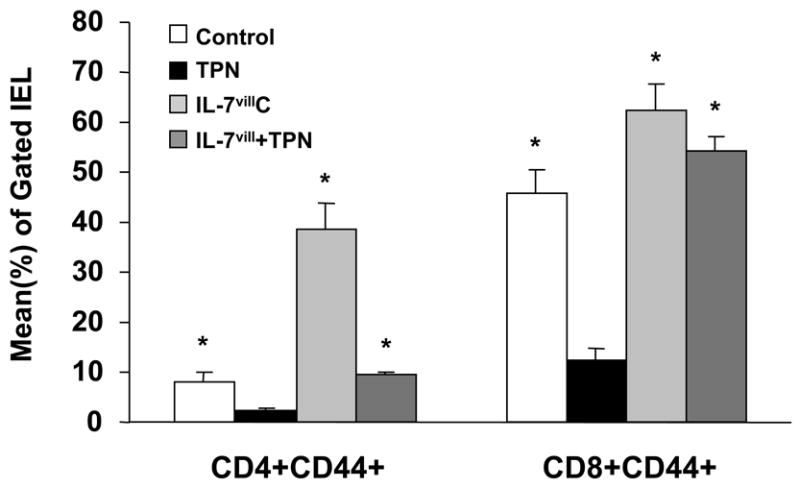
Distribution of the CD44+ IEL. CD44 was used as a marker of IEL maturation. TPN resulted in a significant decrease in the CD4+CD44+ and CD8+CD44+ IEL subtypes; P<0.05 compared to control mice. IL-7vill mice maintained higher CD4+CD44+ and CD8+CD44+ IEL populations with TPN administration than wild-type TPN mice (n=6 each group), *P<0.05 compared to TPN group.
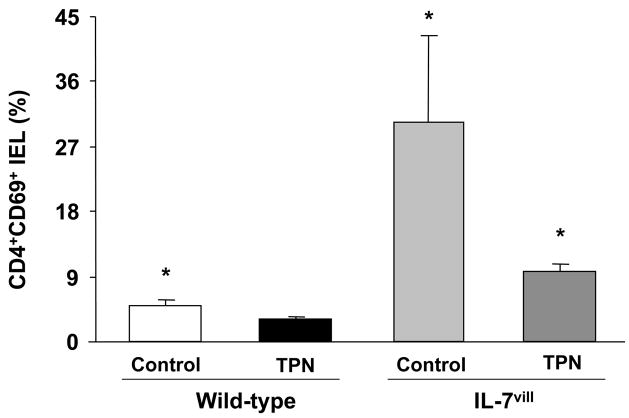
The presence of CD69 was used as a marker of activated T-cells 29; with higher expression of CD69 seen in more activated T-cells. The CD4+,CD69+ IEL sub-population was found to be significantly (P<0.05) lower after TPN administration, and was noted to be significantly increased in IL-7vill mice compared to wild-type mice (P<0.05; Figure 7). The CD4+,CD69+ IEL subtype increased nearly 6-fold in IL-7vill mice compared with wild-type mice. This level of CD4+,CD69+ IEL in TPN transgenics was much larger than wild-type TPN mice, and statistically (P<0.05) similar to wild-type enterally fed control mice (Figure 7).
Figure 7.
Distribution of CD69+ IEL. CD8+,CD69+ double positive populations were found to be significantly (P<0.05) lower in mice receiving TPN compared to enterally fed controls. The CD4+,CD69+ IEL increased nearly 8-fold in IL-7vill mice; whereas, CD8+,CD69+ IEL actually decreased when compared with wild-type mice (57.1±9.9% vs. 76.8±7.8%). IL-7 transgenic mice with TPN administration have higher CD4+,CD69+ population compared to wild-type TPN mice; *P<0.05 compared to TPN group.
IEL Functionality
Changes in IEL proliferation
Because of the profound effect of TPN on IEL phenotypic changes and decline in IEL numbers, IEL proliferation rates were studied. TPN administration resulted in a decrease in IEL proliferation; with rates declining 1.7-fold compared to control mice (P<0.05; Figure 8). IL-7 transgenic mice have higher IEL proliferation levels compared to wild-type mice, and IL-7 transgenic mice receiving TPN have higher proliferation levels compared to wild-type TPN mice (P<0.05; Figure 8). IEL proliferation levels increased 82% in IL-7vill mice with TPN administration when compared with wild-type TPN mice; such that these levels of proliferation were not significantly different from the wild-type enterally fed group.
Figure 8.
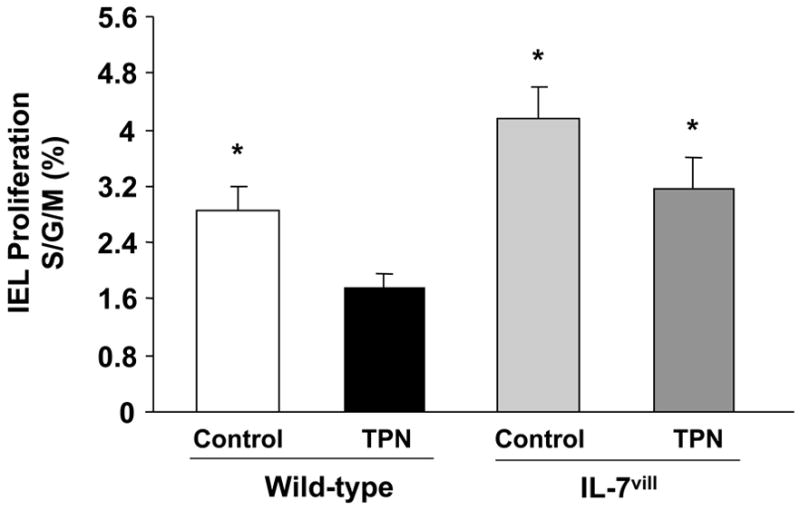
IEL proliferation. IEL were surface-labeled with fluorochrome-conjugated antibody to CD3, and then treated with propidium iodide for detection of cell cycle status using flow cytometric analysis. TPN administration significantly decreased IEL proliferation when compared with controls, P<0.05. IL-7 transgenic mice receiving TPN have much higher proliferation levels compared to wild-type TPN mice; *P<0.05 compared to the TPN group.
Changes in IEL cytokines expression
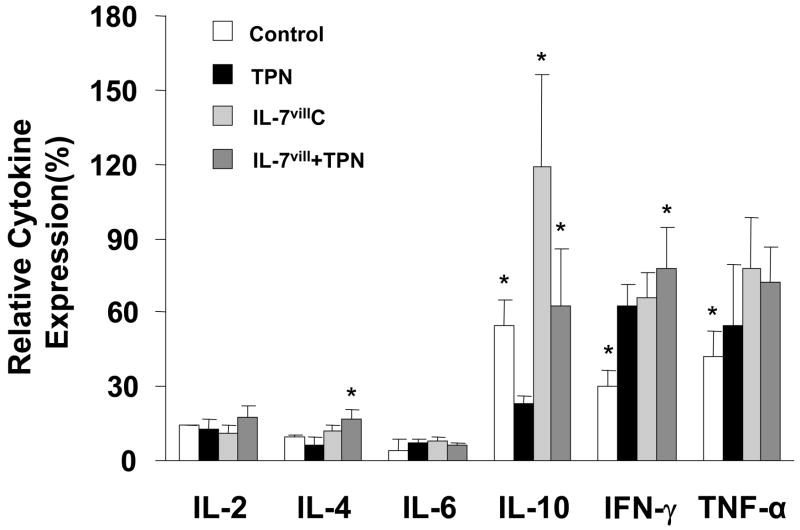
A panel of cytokines was selected to represent the activities responsible for both T-cell activation as well those that could contribute to a down-regulation of T-cell activity 30. Results are shown in Figure 9. IEL cytokine expressions were significantly altered after TPN administration. IL-10 expression declined, whereas IFN-γ, TNF-α showed a significant increase in expression (109% and 30%, respectively; both P<0.05 compared to controls), when compared with the control group. IEL derived IL-10 expression decreased 58% after TPN administration. The remaining cytokines which were examined, including IL-2, IL-4 and IL-6, showed little to no change after TPN administration. Transgenic IL-7vill mice expressed higher levels of IL-10, IFN-γand TNF-α, but not IL-2, IL-4 and IL-6. IL-7vill mice with TPN administration retained significantly higher IL-10 expression level compared to wild-type TPN mice (P<0.05); however, other cytokines, including IL-2, IL-6, and TNF-α, showed no significant difference between wild-type TPN group and IL-7vill TPN group.
Figure 9.
Changes in IEL-derived cytokine mRNA expression. TPN administration significantly changed IEL cytokine expression. IEL derived IL-10, IFN-γ and TNF-α expressions were significantly changed after TPN administration. Transgenic IL-7 mice maintained high levels of IL-10 compared to wild-type TPN mice; however, IL-7vill mice continued to express IFN-γ and TNF-a at levels similar to that of wild-type TPN mice. No significant changes were seen in IL-2, IL-4 and IL-6. Values (mean±SD) are expressed as the ratio of target cytokine vs. expression of β-actin; *P<0.05 compared to the TPN group.
DISCUSSION
In this study, we found that intestinal EC-specific over-expression of IL-7 using IL-7vill mice resulted in a significant expansion of the IEL population and affected IEL phenotype as well as function. IEL functional changes included a significant increase in IEL proliferation and alterations in IEL cytokine expressions. IL-7vill mice given TPN manifested significantly fewer of the TPN-associated IEL alternations compared with wild-type mice given TPN. Of these differences, IL-7vill TPN mice had sustained populations of CD4+, and αβ-TCR+ IEL compared to wild-type TPN mice; and the percent of these populations were similar to the IEL sub-population levels of wild-type control mice. IL-7vill mice receiving TPN also had a much higher CD8αβ+ IEL subpopulation than wild-type TPN mice. Additionally, IL-7vill mice undergoing TPN administration had much higher levels of IEL proliferation level compared to wild-type TPN mice.
Interactions between IEL and intestinal EC are thought to be crucial for maintaining intestinal mucosal immunity. Previous studies by our group have shown that IEL play an important role in the maintenance of the gut barrier function and in the support of intestinal EC growth 6, 7, 11, 13, 31. Several studies have indicated that EC may have an important role in mucosal immune responses by helping to regulate IEL phenotype and function 20, 22. EC have been shown to act as antigen presenting cells for both CD4+ and CD8+ IEL 32, 33. It has also been shown that EC are known to produce a number of immunologically important cytokines, including IL-6 34; IL-7, and IL-10 20, 35. Of these cytokines, the IL-7/IL-7R dependent signaling pathway has been shown to play a crucial role in regulating the immune response in the intestinal mucosa 20, 24, 36–38. Receptors for IL-7 has been identified on CD4+, CD8αα+ and CDαβ+ IEL phenotype, as well as αβ-TCR+ and γδ-TCR+IEL sub-populations 16. Recently, a study from our laboratory showed that IL-7R blockade with anti-IL-7R antibody resulted in several phenotypic IEL changes that were quite similar to those observed in TPN mice 15. These changes include a loss of the CD8αβ, CD4+, CD44+ and CD4+ (including the CD4+,CD8+ and CD4+,CD8−) IEL sub-populations. Additionally, IL-7R blockade reduced the total number of IEL 15. Similarly, other groups have reported that IL-7 knockout and IL-7R knockout mice show distinct declines in absolute numbers of thymocytes and IEL 23. Kanai et al 39 found that anti-IL-7 antibody treatment disturbed the induction of αβ-TCR+ T-cells after athymic nude mice were implanted with fetal thymocytes. In our present study, intestinal EC specific over-expression of IL-7 resulted in a significant increase in the total number of IEL, and significant changes in IEL phenotype and increases in the level of IEL proliferation. It appears that this increase in IEL numbers was at least in part due to an IL-7 induced increase in IEL proliferation. Similar to our findings, a recent paper by Fukatsu, et al noted the importance of IL-7 in immune alterations due to TPN. These authors noted that the exogenous administration of IL-7 reversed GALT associated changes; however, IL-7 was unable to restore diminished levels of immunoglobulin A 17
Administration of TPN led to a significant decrease in the CD4+,CD8−; CD4+,CD8+; and CD8+,CD44+ IEL sub-populations 5, as well as a loss of CD8αβ+ IEL 40. In this current study, we further confirmed these IEL phenotype changes after TPN administration. The precise etiology of these changes after TPN administration remains uncertain. A previous investigation by our laboratory found that IL-7 administration prevented the development of the majority of TPN-associated IEL phenotype changes and the decline in total IEL numbers 16. Furthermore, IL-7 administration in wild-type mice also led to a significant increase in the percent of CD8αβ+ IEL, and significantly increased the absolute numbers of IEL 15. The importance of EC-derived IL-7 is also underscored by a previous study of ours which showed that there is a close physical connection between EC-derived IL-7 and the neighboring IEL 15. These studies suggest that cell-to-cell interactions between EC and IEL, via IL-7, could be an important model of communication for the maintenance and activation of the IEL. Previous attempts at trying to understand the relevance of IL-7 in this TPN model by using exogenous administration of IL-7 has many disadvantages. In particular, this approach will lead to a broad number of IL-7 mediated systemic actions 41–43. One particular disadvantage to systemic over-expression of IL-7 has been the formation of colitis 24 – a potential major obstacle to the utilization of systemic IL-7 for clinical applications. Although not formally studied in our transgenic mice, we have not observed colitis with the confined expression which predominates in the small intestine. These findings may confound the relevance of locally expressed EC-derived IL-7. Similar confounding results could well occur in a previous model, in which IL-7 over-expression is driven by an SRαpromoter, whereby IL-7 expression is seen in a wide array of systemic tissues 24.
To further investigate the role EC-derived IL-7 has in directing IEL lineage and function, we established an intestinal EC specific over-expressing IL-7 transgenic mouse model. We found that the IEL population in these transgenic mice was significantly expanded; consisting of an increase in the CD4+ and CD8αβ+, as well as αβ-TCR+ IEL subtypes. The increase in IEL subtypes is disproportionately biased toward an expansion (compared to wild-type mice) of the CD4+ IEL population; CD4+ IEL increased 9-fold, compared to CD8+ IEL which increased 4-fold. A greater expansion of the αβ-TCR+ IEL compared to the γδ-TCR+ IEL sub-population was also noted. These observations appear consistent with the fact that IL-7R is detected to a much higher level on CD4+ and αβ-TCR+ IEL compared to the CD8 and γδ-TCR+ IEL sub-populations 16.
IL-7 has been shown to enhance peripheral T-cell expression of IL-4 mRNA as well as IL-4 production 44, 45. Jiang et al 46 found that EC-derived IL-7 could enhance human peripheral T-cell IL-4 secretion, but not IFN- in a cell culture model 46. A recent study from our group showed that exogenous IL-7 administration to wild-type health mice can up-regulate IEL-derived IL-2 and IL-10 expression; however, IL-7 had no effect on the expression of IL-4 or IL-6 15. In our present study, we found that IL-7vill mice expressed higher levels of IL-10, IFN-γ, TNF-α; however, we could not find any significant change in IL-2, IL-4 and IL-6 expression between IL-7vill mice and wild-type mice. The reason for this discrepancy is not entirely clear, but may be due to differential action of IL-7 between peripheral T-cells and the IEL. Thus, it is possible that exogenous administration of IL-7 may stimulate extra-mucosal lymphoid populations; and that these extra-mucosal population changes may account for the observed increase in IEL-derived IL-2 which was not found in our transgenic mouse model. A decline in mucosally-derived IL-10 has been observed with TPN administration by other investigators 9. And, the finding of a decline in IL-10 after TPN administration may have significant physiologic relevance in that this cytokine may greatly influence epithelial barrier function 47, 48. Future studies on our IL-7vill mice will need to be done to examine how the sustained expression of IL-10 during TPN administration may affect intestinal barrier function. It is important to note that our IL-7 transgenic mice failed to reverse the observed rise in both IFN-γ and TNF-α which was noted in our TPN wild-type group. This suggests that other mechanisms aside from IL-7 must account for some of the TPN-associated cytokine changes which occur in the IEL, and further studies will need to address the mechanisms which drive these latter changes. TPN administration can impair the ability to fight systemic infections and this is due to a number of derangements in the local gut associated lymphoid tissue 49, 50. Future studies will also be needed to better understand if the sustained expression of IL-7 with TPN in our transgenic mice can prevent this increase in infections.
In conclusion, this study demonstrated that intestinal EC specific over-expression of IL-7 significantly affected IEL phenotype and function, as well as resulting in an increase IEL numbers. Such IL-7vill mice failed to manifest many of the TPN-associated alternations seen in the IEL of wild-type mice. The results of this study suggest that EC-derived IL-7 contribute to several of the alternations observed in the IEL population after TPN. This study also further confirms the close physical and functional cross-communication between EC-derived IL-7 and mucosal IEL.
Acknowledgments
This research was supported by NIH grant 2R01-AI044076-09 (to DHT), and the ASPEN Rhoads Research Foundation Maurice Shils Grant (to HY), and the University of Michigan DNA Core, University of Michigan Transgenic Core.
Footnotes
Presented in part at the Annual Congress of the American Gastroenterology Association, May 20–25, 2006, Los Angeles, CA, U.S.A.
References
- 1.Gogos CA, Kalfarentzos F. Total parenteral nutrition and immune system activity: a review. Nutrition. 1995;11(4):339–44. [see comments] [Review] [74 refs] [PubMed] [Google Scholar]
- 2.Moore F, Moore E, Jones T. TEN vs. TPN following major abdominal trauma: reduced septic morbidity. J Trauma. 1989;29:916–923. doi: 10.1097/00005373-198907000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216(2):172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudsk K, Croce M, Fabian T, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215(5):503–511. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiristioglu I, Teitelbaum DH. Alteration of the intestinal intraepithelial lymphocytes during total parenteral nutrition. Journal of Surgical Research. 1998;79(2):91–96. doi: 10.1006/jsre.1998.5408. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Kiristioglu I, Fan Y, et al. Interferon-gamma expression by intraepithelial lymphocytes results in a loss of epithelial barrier function in a mouse model of total parenteral nutrition. Ann Surg. 2002;236(2):226–34. doi: 10.1097/00000658-200208000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Fan Y, Teitelbaum DH. Intraepithelial lymphocyte-derived interferon-gamma evokes enterocyte apoptosis with parenteral nutrition in mice. Am J Physiol Gastrointest Liver Physiol. 2003;284(4):G629–37. doi: 10.1152/ajpgi.00290.2002. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Gocinski B, Henken B, Kudsk K. Effects of parenteral nutrition on gut associated lymphoid tissue. J Traum. 1995;39:44–52. doi: 10.1097/00005373-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Fukatsu K, Kudsk KA, Zarzaur BL, et al. TPN decreases IL-4 and IL-10 mRNA expression in lipopolysaccharide stimulated intestinal lamina propria cells but glutamine supplementation preserves the expression. Shock. 2001;15(4):318–22. doi: 10.1097/00024382-200115040-00012. [DOI] [PubMed] [Google Scholar]
- 10.Boismenu R, Chen Y, Havran WL. The role of intraepithelial gammadelta T cells: a gut-feeling. Microbes Infect. 1999;1(3):235–40. doi: 10.1016/s1286-4579(99)80039-2. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gammadelta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172(7):4151–8. doi: 10.4049/jimmunol.172.7.4151. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Wildhaber B, Tazuke Y, Teitelbaum DH. Harry M. Vars Research Award. Keratinocyte growth factor stimulates the recovery of epithelial structure and function in a mouse model of total parenteral nutrition. JPEN J Parenter Enteral Nutr. 2002;26(6):333–40. 340–1. doi: 10.1177/0148607102026006333. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Finaly R, Teitelbaum DH. Alteration in epithelial permeability and ion transport in a mouse model of total parenteral nutrition. Critical Care Medicine. 2003;31(4):1118–1125. doi: 10.1097/01.CCM.0000053523.73064.8A. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Spencer A, Teitelbaum DH. IL-7 administration alters the function and phenotype of intestinal intraepithelial lymphocytes in mice. Gastroenterology. 2004;126(4 S2):A271. [Google Scholar]
- 15.Yang H, Spencer AU, Teitelbaum DH. Interleukin-7 administration alters intestinal intraepithelial lymphocyte phenotype and function in vivo. Cytokine. 2005;31(6):419–28. doi: 10.1016/j.cyto.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Sun X, Haxhija EQ, Teitelbaum DH. Intestinal epithelial cell-derived interleukin-7: A mechanism for the alteration of intraepithelial lymphocytes in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G84–91. doi: 10.1152/ajpgi.00192.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukatsu K, Moriya T, Maeshima Y, et al. Exogenous interleukin 7 affects gut-associated lymphoid tissue in mice receiving total parenteral nutrition. Shock. 2005;24(6):541–6. doi: 10.1097/01.shk.0000183237.32256.78. [DOI] [PubMed] [Google Scholar]
- 18.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98(15):8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geiselhart LA, Humphries CA, Gregorio TA, et al. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. Journal of Immunology. 2001;166(5):3019–3027. doi: 10.4049/jimmunol.166.5.3019. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe M, Ueno Y, Yajima T, et al. Interleukin 7 is produced by human intestinal epithelail cells and regulates the proliferation of intestinal mucosal lymphocytes. Journal of Clinical Investigation. 1995;95:2945–2953. doi: 10.1172/JCI118002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe M, Yamazaki M, Okamoto R, et al. Therapeutic approaches to chronic intestinal inflammation by specific targeting of mucosal IL-7/IL-7R signal pathway. Curr Drug Targets Inflamm Allergy. 2003;2(2):119–23. doi: 10.2174/1568010033484269. [DOI] [PubMed] [Google Scholar]
- 22.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2(11):997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 23.Maki K, Sunaga S, Komagata Y, et al. Interleukin 7 receptor-deficient mice lack gammadelta T cells. Proc Natl Acad Sci U S A. 1996;93(14):7172–7. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe M, Ueno Y, Yajima T, et al. Interleukin 7 transgenic mice develop chronic colitis with decreased interleukin 7 protein accumulation in the colonic mucosa. J Exp Med. 1998;187(3):389–402. doi: 10.1084/jem.187.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laky K, Lefrancois L, von Freeden-Jeffry U, et al. The role of IL-7 in thymic and extrathymic development of TCR gamma delta cells. Journal of Immunoloty. 1998:161. [PubMed] [Google Scholar]
- 26.Madison BB, Dunbar L, Qiao XT, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277(36):33275–83. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 27.Witkowski J, Miller R. Increased function of P-glycoprotein in T lymphocyte subsets of aging mice. Journal of Immunology. 1993;150:1296–1306. [PubMed] [Google Scholar]
- 28.Shapiro H. Practical Flow Cytometry. 2. New York: Alan R. Liss, Inc.; 1988. [Google Scholar]
- 29.Yang H, Fan Y, Finaly R, Teitelbaum DH. Alteration of intestinal intraepithelial lymphocytes after massive small bowel resection. J Surg Res. 2003;110(1):276–86. doi: 10.1016/s0022-4804(03)00032-5. [DOI] [PubMed] [Google Scholar]
- 30.Porter BO, Malek TR. Thymic and intestinal intraepithelial T lymphocyte development are each regulated by the gammac-dependent cytokines IL-2, IL-7, and IL-15. Semin Immunol. 2000;12(5):465–74. doi: 10.1006/smim.2000.0264. [DOI] [PubMed] [Google Scholar]
- 31.Yang H, Teitelbaum DH. Novel agents in the treatment of intestinal failure: humoral factors. Gastroenterology. 2006;130(2 Suppl 1):S117–21. doi: 10.1053/j.gastro.2005.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaiserlian D, Vidal K, Revillard JP. Murine enterocytes can present soluble antigen to specific class II-restricted CD4+ T cells. Eur J Immunol. 1989;19(8):1513–6. doi: 10.1002/eji.1830190827. [DOI] [PubMed] [Google Scholar]
- 33.Mayer L, Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987;166(5):1471–83. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung HC, Eckmann L, Yang SK, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95(1):55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galliaerde V, Desvignes C, Peyron E, Kaiserlian D. Oral tolerance to haptens: intestinal epithelial cells from 2,4- dinitrochlorobenzene-fed mice inhibit hapten-specific T cell activation in vitro. Eur J Immunol. 1995;25(5):1385–90. doi: 10.1002/eji.1830250537. [DOI] [PubMed] [Google Scholar]
- 36.Laky K, Lefrancois L, Lingenheld EG, Puddington L. Enterocyte expression of interleukin 7 induces development of gamma delta T cells and Peyer’s patches. Journal of Experiment Medicine. 2000;191(9):1569–1580. doi: 10.1084/jem.191.9.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki K, Oida T, Hamada H, et al. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity. 2000;13(5):691–702. doi: 10.1016/s1074-7613(00)00068-6. [DOI] [PubMed] [Google Scholar]
- 38.Yamazaki M, Yajima T, Tanabe M, et al. Mucosal T cells expressing high levels of IL-7 receptor are potential targets for treatment of chronic colitis. J Immunol. 2003;171(3):1556–63. doi: 10.4049/jimmunol.171.3.1556. [DOI] [PubMed] [Google Scholar]
- 39.Kenai H, Matsuzaki G, Nakamura T, et al. Thymus-derived cytokine(s) including interleukin-7 induce increase of T cell receptor αβ+ CD4−CD8− T cells which are extrathymically differentiated in athymic nude mice. European Journal of Immunology. 1993;23(8):1818–25. doi: 10.1002/eji.1830230813. [DOI] [PubMed] [Google Scholar]
- 40.Kiristioglu I, Antony P, Fan Y, et al. Total parenteral nutrition-associated changes in mouse intestinal intraepithelial lymphocytes. Digestive Diseases and Sciences. 2002;47(5):1147–1157. doi: 10.1023/a:1015066813675. [DOI] [PubMed] [Google Scholar]
- 41.Dobashi H, Seki S, Habu Y, et al. Activation of mouse liver natural killer cells and NK1.1(+) T cells by bacterial superantigen-primed Kupffer cells. Hepatology. 1999;30(2):430–6. doi: 10.1002/hep.510300209. [DOI] [PubMed] [Google Scholar]
- 42.Bhatia SK, Tygrett LT, Grabstein KH, Waldschmidt TJ. The effect of in vivo IL-7 deprivation on T cell maturation. J Exp Med. 1995;181(4):1399–409. doi: 10.1084/jem.181.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kondrack RM, Harbertson J, Tan JT, et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198(12):1797–806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnard JA, Beauchamp RD, Coffey RJ, Moses HL. Regulation of intestinal epithelial cell growth by transforming growth factor type beta. Proc Natl Acad Sci U S A. 1989;86(5):1578–82. doi: 10.1073/pnas.86.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borger P, Kauffman HF, Postma DS, Vellenga E. IL-7 differentially modulates the expression of IFN-gamma and IL-4 in activated human T lymphocytes by transcriptional and post-transcriptional mechanisms. J Immunol. 1996;156(4):1333–8. [PubMed] [Google Scholar]
- 46.Jiang Y, McGee DW. Regulation of human lymphocyte IL-4 secretion by intestinal epithelial cell-derived interleukin-7 and transforming growth factor-belta. Clinical immunology and immunopathology. 1998;88(3):287–296. doi: 10.1006/clin.1998.4586. [DOI] [PubMed] [Google Scholar]
- 47.Madsen KL, Malfair D, Gray D, et al. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5(4):262–70. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Planchon SM, Martins CA, Guerrant RL, Roche JK. Regulation of intestinal epithelial barrier function by TGF-beta 1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol. 1994;153(12):5730–9. [PubMed] [Google Scholar]
- 49.Kang W, Gomez F, Lan J, et al. Parenteral nutrition impairs gut-associated lymphoid tissue and mucosal immunity by reducing lymphotoxin Beta receptor expression. Ann Surg. 2006;244(3):392–9. doi: 10.1097/01.sla.0000234797.42935.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kudsk K. Effect of route and type of nutrition on intestine-derived inflammatory responses. Am J Surg. 2003;185(1):16–21. doi: 10.1016/s0002-9610(02)01146-7. [DOI] [PubMed] [Google Scholar]